Abstract
Purpose
There is limited data on vascular predictors of long-term disability in Hispanics. We hypothesized: 1) functional status declines over time 2) vascular risk factors predict functional decline.
Methods
The Northern Manhattan Study contains a population-based study of 3298 stroke-free individuals ≥40 years of age, followed for median 11 years. The Barthel index (BI) was assessed annually. Generalized estimating equations and Cox models were adjusted for demographic, medical, and social risk factors. Stroke and myocardial infarction occurring during follow-up were censored in sensitivity analysis. Secondarily, motor and non-motor domains of the BI were analyzed.
Results
Mean age (standard deviation) of the cohort (n=3298) was 69.2 (10) years, 37% were male, 52% Hispanic, 22% diabetic, and 74% hypertensive. There was a mean annual decline of 1.02 BI points (p<0.0001). Predictors of decline in BI included age, female sex, diabetes, depression, and normocholesterolemia. Results did not change with censoring. We found similar predictors of BI for motor and non-motor domains.
Conclusion
In this large, population-based, multi-ethnic study with long-term follow-up, we found a 1% mean decline in function per year that did not change when vascular events were censored. Diabetes predicted functional decline in the absence of clinical vascular events.
Keywords: Epidemiology, Disability
Vascular risk factors lead to disability through multiple mechanisms, including clinical and subclinical cerebrovascular events causing impairment, cardiovascular events causing reduced cardiopulmonary fitness, and non-vascular complications of diabetes such as neuropathy. Diabetes in particular is growing in prevalence, and understanding the population impact of diabetes on disability is increasingly important. Several possible mechanisms link diabetes and disability, including decreased cardiovascular function, neuropathy, sarcopenia, and inflammatory processes.1 The interplay between diabetes and particular functional limitations has varied in different populations.2 Previous research on disability in Hispanics, a markedly heterogeneous group in the United States with a disproportionate burden of diabetes, has focused on Mexican-Americans,3–5 and relatively little is known about long-term disability among Caribbean Hispanics.6 Furthermore, the impact of vascular risk factors may vary based upon age subgroups in the elderly.
Prior studies have found that age, cognitive function, mood disorders, and social supports consistently predict long-term disability,7–9 and among diseases stroke is the foremost cause.10–12 However, several questions remain unanswered. First, the relationship between predictors and long-term disability may have been altered by shifts in paradigms of treatment (of cholesterol13 and blood pressure,14 for example), increasing obesity prevalence, and population patterns of aging. Also, in many studies, vascular events such as stroke or MI and vascular risk factors15 did not undergo specialist review and adjudication, resulting in misclassification. Third, most studies have examined predictors of average disability instead of trajectories of change. An explicit analysis of trajectories of disability would distinguish among factors that predict baseline disability, change in function over time, and discrete decrements in function. For this, long term follow-up and repeated measures of disability are needed. However, most studies have included only hospitalized patients with limited follow-up, and few population-based studies have had long-term follow-up.16 Finally, there is limited data of the course and predictors of disability in predominantly Hispanic populations,17 especially in urban, elderly cohorts, in whom the burden of comorbid conditions such as obesity, diabetes and hypertension is high.
We sought to address these questions in the population-based Northern Manhattan Study (NOMAS), an ongoing observational study with annual assessment of functional outcomes and specialist adjudication of vascular events. A prior NOMAS study showed a decline in functional status over the long term after stroke, even in the absence of recurrent vascular events.18, 19 However, we had not examined the course and predictors of long-term functional status in a stroke-free cohort. We hypothesized that functional status declines over time and that vascular risk factors predict decline independent of the occurrence of stroke and heart disease. This study adds to previous literature due to its size, predominantly Caribbean Hispanic cohort, population-based sampling, an explicit focus on modeling trajectories of disability, and the use of 2 complementary modeling strategies to robustly identify predictors and trajectories of disability.
MATERIALS AND METHODS
NOMAS is a prospective, population-based cohort of 3,298 subjects in a community-based sample of a racially and ethnically diverse population. The study was approved by the institutional review boards of Columbia University and the University of Miami, and informed consent was obtained from all participants.
Cohort selection
The cohort was recruited between 1993 and 2001.20, 21 Subjects were enrolled if they were at least 40 years of age, lived in a pre-defined geographic area of northern Manhattan for at least 3 months in a household with a telephone, and did not have prior stroke. Subjects were contacted by random digit dialing of published and unpublished telephone numbers. The telephone response rate was 91%, 87% of eligible subjects indicated willingness to participate, and enrollment response rate was 75%.
Baseline assessment
Baseline examination included comprehensive medical history, physical examination, medical record review, and fasting blood samples. Standardized questions were adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System. Baseline age was calculated by subtracting the date of birth from date of enrollment. Sex was self-reported as male or female. Race-ethnicity was self-identified and modeled after the U.S. census. Smoking was defined as either never, former, and current (within a year). Education was classified as at least versus less than high school education. Marital status was classified as married versus other. Insurance status was characterized as Medicare/private insurance versus Medicaid/no insurance. Hypertension, coded as present or absent, was defined as a systolic blood pressure recording ≥140 mmHg or a diastolic blood pressure recording ≥90 mm Hg (based on the average of two blood pressure measurements) or self-report of history of hypertension or antihypertensive use. Diabetes mellitus was defined by self-report, fasting blood glucose level ≥126 mg/dL, or insulin/oral hypoglycemic use. Fasting total cholesterol was obtained using a Hitachi 705 automated spectrophotometer (Boehringer, Mannheim, Germany). Hypercholesterolemia was defined as self-report of hypercholesterolemia, lipid lowering therapy use, or fasting total cholesterol level >240 mg/dL. Alcohol use was defined as low/no (<1 drink/month), moderate (1 drink/month to 2 drinks/day), and heavy (>2 drinks/day). Physical activity was assessed using a questionnaire adapted from the National Health Interview Survey and classified as any or none, as in previous research in this cohort.22, 23 Number of friends was assessed by asking about the number of individuals whom the participant knows well enough to visit in their homes. Depression was defined as a Hamilton Depression Rating Scale score >10 or history of antidepressant use, as in prior research.24
Prospective follow-up
Subjects are followed annually by telephone, with average annual contact rate of 99%. Only 2 subjects were completely lost to follow-up. The telephone interview assessed change in vital status, neurological symptoms and events, hospitalizations, and functional status via the Barthel Index (BI). The BI25, 26 measures performance in 10 activities of daily living (ADLs) and has been extensively used in geriatric populations,27, 28 stroke observational studies, and clinical trials as a measure of disability.29 The scale ranges from 0 to 100 in 5-point increments, with 100 indicating normal physical functioning. Previous research has demonstrated the reliability of telephone use of the BI.30
A positive screen for any potential neurological or cardiac event was followed by an in-person assessment to determine whether a MI or stroke had occurred. We prospectively screened all admissions and discharges to detect hospitalizations and outcomes that may not have been captured by telephone interview. Nearly 70% of vascular events lead to hospitalizations at Columbia University Medical Center. Hospital records were reviewed to classify all outcomes as previously reported.20 Stroke included ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage, but not transient ischemic attack or venous sinus thrombosis. A consensus of stroke neurologists assessed stroke subtype using modified Stroke Data Bank criteria and all available information, as previously described.31 MI was defined by criteria adapted from the Cardiac Arrhythmia Suppression trial32 and the Lipid Research Clinics Coronary Primary Prevention trial33 as in previous publications,34 and all cases of MI were adjudicated by cardiologists independently after review of all clinical data.
Statistical analysis
Statistical analyses were conducted using SAS 9.1.3 (SAS Institute, Cary, NC). Means were calculated for continuous variables and proportions for categorical variables. Two modeling strategies were used. First, we assessed whether average population functional status declines over time, and whether vascular risk factors predict functional decline over time independently from stroke and heart disease. We used generalized estimating equations (GEE) to test associations with a continuous measure of BI. In the second strategy, we examined predictors of transition from no disability to the first occurrence of disability with Cox proportional hazards models that estimated risk of the first occurrence of dropping below a specific ADL level. Prior research has found that a decrement in function predicts further decline, even if there is recovery to baseline levels.35
GEE models
GEE regression models were chosen due to correlations among repeated measures of BI in the same individual, using an exchangeable working correlation structure. The primary covariate was time of follow-up assessment, and its parameter term signified slope of functional change over time. In model building, we sequentially added groups of confounder variables defined by epidemiological relevance. The first model included no covariates, and successive models included demographic variables, social resource variables, and vascular disease risk factors. To assess whether the main explanatory variables were associated with change in BI over time, we included interaction terms between time of follow-up assessment and every variable. Model diagnostics, including residual plots and goodness of fit measures, were used to evaluate the final model, including linearity of the time trends.
The BI was first treated as a continuous variable. Secondary analysis explored BI categorization at different cutoffs, from 60 to 95 at 5-point increments in successive models, but there were no significant results. Hence, for this analysis, BI was analyzed as a continuous variable in order to increase power to detect associations, describe the course of linear change, and avoid potential misclassification due to crude categorization.36 In a large sample, linear regression coefficient estimates are valid even if the data are not normally distributed37 and even with heteroscedasticity, if the variance of the regression coefficient is obtained using robust variance.38 We insured that predicted values were within the BI range given the observed ranges of predictors. To examine the impact of vascular events on functional decline, we qualitatively compared models without censoring to those in which follow-up was censored at 1) recurrent stroke, and 2) MI or recurrent stroke. As a sensitivity analysis, to further explore the impact of stroke on functional decline, we ran a model in which prevalent stroke was coded as ‘1’ if stroke had occurred before or during a particular follow-up assessment and ‘0’ if it had not.
Although the BI measures a single construct of disability, it consists of mobility and non-mobility domains, and we examined whether the profile of predictors differed for mobility (transfers, mobility, and stair use) and non-mobility (feeding, bathing, grooming, dressing, bowels, bladder, and toilet use) domains, which we analyzed separately. We also performed an analysis stratified by median age to assess a differential impact of covariates upon different age categories.
Cox models
In the second strategy, we used Cox proportional hazards models, adjusting for variables used in the final multivariable GEE model. We modeled time to: 1) incident BI score of ≤90 among those with baseline BI >90, and 2) incident BI score of ≤60 among those with baseline BI>60. We selected these cutoffs based upon prior research in our cohort.18, 39 All models met the proportional hazards assumption. We also created Kaplan-Meier curves of outcomes stratified by diabetes and depression because these were significant predictors of disability with large magnitudes of effect.
RESULTS
Among 3298 participants, median follow-up was 11 years (interquartile range, 7.6–11.3 years); there were 33,477 BI assessments (median 11 per participant, interquartile range, 7–13). Baseline cohort characteristics (Table 1) describe an elderly, urban, predominantly Hispanic population with prevalent vascular risk factors. Table 2 shows the final GEE model examining predictors of baseline BI (left column) and change in BI over time (right column, which shows parameter estimates for interactions with time). Demographic predictors of baseline BI included age, Hispanic ethnicity, and insurance with Medicaid or no insurance. Vascular risk factors included diabetes, depression, coronary artery disease, moderate alcohol use, and any physical activity (left column).
Table 1.
Baseline characteristics of study population.
| Number of participants, No. (%) | 3298 (100) |
|
| |
| Demographics: | |
|
| |
| Age, mean (SD), y | 69.2 (10.3) |
|
| |
| Male, No. (%) | 1227 (37.2) |
|
| |
| Non-Hispanic white, No. (%) | 690 (20.9) |
|
| |
| Non-Hispanic black, No. (%) | 803 (24.4) |
|
| |
| Hispanic, No. (%) | 1726 (52.3) |
|
| |
| Other race, No. (%) | 79 (2.4) |
|
| |
| Highest education achieved, No. (%) | |
| 8th grade or less | 1313 (39.8) |
| Some high school | 473 (14.4) |
| Completed high school | 607 (18.4) |
| Some college | 397 (12.0) |
| College graduate or higher | 507 (15.4) |
|
| |
| Marital status, No. (%) | |
| Single | 523 (15.9) |
| Married | 1042 (31.6) |
| Widowed | 924 (28.0) |
| Divorced | 513 (15.6) |
| Separated | 294 (8.9) |
|
| |
| Insured with Medicaid or uninsured, No. (%) | 1435 (43.8) |
|
| |
| Insured with Medicare or private insurance, No. (%) | 1841 (56.2) |
|
| |
| Number of friends, No. (%) | |
| None | 130 (4.0) |
| 1 or 2 | 367 (11.1) |
| 3 or 4 | 653 (19.8) |
| 5 or more | 2145 (65.1) |
|
| |
| Baseline Barthel index score, No. (%) | |
| 0–60 | 45 (1.4) |
| 65–80 | 108 (3.3) |
| 85–95 | 608 (18.5) |
| 100 | 2532 (76.9) |
|
| |
| Risk factors, No. (%)* | |
|
| |
| Alcohol use, No. (%) | |
| Never | 821 (24.9) |
| Past | 799 (24.2) |
| Light | 421 (12.8) |
| Moderate | 1086 (32.9) |
| Intermediate | 120 (3.6) |
| Heavy | 51 (1.6) |
|
| |
| Physical activity, No. (%) | |
| None | 1388 (42.2) |
| Light | 1622 (49.3) |
| Moderate | 137 (4.2) |
| Heavy | 145 (4.4) |
|
| |
| Hypertension, No. (%) | 2429 (73.7) |
|
| |
| Hypercholesterolemia, No. (%) | 2050 (62.2) |
|
| |
| Diabetes mellitus, No. (%) | 716 (21.8) |
|
| |
| History of coronary artery disease, No. (%) | 705 (21.4) |
|
| |
| Depression, No. (%) | 336 (10.2) |
|
| |
| Hamilton Depression scale score, No. (%) | |
| 0–5 | 2642 (80.4) |
| 6–10 | 456 (13.9) |
| 11–15 | 136 (4.1) |
| 16–20 | 34 (1.0) |
| 21+ | 17 (0.5) |
|
| |
| Smoking status, No. (%) | |
| Never smoked | 1548 (47.0) |
| Past smoking history | 1179 (35.8) |
| Current smoking | 569 (17.3) |
|
| |
| Incident strokes during follow-up, No. | 330 |
as defined in text
Table 2.
Predictors of baseline functional status and change over time as measured by the Barthel index (BI), with all variables included in a generalized estimating equations model.
| Baseline Barthel index score | Change over time in Barthel index score | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Difference in baseline Barthel Index score§ | Std Error | p-value | Annual change in Barthel index score | Std error | p-value |
|
| ||||||
| Overall change | -- | -- | -- | −1.43 | 0.18 | <0.0001 |
|
| ||||||
| Demographic variables | ||||||
|
| ||||||
| Age (per year) | −0.16 | 0.02 | <0.0001 | −0.08 | 0.004 | <0.0001 |
|
| ||||||
| Male sex | 0.39 | 0.37 | 0.3 | 0.26 | 0.07 | 0.0003 |
|
| ||||||
| Race-ethnicity† | ||||||
| Black | −0.64 | 0.56 | 0.3 | −0.15 | 0.11 | 0.2 |
| Hispanic | 0.96 | 0.57 | 0.09 | −0.14 | 0.11 | 0.2 |
|
| ||||||
| At least high school education | 0.73 | 0.42 | 0.08 | 0.02 | 0.08 | 0.8 |
|
| ||||||
| Insured with Medicaid or uninsured | −2.39 | 0.42 | <0.0001 | −0.02 | 0.07 | 0.8 |
|
| ||||||
| Social variables | ||||||
|
| ||||||
| Married | 0.08 | 0.36 | 0.8 | 0.07 | 0.07 | 0.4 |
|
| ||||||
| At least three friends | 1.13 | 0.60 | 0.06 | 0.08 | 0.11 | 0.5 |
|
| ||||||
| Vascular risk factors | ||||||
|
| ||||||
| Diabetes | −1.45 | 0.49 | 0.003 | −0.35 | 0.10 | 0.0005 |
|
| ||||||
| Depression | −2.97 | 0.77 | 0.0001 | −0.24 | 0.13 | 0.06 |
|
| ||||||
| Hypertension | 0.49 | 0.41 | 0.2 | 0.08 | 0.08 | 0.3 |
|
| ||||||
| History of coronary artery disease | −0.91 | 0.46 | 0.0497 | −0.14 | 0.10 | 0.1 |
|
| ||||||
| Hypercholesterolemia | 0.63 | 0.40 | 0.1 | 0.18 | 0.08 | 0.02 |
|
| ||||||
| Any physical activity | 2.27 | 0.38 | <0.0001 | 0.02 | 0.07 | 0.8 |
|
| ||||||
| Smoking status* | ||||||
| Past | −0.64 | 0.42 | 0.1 | 0.06 | 0.08 | 0.5 |
| Current | −0.86 | 0.48 | 0.07 | 0.04 | 0.09 | 0.6 |
|
| ||||||
| Alcohol use‡ | ||||||
| Moderate | 1.00 | 0.34 | 0.003 | 0.13 | 0.07 | 0.07 |
| Heavy | 0.45 | 0.67 | 0.5 | −0.03 | 0.14 | 0.8 |
compared to the referent group as specified in the text, unless otherwise specified;
compared to non-Hispanic white race-ethnicity;
compared to no smoking;
compared to low or no alcohol use
There was an overall annual decline of 1.02 BI points (p<0.0001) in an unadjusted GEE model and 1.43 points (p<0.0001) in the final, fully adjusted model (Table 2). Interaction terms with time (right column) revealed that the annual change significantly differed for those with diabetes (−0.35, p=0.005), males (0.26, p=0.0003), and those with hypercholesterolemia (0.18, p=0.02), compared to their counterparts. The annual decline was significantly greater with increasing age (−0.08 per year, p<0.0001). Hypertension was not a significant predictor of baseline BI or change over time, even when a higher BP cutoff of 160/100 was used to represent Stage 2 hypertension. Results were similar when stroke and MI were censored, and in a sensitivity analysis in which stroke occurring during follow-up was coded as a dichotomous variable (Supplementary Table).
We found similar predictors of BI for mobility and non-mobility domains. Table 3 shows GEE models stratified by median age. Among predictors of baseline BI, several had a greater magnitude of effect above the median age compared to below the median: age (−0.32 versus −0.02, p for difference 0.0002), Medicaid or no insurance compared to Medicare or private insurance (−3.69 versus 1.28, p=0.01), and physical activity (3.56 versus 1.15, p=0.002). The overall mean decline in BI over time was greater among those aged >69 years (change in BI=−2.45, p<0.0001) than ≤69 years (change in BI=−0.45, p=0.007) (p for difference <0.0001). The effects on change in BI over time between those above the median age and those less than or equal to the median were different for the following variables: age (−0.15 per year vs. −0.03; p for difference <0.0001), and moderate alcohol use compared to none or light (0.42 per year vs. 0.01; p=0.02). There was no effect of race or interaction with race in these models.
Table 3.
Separate models, by age group, of predictors of functional status measured by the Barthel index, using generalized estimating equations models.
| Age ≤69 years | Age >69 years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Baseline Barthel index score | Change over time in Barthel index score | Baseline Barthel index score | Change over time in Barthel | |||||||||
|
| ||||||||||||
| Variable | Differenc e in baseline Barthel Index score§ | Std Error | p-value | Annual change in Barthel index score | Std error | p-value | Difference in baseline Barthel Index score§ | Std Error | p-value | Annual change in Barthel index score | Std error | p-value |
|
| ||||||||||||
| Overall change | -- | -- | -- | −0.45 | 0.17 | 0.007 | -- | -- | -- | −2.45 | 0.35 | <0.0001 |
|
| ||||||||||||
| Demographic variables | ||||||||||||
|
| ||||||||||||
| Age (centered on mean age) | −0.02 | 0.03 | 0.5 | −0.03 | 0.005 | <0.0001 | −0.32 | 0.07 | <0.0001 | −0.15 | 0.01 | <0.0001 |
|
| ||||||||||||
| Male sex | 0.24 | 0.34 | 0.5 | 0.18 | 0.07 | 0.008 | 0.12 | 0.75 | 0.9 | 0.43 | 0.16 | 0.008 |
|
| ||||||||||||
| Race-ethnicity† | ||||||||||||
| Black | 0.14 | 0.56 | 0.8 | −0.12 | 0.11 | 0.3 | −0.87 | 0.85 | 0.3 | −0.20 | 0.18 | 0.3 |
| Hispanic | 1.00 | 0.51 | 0.05 | −0.08 | 0.10 | 0.4 | 1.25 | 0.99 | 0.2 | −0.30 | 0.22 | 0.2 |
|
| ||||||||||||
| At least high school education | 0.38 | 0.41 | 0.4 | 0.04 | 0.07 | 0.5 | 1.14 | 0.75 | 0.1 | 0.02 | 0.17 | 0.9 |
|
| ||||||||||||
| Insured with Medicaid or uninsured | −1.28 | 0.33 | 0.0001 | −0.03 | 0.07 | 0.6 | −3.69 | 0.89 | <0.0001 | 0.11 | 0.18 | 0.5 |
|
| ||||||||||||
| Social variables | ||||||||||||
|
| ||||||||||||
| Married | 0.06 | 0.34 | 0.9 | −0.009 | 0.08 | 0.9 | 3.56 | 0.69 | <0.0001 | 0.05 | 0.16 | 0.8 |
|
| ||||||||||||
| At least one friend | 1.26 | 0.63 | 0.048 | −0.08 | 0.10 | 0.4 | 1.01 | 0.99 | 0.3 | 0.14 | 0.20 | 0.5 |
|
| ||||||||||||
| Vascular risk factors | ||||||||||||
|
| ||||||||||||
| Diabetes | −1.00 | 0.57 | 0.08 | −0.42 | 0.11 | 0.0002 | −2.64 | 0.82 | 0.001 | −0.46 | 0.19 | 0.02 |
|
| ||||||||||||
| Depression | −2.47 | 0.82 | 0.003 | −0.12 | 0.13 | 0.3 | −3.59 | 1.42 | 0.01 | −0.54 | 0.28 | 0.0495 |
|
| ||||||||||||
| Hypertension | −0.36 | 0.31 | 0.2 | −0.07 | 0.06 | 0.2 | 0.73 | 0.85 | 0.4 | 0.22 | 0.18 | 0.2 |
|
| ||||||||||||
| History of coronary artery disease | −0.64 | 0.52 | 0.2 | −0.15 | 0.10 | 0.1 | −1.15 | 0.72 | 0.1 | −0.16 | 0.17 | 0.3 |
|
| ||||||||||||
| Hypercholesterolemia | 0.28 | 0.37 | 0.4 | 0.16 | 0.08 | 0.03 | 0.84 | 0.76 | 0.3 | 0.16 | 0.15 | 0.3 |
|
| ||||||||||||
| Any physical activity | 1.15 | 0.37 | 0.002 | 0.06 | 0.07 | 0.4 | 3.56 | 0.69 | <0.0001 | −0.07 | 0.14 | 0.6 |
|
| ||||||||||||
| Smoking status** | ||||||||||||
| Past | −0.68 | 0.40 | 0.09 | 0.09 | 0.08 | 0.3 | −0.86 | 0.76 | 0.3 | −0.05 | 0.16 | 0.7 |
| Current | −1.50 | 0.51 | 0.003 | 0.06 | 0.09 | 0.5 | 0.33 | 0.97 | 0.7 | −0.09 | 0.20 | 0.7 |
|
| ||||||||||||
| Alcohol use‡ | ||||||||||||
| Moderate | 0.65 | 0.32 | 0.04 | 0.01 | 0.07 | 0.9 | 1.26 | 0.66 | 0.06 | 0.42 | 0.15 | 0.007 |
| Heavy | 0.96 | 0.76 | 0.2 | −0.17 | 0.13 | 0.2 | −0.58 | 1.39 | 0.7 | 0.57 | 0.36 | 0.1 |
compared to the referent group as specified in the text, unless otherwise specified;
compared to non-Hispanic white race-ethnicity;
compared to no smoking;
compared to low or no alcohol use
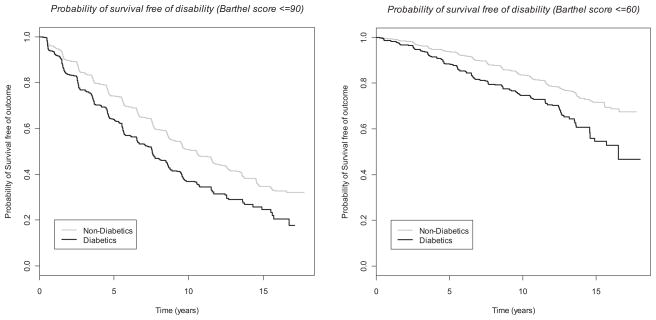
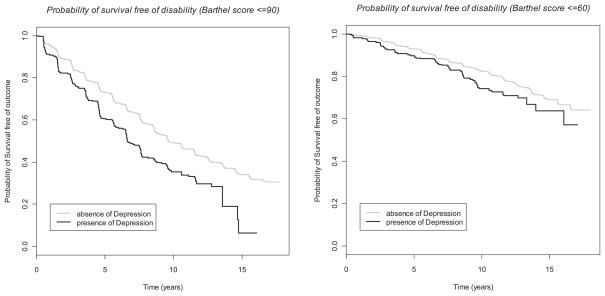
The multivariable Cox models (Table 4) confirm findings from the GEE models. In particular, for both BI cutoffs, there was a significant, markedly increased risk of disability for diabetes (1.4-fold increased incidence of BI ≤90 and 1.85-fold increased incidence of BI ≤60 compared to non-diabetics) and depression (1.5-fold increased incidence of BI ≤90 and BI ≤60 compared to absence of depression). The Figures illustrate the course of increasing disability over time, and the steeper decline among those with diabetes (Figure 1) and depression (Figure 2).
Table 4.
Cox proportional hazards models examining predictors of disability.
| Incidence of Barthel index score ≤90 (n=2858) | Incidence of Barthel index score ≤60 (n=3150) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Hazard ratio | 95% confidence interval | p-value | Hazard ratio | 95% confidence interval | p-value |
|
| ||||||
| Age | 1.07 | 1.07–1.08 | <0.0001 | 1.12 | 1.11–1.13 | <0.0001 |
|
| ||||||
| Male sex | 0.77 | 0.68–0.88 | <0.0001 | 0.93 | 0.76–1.14 | 0.5 |
|
| ||||||
| Race-ethnicity† | ||||||
| Black | 1.00 | 0.86–1.18 | 0.98 | 1.22 | 0.97–1.54 | 0.09 |
| Hispanic | 1.05 | 0.88–1.25 | 0.6 | 1.03 | 0.78–1.35 | 0.8 |
|
| ||||||
| At least high school education | 0.84 | 0.74–0.96 | 0.008 | 0.91 | 0.75–1.10 | 0.3 |
|
| ||||||
| Insured with Medicaid or uninsured | 1.16 | 1.03–1.32 | 0.02 | 1.28 | 1.05–1.56 | 0.01 |
|
| ||||||
| Married | 0.96 | 0.86–1.09 | 0.6 | 0.98 | 0.80–1.19 | 0.8 |
|
| ||||||
| At least one friend | 0.82 | 0.71–0.95 | 0.007 | 0.86 | 0.70–1.06 | 0.2 |
|
| ||||||
| Diabetes | 1.46 | 1.29–1.64 | <0.0001 | 1.85 | 1.54–2.22 | <0.0001 |
|
| ||||||
| Depression | 1.50 | 1.27–1.77 | <0.0001 | 1.52 | 1.19–1.94 | 0.0009 |
|
| ||||||
| Hypertension | 1.10 | 0.97–1.24 | 0.1 | 1.03 | 0.84–1.26 | 0.8 |
|
| ||||||
| History of coronary artery disease | 1.52 | 1.35–1.72 | <0.0001 | 1.30 | 1.08–1.57 | 0.005 |
|
| ||||||
| Hypercholesterolemia | 0.86 | 0.77–0.96 | 0.009 | 0.85 | 0.72–1.01 | 0.06 |
|
| ||||||
| Any physical activity | 0.78 | 0.70–0.87 | <0.0001 | 0.81 | 0.69–0.96 | 0.01 |
|
| ||||||
| Smoking status** | ||||||
| Past | 0.98 | 0.87–1.1 | 0.7 | 1.05 | 0.88–1.26 | 0.6 |
| Current | 1.13 | 0.97–1.32 | 0.1 | 1.19 | 0.92–1.53 | 0.2 |
|
| ||||||
| Alcohol use‡ | ||||||
| Moderate | 0.86 | 0.76–0.96 | 0.01 | 0.67 | 0.55–0.83 | 0.0001 |
| Heavy | 0.82 | 0.62–1.08 | 0.2 | 1.02 | 0.67–1.55 | 0.9 |
compared to non-Hispanic white race-ethnicity;
compared to no smoking;
compared to low or no alcohol use
Figure 1.
Kaplan-Meier curves stratified by presence or absence of diabetes.
Figure 2.
Kaplan-Meier curves stratified by presence or absence of depression.
DISCUSSION
In this large, population-based study with long-term follow-up, we examined predictors not only of baseline function but also change over time. Findings were similar in 2 complementary modeling approaches that addressed distinct but overlapping hypotheses. We examined a predominantly Caribbean Hispanic, urban population, which has been under-represented in previous research on disability. Hispanics, rather than a genetically homogeneous group with similar health characteristics, are diverse in terms of genetic constitution and cultural, contextual, and behavioral characteristics. The interplay between risk factors and disability may differ in different Hispanic communities. In this study, we did not find significant race-ethnic disparities in results, and further research would clarify the etiologic relationship between predictors and disability in this population.
We found that baseline functional status was associated with demographics, social support, and vascular risk factors. In particular, the effect of diabetes on functional decline was equivalent to ~5 years of age. Diabetes may accelerate functional decline through cumulative effects on atherosclerosis, microvascular disease, and non-vascular diabetic complications such as neuropathy, retinopathy, nephropathy, and limb loss.40 These processes affect mobility (walking, reaching, carrying), cognitive function (instrumental ADLs such as managing money), and cardiovascular function (walking and endurance activities). We also found that lower socioeconomic status (indicated by a proxy insurance variable) predicted more disability, possibly reflecting reduced access to care or less knowledge of self-care and health literacy,41 which are particularly important for complex chronic conditions such as diabetes. In this cohort, as in previous research, there was a large effect of depression on disability.42 Prior research has shown a synergy between depression and diabetes in Mexican-Americans, and further research will clarify this relationship in this cohort.43
We also explicitly modeled trajectories of change in function over time and found a 1% mean decline in function per year, similar to previous research among 547 non-institutionalized 70- and 80-year old Americans studied over 3 years.44 This extent of decline, seen among >3000 participants in our study over 11 years of follow-up, did not change when the effect of major vascular events was excluded by censoring, or, in sensitivity analysis, explicitly modeled with a stroke indicator variable. One possible explanation, considering the steeper decline in the presence of diabetes, is the accumulation of silent brain infarcts. Subclinical infarcts are at least 5 times as prevalent as clinical strokes45 and are associated with stroke and cognitive impairment,46, 47 and white matter disease has been associated with multiple outcomes including functional status.48–53 Future analyses in this cohort will examine the relationship between subclinical infarcts and disability. It is also possible that non-vascular conditions, such as neurodegenerative dementia, depression, and arthritis, may have a stronger impact than vascular conditions on average function in populations.54
Hypercholesterolemia showed an unexpected association with better functional outcomes, which may reflect better nutritional or health status among those with higher total cholesterol,55–57 or a protective effect of statins on vascular events (20% of those diagnosed with hypercholesterolemia used statins).58
A limitation of this primarily vascular epidemiology study was that we were not able to reliably ascertain non-vascular conditions that may have an impact on disability during follow-up. We did not measure risk factors repeatedly during follow-up, which would have allowed analysis of time-varying covariates. There was no information about use of physical therapy, the development of arthritis during follow-up, and pain, all of which influence disability. Also, the BI is subject to ceiling effects and is insensitive to small changes in disability.59 Despite these limitations, this study employs innovative methods, including the analysis of trajectories of repeated measures of disability over long term follow-up. We also used 2 modeling strategies to confirm the robustness of the findings and employed detailed adjudication of vascular events, which strengthens construct validity.
In conclusion, findings suggest a strong effect of several modifiable individual-level factors, including insurance coverage, social support, physical activity, depression, and diabetes. Further research would clarify the mechanisms of effect and the relationship between vascular disease and function, which would facilitate interventions to improve function among those at increased risk.60
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (R01 NS48134, MSVE; R37 29993, RLS/MSVE; K23NS079422, MSD).
Footnotes
None of the authors has a financial relationship relevant to the topic of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and a1c with functional disability in older adults: Results from the national health and nutrition examination survey (nhanes), 1999–2006. Diabetes Care. 2010;33:1055–1060. doi: 10.2337/dc09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu CJ, Wray LA. Gender differences in functional limitations in adults living with type 2 diabetes: Biobehavioral and psychosocial mediators. Ann Behav Med. 2011;41:71–82. doi: 10.1007/s12160-010-9226-0. [DOI] [PubMed] [Google Scholar]
- 3.Palmer RF, Espino DV, Dergance JM, Becho J, Markides K. The role of physical activity and diabetes status as a moderator: Functional disability among older mexican americans. Age Ageing. 2012;41:752–758. doi: 10.1093/ageing/afs106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beard HA, AlGhatrif M, Samper-Ternent R, Gerst K, Markides KS. Trends in diabetes prevalence and diabetes-related complications in older mexican americans from 1993–1994 to 2004–2005. Diabetes Care. 2009;32:2212–2217. doi: 10.2337/dc09-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ottenbacher KJ, Graham JE, Al Snih S, Raji M, Samper-Ternent R, Ostir GV, Markides KS. Mexican americans and frailty: Findings from the hispanic established populations epidemiologic studies of the elderly. Am J Public Health. 2009;99:673–679. doi: 10.2105/AJPH.2008.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariza MA, Vimalananda VG, Rosenzweig JL. The economic consequences of diabetes and cardiovascular disease in the united states. Rev Endocr Metab Disord. 2010;11:1–10. doi: 10.1007/s11154-010-9128-2. [DOI] [PubMed] [Google Scholar]
- 7.Tas U, Steyerberg EW, Bierma-Zeinstra SM, Hofman A, Koes BW, Verhagen AP. Age, gender and disability predict future disability in older people: The rotterdam study. BMC Geriatr. 2011;11:22. doi: 10.1186/1471-2318-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tas U, Verhagen AP, Bierma-Zeinstra SM, Odding E, Koes BW. Prognostic factors of disability in older people: A systematic review. Br J Gen Pract. 2007;57:319–323. [PMC free article] [PubMed] [Google Scholar]
- 9.Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Soc Sci Med. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 10.Verbrugge LM, Lepkowski JM, Imanaka Y. Comorbidity and its impact on disability. Milbank Q. 1989;67:450–484. [PubMed] [Google Scholar]
- 11.Boult C, Altmann M, Gilbertson D, Yu C, Kane RL. Decreasing disability in the 21st century: The future effects of controlling six fatal and nonfatal conditions. Am J Public Health. 1996;86:1388–1393. doi: 10.2105/ajph.86.10.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HP, Jr, Jackson CM, Pullicino P. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 13.Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. Jama. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: A cohort study of older persons. Ann Intern Med. 2012;156:131–140. doi: 10.1059/0003-4819-156-2-201201170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putman K, De Wit L, Schoonacker M, Baert I, Beyens H, Brinkmann N, Dejaeger E, De Meyer AM, De Weerdt W, Feys H, Jenni W, Kaske C, Leys M, Lincoln N, Schuback B, Schupp W, Smith B, Louckx F. Effect of socioeconomic status on functional and motor recovery after stroke: A european multicentre study. J Neurol Neurosurg Psychiatry. 2007;78:593–599. doi: 10.1136/jnnp.2006.094607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill TM, Guo Z, Allore HG. The epidemiology of bathing disability in older persons. J Am Geriatr Soc. 2006;54:1524–1530. doi: 10.1111/j.1532-5415.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 18.Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, Elkind MS. Long-term functional recovery after first ischemic stroke: The northern manhattan study. Stroke. 2009;40:2805–2811. doi: 10.1161/STROKEAHA.109.549576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhamoon MS, Moon YP, Paik MC, Sacco RL, Elkind MS. Trajectory of functional decline before and after ischemic stroke: The northern manhattan study. Stroke. 2012;43:2180–2184. doi: 10.1161/STROKEAHA.112.658922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a triethnic cohort: The northern manhattan study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 21.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL. Elevated white blood cell count and carotid plaque thickness: The northern manhattan stroke study. Stroke. 2001;32:842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 22.Sacco RL, Gan R, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. Leisure-time physical activity and ischemic stroke risk: The northern manhattan stroke study. Stroke. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 23.Willey JZ, Paik MC, Sacco R, Elkind MS, Boden-Albala B. Social determinants of physical inactivity in the northern manhattan study (nomas) J Community Health. 2010;35:602–608. doi: 10.1007/s10900-010-9249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Economos A, Wright CB, Moon YP, Rundek T, Rabbani L, Paik MC, Sacco RL, Elkind MS. Interleukin 6 plasma concentration associates with cognitive decline: The northern manhattan study. Neuroepidemiology. 2013;40:253–259. doi: 10.1159/000343276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahoney FI, Barthel DW. Functional evaluation: The barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 26.Granger CV, Dewis LS, Peters NC, Sherwood CC, Barrett JE. Stroke rehabilitation: Analysis of repeated barthel index measures. Arch Phys Med Rehabil. 1979;60:14–17. [PubMed] [Google Scholar]
- 27.de Morton NA, Keating JL, Davidson M. Rasch analysis of the barthel index in the assessment of hospitalized older patients after admission for an acute medical condition. Arch Phys Med Rehabil. 2008;89:641–647. doi: 10.1016/j.apmr.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Richards SH, Peters TJ, Coast J, Gunnell DJ, Darlow MA, Pounsford J. Inter-rater reliability of the barthel adl index: How does a researcher compare to a nurse? Clin Rehabil. 2000;14:72–78. doi: 10.1191/026921500667059345. [DOI] [PubMed] [Google Scholar]
- 29.Sulter G, Steen C, De Keyser J. Use of the barthel index and modified rankin scale in acute stroke trials. Stroke. 1999;30:1538–1541. doi: 10.1161/01.str.30.8.1538. [DOI] [PubMed] [Google Scholar]
- 30.Shinar D, Gross CR, Bronstein KS, Licata-Gehr EE, Eden DT, Cabrera AR, Fishman IG, Roth AA, Barwick JA, Kunitz SC. Reliability of the activities of daily living scale and its use in telephone interview. Arch Phys Med Rehabil. 1987;68:723–728. [PubMed] [Google Scholar]
- 31.Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: The northern manhattan stroke study experience. Neurology. 1997;48:1204–1211. doi: 10.1212/wnl.48.5.1204. [DOI] [PubMed] [Google Scholar]
- 32.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The cardiac arrhythmia suppression trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer EJ, Lamon-Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, Abolafia JM, Lippel K, Levy RI. Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid research clinics coronary primary prevention trial. Jama. 1994;271:999–1003. doi: 10.1001/jama.1994.03510370051031. [DOI] [PubMed] [Google Scholar]
- 34.Dhamoon MS, Tai W, Boden-Albala B, Rundek T, Paik MC, Sacco RL, Elkind MS. Risk of myocardial infarction or vascular death after first ischemic stroke: The northern manhattan study. Stroke. 2007;38:1752–1758. doi: 10.1161/STROKEAHA.106.480988. [DOI] [PubMed] [Google Scholar]
- 35.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. Jama. 2004;291:1596–1602. doi: 10.1001/jama.291.13.1596. [DOI] [PubMed] [Google Scholar]
- 36.Bath PM, Lees KR, Schellinger PD, Altman H, Bland M, Hogg C, Howard G, Saver JL. Statistical analysis of the primary outcome in acute stroke trials. Stroke. 2012;43:1171–1178. doi: 10.1161/STROKEAHA.111.641456. [DOI] [PubMed] [Google Scholar]
- 37.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 38.Carroll R, Ruppert D. Robust estimation in heteroscedastic linear models. Ann Statist. 1982;10:429–441. [Google Scholar]
- 39.Willey JZ, Disla N, Moon YP, Paik MC, Sacco RL, Boden-Albala B, Elkind MS, Wright CB. Early depressed mood after stroke predicts long-term disability: The northern manhattan stroke study (nomass) Stroke. 2010;41:1896–1900. doi: 10.1161/STROKEAHA.110.583997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev. 2010;6:134–143. doi: 10.2174/157339910791162961. [DOI] [PubMed] [Google Scholar]
- 41.Chiu CJ, Wray LA. Physical disability trajectories in older americans with and without diabetes: The role of age, gender, race or ethnicity, and education. Gerontologist. 2011;51:51–63. doi: 10.1093/geront/gnq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathers CD, Lopez AD, Murray CJL. The burden of disease and mortality by condition: Data, methods, and results for 2001. 2006 [PubMed] [Google Scholar]
- 43.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older mexican americans with type 2 diabetes. Diabetes Care. 2003;26:2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 44.Royall DR, Palmer R, Chiodo LK, Polk MJ. Normal rates of cognitive change in successful aging: The freedom house study. J Int Neuropsychol Soc. 2005;11:899–909. doi: 10.1017/s135561770505109x. [DOI] [PubMed] [Google Scholar]
- 45.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke. 2002;33:21–25. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- 46.Hachinski V. World stroke day 2008: “Little strokes, big trouble”. Stroke. 2008;39:2407–2420. doi: 10.1161/STROKEAHA.108.531681. [DOI] [PubMed] [Google Scholar]
- 47.Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O’Leary D, Carr J, Furberg CD. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: The cardiovascular health study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 48.Hajjar I, Quach L, Yang F, Chaves PH, Newman AB, Mukamal K, Longstreth W, Jr, Inzitari M, Lipsitz LA. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: The cardiovascular health study. Circulation. 2011;123:858–865. doi: 10.1161/CIRCULATIONAHA.110.978114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ., Jr White matter hyperintensity on cranial magnetic resonance imaging: A predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 50.Kuller LH, Arnold AM, Longstreth WT, Jr, Manolio TA, O’Leary DH, Burke GL, Fried LP, Newman AB. White matter grade and ventricular volume on brain mri as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Baune BT, Schmidt WP, Roesler A, Berger K. Functional consequences of subcortical white matter lesions and mri-defined brain infarct in an elderly general population. J Geriatr Psychiatry Neurol. 2009;22:266–273. doi: 10.1177/0891988709342722. [DOI] [PubMed] [Google Scholar]
- 52.Pohjasvaara TI, Jokinen H, Ylikoski R, Kalska H, Mantyla R, Kaste M, Erkinjuntti T. White matter lesions are related to impaired instrumental activities of daily living poststroke. J Stroke Cerebrovasc Dis. 2007;16:251–258. doi: 10.1016/j.jstrokecerebrovasdis.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 54.Schmitz N, Wang J, Malla A, Lesage A. Joint effect of depression and chronic conditions on disability: Results from a population-based study. Psychosom Med. 2007;69:332–338. doi: 10.1097/PSY.0b013e31804259e0. [DOI] [PubMed] [Google Scholar]
- 55.Araújo JP, Friões F, Azevedo A, Lourenço P, Rocha-Gonçalves F, Ferreira A, Bettencourt P. Cholesterol — a marker of nutritional status in mild to moderate heart failure. International Journal of Cardiology. 2008;129:65–68. doi: 10.1016/j.ijcard.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 56.Akerblom JL, Costa R, Luchsinger JA, Manly JJ, Tang MX, Lee JH, Mayeux R, Schupf N. Relation of plasma lipids to all-cause mortality in caucasian, african-american and hispanic elders. Age Ageing. 2008;37:207–213. doi: 10.1093/ageing/afn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Onder G, Volpato S, Liperoti R, D’Arco C, Maraldi C, Fellin R, Bernabei R, Landi F. Total serum cholesterol and recovery from disability among hospitalized older adults. J Gerontol A Biol Sci Med Sci. 2006;61:736–742. doi: 10.1093/gerona/61.7.736. [DOI] [PubMed] [Google Scholar]
- 58.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 59.Dromerick AW, Edwards DF, Diringer MN. Sensitivity to changes in disability after stroke: A comparison of four scales useful in clinical trials. J Rehabil Res Dev. 2003;40:1–8. doi: 10.1682/jrrd.2003.01.0001. [DOI] [PubMed] [Google Scholar]
- 60.Suijker JJ, Buurman BM, ter Riet G, van Rijn M, de Haan RJ, de Rooij SE, Moll van Charante EP. Comprehensive geriatric assessment, multifactorial interventions and nurseled care coordination to prevent functional decline in community-dwelling older persons: Protocol of a cluster randomized trial. BMC Health Serv Res. 2012;12:85. doi: 10.1186/1472-6963-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.