Abstract
Background
NSABP P-1 provides an opportunity to examine the association of behavioral factors with prospectively monitored cancer incidence and interactions with tamoxifen.
Methods
From 1992–1997, 13,388 women with estimated 5-year breast cancer (BC) risk greater than 1.66% or a history of lobular carcinoma in situ (87% under age 65; 67% post-menopausal) were randomly assigned to tamoxifen versus placebo. Invasive BC, lung (LC), colon (CC), and endometrial cancers (EC) were analyzed with Cox regression. Predictors were baseline cigarette smoking, leisure-time physical activity, alcohol consumption, and established risk factors.
Results
At median 7 years follow-up, we observed 395, 66, 35, and 74 BC, LC, CC, and EC, respectively. Women who had smoked were at increased risk of BC (P=.007; hazard ratio (HR)=1.3 for 15–35 years smoking, HR=1.6 for ≥35 years), LC (P<.001; HR=3.9 for 15–35 years; HR=18.4 for ≥35 years), and CC (P<.001; HR=5.1 for ≥35 years) versus never-smokers. Low activity predicted increased BC risk only among women assigned to placebo (P=.021 activity main effect, P=.013 activity-treatment interaction; HR=1.4 for placebo group) and EC among all women (P=.026, HR=1.7). Moderate alcohol (>0–1 drink/day) was associated with decreased risk of CC (P=.019; HR=.35) versus no alcohol. There were no other significant associations between these behaviors and cancer risk.
Conclusion
Among women with elevated risk of BC, smoking has an even greater impact on BC risk than observed in past studies in the general population.
Impact
Women who smoke or are inactive should be informed of the increased risk of multiple types of cancer.
Keywords: Breast cancer, tobacco, physical activity, alcohol, colon cancer, endometrial cancer, lung cancer
Introduction
Cigarette smoking, physical activity, and alcohol consumption have been implicated in previous studies as risk or protective factors for cancer at a number of organ sites. Cigarette smoking, long known to increase the risk of lung cancer, is also associated with increased risk of many other cancers including those of the colon and breast (1–10). There is an inverse effect of cigarette smoking for endometrial cancer, especially among postmenopausal overweight or obese women (due possibly to an anti-estrogenic effect of smoking) (11–16). Physical activity appears to be a protective factor for many cancers (17–21). Alcohol consumption has been associated with increased risks of breast, lung, and colon cancer, among others (22–24). The evidence is stronger for some of these associations than others, and the generalizability across populations varies as well.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial (BCPT, also known as P-1) tested the drug tamoxifen for the reduction of the rate of breast cancer in high-risk women without a history of breast cancer. NSABP P-1 results indicated that a 5-year course of daily tamoxifen was associated with a 50% reduction in the primary incidence of invasive breast cancer relative to a placebo (25). In NSABP P-1, participants reported their baseline cigarette smoking history, leisure-time physical activity, and alcohol consumption. NSABP P-1 provides an important opportunity to examine the association of behavioral risk factors with prospectively monitored cancer incidence in a cohort of women at high risk of breast cancer. In this primary report of behavioral risk factors and cancer incidence in NSABP P-1, we examine the associations of baseline cigarette smoking history, leisure-time physical activity, and alcohol consumption, with the four most commonly occurring cancers among NSABP P-1 participants: invasive cancer of the breast, lung, colon, and endometrium.
Materials and Methods
Participants
This is a secondary analysis of the NSABP P-1 database. NSABP P-1, which was funded by the National Cancer Institute, was a double-blinded, placebo-controlled clinical trial that was open for accrual at several hundred clinical centers throughout North America from June 1, 1992, through September 30, 1997. During this interval, 13,388 women were randomly assigned to receive either 20 mg/day of tamoxifen or placebo for a duration of five years (25). The risk of breast cancer was estimated using the Gail model, which incorporates a woman’s age at menarche, number of benign breast biopsies, histological diagnosis of atypical hyperplasia, nulliparity or age at first live birth, and number of first-degree relatives with breast cancer (26). Participants were required to have an estimated 5-year risk greater than 1.66% or a history of lobular carcinoma in situ (LCIS). They must have discontinued hormone use (hormone replacement or oral contraceptives) 3 months prior to enrollment. Exclusion criteria included a history of cancer (other than basal or squamous cell carcinoma of the skin or carcinoma in situ of the cervix) within the prior 10 years, or any history of breast cancer other than LCIS. All participants provided informed consent, which was approved by the Institutional Review Boards of all participating institutions.
Cancer surveillance
Participants underwent clinical examinations (including medical history since last visit, height, weight, physical breast exam, complete blood count, platelet count, alkaline phosphatase, calcium, liver functions, and renal tests) every 6 months, and gynecologic examinations and mammograms annually. Documentation of all cancer events and all hospitalizations was reviewed centrally to verify cancer diagnoses. After a first diagnosis of cancer, surveillance continued, so that the first invasive event at each organ site was reported independently.
Measures
A patient-reported instrument administered upon enrollment collected cigarette smoking data (at least 100 cigarettes in lifetime, age of initiation, current smoking frequency and intensity, past intensity, and age at quitting if a former smoker). The instrument also provided descriptions of physical inactivity, and light, moderate, or vigorous physical activity. Participants were asked to select one of these 4 levels to describe their activity during their leisure time in the previous 12 months. Frequency and quantity (serving size and number of servings consumed) of beer, wine, and liquor over the previous 12 months were reported in separate items. Items were adapted from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial (27).
Statistical considerations
Separate multivariable Cox proportional hazards regression analyses were conducted for the time from random assignment to the diagnosis of invasive cancer of the breast, lung, colon, and endometrium. Time was censored at the date of last follow-up or date of death without evidence of the particular cancer. We provide results for smoking duration, classified as never, <15 years, 15–35 years, and ≥35 years [using cutoffs of 15 and 35 years based on an example colon cancer prediction tool (28) and the PDQ Smoking in Cancer Care evidence review published by the National Cancer Institute that found increased colorectal cancer risk after 35 years of smoking]. Because there is not a consensus regarding the best measures of cigarette smoking history (29), we provide results for candidate alternative measures in the Appendix: smoking status at baseline (current, former, never); intensity (none, 0–1 pack per day, or >1 pack per day);and additional candidate variables derived from duration, status, and intensity. Physical activity was classified a priori as inactive/light versus moderate/heavy. The quantities of alcoholic beverages were first converted to a common unit “drink” (12 oz. [355 ml] beer, 5 oz. [148 ml] wine, or 1.5 oz. [44 ml] spirits) and then added across beer, wine, and liquor. Alcohol consumption was classified a priori as none, 1, or more than 1 drink/day, on average, distinguishing healthy or unhealthy consumption based on the 2005 U.S. Department of Agriculture recommendation that women who choose to drink limit their consumption to up to 1 drink/day on average, following the approach used in a recent publication (30). We fit one model for each cancer disease site, including smoking duration, physical activity, alcohol consumption, and other candidate predictors that were selected a priori based on existing literature on risk factors and primary cancer incidence. The variables included in each disease site model are indicated in Table 1 (characteristics column). Assigned treatment group, age (≥65), participant-reported race/ethnicity, menopausal status, and prior estrogen use were included as candidate predictors of risk for the analysis of all of the organ sites of cancer being assessed. Other candidate predictors included for analyses differed by cancer site being assessed. Estimated breast cancer risk (Gail score analyzed as a continuous variable) and diabetes were also included for the analysis of breast cancer incidence. Family cancer history and personal history of tuberculosis were included for the analysis of lung cancer. For the analysis of colon cancer, family cancer history and current aspirin use were included. Age at menarche, number of past pregnancies, family cancer history, and diabetes were included for the analysis of endometrial cancer. Pre-treatment values were used for all explanatory variables. Stepwise selection determined the medical/demographic factors to include in the final model for each organ site. Candidate interactions tested were: two- and three-way interactions between treatment group, smoking, and menstrual status. In the model for breast cancer, we also tested whether the effect of tamoxifen on breast cancer incidence was modified by alcohol consumption or physical activity at the time of random assignment. The significance of explanatory variables was tested at two-sided alpha level 0.05.
Table 1.
Participant characteristics: NSABP P-1
| Characteristica | Placebo N=6707 (50.1%) |
Tamoxifen N=6681 (49.9%) |
Total (N=13388) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| AGE, years (B, L, C, E) | ||||||
| < 65 | 5797 | 86.4 | 5783 | 86.6 | 11580 | 86.5 |
| ≥ 65 | 910 | 13.6 | 898 | 13.4 | 1808 | 13.5 |
| RACE (B, L, C, E) | ||||||
| White | 6352 | 94.7 | 6337 | 94.8 | 12689 | 94.8 |
| Black | 133 | 2 | 143 | 2.1 | 276 | 2.1 |
| Hispanic | 94 | 1.4 | 73 | 1.1 | 167 | 1.2 |
| Other/Unknown | 128 | 1.9 | 128 | 2 | 256 | 1.9 |
| LEISURE TIME - PHYSICAL ACTIVITY (B, L, C, E) | ||||||
| Moderate - Heavy | 3058 | 45.6 | 3067 | 45.9 | 6125 | 45.7 |
| Inactive - Light | 3626 | 54.1 | 3586 | 53.7 | 7212 | 53.9 |
| Unknown | 23 | 0.3 | 28 | 0.4 | 51 | 0.4 |
| BASELINE BODY MASS INDEX (BMI; Bb, Eb) | ||||||
| Healthy (BMI < 25) | 2603 | 38.8 | 2635 | 39.4 | 5238 | 39.1 |
| Overweight (BMI ≥ 25 to < 30) | 2233 | 33.3 | 2157 | 32.3 | 4390 | 32.8 |
| Obese (BMI ≥ 30) | 1871 | 27.9 | 1889 | 28.3 | 3760 | 28.1 |
| ALCOHOL CONSUMPTION (B, L, C, E) | ||||||
| None | 1421 | 21.2 | 1366 | 20.5 | 2787 | 20.8 |
| 1 drink/day | 4383 | 65.3 | 4439 | 66.4 | 8822 | 65.9 |
| > 1 drink/day | 877 | 13.1 | 847 | 12.7 | 1724 | 12.9 |
| Unknown | 26 | 0.4 | 29 | 0.4 | 55 | 0.4 |
| SMOKING STATUS (B, L, C, E) | ||||||
| Never smoked | 3707 | 55.3 | 3612 | 54.1 | 7319 | 54.7 |
| Current smoker | 827 | 12.3 | 847 | 12.7 | 1674 | 12.5 |
| Former smoker | 2150 | 32.1 | 2195 | 32.8 | 4345 | 32.5 |
| Unknown | 23 | 0.3 | 27 | 0.4 | 50 | 0.4 |
| SMOKING DURATION (B, L, C, E) | ||||||
| Never smokedc | 3715 | 55.4 | 3620 | 54.2 | 7335 | 54.8 |
| < 15 smoking years | 839 | 12.5 | 825 | 12.3 | 1664 | 12.4 |
| ≥ 15 to < 35 smoking years | 1607 | 24 | 1707 | 25.6 | 3314 | 24.8 |
| ≥ 35 smoking years | 514 | 7.7 | 497 | 7.4 | 1011 | 7.6 |
| Unknown | 32 | 0.5 | 32 | 0.5 | 64 | 0.5 |
| SMOKING INTENSITY (B, L, C, E) | ||||||
| None | 3707 | 55.3 | 3612 | 54.1 | 7319 | 54.7 |
| 1 pack/day | 2008 | 29.9 | 2069 | 31 | 4077 | 30.5 |
| > 1 packs/day | 968 | 14.4 | 972 | 14.5 | 1940 | 14.5 |
| Unknown | 24 | 0.4 | 28 | 0.4 | 52 | 0.4 |
| 5-YEAR BREAST CANCER RISK (B)d | ||||||
| ≤ 2% | 1682 | 25.1 | 1677 | 25.1 | 3359 | 25.1 |
| > 2% and ≤ 5% | 3895 | 58.1 | 3823 | 57.2 | 7718 | 57.6 |
| > 5% | 1130 | 16.8 | 1181 | 17.7 | 2311 | 17.3 |
| FAMILY CANCER HISTORY (# family members who developed cancer; L, C, E) | ||||||
| None | 759 | 11.3 | 731 | 10.9 | 1490 | 11.1 |
| 1 | 2709 | 40.4 | 2717 | 40.7 | 5426 | 40.5 |
| > 1 | 3183 | 47.5 | 3182 | 47.6 | 6365 | 47.5 |
| Unknown | 56 | 0.8 | 51 | 0.8 | 107 | 0.8 |
| MENSTRUAL PERIOD STOPPED (B, L, C, E) | ||||||
| No | 2254 | 33.6 | 2159 | 32.8 | 4449 | 33.2 |
| Yes | 4453 | 66.4 | 4486 | 67.2 | 8939 | 66.8 |
| ESTROGEN USE (B, L, C, E) | ||||||
| Never used | 1619 | 24.1 | 1542 | 23.1 | 3161 | 23.6 |
| Ever used | 5088 | 75.9 | 5139 | 76.9 | 10227 | 76.4 |
| AGE AT MENARCHE (years; E) | ||||||
| 7–10 | 567 | 8.5 | 576 | 8.6 | 1143 | 8.5 |
| 11–13 | 4868 | 72.6 | 4902 | 73.4 | 9770 | 73 |
| ≥ 14 | 1243 | 18.5 | 1180 | 17.7 | 2423 | 18.1 |
| Unknown | 29 | 0.4 | 23 | 0.3 | 52 | 0.4 |
| EVER HAD DIABETES (B, E) | ||||||
| No | 6443 | 96.1 | 6402 | 95.8 | 12845 | 95.9 |
| Yes | 264 | 3.9 | 279 | 4.2 | 543 | 4.1 |
| PAST PREGNANCY (E) | ||||||
| No | 1011 | 15.1 | 1009 | 15.1 | 2020 | 15.1 |
| Yes | 5690 | 84.8 | 5669 | 84.9 | 11359 | 84.8 |
| Unknown | 6 | 0.1 | 3 | 0 | 9 | 0.1 |
| CURRENT REGULAR ASPIRIN USE (C) | ||||||
| No | 5651 | 84.3 | 5610 | 84.0 | 11261 | 84.1 |
| Yes | 1056 | 15.7 | 1071 | 16.0 | 2127 | 15.9 |
| PERSONAL HISTORY OF TUBERCULOSIS (L) | ||||||
| No | 6629 | 98.8 | 6581 | 98.5 | 13210 | 98.7 |
| Yes | 78 | 1.2 | 100 | 1.5 | 178 | 1.3 |
Characteristic included in disease site models in parentheses: breast (B), lung (L), colon (C), and endometrial (E)
Overweight/obesity was included in secondary analyses for models with significant effects of physical activity. These were the models for breast and endometrial cancers.
Includes former smokers who indicated 0 years of smoking history
Estimated breast cancer risk is included as a continuous variable in models.
Height and weight were also measured at each follow-up, from which we computed body mass index (BMI). However, BMI is not a focus of the present report, as it has been examined in detail as a predictor of breast cancer separately (31). Overweight/obesity has also been identified in previous literature as a risk factor for endometrial cancers. However, it may be on the same causal pathway as physical activity, and we were interested in physical activity as a factor in cancer prevention, whether or not it acts through obesity reduction. Therefore, our primary analyses did not adjust for overweight/obesity. (For further discussion of this issue, see Moore et al (32)). Secondary analyses were performed to examine whether findings for physical activity were independent of being overweight/obese. In the model for endometrial cancer that accounted for being overweight/obese, we tested two and three-way interactions between being overweight/obese, menopausal status, and smoking duration.
Computations were performed in SAS 9.2 (Cary, NC).
Results
Of 13,388 enrolled participants, clinical follow-up was available for 13,208 (98.7%). Forty-seven participants (<1%) withdrew from study before starting therapy; 95 (<1%) withdrew from study after starting therapy; and 38 (<1%) were without follow-up for other reasons. The present report is based on a cut-off of 7 years of follow-up, in keeping with the most recent update of breast cancer incidence (33). Participants’ mean age was 54; 8,939 (67%) were post-menopausal. (See Table 1.) During follow-up, invasive cancers of the breast, lung, colon, and endometrium were reported for 395, 66, 35, and 74 women, respectively. Very few women had more than one cancer of these sites: 1 had both colon and breast; 3 had endometrial and breast cancers.
Cigarette smoking
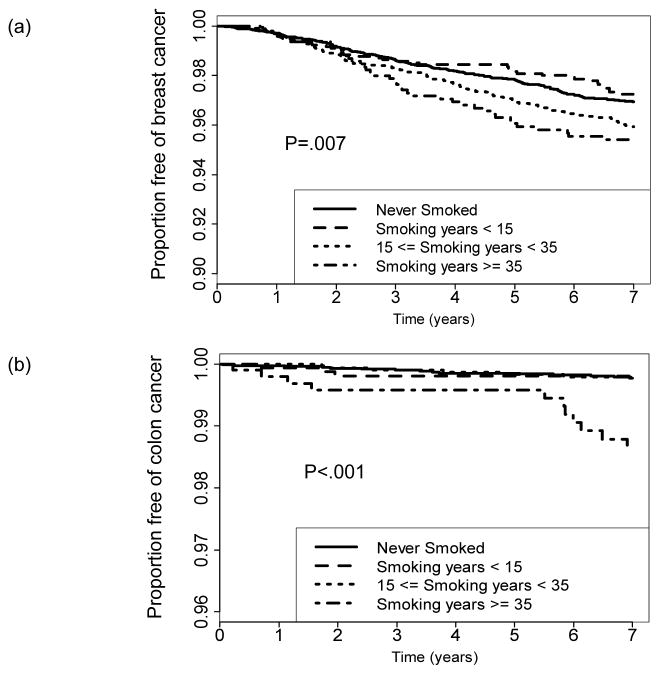
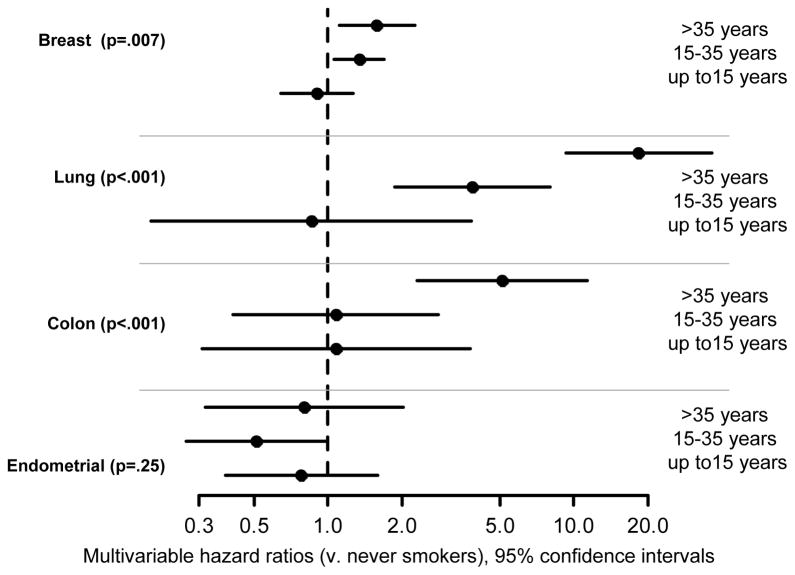
There were 7,335 participants (54.8%) who had never smoked cigarettes or reported smoking 0 years; 1,664 (12.4%) former or current smokers who smoked >0 to 15 years; 3,314 (24.8%) who smoked 15–35 years; and 1,011 (7.6%) who smoked at least 35 years. Smoking duration was unknown for 64 women. In the multivariable model (accounting for treatment, alcohol consumption, leisure time physical activity, and major risk factors), smoking duration was associated with an increased risk of breast cancer (P=.007; multivariable hazard ratio [HR]=1.34, 95% confidence interval [CI] 1.06–1.69 for women who had smoked 15–35 years and HR=1.58, CI 1.11–2.26 for women who smoked at least 35 years, both as compared with never smokers). (See Appendix Table A1 and Figures 1 and 2.) Smoking duration was also associated with an increased risk of lung cancer (p<.001; HR=3.89, CI 1.88–8.06 for women who smoked 15–35 years and HR=18.45, CI 9.35–36.41 for women who smoked for ≥35 years, as compared with never smokers; see Appendix Table A1 and Figure 2). The multivariable association of cigarette smoking with colon cancer was significant (p<.001), but was evident only for women who smoked ≥35 years (HR=5.11, 2.30–11.37; see Appendix Table A1 and Figure 1b). Smoking duration was not significantly associated with the risk of endometrial cancer (P=.25; see Appendix Table A1 and Figure 2). There was no significant two- or three-way interaction between tamoxifen assignment, smoking duration, and menstrual status in the full multivariable analyses of breast, colon, lung, or endometrial cancers. The interaction between treatment assignment and smoking status in the analysis of breast cancer was also non-significant (P=0.56, see Appendix). In the secondary analysis for endometrial cancer that accounted for overweight/obesity, there were no significant two or three-way interactions between overweight/obesity, menopausal status, and smoking duration. (Data not shown.) Analyses with alternative measures of smoking status and history are provided in the Appendix. Of note, smoking status at baseline did not significantly predict breast cancer risk (p=.074).
Figure 1.
Kaplan-Meier graph of the time to breast cancer event (a) or colon cancer (b), grouped according to smoking duration. P-values are based on multivariable Cox regression analysis.
Figure 2.
Hazard ratios and 95% confidence intervals for the association of cigarette smoking history with time to invasive cancer of the breast, lung, colon, and endometrium.
Leisure-time physical activity
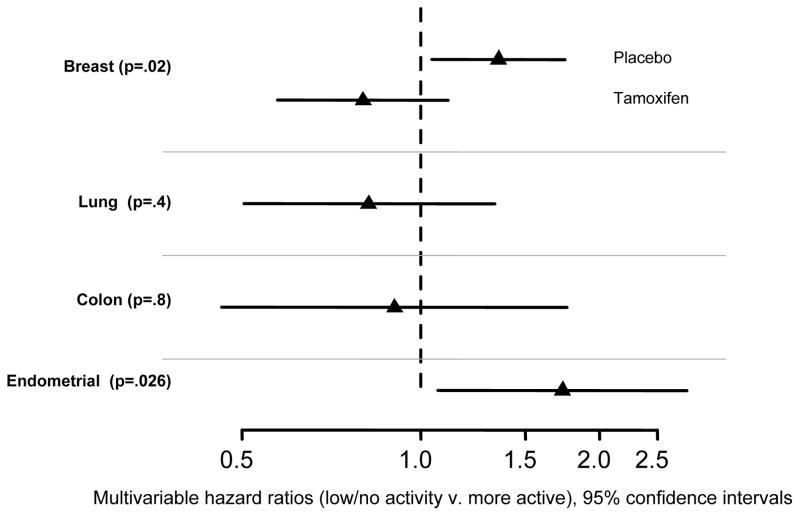
7212 women (53.9%) reported that they typically engaged in low levels of leisure-time physical activity or were inactive. In the multivariable model that accounted for treatment, alcohol consumption, smoking duration, and major risk factors, less active women were at significantly greater risk of breast cancer (main effect P=.021), and there was a significant interaction between physical activity and treatment (P=.013). Specifically, low/no activity was associated with a physical activity (PA) hazard ratio HRPA=1.35, CI 1.05–1.75 for women assigned to placebo, as compared with more active women; but for women assigned to tamoxifen, HRPA was non-significant (HRPA=0.80, CI 0.58–1.11). The effect of tamoxifen on breast cancer risk was stronger in less active women (HRTAM=0.45, CI 0.34–0.59) than in more active women (HRTAM=0.75, CI 0.56–1.02). The risks of lung and colon cancers were not associated with activity (P=.4, HR=0.8; P=.8, HR=0.9, for lung and colon, respectively). Women with low/no physical activity were at a 70% greater risk of endometrial cancer (P=.026; HR=1.73, CI 1.07–2.80). (See Figure 3.) Significant findings were re-tested in secondary analyses that accounted for overweight/obesity (BMI>25). Results for breast cancer were essentially unchanged (data not shown). Results for physical activity and endometrial cancer, that accounted for overweight/obesity, were suggestive (HR=1.58, CI 0.97–2.57, P=.068).
Figure 3.
Hazard ratios and 95% confidence intervals for the association of physical activity with time to invasive cancer of the breast, lung, colon, and endometrium.
Alcohol consumption
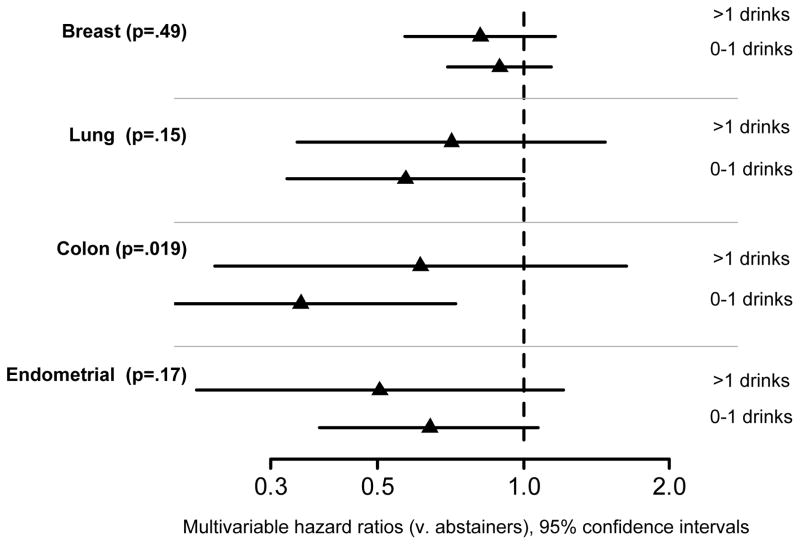
The majority of women (8822, 65.9%) drank in moderation (on average >0 to 1 alcoholic drinks per day); 1724 (12.9%) drank more; and 2787 (20.8%) did not drink. In the multivariable models that accounted for treatment, smoking duration, leisure time physical activity, and major risk factors, the risk of breast, lung, and endometrial cancer was not significantly different among alcohol consumption groups (P=.49, .15, .17, respectively; see Figure 4). The interaction between tamoxifen assignment and alcohol consumption in the analysis of breast cancer was not statistically significant. The risk of colon cancer was significantly different (P=.019), with lower risk for women who drank in moderation (HR=0.35, CI 0.17–0.73) versus non-drinkers. Women who drank more heavily did not have an increased risk of colon cancer (HR=0.61, CI 0.23–1.63) relative to non-drinkers. Exploratory analyses compared the risk of breast and colon cancer across higher levels of alcohol consumption (>3 drinks/day) to non-drinkers. Results were non-significant, and estimated HRs were <1. (Data not shown.)
Figure 4.
Hazard ratios and 95% confidence intervals for the association of alcohol consumption with time to invasive cancer of the breast, lung, colon, and endometrium.
Discussion
Our understanding of the associations between smoking and the risk of breast cancer has evolved considerably in the past decade. There has been considerable research on this topic for decades, but earlier research had produced equivocal or null results,(34–35) and in 2004 the U.S. Surgeon General concluded that evidence suggested no causal relationship between active smoking and breast cancer (16). However, evidence has emerged from studies over the past decade,(2, 36–40) and by 2009 a Canadian expert panel concluded that the relationship between active smoking and breast cancer was consistent with causality (41). Since 2011, additional evidence of association has been reported from three large cohort studies. The Women’s Health Initiative reported increased breast cancer rates in their population of post-menopausal women although the effect sizes were modest (multivariable HR=1.09, CI 0.97–1.22 for women who smoked 20–29 years and HR=1.21, CI 1.07–1.36 for women who smoked 30–39 years versus never smokers) (3). The Nurses’ Health Study also reported higher breast cancer incidence rates with ever smoking and with longer duration of smoking among their population of pre- and post-menopausal women, but the effect sizes and significance levels were again modest (multivariable HR=1.07, CI 1.00–1.14 for women who smoked 20–39 years versus never smokers) (4). The American Cancer Society Cancer Prevention Study II reported HR=1.26, CI 1.00–1.58 for women who smoked 1–40 years (9). Based on literature published through October 2012, the U.S. Surgeon General concluded that evidence was “sufficient to identify mechanisms by which cigarette smoking may cause breast cancer,” but that evidence was “suggestive but not sufficient to infer a causal relationship between active smoking and breast cancer” (10). The present NSABP P-1 analysis found larger effects for smoking duration than had been seen in those previous studies (e.g., HR=1.34, CI 1.06–1.69 for women who had smoked 15–35 years). We also note that women who were current smokers at baseline in P-1 had a higher estimated risk of breast cancer than never-smokers (HR=1.32, CI .98–1.78), as did former smokers (HR=1.24, CI .99–1.54) although smoking status was not statistically significant (p=.074). Among participants assigned to placebo, the HR was 1.2 (CI 0.8–1.7) for current versus never smokers, and the HR was 1.3 (CI 1.0–1.7) for former versus never smokers. (See Appendix.) These estimates are larger than comparators 1.12 and 1.09 estimated in a recent meta-analysis (9). This might indicate that smoking had an even greater impact on risk of breast cancer among women in NSABP P-1 than in other studies, perhaps because women in NSABP P-1 were already at an elevated risk of breast cancer due to family history and other factors. For women assigned to tamoxifen, an increased hazard of breast cancer associated with smoking might partly be explained by the decrease in adherence to tamoxifen that was observed among current smokers in P-1 (30).
The evidence for smoking as a risk factor for colon cancer had been inconsistent until recently. A study that examined colon and rectal cancers separately in women found that the risk is largely that of rectal rather than colon cancer (42). A meta-analysis in 2008 demonstrated a pooled relative risk (RR) of colorectal cancers of RR=1.18, CI 1.11–1.25, for ever-smokers versus never-smokers. As in the present report, that analysis of cumulative exposure found a significant association only for long-term smoking (more than 30 years) (5). The International Agency for Research on Cancer (IARC) has concluded that tobacco smoking is a cause of colon cancer (43). In contrast to the other three cancers examined, smoking may be associated with a decreased risk of endometrial cancer, though our results were not confirmatory in that regard (14).
One strength of NSABP P-1 was the relatively detailed cigarette smoking history assessment. These data permitted analyses using several alternative variables to capture smoking exposure (see Appendix). A shortcoming of P-1, typical of trials in cancer prevention and treatment, is that follow-up tobacco use data were not collected. The increasing evidence of the clinical impact of tobacco use on cancer prevention and prognosis across a range of disease sites warrants more widespread assessment in clinical trials than has typically been conducted (10, 29, 44–47). Biochemical validation of smoking status was not conducted in P-1. Validation may not be feasible in many trials, but concern exists that smokers may under-represent their tobacco use, particularly in the setting of cancer care delivery (48). Such under-reporting would reduce statistical power to detect associations with tobacco use. Our analysis of breast cancer incidence does not provide evidence of interactions between tamoxifen assignment and smoking; that is, the results do not confirm that tamoxifen is less effective in women who are current smokers or who have more past smoking exposure. The estimated hazard ratios, however, are consistent with a possible modest interaction.
The U.S. Department of Health and Human Services (DHHS) Physical Activity Guidelines Committee concluded that active women have a reduced risk of breast and colon cancer, and may also have a reduced risk of lung cancer (49). However, the associations were complex. For example, some of the studies they reviewed had reported that the association between physical activity and breast cancer risk is weaker in women with a family history of breast cancer. We found that leisure-time physical activity was associated with decreased risk of breast cancer, but that this association was limited to women assigned to placebo. That interaction between treatment and physical activity would need to be replicated in other studies. We did not detect significant increases in risk of colon or lung cancer with low physical activity. Our results might differ from the DHHS report due to the limitations of our data: physical activity was self-reported, using broad measures, reported only at baseline, and was most often at levels that may have been below the thresholds that past studies have found associated with improvement in cancer risk (see the “Physical Activity and Cancer Factsheet” on www.cancer.gov). We did confirm a previously reported protective effect of physical activity on endometrial cancer. That association seems to be partly mediated by obesity because when obesity was included in the model, the significance of physical activity was diminished.
Past studies have indicated an increased risk of breast and colon cancer with heavy alcohol consumption (50–51). With respect to breast cancer, a reanalysis of data from 53 epidemiological studies found elevated risk among women who drank 3 to 3.5 drinks per day (RR=1.32, CI 1.19–1.45) or more (RR=1.46, CI 1.33–1.61) versus non-drinkers (35). A review found that the increase in risk was for hormone receptor-positive, and not for hormone receptor-negative, breast cancers (52). IARC concluded that there was sufficient evidence that alcohol was causally related to female breast cancer (51). A recent meta-analysis found an increased risk even for women classified as light drinkers (up to 1 drink per day) versus non-drinkers, but the increase was small (pooled RR=1.03, CI 1.00–1.07 adjusted for major risk factors) (50). A large cohort study also reported increased risk for moderate levels of alcohol drinking (RR=1.15, CI 1.06–1.24 for women who drank roughly 1/3–2/3 drinks per day) (53). With respect to colon cancer, a pooled analysis of 8 cohort studies in the U.S. and Europe demonstrated a RR of 1.45 for colon cancer among people who consumed at least 3 drinks per day on average (54). However, the same study found a slight decrease in risk of colon cancer that almost reached statistical significance (RR=0.92, CI 0.84–1.01) among the lightest drinkers (0 – 5 grams of alcohol per day, or roughly one-third of one drink) relative to non-drinkers. A European cohort study found significantly increased risk of colon cancer only at levels of alcohol consumption above 4 drinks per day, and no increased risk of colon cancer with modest drinking (55). Our results for alcohol consumption show decreased risk for all four cancer sites, with significant decreases only for colon cancer. NSABP P-1 included few women who reported drinking at very high levels (129 and 94 women who drank 3–4, or more than 4 drinks per day, respectively). Furthermore, women classified as drinking up to one drink per day actually drank very modestly, with a median of 0.13 drinks per day. Therefore, our data do not necessarily constitute evidence against past studies, which found increased risk at high levels of alcohol consumption and either no increase or small increases in risk among those who drink in moderation.
Differences between results from NSABP P-1 and other studies may be due to the systematic prospective detection of cancers at all organ sites, to the unique population in NSABP P-1, or to statistical variation. Our findings must be interpreted within the context of the special characteristics of the women who volunteered for this clinical trial in the early 1990s. Women in NSABP P-1 differed from the general population by definition, having been selected based on their elevated risk of breast cancer. They had to perceive themselves as being at very high risk for breast cancer to consider enrolling in what was portrayed as a risky study, in which healthy women were taking a drug used for the treatment of cancer. Second, if a woman considered herself at high risk for breast cancer and was willing to enter this clinical trial, she had to accept the 50% chance that she would be given a placebo. Third, the demographic characteristics of the women who volunteered reflected a higher socioeconomic status and highly educated population, typically less likely to have unhealthy behaviors. For example, they were less likely to smoke cigarettes than women of a comparable age during the 1990’s. In the 1992 National Health Interview Survey (NHIS), of adult women age >65, 12.9% reported smoking cigarettes either every day or some days; of women age >65 enrolling in NSABP P-1 in the same year, only 6.4% were current smokers (56). For women aged 45–64, the rates were 26.5% in the NHIS versus 13.3% in NSABP P-1. And, as noted above, participants were not heavy alcohol consumers. Thus, conclusions that we make about behaviors and their influence on cancer risk should be interpreted in the context of this unique population. In addition, a strength of our analysis is that we included the major known risk factors for each cancer in the multivariable models, which may not have been possible in some prior research.
These new findings contribute to the growing evidence base and provide a richer understanding of the complex impact of risk behaviors of cigarette smoking, alcohol consumption, and physical activity on primary cancer incidence. Our findings indicate that women who are at elevated risk of breast cancer, due to family history or other risk factors used for NSABP P-1 eligibility, should have their smoking status and physical activity assessed by their health care providers. Results indicated higher risks of breast, lung, and colon cancers with higher intensity and/or duration of smoking, and with lower baseline leisure-time physical activity, which suggests that smoking cessation and an increase in physical activity may provide a reduction in risk. Our study therefore provides further support that smoking cessation and regular physical activity are important means to reduce cancer risk. Those who smoke or are inactive, should be informed, encouraged, and assisted in behavioral change to reduce the risk of cancer.
Supplementary Material
Acknowledgments
Authors’ research support: Public Health Service Grants U10-CA-37377 (S Land, Q Liu, DL Wickerham, J Costantino, P Ganz) U10-CA-69974 (S Land, Q Liu, DL Wickerham, J Costantino, P Ganz), and R03CA134199-02 (S Land, P Ganz) from the National Cancer Institute, Department of Health and Human Services.
Footnotes
Trial registration: PDQ: NSABP-P-1
Potential conflict(s) of interest: DL Wickerham: Astra Zeneca Speakers’ Bureau and Eli Lilly Consultant/Advisory Board. All other authors declare no potential conflicts of interest.
References
- 1.Terry MB, Neugut AI, Bostick RM, Sandler RS, Haile RW, Jacobson JS, et al. Risk factors for advanced colorectal adenomas: a pooled analysis. Cancer Epidemiol Biomarkers Prev. 2002;11:622–9. [PubMed] [Google Scholar]
- 2.Terry PD, Miller AB, Rohan TE. Cigarette smoking and breast cancer risk: a long latency period? Int J Cancer. 2002;100:723–8. doi: 10.1002/ijc.10536. [DOI] [PubMed] [Google Scholar]
- 3.Luo J, Margolis KL, Wactawski-Wende J, Horn K, Messina C, Stefanick ML, et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. BMJ. 2011;342:d1016. doi: 10.1136/bmj.d1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB. Cigarette smoking and the incidence of breast cancer. Arch Intern Med. 2011;171:125–33. doi: 10.1001/archinternmed.2010.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–78. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 6.Tsoi KK, Pau CY, Wu WK, Chan FK, Griffiths S, Sung JJ. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2009;7:682–8. e1–5. doi: 10.1016/j.cgh.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Viswanath K, Herbst RS, Land SR, Leischow SJ, Shields PG Writing Committee for the AACR Task Force on Tobacco and Cancer. Tobacco and cancer: an American Association for Cancer Research policy statement. Cancer Res. 2010;70:3419–30. doi: 10.1158/0008-5472.CAN-10-1087. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- 9.Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Inst. 2013;105:515–25. doi: 10.1093/jnci/djt023. [DOI] [PubMed] [Google Scholar]
- 10.Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 2014. [Google Scholar]
- 11.Polesel J, Serraino D, Zucchetto A, Lucenteforte E, Dal Maso L, Levi F, et al. Cigarette smoking and endometrial cancer risk: the modifying effect of obesity. Eur J Cancer Prev. 2009;18:476–81. doi: 10.1097/CEJ.0b013e32832f9bc4. [DOI] [PubMed] [Google Scholar]
- 12.Loerbroks A, Schouten LJ, Goldbohm RA, van den Brandt PA. Alcohol consumption, cigarette smoking, and endometrial cancer risk: results from the Netherlands Cohort Study. Cancer Causes Control. 2007;18:551–60. doi: 10.1007/s10552-007-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Zoughool M, Dossus L, Kaaks R, Clavel-Chapelon F, Tjonneland A, Olsen A, et al. Risk of endometrial cancer in relationship to cigarette smoking: results from the EPIC study. Int J Cancer. 2007;121:2741–7. doi: 10.1002/ijc.22990. [DOI] [PubMed] [Google Scholar]
- 14.Terry PD, Miller AB, Rohan TE. A prospective cohort study of cigarette smoking and the risk of endometrial cancer. Br J Cancer. 2002;86:1430–5. doi: 10.1038/sj.bjc.6600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terry PD, Rohan TE, Franceschi S, Weiderpass E. Cigarette smoking and the risk of endometrial cancer. Lancet Oncol. 2002;3:470–80. doi: 10.1016/s1470-2045(02)00816-1. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 17.Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170:1758–64. doi: 10.1001/archinternmed.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–6. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki R, Iwasaki M, Yamamoto S, Inoue M, Sasazuki S, Sawada N, et al. Leisure-time physical activity and breast cancer risk defined by estrogen and progesterone receptor status--the Japan Public Health Center-based Prospective Study. Prev Med. 2011;52:227–33. doi: 10.1016/j.ypmed.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Spence RR, Heesch KC, Brown WJ. A systematic review of the association between physical activity and colorectal cancer risk. Scand J Med Sci Sports. 2009;19:764–81. doi: 10.1111/j.1600-0838.2009.00992.x. [DOI] [PubMed] [Google Scholar]
- 21.Wolin KY, Yan Y, Colditz GA. Physical activity and risk of colon adenoma: a meta-analysis. Br J Cancer. 2011;104:882–5. doi: 10.1038/sj.bjc.6606045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 23.Bagnardi V, Randi G, Lubin J, Consonni D, Lam TK, Subar AF, et al. Alcohol consumption and lung cancer risk in the Environment and Genetics in Lung Cancer Etiology (EAGLE) study. Am J Epidemiol. 2009;171:36–44. doi: 10.1093/aje/kwp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagnardi V, Rota M, Botteri E, Scotti L, Jenab M, Bellocco R, et al. Alcohol consumption and lung cancer risk in never smokers: a meta-analysis. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:2631–9. doi: 10.1093/annonc/mdr027. [DOI] [PubMed] [Google Scholar]
- 25.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 26.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 27.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 28.Freedman AN, Slattery ML, Ballard-Barbash R, Willis G, Cann BJ, Pee D, et al. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol. 2009;27:686–93. doi: 10.1200/JCO.2008.17.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Land SR. Methodologic barriers to addressing critical questions about tobacco and cancer prognosis. J Clin Oncol. 2012;30:2030–2. doi: 10.1200/JCO.2012.41.7402. [DOI] [PubMed] [Google Scholar]
- 30.Land SR, Cronin WM, Wickerham DL, Costantino JP, Christian NJ, Klein WM, et al. Cigarette smoking, obesity, physical activity, and alcohol use as predictors of chemoprevention adherence in the National Surgical Adjuvant Breast and Bowel Project P-1 Breast Cancer Prevention Trial. Cancer Prev Res (Phila) 2011;4:1393–400. doi: 10.1158/1940-6207.CAPR-11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cecchini RS, Costantino JP, Cauley JA, Cronin WM, Wickerham DL, Land SR, et al. Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res. 2012;5:583–92. doi: 10.1158/1940-6207.CAPR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore SC, Gierach GL, Schatzkin A, Matthews CE. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br J Cancer. 2010;103:933–8. doi: 10.1038/sj.bjc.6605902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 34.Terry PD, Rohan TE. Cigarette smoking and the risk of breast cancer in women: a review of the literature. Cancer Epidemiol Biomarkers Prev. 2002;11:953–71. [PubMed] [Google Scholar]
- 35.Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Jr, et al. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–45. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds P, Hurley S, Goldberg DE, Anton-Culver H, Bernstein L, Deapen D, et al. Active smoking, household passive smoking, and breast cancer: evidence from the California Teachers Study. J Natl Cancer Inst. 2004;96:29–37. doi: 10.1093/jnci/djh002. [DOI] [PubMed] [Google Scholar]
- 37.Olson JE, Vachon CM, Vierkant RA, Sweeney C, Limburg PJ, Cerhan JR, et al. Prepregnancy exposure to cigarette smoking and subsequent risk of postmenopausal breast cancer. Mayo Clin Proc. 2005;80:1423–8. doi: 10.4065/80.11.1423. [DOI] [PubMed] [Google Scholar]
- 38.Gram IT, Braaten T, Terry PD, Sasco AJ, Adami HO, Lund E, et al. Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol Biomarkers Prev. 2005;14:61–6. [PubMed] [Google Scholar]
- 39.Cui Y, Miller AB, Rohan TE. Cigarette smoking and breast cancer risk: update of a prospective cohort study. Breast Cancer Res Treat. 2006;100:293–9. doi: 10.1007/s10549-006-9255-3. [DOI] [PubMed] [Google Scholar]
- 40.Ha M, Mabuchi K, Sigurdson AJ, Freedman DM, Linet MS, Doody MM, et al. Smoking cigarettes before first childbirth and risk of breast cancer. Am J Epidemiol. 2007;166:55–61. doi: 10.1093/aje/kwm045. [DOI] [PubMed] [Google Scholar]
- 41.Cooley ME, Emmons KM, Haddad R, Wang Q, Posner M, Bueno R, et al. Patient-reported receipt of and interest in smoking-cessation interventions after a diagnosis of cancer. Cancer. 2011;117:2961–9. doi: 10.1002/cncr.25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terry PD, Miller AB, Rohan TE. Prospective cohort study of cigarette smoking and colorectal cancer risk in women. Int J Cancer. 2002;99:480–3. doi: 10.1002/ijc.10364. [DOI] [PubMed] [Google Scholar]
- 43.IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. Personal habits and indoor combustions. Lyon, France: International Agency for Research on Cancer (IARC) Working Group; 2010. p. 100. Part E. [PMC free article] [PubMed] [Google Scholar]
- 44.Peters EN, Torres E, Toll BA, Cummings KM, Gritz ER, Hyland A, et al. Tobacco Assessment in Actively Accruing National Cancer Institute Cooperative Group Program Clinical Trials. Journal of Clinical Oncology. 2012;30:2869–75. doi: 10.1200/JCO.2011.40.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganz PA. Host factors, behaviors, and clinical trials: opportunities and challenges. J Clin Oncol. 2012;30:2817–9. doi: 10.1200/JCO.2012.43.6576. [DOI] [PubMed] [Google Scholar]
- 46.Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:1941–8. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanna N, Mulshine J, Wollins DS, Tyne C, Dresler C. Tobacco cessation and control a decade later: American society of clinical oncology policy statement update. J Clin Oncol. 2013;31:3147–57. doi: 10.1200/JCO.2013.48.8932. [DOI] [PubMed] [Google Scholar]
- 48.Morales NA, Romano MA, Michael Cummings K, Marshall JR, Hyland AJ, Hutson A, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control. 2013;24:1223–30. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Physical Activity Guidelines Advisory Committee; DHHS, editor. Physical Activity Guidelines Advisory Committee Report. Washington, DC: 2008. [Google Scholar]
- 50.Seitz HK, Pelucchi C, Bagnardi V, La Vecchia C. Epidemiology and pathophysiology of alcohol and breast cancer: update 2012. Alcohol Alcohol. 2012;47:204–12. doi: 10.1093/alcalc/ags011. [DOI] [PubMed] [Google Scholar]
- 51.IARC. Alcohol Consumption and Ethyl Carbamate. Lyon, France: International Agency for Research on Cancer (IARC) Working Group; 2010. Monographs on the Evaluation of Carcinogenic Risks to Humans; p. 96. [PMC free article] [PubMed] [Google Scholar]
- 52.Chen WY, Colditz GA. Risk factors and hormone-receptor status: epidemiology, risk-prediction models and treatment implications for breast cancer. Nat Clin Pract Oncol. 2007;4:415–23. doi: 10.1038/ncponc0851. [DOI] [PubMed] [Google Scholar]
- 53.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–90. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140:603–13. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 55.Ferrari P, Jenab M, Norat T, Moskal A, Slimani N, Olsen A, et al. Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC) Int J Cancer. 2007;121:2065–72. doi: 10.1002/ijc.22966. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (CDC). . Health Objectives for the Nation: Cigarette Smoking Among Adults --United States, 1993. MMWR Morb Mortal Wkly Rep. 1994;45:925–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.