Abstract
Purpose
To evaluate the use of Bowman’s layer (BL) vertical topographic thickness maps in diagnosing keratoconus (KC).
Design
Prospective, case control, interventional case series.
Participants
42 eyes; 22 eyes of 15 normal subjects and 20 eyes of 15 KC patients.
Intervention
BL 2-dimensional 9 mm vertical topographic thickness maps were created using custom-made ultra high-resolution optical coherence tomography.
Main Outcome Measures
BL average and minimum thicknesses of the inferior half of the cornea, Bowman’s ectasia index (BEI; defined as BL minimum thickness of the inferior half of the cornea divided by BL average thickness of the superior half of the cornea multiplied by 100), BEI-Max (defined as BL minimum thickness of the inferior half of the cornea divided by BL maximum thickness of the superior half of the cornea multiplied by 100), KC patients’ Keratometric astigmatism (Ast-K) and average keratometric readings (Avg-K).
Results
In KC patients, BL vertical thickness maps disclosed localized relative inferior thinning of the BL. Inferior BL average thickness (normal=15±2, KC=12±3 μm), inferior BL minimum thickness (normal=13±2, KC=7±3 μm), BEI (normal=91±7, KC=48±14) and BEI-Max (normal=75±8; KC=40±13) all showed highly significant differences in KC compared to normal subjects (P<0.001). Receiver-operating characteristics (ROC) curve analysis showed excellent predictive accuracy for BEI and BEI-max with 100% sensitivity and specificity (area under the curve or AUC of 1) with cut-off values of 80 and 60, respectively. AUC of inferior BL average thickness and minimum thickness were 0.87 and 0.96 with sensitivity of 80% and 93%, respectively and specificity of 93% and 93%, respectively. Inferior BL average thickness, inferior BL minimum thickness, BEI and BEI-Max correlated highly to Ast-K (R=−0.72; −0.82; −0.84 and −0.82, respectively; P<0.001) and to Avg-K (R=−0.62; P<0.001, R=−0.59; P=0.001, R=−0.60; P<0.001 and R=−0.59, P=0.001, respectively).
Conclusions
BL vertical topographic thickness maps of KC patients disclose characteristic localized relative inferior thinning. Inferior BL average thickness, inferior BL minimum thickness, BEI and BEI-max are qualitative and quantitative indices for the diagnosis of KC that accurately correlate with the severity of KC. In our pilot study, BEI and BEI-max showed excellent accuracy, sensitivity and specificity in the diagnosis of KC.
Introduction
Bowman’s layer (BL) is an acellular condensation of the anterior stroma of the cornea lying between the epithelial basement membrane and anterior cellular stroma. It is formed of collagen fibrils that are randomly interwoven to form a dense felt-like sheet.2 Light and electron microscopy studies have shown that in keratoconus (KC), BL undergoes disintegration that leads to irregular thinning, fragmentation and then breaks within the layer.3–8 Those structural changes are noted when the stroma is only minimally affected suggesting that BL changes are possibly early pathological changes in the disease process.4, 9 Ocular pathologists have long used those signs for the in-vitro diagnosis of KC in the pathology laboratory under light microscopy.3 Nevertheless, those signs are not useful for clinicians owing to the simple fact that clinicians do not have the capability to visualize this layer in vivo.
The in vivo study of BL structural characteristics and thickness is essential to understand the role it plays in KC and to evaluate its use in the diagnosis of the disease. Nevertheless, lack of enough resolution in current imaging techniques has limited that to in-vitro studies. With the introduction of new imaging techniques, namely spectral-domain optical coherence tomography (SD-OCT), it has become possible to visualize the finest layers of the cornea down to the resolution of a few microns. This technology has allowed for the in vivo visualization of structural changes that occur in KC corneas, for better understanding of the disease and perhaps, for better techniques to diagnose it. The technical issues inherent to SD-OCT have limited its ability to map BL out to the peripheral cornea, due to distortions of the images and loss of resolution in the periphery.10 Until now, those limitations have restricted the study of BL to the central cornea limiting the usefulness of SD-OCT in diagnosing diseases that typically start in the periphery such as KC.11
In our pilot study, we used custom-made ultra high resolution SD-OCT (UHR-OCT) with a resolution of 3 μm and adopted an imaging technique that allowed us to image and map BL out to the periphery of the cornea. We are able for the first time in the literature to demonstrate and quantify the in vivo structural changes of BL in KC. We report BL indices that are highly sensitive and specific in the diagnosis of KC. Moreover, these indices have highly significant correlations with the severity of KC suggesting that they are quantitative indices as well and able to accurately describe the severity of the disease.
Methods
Study Population
Our study included 42 eyes, 22 eyes of 15 normal subjects and 20 eyes of 15 KC patients. Written informed consent, approved by University of Miami Institutional Review Board (IRB), was obtained from all patients. Keratoconus was diagnosed based on clinical examination with slit lamp biomicroscopy, characteristic inferior steepening on placido based topography (TMS-3; Tomey, Erlangen, Germany) and posterior corneal elevation on Scheimpflug imaging (Pentacam; Oculus, Wetzlar, Germany). Eyes with corneal scars or status post-corneal or refractive surgery were excluded from the study.
Instrumentation
In our study, all subjects were imaged using custom-built prototype high-speed ultra-high resolution spectral domain optical coherence tomography (UHR-OCT). Details of the UHR-OCT device were described in our previous reports.12, 13 Briefly, the light source used was a three-module superluminescent diode (SLD, Broadlighter, T840-HP, Superlumdiodes Ltd, Moscow, Russia) with a center wavelength of 840 nm and a full width at half maximum bandwidth of 100 nm. The A-line (depth scan) rate of the OCT system was set to be 24 kHz. The calibrated axial resolution of the system was 3 μm in tissue (the refractive index is ~1.39).14
Image Acquisition and Processing
In order to create 9 mm 2-dimensional (2D) vertical topographic thickness maps of the BL, vertical images of the superior, central and inferior one-thirds of the cornea were captured then combined to create a 9 mm composite vertical image of the cornea. Details of the imaging and image analysis techniques were described in previous reports.15–17 Briefly, each subject was asked to look at a central fixation target to capture a vertical 2D image of central cornea centered on the corneal vertex. The presence of a visible specular reflection confirmed that the OCT scanning probe was perpendicular on the cornea. Subjects were then asked to look up and then down at calibrated fixation targets to capture vertical 2D images of the lower and upper regions of the cornea, respectively. Custom-made software was then used to extract the central 3 mm region of the images and combine them to a vertical 9 mm composite image of the cornea. The custom made software semi-automatically delineated BL and created a 2D topographic thickness map.
Validation and repeatability studies of our imaging and image analysis techniques to image and calculate the thickness of BL up to the corneal periphery were described in previous reports.15–17 In order to further validate the imaging technique, a validation and repeatability experiment was carried out. In our experiment, 8 eyes of 4 normal subjects received corneal marking 3 mm superior and 3 mm inferior to visual axis using a slit lamp and a marking pen (Devon surgical marker, Tyco Healthcare, Mansfield, Massachusetts, USA). We then imaged those eyes with the UHR-OCT. The marked spots were visible on the OCT acquisition monitor as hyper-reflective spots on the surface of the cornea. Subjects were instructed to look up and their up-gaze position was adjusted so that the specular reflection of the OCT image coincided with the inferior hyper-reflective marking point on the cornea denoting that the OCT image was centered on the point 3 mm inferior to the corneal vertex. Subjects were given a pinpoint red dot sticker to mark where they were fixing on a fixation card attached to the UHR-OCT machine. The same procedure was repeated for the superior fixation point. That experiment was repeated for the 4 subjects. There was excellent repeatability as the 4 subjects confirmed that they were looking at same fixation points when the corresponding mark on the cornea was aligned with the specular reflections of the OCT image. Those fixation targets were used for all other subjects in the study.
Bowman’s layer diagnostic indices
In order to quantify the differences between the topographic thickness maps of BL of KC and normal subjects, we formulated different indices and tested them statistically to determine which best differentiated between the diseased and normal eyes and correlated with the severity of the disease.
Statistical Analysis
Statistical analyses with SPSS software version 20.0 (SPSS, Chicago, IL, USA) were performed to calculate descriptive statistics for all eyes. One eye per patient was included in the analysis and if both eyes had measurements available, one was randomly selected. Means of all indices were compared between normal and KC subjects with two sample t-tests. The sensitivity and specificity of BL and epithelial indices in differentiating between KC and normal subjects were determined by generating a receiver operating characteristic curve (ROC). In order to determine if the indices that successfully differentiated between KC and normal eyes would be descriptive of the severity of KC, linear regression analysis of the indices and disease severity described by average keratometry (Avg-K) and astigmatic keratometry (Ast-K) obtained from corneal topography were computed. Two sided p-values less than 0.05 were considered statistically significant. Values are presented as means ± standard deviation.
Results
Our study included 42 eyes: 22 eyes of 15 normal subjects and 20 eyes of 15 KC patients. Table 1 summarizes the different characteristics of the two groups.
Table 1.
Characteristics of the control and keratoconus groups:
| Control group * | Keratoconus group * | P value | ||
|---|---|---|---|---|
| Age | 33±5 years | 37±10 years | 0.19 | |
| Gender | Female | 8 | 8 | 1.00 |
| Male | 7 | 7 | 1.00 | |
| Mean best spectacle corrected log MAR visual acuity | 0 (20/20) | 0.17±0.12 (approximately 20/30) | .001 | |
| Thinnest corneal thickness | 526±25 μm | 445±36 μm | .001 | |
| Average keratometry | 43.2±1.6 diopters | 48.5±7.3 diopters | .011 | |
| Astigmatic keratometry | 0.8±0.2 diopters | 5.2±3.0 diopters | .001 | |
One randomly selected eye per patient was included in the analysis. Values are presented as means ± standard deviation.
log MAR: Logarithm of the Minimum Angle of Resolution.
Two-dimensional vertical topographic thickness maps of the BL were created successfully for all subjects. BL maps measured 9 mm and were centered on the corneal apex. (Figure 1) In the control group, the total average thickness of the BL as well as the average BL thickness measured on the superior and the inferior halves of the normal corneas were all 15 μm. This showed that in normal subjects, there was no difference in the BL thickness between the superior and inferior halves of the cornea. In the KC group, the BL topographic thickness maps showed a different pattern than normal subjects. It was evident from the topographic maps that there was a characteristic localized thinning in the inferior cornea of KC patients that did not exist in the normal group. (Figure 2) In the KC group, the average BL thickness of the inferior halves of the KC corneas was significantly less than that of the average thickness measured on their superior halves (12±3 and 15±2 μm, respectively; P<0.001).
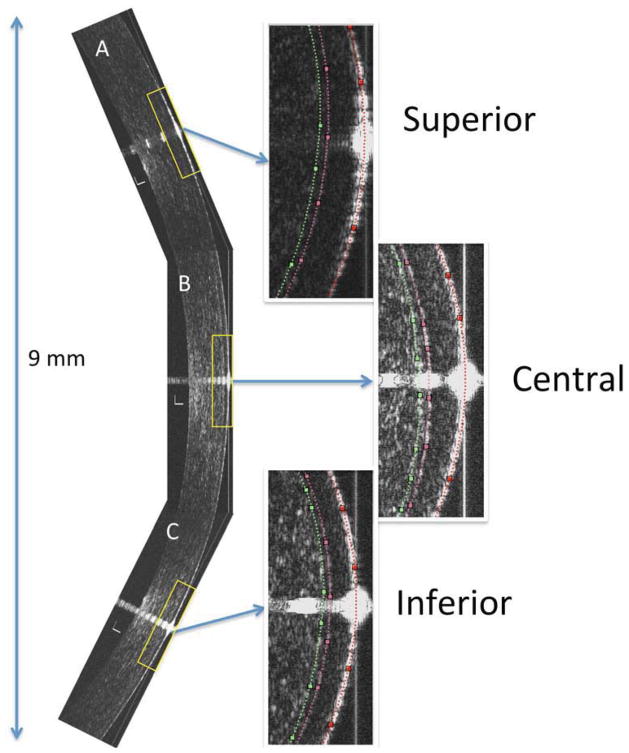
Figure 1.
9 mm vertical 2-dimensional ultra-high resolution optical coherence tomography (UHR-OCT) composite image of the cornea of a keratoconus patient: Note that the composite image consists of three images, A (superior), B (central) and C (inferior) images of the respective parts of the cornea. Specular reflection is present through the center of each image denoting that UHR-OCT scanning probe is perpendicular on the area of the cornea under study. The 3 presets from each part of the UHR-OCT image show a magnified image of the anterior part of the cornea. In the presets, doted lines represent the demarcation lines for the anterior surface of the epithelium (red), interface between the epithelium and Bowman’s layer (pink) and the interface between the Bowman’s layer and the anterior stroma (green). Bars are 100 μm.
Figure 2.
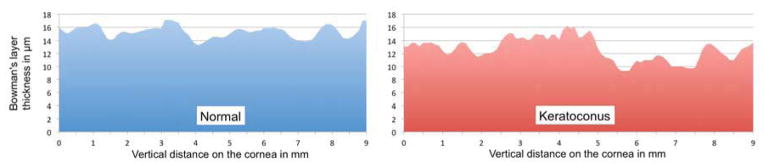
Topographic thickness maps of the Bowman’s layer (BL) of a control subject (blue) and a keratoconus patient (red; same patient as in figure 1). The BL thickness map of the keratoconus patient discloses an inferior localized thinning of the layer, whereas the normal subject map discloses a more uniform thickness without that inferior localized thinning. In the normal subject, BL average thickness on the inferior half of the cornea, BL minimum thickness on the inferior half of the cornea, Bowman’s ectasia index (BEI) and Bowman’s ectasia index-maximum (BEI-Max) are 15 μm, 14 μm, 90 and 81, respectively, whereas in the keratoconus patient, those are 12 μm, 9 μm, 68 and 53, respectively.
In order to quantify this specific thinning pattern, different diagnostic indices were computed and tested to evaluate their utility as qualitative and quantitative indices for the diagnosis of KC. It was evident that the average total thickness of the BL was not a good descriptor of the localized pattern of thinning of the BL. That was highlighted by the fact that the average thickness of the BL in the KC patients was not significantly different than that of the normal group (13±2 and 15±1 μm, respectively; P=0.056). We then examined different indices to better describe the specific localized pattern of thinning of the BL. Those indices included the average thickness of the BL of the inferior half of the cornea and minimum thickness of the BL of the inferior half of the cornea. We also computed an index that we named the Bowman’s ectasia index (BEI) and defined it as the minimum thickness of the BL on the inferior cornea divided by the average thickness of the BL on the superior cornea multiplied by 100. BEI-Max was the fourth index we computed and we defined it as the minimum thickness of the BL on the inferior cornea divided by the maximum BL thickness on the superior cornea multiplied by 100.
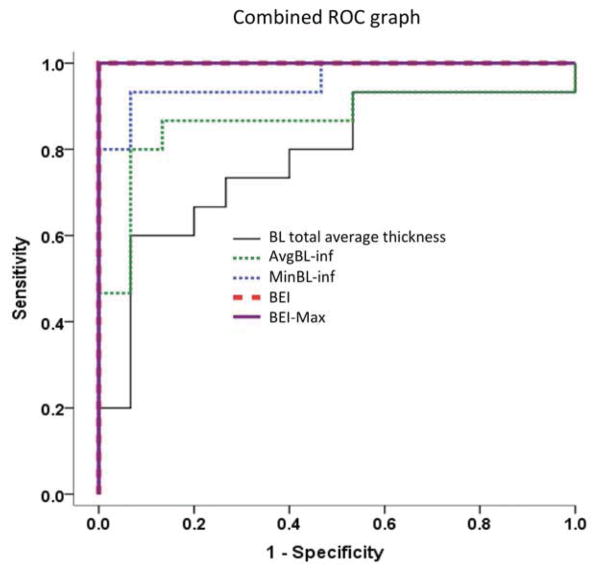
To evaluate the use of BL indices in the diagnosis of KC and to test which of them has the highest predictive accuracy, we compared the means of the indices between the two groups and created ROC curves for each. Mean inferior BL average thickness, inferior BL minimum thickness, BEI and BEI-Max were all highly significantly less in the KC group when compared to the control group (P<0.001). The area under the curve (AUC) for the inferior BL average thickness and the minimum thickness were found to be 0.87 and 0.96, respectively. An inferior BL average thickness cutoff value of 13.5 μm achieved 80% sensitivity and 93% specificity, whereas an inferior BL minimum thickness cutoff value of 11.5 μm achieved 93% sensitivity and specificity. On the other hand, BEI and BEI-Max showed excellent predictive accuracy for the diagnosis of keratoconus with AUC of 1. Cutoff values of 80 for BEI and 60 for BEI-Max achieved 100% sensitivity and specificity in differentiating KC from controls in our study. (Table 2; Figure 3) The excellent discriminating power of BEI and BEI-max was not reduced in an ancillary analysis that included both eyes of all patients and controls.
Table 2.
Characteristics of Bowman’s layer indices calculated on vertical topographic thickness maps of the layer in normal and keratoconus patients:
| Control | KC | P value | AUC | Sensitivity* | Specificity* | Cut-off* | Correlation to Avg-K | Correlation to Ast-K | |
|---|---|---|---|---|---|---|---|---|---|
| BL total average thickness | 15±1 μm | 13±2 μm | 0.056 | 0.78 | 60% | 93% | 13.3 μm | R=−0.48; P=0.007 | R=−0.53; P=0.002 |
| BL average thickness of the inferior half of the cornea | 15±2 μm | 12±3 μm | <0.001 | 0.87 | 80% | 93% | 13.5 μm | R=−0.62;P<0.001 | R=−0.72; P<0.001 |
| BL minimum thickness of the inferior half of the cornea | 13±2 μm | 7±3 μm | <0.001 | 0.96 | 93% | 93% | 11.5 μm | R=−0.59; P=0.001 | R=−0.82; P<0.001 |
| BEI | 91±7 | 48±14 | <0.001 | 1.00 | 100 | 100 | 80 | R=−0. 60; P<0.001 | R=−0.84; P<0.001 |
| BEI-Max | 75±8 | 40±13 | <0.001 | 1 | 100 | 100 | 60 | R=−0.59; P=0.001) | R=−0.82; P<0.001 |
-KC: keratoconus.
-AUC: area under the curve.
-BL: Bowman’s layer.
-BEI: Bowman’s ectasia index; defined as BL minimum thickness of the inferior half of the cornea divided by BL average thickness of the superior half of the cornea multiplied by 100.
-BEI-Max: defined as BL minimum thickness of the inferior half of the cornea divided by BL maximum thickness of the superior half of the cornea multiplied by 100.
-Avg-K: average keratometric readings.
-Ast-K: Keratometric astigmatism.
Chosen to maximize total diagnostic accuracy (minimize total number of errors).
Figure 3.
Combined receiver operating characteristics (ROC) graph of Bowman’s layer (BL) total average thickness, BL average thickness of the inferior half of the cornea, BL minimum thickness of the inferior half of the cornea, Bowman’s ectasia index (BEI) and Bowman’s ectasia index-Maximum (BEI-Max). Note that area under the curve for BEI and BEI-Max is 1.
To test if those BL indices could be used as quantitative indices to describe the severity of KC, we correlated each of them to the Avg-K and Ast-K of KC patients. The inferior BL average thickness and minimum thickness showed highly statistically significant correlation to Ast-K (R=−0.72; P<0.001 and R=−0.82, P<0.001, respectively) and to Avg-K (R=−0.62; P<0.001 and R=−0.59, P=0.001, respectively). Similarly, BEI and BEI-Max showed highly statistically significant correlation to Ast-K (R=−0.84; P<0.001 and R=−0.82, P<0.001, respectively) as well as to Avg-K (R=−0.60; P<0.001 and R=−0.59, P=0.001, respectively). (Table 2)
Discussion
Histopathological light and electron microscopy studies of KC patients have disclosed thinning, disintegration and breakage of Bowman’s layer. 6, 7,4, 5, 8 Those BL changes have been shown to occur earlier than corneal stromal changes suggesting that they might potentially represent earlier signs for the diagnosis of KC.4, 9 Those in vitro diagnostic signs have had clear value in the pathology laboratory to diagnose KC in corneal specimens; nevertheless, they are of limited value in the clinic since BL is too fine to be seen or measured by the available in vivo imaging techniques.18 Those in vitro histopathological changes were seen in corneal specimens obtained during penetrating keratoplasty from patients who had keratoconus severe enough to require surgery. Thus, in vitro studies did not describe the complete story of the BL structural changes in KC especially in the early stages of the disease. Never did those studies map the layer to produce thickness maps that can disclose localized specific changes of BL in KC or answer the question of whether those changes correlate with the severity of the disease.
In our study, we report novel BL diagnostic indices for the in vivo diagnosis of KC obtained from novel 2D vertical topographic thickness maps of BL out to a diameter of 9 mm. These maps show for the first time in the literature the in-vivo structural changes that BL undergoes in KC. It was evident from our BL maps that the BL in KC developed a localized inferior thinning as compared to BL maps of normal subjects, which disclosed a relatively smooth and consistently thick BL. The fact that the average thickness of the layer is not statistically different in KC patients when compared to normal subjects underscores that it is a localized rather than a generalized thinning of the layer, at least in our patients who did not have the severe form of the disease.
Our group has proposed different diagnostic indices to quantify that localized thinning and tested them to evaluate their use as qualitative and quantitative indices for the diagnosis of KC. The average thickness of the layer on the inferior half of the cornea was the first to be evaluated. The inferior BL average thickness was highly significantly different in KC when compared to normal subjects. Nevertheless, AUC was only 0.87 and the sensitivity and specificity were only 80 and 93, respectively. The inferior BL average thickness correlated significantly with Ast-K and Avg-K indicating that it is also an indicator of the severity of KC. The inferior BL average thickness described the generalized thinning of the BL in the inferior cornea. Nonetheless, it was evident to us that the thinning of BL is a localized rather than generalized inferior thinning at least in the early stages of the disease. Based on this observation, we then proposed the inferior BL minimum thickness index. Similarly, the inferior BL minimum thickness was highly significantly different in KC when compared to normal subjects and showed comparable highly significant correlation to Avg-K and Ast-K. The main improvement was seen when constructing the ROC curve that showed that the AUC for the inferior BL minimum thickness was 0.96 with 93% sensitivity and specificity. Thus, the inferior BL minimum thickness index has shown to be a valuable qualitative and quantitative index for the diagnosis of KC.
Further analysis of our BL thickness maps revealed that we could significantly improve our diagnostic indices by attempting to compute the relative rather than the absolute localized inferior thinning of the BL. In other words, computing an index that compares the thinning inferiorly to the patient’s own BL thickness, in this case the thickness of the superior half of the BL which is typically spared in KC. That led us to propose an index named the Bowman’s ectasia index (BEI), defined as the minimum BL thickness of the inferior cornea divided by average thickness of BL measured on the superior cornea multiplied by 100. BEI was highly significantly different in KC when compared to normal subjects. The ROC curve for BEI has, in our study, an AUC of 1 with 100% sensitivity and specificity in the diagnosis of KC using a cut-off value of 80. Additionally, BEI has shown to be an excellent quantitative index as well as it correlated highly with Avg-K and Ast-K. We have also proposed another index that has shown similar excellent results named BEI-max. BEI-max was defined as the minimum thickness of the BL on the inferior cornea divided by the maximum BL thickness on the superior cornea multiplied by 100. With a cut-off value of 60 it has also shown 100% sensitivity and specificity. BEI-max has shown highly significant correlations with Avg-K and Ast-K denoting that it is descriptive of the severity of KC as well.
Based on light and electron microscopy histopathological studies of KC, it is evident that BL plays an important role in the diagnosis of KC. In vivo imaging of BL with confocal microscopy in KC has demonstrated disruptions and breaks in BL in the area of the cone.19, 20 Nevertheless, confocal microscopy does not create cross-sectional images of the cornea and thus unable to create maps of its different layers. Thus, using confocal microscopy, it was only possible to demonstrate the breaks in advanced cases of KC but not the actual thinning that can be quantified and used in clinical settings for the diagnosis of the disease.
The introduction of UHR-OCT in ophthalmology allowed Yatav et al11 to reattempt capturing the changes BL undergoes in KC. Nevertheless, secondary to limitation in technology, they were only able to image the central 4 mm of the layer. They studied 9 KC eyes and showed that the average thickness of the layer is significantly less than in control subjects. They were not able, however, to demonstrate the key structural change in BL, which is, according to our study, the localized inferior thinning that our 9 mm BL maps have shown. In our study, average BL thickness did not show a statistically significant difference between control and KC eyes. That is most likely secondary to differences in the severity of keratoconus between the two studies or merely because different scan widths were used. It is worth noting that in Yatav’s study the average BL thickness did not correlate to the severity of the disease. On the other hand, as we have extended BL maps to 9 mm, we were able to show the localized structural changes in BL that occur in the periphery, which were not demonstrated in their central scans. Thus, we were able to create diagnostic indices that were found to be highly statistically significant in KC when compared to controls with excellent predictive accuracy and showing significant correlation to the severity of the disease; something that the central average thickness might not be able to demonstrate in a disease that typically affects the periphery.
Our study is not without its limitations. In our study, we used three images to create a composite image of the cornea. Overlapping or gapping between the three segments of the map can theoretically occur despite the calibrated fixation targets. Future development of UHR-OCT with extra wide scanning can overcome this limitation and can allow for creating three-dimensional maps (3D) of the BL instead of the 2D maps. In our study, we excluded KC patients with apical scarring as this was seen to distort the tissue planes. Nonetheless, the shortcomings of the currently available diagnostic techniques are in the diagnosis of mild disease, not severe disease with apical scarring. It is important to highlight that all our subjects had topographic evidences of keratoconus. Future studies of forme fruste keratoconus eyes and fellow eyes of keratoconus patients that do not show the typical topographic changes are needed to evaluate if BL changes precede the typical topographic findings. In our study, BEI and BEI-max have demonstrated similar excellent performance in the diagnosis of KC. Futures studies of KC and forme fruste KC might be able to reveal the superiority of one of the indices over the other.
In conclusion, our pilot study has shown for the first time in the literature; the in vivo relative localized inferior thinning of BL in KC as measured on a 2D 9 mm vertical topographic thickness map of the layer. Diagnostic indices measured on BL maps, namely the inferior BL average thickness, the inferior BL minimum thickness, BEI and BEI-max have shown to be valuable qualitative as well as quantitative diagnostic indices for the diagnosis of KC. BEI and BEI-max showed excellent accuracy in differentiating KC from normal subjects with 100% sensitivity and specificity in our case series. Moreover, BEI and BEI-max have shown to be valuable quantitative indices that accurately correlate with the severity of KC.
Acknowledgments
Financial Support: This study was supported by a NEI core center grant to the University of Miami (P30 EY014801) and Research to Prevent Blindness (RPB).
The funding organization had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: United States Provisional Patent Application. Application No.: 61809518 (MA, VLP, JW and SHY). Patent is owned by University of Miami.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 2.Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244–58. [PubMed] [Google Scholar]
- 3.Sykakis E, Carley F, Irion L, et al. An in depth analysis of histopathological characteristics found in keratoconus. Pathology. 2012;44:234–9. doi: 10.1097/PAT.0b013e3283511b42. [DOI] [PubMed] [Google Scholar]
- 4.Sawaguchi S, Fukuchi T, Abe H, et al. Three-dimensional scanning electron microscopic study of keratoconus corneas. Arch Ophthalmol. 1998;116:62–8. doi: 10.1001/archopht.116.1.62. [DOI] [PubMed] [Google Scholar]
- 5.Sawaguchi S, Fukuchi T, Shirakashi M, et al. Three dimensional architecture of Bowman’s collagen fibrils in diseased corneas: a scanning electron microscopic study [in Japanese] Nihon Ganka Kiyo. 1995;46:1261–5. [Google Scholar]
- 6.McPherson SD, Jr, Kiffney GT., Jr Some histologic findings in keratoconus. Arch Ophthalmol. 1968;79:669–73. doi: 10.1001/archopht.1968.03850040671004. [DOI] [PubMed] [Google Scholar]
- 7.Chi HH, Katzin HM, Teng CC. Histopathology of keratoconus. Am J Ophthalmol. 1956;42:847–60. [Google Scholar]
- 8.Bourges JL, Savoldelli M, Dighiero P, et al. Recurrence of keratoconus characteristics: a clinical and histologic follow-up analysis of donor grafts. Ophthalmology. 2003;110:1920–5. doi: 10.1016/S0161-6420(03)00617-1. [DOI] [PubMed] [Google Scholar]
- 9.Tuori AJ, Virtanen I, Aine E, et al. The immunohistochemical composition of corneal basement membrane in keratoconus. Curr Eye Res. 1997;16:792–801. doi: 10.1076/ceyr.16.8.792.8989. [DOI] [PubMed] [Google Scholar]
- 10.Podoleanu A, Charalambous I, Plesea L, et al. Correction of distortions in optical coherence tomography imaging of the eye. Phys Med Biol. 2004;49:1277–94. doi: 10.1088/0031-9155/49/7/015. [DOI] [PubMed] [Google Scholar]
- 11.Yadav R, Kottaiyan R, Ahmad K, Yoon G. Epithelium and Bowman’s layer thickness and light scatter in keratoconic cornea evaluated using ultrahigh resolution optical coherence tomography. J Biomed Opt. 2012;17:116010. doi: 10.1117/1.JBO.17.11.116010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shousha MA, Karp CL, Canto AP, et al. Diagnosis of ocular surface lesions using ultra-high resolution optical coherence tomography. Ophthalmology. 2012;120:883–91. doi: 10.1016/j.ophtha.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shousha MA, Perez VL, Wang J, et al. Use of ultra-high-resolution optical coherence tomography to detect in vivo characteristics of Descemet’s membrane in Fuchs’ dystrophy. Ophthalmology. 2010;117:1220–7. doi: 10.1016/j.ophtha.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin RC, Shure MA, Rollins AM, et al. Group index of the human cornea at 1. 3-microm wavelength obtained in vitro by optical coherence domain reflectometry. Opt Lett. 2004;29:83–5. doi: 10.1364/ol.29.000083. [DOI] [PubMed] [Google Scholar]
- 15.Tao A, Wang J, Chen Q, et al. Topographic thickness of Bowman’s layer determined by ultra-high resolution spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:3901–7. doi: 10.1167/iovs.09-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du C, Wang J, Cui L, et al. Vertical and horizontal corneal epithelial thickness profiles determined by ultrahigh resolution optical coherence tomography. Cornea. 2012;31:1036–43. doi: 10.1097/ICO.0b013e31823f8d56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lian Y, Shen M, Jiang J, et al. Vertical and horizontal thickness profiles of the corneal epithelium and Bowman’s layer after orthokeratology. Invest Ophthalmol Vis Sci. 2013;54:691–6. doi: 10.1167/iovs.12-10263. [DOI] [PubMed] [Google Scholar]
- 18.Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. SD-OCT analysis of regional epithelial thickness profiles in keratoconus, postoperative corneal ectasia, and normal eyes. J Refract Surg. 2013;29:173–9. doi: 10.3928/1081597X-20130129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Efron N, Hollingsworth JG. New perspectives on keratoconus as revealed by corneal confocal microscopy. Clin Exp Optom. 2008;91:34–55. doi: 10.1111/j.1444-0938.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- 20.Hollingsworth JG, Bonshek RE, Efron N. Correlation of the appearance of the keratoconic cornea in vivo by confocal microscopy and in vitro by light microscopy. Cornea. 2005;24:397–405. doi: 10.1097/01.ico.0000151548.46231.27. [DOI] [PubMed] [Google Scholar]