Abstract
Autophagy, an evolutionally conserved process of controlled cellular cannibalization, plays a vital role in cardiac physiology. Perturbations in cardiomyocyte autophagy contribute to the pathogenesis of a wide range of cardiac diseases, many of which culminate in heart failure. With recent advances in cancer chemotherapy and consequent improvements in cancer survival, drug-induced toxicity to the heart has assumed greater importance. As a number of prominent cellular pathways are critical to the survival of both tumor cells and heart cells, it comes as little surprise that therapies targeting those pathways have consequences in both tissues. Little is known presently about cardiomyocyte autophagy, a prominent cellular response to stress, in the setting of chemotherapy, but preliminary evidence suggests an important and context-dependent role. Dissecting the role of autophagy in “onco-cardiology” will likely yield insights into mechanisms underlying cardiomyopathy and may lead to novel means to protect the myocardium from chemotherapy-induced injury.
INTRODUCTION
Recent advances in oncologic medicine, including early diagnosis and novel therapies, have significantly improved the long-term survival of patients with cancer [1]. This is especially true in pediatric oncology, as most children diagnosed with cancer today are expected to be long-term survivors [2]. However, as a consequence of these successes, cancer therapy-related complications are replacing tumor recurrence and secondary neoplasia as major clinical issues. Among those complications, cardiotoxicity has emerged as a prominent cause of chemotherapy-related co-morbidity and mortality [3], presenting as a spectrum of clinical manifestations that includes left ventricular dysfunction, arrhythmia, ischemia, and pericarditis. In addition, in many cancer patients concomitant cardiovascular comorbidities exist which synergize with the stress of chemotherapy. Also, emergence of new anti-cancer drugs and the prominence of combination therapies together heighten concern for potential untoward cardiac toxicities.
Our understanding of mechanisms underlying chemotherapy-induced cardiotoxicity is limited. Further complicating the picture is the fact that these mechanisms vary widely. Drugs such as anthracyclines and HER-2 receptor inhibitors provoke direct cardiomyocyte injury, while others such as anti-metabolics cause indirect cardiac effects by inducing hypertension or thrombotic events. Among the direct toxicities, accumulation of reactive oxygen species (ROS), mitochondrial damage, endoplasmic reticulum (ER) stress, disruption of pro-survival signaling pathways, and metabolic alterations have been implicated [4, 5]. Recent reviews have discussed molecular mechanisms associated with cancer chemotherapy [6]. Here, we focus specifically on autophagy, a less appreciated aspect of chemotherapy-induced cardiotoxicity. Although the possible role of cardiomyocyte autophagy in cancer therapy-induced cardiotoxicity is uncertain and numerous contradictory observations have been reported in literature, there are strong hints suggesting a significant contribution.
Autophagy and its molecular regulation
Autophagy, an evolutionarily conserved cellular cannibalization process, has gained increasing recognition in recent years for its vital role in cardiac physiology and pathology [7]. Autophagy is a generic name for different routes of delivery of cytosolic materials to the lysosome for degradation [8]. Three major forms of autophagy have been described: macroautophagy, microautophagy, and chaperone-mediated autophagy [8]. Macroautophagy, the most extensively studied type and hereafter termed autophagy, involves sequestration of cellular contents into double-membrane autophagosomes followed by cargo delivery to lysosomes for bulk degradation. At present, nothing is known about possible involvement of microautophagy or chaperone-mediated autophagy in chemotherapy-induced cardiomyopathy.
Autophagy is critical to cellular survival under baseline, resting conditions, serving to maintain cellular homeostasis, recycle cellular constituents such as mitochondria and ER, and eliminate misfolded, dysfunctional proteins. In response to cellular stress, such as starvation, autophagic activation is up-regulated, recycling macromolecules to replenish essential substrates for energy production [8, 9]. In animal models, defective autophagy leads to perinatal death due to severe nutritional deficiency prior to proper feeding [9]. In later stages of life, defective autophagy accelerates aging, promoting end-organ damage and reduced lifespan [10–12].
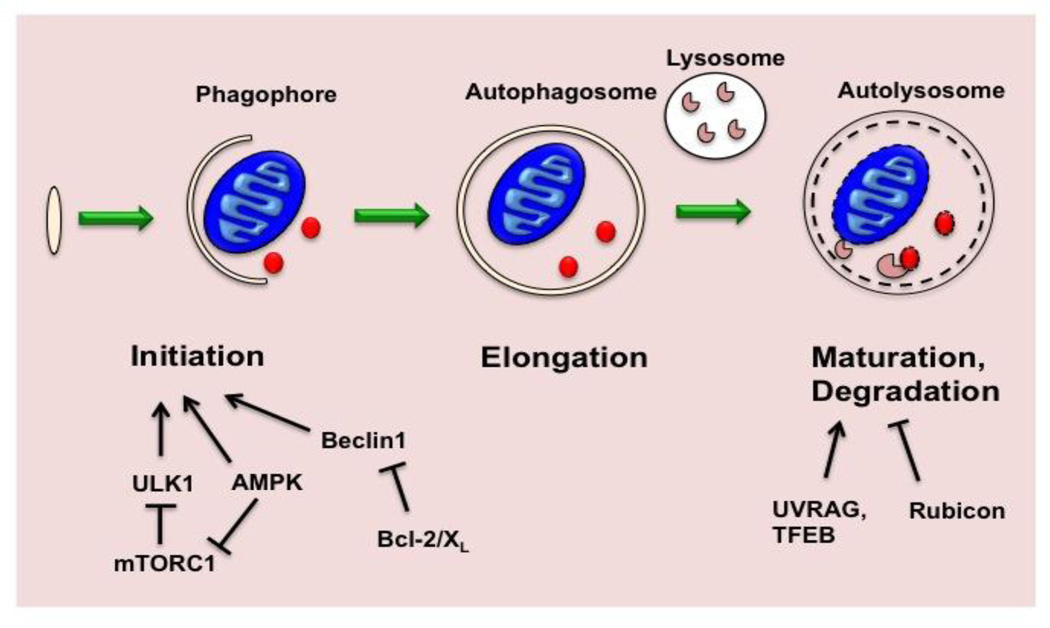
Molecular mechanisms of autophagy are highly conserved from yeast to human. Autophagy is initiated by formation of a phagophore, an isolated membrane that originates from the ER or other cellular membranes, such as mitochondria and plasma membrane [9, 13]. The process initiates with formation of a multiprotein complex containing Beclin 1, Atg14L, Vps34 and Vps15 (p150). Next, phagophore elongation is initiated by two ubiquitin-like conjugation cascades: a) the Atg5-Atg12 conjugation system, and b) the microtubule-associated light chain 3 (MAP-LC3/Atg8/LC3) conjugation system. As the phagophore elongates, it progressively engulfs a portion of the cytoplasm, including proteins and organelles. Ultimately, the phagophore membrane fuses on itself, forming the double-membrane autophagosome. Next, fusion of the autophagosome with a lysosome leads to formation of an autolysosome and degradation of intravesicular materials together with the inner membrane (Figure 1).
Figure 1. Regulation of autophagy.
Autophagy starts with nucleation of an intracellular membrane structure termed the phagophore. Elongation and closure of the phagophore leads to the formation of the autophagosome which harbors cytoplasmic material, such as protein aggregates or a mitochondrion. Subsequent fusion of the autophagosome with a lysosome (maturation) leads to degradation of the autophagosomal cargo and inner membrane by lysosomal enzymes. Initiation of the autophagic cascade is regulated by multiple elements, including mTORC1 and AMPK. Beclin1, a core component of the class III phosphatidylinositol 3-kinase VPS34 complex, can be sequestered by its binding to BCL-2 or BCL-XL, leading to inhibition of autophagy. Autophagosome maturation can also be regulated. TFEB, a transcription factor that governs lysosomal biogenesis as well as expression of genes coding for multiple autophagic proteins, promotes autophagic flux. Binding of Beclin1 to UVRAG promotes autophagosome maturation, while the Rubicon-Beclin1-UVRAG complex acts oppositely.
Several signaling pathways regulate the induction of autophagy [14]. The mammalian target of rapamycin complex 1 (mTORC1) is a major regulator of starvation-induced autophagy. mTORC1 suppresses autophagy induction mainly by phosphorylating ULK1/2, thereby inhibiting the cascade of autophagy induction. mTORC1 activity, in turn, is tightly controlled by availability of nutrients (e.g. amino acids), growth factors, and energy status as sensed by AMP-activated protein kinase (AMPK). AMPK also regulates autophagy by inhibiting mTORC1 and by phosphorylating Beclin 1 [15]. Further, phosphorylation of Bcl-2 and Bcl-XL disrupts their interaction with Beclin 1, leading to release of Beclin 1 for autophagy induction [16, 17].
Though less well characterized, autophagosome turnover, the second half of autophagy, is fundamental to the process. Disruption of autophagosome turnover has been observed in various human diseases, including Danon cardiomyopathy [18, 19]. Recent studies have shown that late events in autophagosome processing are regulated. Transcription factor EB (TFEB) induces expression of genes coding for both autophagic and lysosomal proteins. As a consequence, TFEB governs autophagic initiation, autophagosome-lysosome fusion, and lysosomal degradation of internal cargo [20, 21]. Other proteins, such as UVRAG [22], Rubicon [23], Rab7 [24], and Lamp2 [18], have also been suggested to play important roles in autophagosome-lysosome fusion.
Monitoring autophagy
There are multiple ways to measure autophagy, including assays using for both in vitro and in vivo studies [25]. Atg8/LC3 detection either by Western blot or by cellular imaging, and protein turnover assays, are commonly used.
Atg8/LC3 detection assays serve as a measure of autophagosomes abundance. Conversely, conversion of cytosolic LC3-I to phosphotidylethanolamine-conjugated LC3-II is used as a marker for autophagosome formation. However, it is critical to bear in mind that autophagy is a highly dynamic process; abundance of autophagosomes can reflect either induction of autophagosome formation or a defect in downstream autophagosome degradation (or both occurring together). Obviously, the implications of these two different events are vastly dissimilar. Therefore, it is of critical importance to assess autophagic flux, as opposed to relying on a single “snapshot in time” of autophagosomes levels within the cell.
Turnover of long-lived proteins is the oldest and “classical” means of assessing autophagic activity. More recently, however, assays of long-lived protein degradation have been supplanted largely by monitoring degradation of protein p62. However, given this protein’s multiple biological functions [26, 27], its transcriptional regulation by stress [28], and its decreased solubility in some contexts [25], p62 levels cannot be used reliably in every scenario as a reflection of autophagic flux.
Another common method to probe autophagic flux involves autophagosome quantification in the presence versus absence of pharmaceutical manipulations to block autophagic degradation. E64D, pepstatinA, chloroquine and bafilomycin A are commonly used for this purpose.
A third method to quantify autophagic flux involves usage of tandem fluorescence probes of LC3, such as mRFP/mCherry-GFP-LC3 [29]. As GFP fluorescence is selectively quenched in the acidic environment of the lysosome, autolysosomes emit a red signal and autophagosomes shine yellow (red + green).
Autophagy in cardiovascular disease
The heart is a dynamic organ marked by a high metabolic rate, requiring continuous production of ATP to maintain a healthy contractile state. Under conditions of nutrient insufficiency, autophagy is required for energy production [30]. Viewed from another angle, the cardiomyocyte’s abundance of mitochondria and high levels of oxidative phosphorylation render it susceptible to accumulation of ROS and injury from oxidative damage. Accumulation of ROS can lead to protein and membrane oxidation, organelle dysfunction, and ultimately cell injury and death. Working in concert with anti-oxidant enzymes, autophagy serves as an efficient housekeeper, eliminating misfolded proteins and damaged mitochondria.
Defective cardiomyocyte autophagy can result in cardiomyopathy and clinical heart failure, as well as premature cardiac aging [12]. Danon disease in humans is a well characterized example in which defective autophagic flux arising from Lamp2 deficiency leads to accumulation of autophagic vacuoles and ultimately heart failure [18, 19]. In addition, mice with cardiomyocyte-specific deletion of Atg5 (αMHC-Cre, Atg5 flox/flox) develop dilated cardiomyopathy in adulthood, again consistent with the notion that basal autophagic flux is critical for maintaining normal cardiomyocyte homeostasis and functional integrity [12].
Altered levels of autophagy have been observed in a wide spectrum of cardiac diseases. Whether autophagy is adaptive or maladaptive in these cases is unclear and likely depends on the context, extent of flux activation, efficiency of downstream lysosomal processing, and other aspects of the biology [7].
Increased numbers of autophagic vacuoles have been observed in heart samples from patients with dilated cardiomyopathy and heart failure [31]. However, due to technical limitations in assessing the dynamics of the autophagic cascade in human endomyocardial biopsy samples, it is not known whether the accumulation of vesicles within cardiomyocytes is due to increased activation of autophagic flux, defective downstream lysosomal degradation of autophagic cargo, or both. Also, whether autophagy is a culprit that promotes disease progression, or rather a failed compensatory strategy arising in response to disease-related stress, is not known. Finally, as heart failure is a syndrome defined on clinical terms and stemming from a wide range of cardiovascular diseases, it is likely that autophagy plays different roles in heart failure deriving from different etiologies.
Autophagy and chemotherapy-induced cardiomyopathy
Chemotherapeutic drugs induce cardiotoxicity via a variety of mechanisms, some with direct toxicity to the cardiomyocyte, while others affect other cell types (e.g. endothelial cells), or indirectly disrupt cardiac function by affecting blood pressure or the coagulation system. However, here we focus on potential direct effects of various drugs on cardiomyocyte autophagy and how autophagy might play a role in drug-induced cardiomyopathy. Clear conclusions have not been drawn so far in many cases. Yet, a handful of studies provide hints that autophagy may be important in this process.
Anthracyclines
Anthracyclines, including doxorubicin and epirubicin, are among the most efficacious anti-cancer chemotherapeutics. Anthracyclines are used in treatment of a variety of solid and liquid tumors, including hematologic malignancies, carcinoma of the breast, sarcomas, and more. However, clinical use of anthracycline is limited by dose-dependent and cumulative cardiotoxicity [32]. The incidence of cardiomyopathy is reported to be 5% at a cumulative dose of 400 mg/m2, 26% at 550 mg/m2, and 48% at 700 mg/m2. Subclinical cardiac dysfunction can be observed at low cumulative exposures, as well [32].
Despite extensive studies of anthracycline-induced cardiomyopathy, critical aspects of underlying mechanisms remain unclear. Generally ROS production is considered a major culprit of cardiomyocyte damage triggered by doxorubicin [33]. One recent study demonstrated that topoisomerase IIβ-dependent DNA damage in cardiomyocytes is critical in doxorubicin-induced cardiomyocyte damage [34]. Also, in recent years a number of studies have emerged focusing on the role of autophagy in doxorubicin-induced cardiotoxicity. However, studies employing either in vitro or in vivo platforms have generated conflicting results. Overcoming the challenges inherent to experimental models of doxorubicin cardiotoxicity will be required to facilitate emergence of new clinically relevant insights (Table 1).
Table 1.
In Vitro and in vivo models of autophagy in doxorubicin cardiotoxicity
| Model(s) | Description | Features | Ref | Advantage | Disadvantage |
|---|---|---|---|---|---|
| H9c2 | Embryonic rat BDIX heart myoblasts | Active replication | [36] | Can be passaged; used for cardiac biology studies | Morphologically different from cardiomyocytes; can differentiate into mytube-like structures |
| HL1 | AT-1 mouse atrial cardiomyocyte tumor lineage | Spontaneously contract; sarcomere structures | [33] | Can be passaged; Structural similarity to cardiomyocytes | Glycolytic metabolism, lack complex I activity; derived from atrial not ventricular cells. |
| NRVMs | Neonatal rat ventricular myocytes | Contractile | [34] | Well characterized for cardiac studies, readily available | Susceptible to apoptosis; |
| ARVMs | Adult rat ventricular myocytes | Cardiomyocyte morphology and biological features | [35] | Mostly resemble ventricular cardiomyocyte physiology | Relatively difficult to maintain in culture; relatively low cell yield |
| Rodents | Acute toxicity model | 1 large dose | [33, 41–44] | Study direct and acute responses and molecular changes | Less resemblance to clinal doxorubicin-cardiotoxity |
| Rodents | Chronic toxicity model | Serial small dose injections | [33, 45] | More clinically relevant; study cardiac function changes | Variable drug administration protocols. |
In vitro studies
Involvement of autophagy in doxorubicin cardiotoxicity has been tested in a variety of cell types in vitro, including neonatal rat ventricular myocytes (NRVMs), adult rat ventricular myocytes (ARVMs), and H9c2 cells (rat embryonic myoblast cell line) [35–37]. In general, ratios of LC3-II/LC3-I and p62 protein abundance were evaluated as the major criteria of autophagic activation. Kobayashi et al [35] and Dimitrakis et al [36] reported an increase in autophagy in doxorubicin-treated NRVMs (1 µM for 18 hours) and ARVMs (10–20 µM for 48 hours) respectively. In contrast, Sishi et al reported that LC3-II/LC3-I ratios in H9c2 cells were not altered by doxorubicin treatment (3 µM for 24 hours) [37]. However, autophagic flux was not rigorously studied in the latter two studies. Thus, involvement of autophagy in doxorubicin-exposed cardiomyocytes and cardiomyocyte-like cell lines remains inconclusive.
Several in vitro studies have yielded conflicting data regarding whether autophagy alters the cardiomyocyte response to doxorubicin. The Kobayashi group suggested that autophagy activation increased cell injury and death in doxorubicin-treated NRVMs [35]. However, Sishi et al reported opposite results in H9c2 cells [37].
Apart from the obvious differences in cell type, discrepancies observed among these studies might be explained by: 1) differing treatment strategies: co-incubating rapamycin or 3MA simultaneously with doxorubicin versus pre-stimulating autophagy before doxorubicin exposure; 2) Off-target effects of compounds used to modulate autophagy: rapamycin, 3-MA, chloroquine, and bafilomycin A1 all have biological actions beyond their effects on autophagy [38]. Even beclin1 1 levels affect events well beyond autophagosome formation, such as autophagosome/endosome maturation and endosome trafficking [39]. Therefore, further analyses with rigorous controls are required.
From another point of view, discrepancies observed in multiple studies may also reflect the complexity of studying drug-induced cardiotoxicity. No cell line optimally mimics human cardiac biology [40]. H9c2 cells do not resemble cardiomyocytes morphologically, and they actively proliferate; hence, they could be more prone to doxorubicin-induced cytotoxicity and cell death. NRVMs, although widely used and well characterized, differ from adult cardiomyocytes in many ways. Doxorubicin at 1–3 µM, a concentration used in many in vitro studies and reflective of plasma drug concentrations in patients receiving doxorubicin, triggers robust cell death in NRVMs and H9c2 cells within 24 hours, but a much higher concentration of doxorubicin (>20 µM) is required to induce cell death in ARVMs [41]. Indeed, acute cardiomyocyte death is not a frequent event in animal hearts even on exposure to lethal-dose doxorubicin, suggesting that cell death assays may not be accurately reflective of doxorubicin-induced cardiotoxicity.
In vivo studies
In vivo studies of doxorubicin-induced cardiomyopathy have employed rabbits, rodents, and zebrafish. In general, two modes of drug administration have been studied in rodent models: high-dose drug (10 mg/kg-20 mg/kg × 1–2 injections) and chronic exposure to serial low-dose drug (1–5 mg/kg × 3–15 injections). While early studies employed intravenous (i.v.) injections, some more recent studies have employed intraperitoneal (i.p.) injections, largely for sake of convenience. One limitation of i.p. doxorubicin exposure is local peritoneal inflammation which might alter bowel function and food intake, confounding the evaluation of autophagy.
Multiple in vivo studies arrived at dissimilar conclusions regarding how autophagy is regulated by doxorubicin in animal hearts. Several studies reported increases in LC3-II/LC3-I ratios in heart tissue after doxorubicin exposure, yet autophagic flux was not rigorously evaluated [42–44]. In contrast, Kawaguchi et al [45] reported that whereas steady state levels of LC3-II/LC3-I increased, inhibition of downstream lysosomal processing with bafilomycin A1 failed to increase those ratios further, suggesting a defect in downstream autophagosome processing. Interestingly, early studies reported that doxorubicin affects lysosome function in mouse heart chronically exposed to doxorubicin [46], which highlights the importance of evaluating autophagosome turnover and lysosome function in doxorubicin-treated hearts.
In a zebrafish model of doxorubicin cardiomyopathy [47], steady state LC3-II levels increased acutely upon doxorubicin exposure and later declined. It is plausible that the autophagic response differs in the acute phase relative to later phases. In aggregate, it is still debatable whether cardiac autophagy is up-regulated or rather blocked by doxorubicin. While maladaptive autophagy exacerbates cardiac function under stress [48], perturbed downstream autophagosome processes will also lead to cellular damage resulting from the accumulation of damaged mitochondria as well as ROS-damaged proteins [9].
The functional role of autophagy in the pathogenesis of doxorubicin cardiotoxicity has been explored in multiple in vivo studies. However, a wide range of sometimes-conflicting conclusions has been drawn. Lu et al [42] reported that 3MA treatment minimized mitochondrial damage and preserved cardiac function after exposure to high-dose doxorubicin. However, several studies have reported that boosting autophagy by either rapamycin or fasting is protective of doxorubicin-induced cardiac dysfunction. Other studies reported correlations between autophagic levels and the severity of doxorubicin cardiotoxicity [43, 44]; again, these studies cannot be used to infer causal mechanisms. In light of mTORC1’s regulation of autophagy, two studies evaluated mTORC1’s role in doxorubicin cardiotoxicity in rodents and zebrafish respectively, but also with conflicting results [43, 47].
At present, a consensus has not emerged regarding how cardiomyocyte autophagy is altered by doxorubicin or its possible role in the pathogenesis of anthracycline cardiomyopathy. To address this, additional assays in vitro, beyond simple cell death, are required: measurements of ROS levels and mitochondrial function should be examined. Further, it is critical to probe autophagic flux by inhibiting lysosomal processing, especially given that p62 expression can be induced by oxidative stress [28]. Tandem fluorescence probes can be informative, as well [29]. For in vivo models, it is critical to avoid the systemic effects of doxorubicin exposure, including lethargy, anorexia, and weight loss, as these are likely to alter autophagy secondarily. Careful time course analyses are needed, as acute responses may differ from more chronic events. As with in vitro experiments, autophagic flux must be tested.
Tyrosine kinase inhibitors
The advent of tyrosine kinase inhibitors (TKIs) has revolutionized cancer therapy. Compared with classical chemotherapeutics that target highly proliferating cells, TKIs are more selective of cancer cells that often manifest aberrant tyrosine kinase activities [49]. However, clinical use of several TKIs has uncovered negative cardiac side effects. Whereas cardiotoxicity is seen less frequently and is often less severe relative to doxorubicin-induced cardiotoxicity [50], it can be clinically meaningful in patients with comorbidities or receiving combination therapies.
TKIs fall into two categories: monoclonal antibodies, such as trastuzumab, and small molecules, such as imatinib and sunitinib. To date, cardiotoxicity has been reported with trastuzumab, lapatinib, imatinib, sorafenib, sunitinib, dasatinib, and bevicizumab in clinical trials and clinical practice [50]. Many of the TKIs are reported to alter autophagy in various tumor cell types as well as in noncancerous mammalian cells.
1. Abl inhibitors: imatinib and dasatinib
Initially developed to target the Bcr-Abl fusion protein for treating chronic myelogenous leukemia (CML), imatinib was eventually found to target multiple other kinases, including c-Abl, Arg, c-Kit, and the PDGF receptor [51]. Dasatinib and nilotinib are more recently developed Abl inhibitors that target the same kinases, and cardiotoxicity has been reported in clinical trials of each agent [50, 51]. It remains controversial whether cardiotoxicity is due specifically to Abl inhibition or suppression of other kinases vital to cardiomyocyte homeostasis.
Imatinib has been reported to induce autophagy in multiple human cancer cell lines, as well as in several mammalian cell lines, possibly by increasing lysosomal activity [52]. Furthermore, it has been suggested that autophagy promotes survival in CML cells, allowing them to evade imatinib-induced apoptosis [53]. However, exposure of neonatal cardiomyocytes to imatinib results in an increase in p62 levels along with autophagosome accumulation, together suggesting inhibition of autophagosome degradation [54]. Some evidence suggests that imatinib accumulates in lysosomes, perturbing their function and thereby blocking autophagy [54]. However, in the absence of careful measures of autophagic flux, the molecular effects of imatinib on autophagy remain unknown.
In a rat model of imatinib-induced cardiomyopathy [55], increased levels of LAMP-2 immunoreactivity were reported, hinting at a possible increase in lysosome function and autophagic flux. In other studies, imatinib has been reported to promote calcium-induced opening of the mitochondrial permeability transition pore, mitochondrial dysfunction, and compromised ATP production in mouse heart [51]. As autophagy is strongly induced by energy depletion, increases in flux in this context may be entirely secondary.
2. Multi-kinase inhibitors: sunitinib and sorafenib
Multi-kinase inhibitors target multiple tyrosine kinases that govern both angiogenesis and tumor proliferation. Sunitinib and sorafenib improve survival in patients with renal cell carcinoma and gastrointestinal stromal tumors [56]. However, both were associated with left ventricular dysfunction and congestive heart failure during clinical trials [56, 57].
Increased autophagosome number as well as increased LC3-II levels on Western blot are observed in H9c2 cells treated with sunitinib [58], although autophagic flux measurements were not performed. Sunitinib provokes energy compromise in NRVMs [59], which could trigger autophagy. Endomyocardial biopsy specimens from a patient with sunitinib-induced congestive heart failure revealed widespread swollen mitochondria, hinting at opening of the mitochondria permeability transition pore and ATP compromise [57]. Hence, the induction of autophagy could derive as a secondary consequence of energy compromise. Sorafenib, on the other hand, has been reported to up-regulate autophagy in multiple cancer cell types [60–62], but its effects on cardiomyocyte autophagy remain unknown.
3. Trastuzumab and lapatinib
These drugs target the human epidermal growth factor receptor 2 (HER-2), which is amplified in a subset of breast cancer cells. Trastuzumab is a recombinant monoclonal antibody against HER-2, while lapatinib is a dual TKI inhibiting both HER-2 and the epidermal growth factor receptor (EGFR) pathway. Due to robust clinical efficacy, trastuzumab has emerged as a key adjuvant therapeutic for both early and advanced stage breast cancer. However, cardiotoxicity ranging from subclinical functional declines to overt congestive heart failure has been observed in 0.4–4.1% of breast cancer patients receiving trastuzumab [63]. Moreover, when combined with anthracycline, the incidence of cardiac toxicity increases substantially. It is well established that neuregulin signaling through HER-2 plays an important role in cardiomyocyte homeostasis and survival; mice with cardiomyocyte-specific knockout of ERBB2, the gene coding for HER-2, develop dilated cardiomyopathy and heart failure [64]. However, lapatinib manifests much less cardiotoxicity relative to trastuzumab [65]. The difference in cardiotoxicity between the two drugs might be at least partially explained by AMPK activation: lapatinib activates AMPK in cardiomyocytes while trastuzumab does not [66].
Again, the possible role of autophagy here is unknown, although it is intriguing to speculate that it plays a differential role. First, AMPK, activated by lapatinib, is a robust regulator of autophagy. Consistent, lapatinib activates autophagy in breast cancer cells [67] and leukemic cells [68], whereas this has not been reported for trastuzumab. Second, recent work has revealed that HER-2 physically interacts with Beclin 1 in breast cancer cells, although the biological significance of this interaction is unclear [69].
Autophagy-targeting cancer therapies
In recent years, autophagy has emerged as a “hot topic” in cancer biology, even though its role remains incompletely characterized: it may promote tumorigenesis, or it could serve a protective mechanism promoting tumor cell survival, depending on the context and stage of the tumor.
On one hand, autophagy appears to suppress tumorigenesis. Beclin1+/− mice develop multiple neoplasms spontaneously [70]. Also, both hepatocyte-specific Atg7 knockout mice and mosaic Atg5-deficient mice develop benign hepatic adenomas [71]. One potential mechanism could be that defective autophagy leads to accumulation of p62, which promotes toxic accumulation of ROS, chromosomal instability, and activation of NF-κB [72]. On the other hand, autophagy promotes cancer cell survival by antagonizing metabolic stress. Tumor cells typically experience metabolic stress, such as hypoxia, ROS accumulation, and nutrient deprivation due to rapid growth, lack of blood supply, and a metabolic switch to glycolysis [73]. Each of these is capable of activating autophagy, which could replenish bioenergetic substrates and clear damaged organelles and misfolded protein aggregates. Therefore, cancer cells, especially the ones with defects in apoptosis, rely heavily on autophagy for survival. It is also speculated that autophagy is a key mechanism whereby cancer cells withstand chemotherapeutic attack and might account for cancer relapse after anti-cancer therapy [74].
As autophagy is a universal biological process, autophagy-targeting therapy is likely to have untoward actions in other systems, including the heart. As discussed, autophagy is vital in sustaining cardiomyocyte homeostasis and serves a protective role in response to multiple cardiac stresses; inhibiting autophagy might have detrimental cardiac effects, especially in patients with pre-existing heart disease. Thus, future development of drugs targeting autophagy warrants careful evaluation of cardiac effects.
1. mTORC1 inhibitors
A myriad of tumors manifest aberrant activation of the mTORC1 pathway [75]. Allosteric inhibitors of mTORC1, such as sirolimus/rapamycin, exert significant anti-tumor effects [76]. Temsirolimus and everolimus are derivatives of sirolimus and have been approved to treat advanced renal cell carcinoma and mantle cell lymphoma. As mTORC1 is a major regulator of autophagy, it is plausible that cardiac autophagy is regulated by these mTORC1-targeting agents. In fact, both temsirolimus and everolimus have been reported to increase cardiac autophagy in lamin A/C gene mutation-induced cardiomyopathy and cardiac remodeling postmyocardial infarction, respectively [77, 78]. Cardiotoxicity has not been reported in clinical trials of mTORC1 inhibitors; on the contrary, sirolimus and everolimus have also been used for immunosuppression after heart transplantation as well as in drug eluting coronary stents (although this delivery is highly localized within the coronary artery). In addition, temsirolimus and everolimus have been suggested to ameliorate cardiomyopathy caused by lamin A/C gene mutation in mice and left ventricular remodeling after MI in rats [77, 78].
2. Chloroquine and hydrochloroquine
Experience with the autophagy-targeting drugs chloroquine (CQ) and hydrochloroquine (HCQ) is informative of how autophagy-targeting cancer drugs might affect cardiac biology. Standard therapy for malaria and rheumatic diseases, both drugs permeate into and accumulate within lysosomes after protonation; as weak bases, they alter lysosomal pH and inhibit the activities of lysosomal enzymes, leading to suppression of autophagosome turnover. CQ enhances cytotoxicity of some chemotherapeutic drugs, such as SAHA and imatinib, in cancer cell lines [53, 79]. HCQ promotes tumor cell death triggered by alkylating drugs in a mouse model of myc-driven lymphoma [80]. Finally, in a small clinical trial, adding CQ to conventional therapy improved mid-term survival of patients with glioblastoma multiforme [81]. Presently, several clinical trials are underway to assess CQ and HCQ’s effects in various cancers, including lung (NCT00809237), prostate (NCT00786682), and breast (NCT00765765).
While CQ and HCQ are promising therapeutics for various cancers, cardiac concerns have been well described in long-term use of these drugs. Long-term exposure to CQ/HCQ can lead to damage in the cardiac conduction system and conduction block; less commonly, cardiomyopathy, either dilated or restrictive, has been reported [82]. In cases of CQ/HCQ-induced cardiomyopathy, the duration of drug use ranged from 4 months to 27 years, and the cumulative dosage ranged from 100g to >9,000g for CQ and 290g to >4,000g for HCQ[83]. Electron microscopy of endomyocardial biopsy samples from patients with CQ/HCQ-induced cardiomyopathy revealed myelin figures, large lysosomes, and curvilinear bodies, features consistent with perturbed autophagy [83]. Cardiotoxicity has been reported in lupus patients, suggesting higher risk of CQ/HCQ-induced cardiomyopathy in the setting of preexisting cardiac damage, auto-immune related or not. In a phase II clinical trial testing CQ for glioblastoma [81], it was used at 150 mg daily for 12 months; in a phase I study, HCQ was recommended at 1,000 mg per day. Persistent use of CQ/HCQ in cancer patients, therefore, could ultimately reach a range associated with CQ/HCQ cardiotoxicity.
Another concern is combination chemotherapy. Since synthetic lethality is an established mechanism in cancer treatment, combination therapy with autophagy inhibitors and other chemotherapeutic agents appears to be an attractive and promising strategy for the future. However, with combined therapy, autophagy levels in the heart could be altered extensively, posing increased risk of cardiac side effects.
Combined use of autophagy inhibitors and proteasomal inhibitors is intriguing, given their synergy in protein degradation and maintenance of cellular homeostasis. In cancer cells, inhibition of the proteasome activates autophagy, possibly due to ER stress and accumulation of aggregated misfolded protein. Autophagy serves a compensatory function in the setting of proteasome inhibition. Therefore, dual targeting of both the proteasome and autophagy may be particularly lethal to cancer cells, especially ones with high metabolic rate. However, the same phenomenon may exist in cardiomyocytes. Accumulation of ubiquitinated proteins and ER stress are observed in mouse hearts with afterload-induced failure, with increased proteasomal activity and autophagy levels [84]. Inhibition of the proteasome also induces autophagy in cardiomyocytes in vitro [84, 85]. Collectively, these facts suggest that both autophagy and the proteasome are critical to protein quality control, particularly in the stressed heart. In addition, cardiotoxicity has been reported in the literature, albeit rarely, with the use of the proteasome inhibitor bortezomib [86]. Therefore, the possibility exists that combination therapy with autophagy inhibitors and proteasome inhibitors could manifest cardiotoxicity.
Conclusion and perspective
Mounting evidence points to a vital role of autophagy in normal heart physiology and in a wide spectrum of cardiac diseases. Meanwhile, autophagy has emerged as a promising target for cancer therapy, as it has been shown to be a mechanism whereby various cancer cells withstand metabolic stress and cancer therapy cytotoxicity. Many of the chemotherapeutics currently in clinical use alter autophagy levels in cancer cells and/or cardiomyocytes, and it is quite plausible that autophagy plays an important role in cardiotoxicity caused by these drugs. Elucidation of this biology may help prevent or treat chemotherapy-induced cardiotoxicity. Additionally, development of future autophagy-targeting anti-cancer drugs warrants caution for cardiac side effects, especially when used in combination with other drugs known to affect autophagy levels.
The limitations and complexities of both in vitro and in vivo models of heart disease have plagued this field, leading to a literature replete with conflicting results. As we move this biology forward, new state-of-the-art experimental strategies must be harnessed to determine the functional role of autophagic flux in chemotherapy-stressed cardiomyocytes. While quantification of LC3-II and p62 levels and autophagosome numbers in imaging studies has revolutionized the study of autophagy, this is not sufficient to assess flux. Rigorous strategies to evaluate and modulate autophagic flux will be critical to elucidation of these complex biological questions and assist in advancing this biology to achieve enhanced clinical efficacy and minimal cardiac complications.
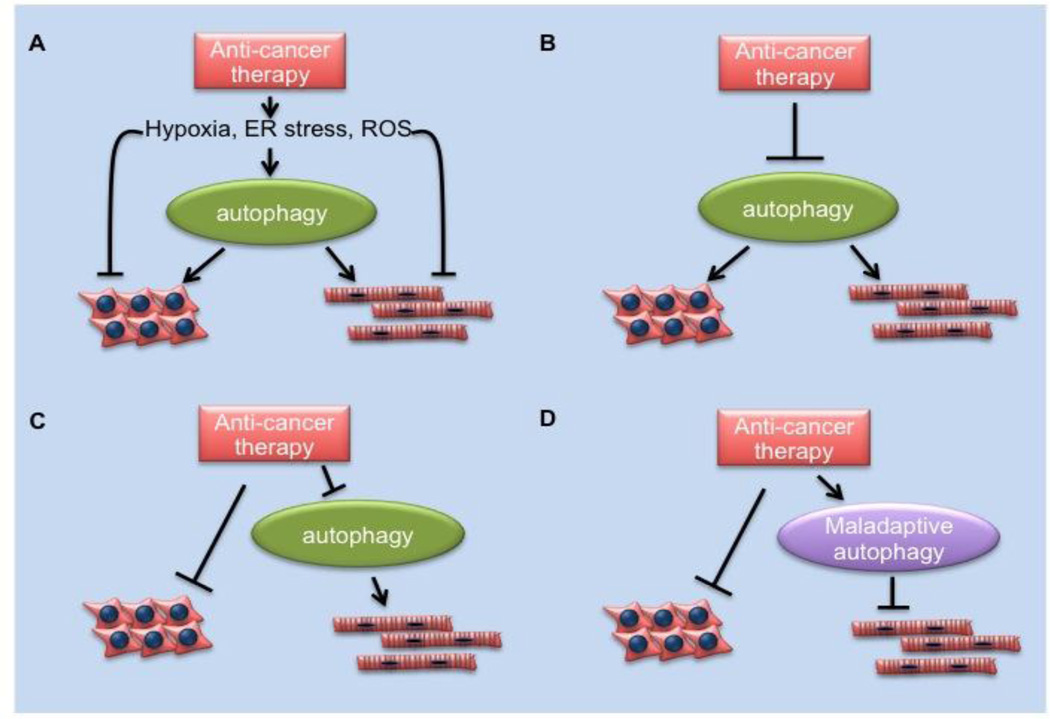
Figure 2. Possible roles of autophagy in chemotherapy-induced cardiomyopathy.
A. Chemotherapies (e.g. imatinib, sunitinib) induce cellular stress, such as hypoxia, ER stress, organelle damage, etc., which trigger autophagy. Autophagy functions as a protective mechanism in both cancer cells and cardiomyocytes. B. Anti-cancer agents inhibit autophagy directly (e.g. chloroquine) in both cancer cells and cardiomyocytes, causing damage to both cell types. C. Anti-cancer agents (e.g. possibly doxorubicin) have no clear effect on cancer cell autophagy but might block autophagy in cardiomyocytes, promoting cardiomyopathy. D. anticancer agents (e.g. possibly doxorubicin) might stimulate maladaptive autophagy in cardiomyocytes, promoting cardiomyopathy.
Table 2.
Summary of in vivo studies of autophagy in chemotherapy-induced cardiotoxicity
| Drug | Refs | Animal model |
Drug administration |
Endpoint (after last injection) |
Autophagy change | Autophagy’s role |
|---|---|---|---|---|---|---|
| Doxorubicin | [41] | rat | 6 × 2.5 mg/kg b.w., i.p. | 1 day | Increase in beclin 1 | 3MA administration reduced cardiac injury by doxorubicin |
| Doxorubicin | [44] | mouse | 2 × 10 mg/kg at 3 days interval | 2 days | Decrease in autophagic flux by LC3 immunobloting and GFP-LC3 puncta with chloroquine injection | pre-starvation partially rescued cardiac injury by doxorubicin |
| Doxorubicin | [43] | mouse | 20 mg/kg i.p. | 4 days | Increase in LC3-II/LC3-I by western blot | NA |
| Doxorubicin | [42] | mouse | 8 × 3.5 mg/kg i.p. weekly | 47 days | Increase in LC3-II/LC3-I, Beclin1, LAMP-1, p62 by western blot | NA |
| Doxorubicin | [46] | zebrafish | 20 µg/gbm i.p. | 3 days and 12 weeks | GFP-LC3 puncta increased 3 days post injection, decreased at 12 weeks | ztor heterozygous fish are partially protected from doxorubicin induced cardiac damage |
| Imatinib | [54] | rat | 10–100 mg/kg i.p. | 10–14 days | Increased lamp-2 immunoreactivity | NA |
Highlights.
We review studies of autophagy in chemotherapy-induced cardiotoxicity.
We discuss controversies and shortcomings of existing knowledge within the field.
We highlight the importance of preclinical models designed with clinical relevance.
We discuss the critical importance of measuring autophagic flux.
Acknowledgements
We thank members of the Hill lab for helpful discussion and comments.
Source of Funding
This work was supported by grants from the NIH (HL-0980842, HL-120732, HL-100401), Cancer Prevention and Research Institute of Texas (CPRIT, RP110486P3), the American Heart Association-DeHaan Foundation (0970518N), and the Fondation Leducq (11CVD04).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Disclosures
None
References
- 1.Valdivieso M, Kujawa AM, Jones T, Baker LH. Cancer survivors in the United States: a review of the literature and a call to action. International journal of medical sciences. 2012;9:163–173. doi: 10.7150/ijms.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Pal HJ, van Dalen EC, van Delden E, van Dijk IW, Kok WE, Geskus RB, et al. High risk of symptomatic cardiac events in childhood cancer survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 3.Khakoo AY, Yeh ET. Therapy insight: Management of cardiovascular disease in patients with cancer and cardiac complications of cancer therapy. Nature clinical practice Oncology. 2008;5:655–667. doi: 10.1038/ncponc1225. [DOI] [PubMed] [Google Scholar]
- 4.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. Journal of the National Cancer Institute. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sereno M, Brunello A, Chiappori A, Barriuso J, Casado E, Belda C, et al. Cardiac toxicity: old and new issues in anti-cancer drugs. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2008;10:35–46. doi: 10.1007/s12094-008-0150-8. [DOI] [PubMed] [Google Scholar]
- 6.Ky B, Vejpongsa P, Yeh ET, Force T, Moslehi JJ. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circulation research. 2013;113:754–764. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavandero S, Troncoso R, Rothermel BA, Martinet W, Sadoshima J, Hill JA. Cardiovascular autophagy: Concepts, controversies and perspectives. Autophagy. 2013;9 doi: 10.4161/auto.25969. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 11.Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, et al. Autophagy is required to maintain muscle mass. Cell metabolism. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 13.Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–393. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nature cell biology. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, et al. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. The EMBO journal. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 19.Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 20.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 21.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, et al. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nature cell biology. 2008;10:776–787. doi: 10.1038/ncb1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nature cell biology. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 24.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, et al. Role for Rab7 in maturation of late autophagic vacuoles. Journal of cell science. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 25.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Molecular cell. 2013;51:283–296. doi: 10.1016/j.molcel.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nature cell biology. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 28.Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. The Journal of biological chemistry. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 30.Kanamori H, Takemura G, Maruyama R, Goto K, Tsujimoto A, Ogino A, et al. Functional significance and morphological characterization of starvation-induced autophagy in the adult heart. The American journal of pathology. 2009;174:1705–1714. doi: 10.2353/ajpath.2009.080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Japanese circulation journal. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 32.Sheppard RJ, Berger J, Sebag IA. Cardiotoxicity of cancer therapeutics: current issues in screening, prevention, and therapy. Frontiers in pharmacology. 2013;4:19. doi: 10.3389/fphar.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Progress in cardiovascular diseases. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nature medicine. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. The Journal of biological chemistry. 2010;285:793–804. doi: 10.1074/jbc.M109.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimitrakis P, Romay-Ogando MI, Timolati F, Suter TM, Zuppinger C. Effects of doxorubicin cancer therapy on autophagy and the ubiquitin-proteasome system in long-term cultured adult rat cardiomyocytes. Cell and tissue research. 2012;350:361–372. doi: 10.1007/s00441-012-1475-8. [DOI] [PubMed] [Google Scholar]
- 37.Sishi BJ, Loos B, van Rooyen J, Engelbrecht AM. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochemical pharmacology. 2013;85:124–134. doi: 10.1016/j.bcp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nature reviews Molecular cell biology. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He C, Levine B. The Beclin 1 interactome. Current opinion in cell biology. 2010;22:140–149. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Force T, Kolaja KL. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nature reviews Drug discovery. 2011;10:111–126. doi: 10.1038/nrd3252. [DOI] [PubMed] [Google Scholar]
- 41.Konorev EA, Vanamala S, Kalyanaraman B. Differences in doxorubicin-induced apoptotic signaling in adult and immature cardiomyocytes. Free radical biology & medicine. 2008;45:1723–1728. doi: 10.1016/j.freeradbiomed.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Wu W, Yan J, Li X, Yu H, Yu X. Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. International journal of cardiology. 2009;134:82–90. doi: 10.1016/j.ijcard.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 43.Ma Y, Zhang X, Bao H, Mi S, Cai W, Yan H, et al. Toll-like receptor (TLR) 2 and TLR4 differentially regulate doxorubicin induced cardiomyopathy in mice. PloS one. 2012;7:e40763. doi: 10.1371/journal.pone.0040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Kang YM, Tian C, Zeng Y, Jia LX, Ma X, et al. Overexpression of Nrdp1 in the heart exacerbates doxorubicin-induced cardiac dysfunction in mice. PloS one. 2011;6:e21104. doi: 10.1371/journal.pone.0021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawaguchi T, Takemura G, Kanamori H, Takeyama T, Watanabe T, Morishita K, et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovascular research. 2012;96:456–465. doi: 10.1093/cvr/cvs282. [DOI] [PubMed] [Google Scholar]
- 46.Singal PK, Segstro RJ, Singh RP, Kutryk MJ. Changes in lysosomal morphology and enzyme activities during the development of adriamycin-induced cardiomyopathy. The Canadian journal of cardiology. 1985;1:139–147. [PubMed] [Google Scholar]
- 47.Ding Y, Sun X, Huang W, Hoage T, Redfield M, Kushwaha S, et al. Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circulation research. 2011;109:658–669. doi: 10.1161/CIRCRESAHA.111.248260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. The Journal of clinical investigation. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen MH, Kerkela R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118:84–95. doi: 10.1161/CIRCULATIONAHA.108.776831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nature reviews Cancer. 2007;7:332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 51.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nature medicine. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 52.Ertmer A, Huber V, Gilch S, Yoshimori T, Erfle V, Duyster J, et al. The anticancer drug imatinib induces cellular autophagy. Leukemia. 2007;21:936–942. doi: 10.1038/sj.leu.2404606. [DOI] [PubMed] [Google Scholar]
- 53.Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M, et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. The Journal of clinical investigation. 2009;119:1109–1123. doi: 10.1172/JCI35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu W, Lu S, McAlpine I, Jamieson JD, Lee DU, Marroquin LD, et al. Mechanistic investigation of imatinib-induced cardiac toxicity and the involvement of c-Abl kinase. Toxicological sciences : an official journal of the Society of Toxicology. 2012;129:188–199. doi: 10.1093/toxsci/kfs192. [DOI] [PubMed] [Google Scholar]
- 55.Herman EH, Knapton A, Rosen E, Thompson K, Rosenzweig B, Estis J, et al. A multifaceted evaluation of imatinib-induced cardiotoxicity in the rat. Toxicologic pathology. 2011;39:1091–1106. doi: 10.1177/0192623311419524. [DOI] [PubMed] [Google Scholar]
- 56.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 57.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y, Xue T, Yang X, Zhu H, Ding X, Lou L, et al. Autophagy plays an important role in sunitinib-mediated cell death in H9c2 cardiac muscle cells. Toxicology and applied pharmacology. 2010;248:20–27. doi: 10.1016/j.taap.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Kerkela R, Woulfe KC, Durand JB, Vagnozzi R, Kramer D, Chu TF, et al. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clinical and translational science. 2009;2:15–25. doi: 10.1111/j.1752-8062.2008.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimizu S, Takehara T, Hikita H, Kodama T, Tsunematsu H, Miyagi T, et al. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. International journal of cancer Journal international du cancer. 2012;131:548–557. doi: 10.1002/ijc.26374. [DOI] [PubMed] [Google Scholar]
- 61.Lin JC, Liu CL, Lee JJ, Liu TP, Ko WC, Huang YC, et al. Sorafenib induces autophagy and suppresses activation of human macrophage. International immunopharmacology. 2013;15:333–339. doi: 10.1016/j.intimp.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW, et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7:1159–1172. doi: 10.4161/auto.7.10.16818. [DOI] [PubMed] [Google Scholar]
- 63.Popat S, Smith IE. Therapy Insight: anthracyclines and trastuzumab--the optimal management of cardiotoxic side effects. Nature clinical practice Oncology. 2008;5:324–335. doi: 10.1038/ncponc1090. [DOI] [PubMed] [Google Scholar]
- 64.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nature medicine. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 65.Azim H, Azim HA, Jr, Escudier B. Trastuzumab versus lapatinib: the cardiac side of the story. Cancer treatment reviews. 2009;35:633–638. doi: 10.1016/j.ctrv.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Spector NL, Yarden Y, Smith B, Lyass L, Trusk P, Pry K, et al. Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cardiac cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10607–10612. doi: 10.1073/pnas.0701286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu X, Wu L, Qiao H, Han T, Chen S, Liu X, et al. Autophagy stimulates apoptosis in HER2-overexpressing breast cancers treated by lapatinib. Journal of cellular biochemistry. 2013 doi: 10.1002/jcb.24611. [DOI] [PubMed] [Google Scholar]
- 68.Huang HL, Chen YC, Huang YC, Yang KC, Pan H, Shih SP, et al. Lapatinib induces autophagy, apoptosis and megakaryocytic differentiation in chronic myelogenous leukemia K562 cells. PloS one. 2011;6:e29014. doi: 10.1371/journal.pone.0029014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han J, Hou W, Lu C, Goldstein LA, Stolz DB, Watkins SC, et al. Interaction between Her2 and Beclin-1 Proteins Underlies a New Mechanism of Reciprocal Regulation. The Journal of biological chemistry. 2013;288:20315–20325. doi: 10.1074/jbc.M113.461350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. The Journal of clinical investigation. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, et al. Autophagy-deficient mice develop multiple liver tumors. Genes & development. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nature reviews Clinical oncology. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 74.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nature reviews Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. The Journal of clinical investigation. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nature reviews Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 77.Choi JC, Muchir A, Wu W, Iwata S, Homma S, Morrow JP, et al. Temsirolimus activates autophagy and ameliorates cardiomyopathy caused by lamin A/C gene mutation. Science translational medicine. 2012;4:144ra02. doi: 10.1126/scitranslmed.3003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. Journal of the American College of Cardiology. 2009;54:2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 79.Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maclean KH, Dorsey FC, Cleveland JL, Kastan MB. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. The Journal of clinical investigation. 2008;118:79–88. doi: 10.1172/JCI33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Annals of internal medicine. 2006;144:337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- 82.Teixeira RA, Martinelli Filho M, Benvenuti LA, Costa R, Pedrosa AA, Nishioka SA. Cardiac damage from chronic use of chloroquine: a case report and review of the literature. Arquivos brasileiros de cardiologia. 2002;79:85–88. doi: 10.1590/s0066-782x2002001000009. [DOI] [PubMed] [Google Scholar]
- 83.Soong TR, Barouch LA, Champion HC, Wigley FM, Halushka MK. New clinical and ultrastructural findings in hydroxychloroquine-induced cardiomyopathy--a report of 2 cases. Human pathology. 2007;38:1858–1863. doi: 10.1016/j.humpath.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 84.Tannous P, Zhu H, Nemchenko A, Berry JM, Johnstone JL, Shelton JM, et al. Intracellular protein aggregation is a proximal trigger of cardiomyocyte autophagy. Circulation. 2008;117:3070–3078. doi: 10.1161/CIRCULATIONAHA.107.763870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nowis D, Maczewski M, Mackiewicz U, Kujawa M, Ratajska A, Wieckowski MR, et al. Cardiotoxicity of the anticancer therapeutic agent bortezomib. The American journal of pathology. 2010;176:2658–2668. doi: 10.2353/ajpath.2010.090690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bockorny M, Chakravarty S, Schulman P, Bockorny B, Bona R. Severe heart failure after bortezomib treatment in a patient with multiple myeloma: a case report and review of the literature. Acta haematologica. 2012;128:244–247. doi: 10.1159/000340050. [DOI] [PubMed] [Google Scholar]