Abstract
HIV-1-associated neuroinflammation persists even with effective combined anti-retroviral therapy (cART), and is associated with the presence of activated monocytes/macrophages within the CNS. In order to infiltrate the CNS, monocytes transmigrate across the selectively permeable blood brain barrier (BBB), which is compromised during HIV infection. Interestingly, platelet-derived excess soluble CD40L (sCD40L) found in the plasma and cerebrospinal fluid (CSF) of HIV-1 infected individuals with cognitive impairment has previously been implicated in increased BBB permeability. Here we show that sCD40L also promotes the formation of complexes between inflammatory monocytes and activated platelets (PMCs), which are detected by flow cytometry as monocytes that express excess of CD61, a platelet marker and that these complexes are increased in individuals with HIV infection. PMCs exhibit an enhanced ability to adhere to human brain microvascular endothelial cells as compared to monocytes alone and migrate across transendothelial barrier. These complexes can be found marginalized in the lumen of post-capillary venules in post-mortem brain tissue derived from cases of HIV-1-associated encephalitis (HIV-E). The extravasation of monocytes across the brain endothelium may exacerbate neuroinflammation, indicating that enhancing this event via platelet interaction may be a contributing factor in the development of cognitive impairment. Thus, dampening platelet activation, and in turn PMC formation, with anti-platelet agents may prove beneficial in developing adjunctive therapies for use in combination with cART in an effort to reduce HIV-1-associated neurological deficit
Introduction
Human Immunodeficiency Virus Type 1 (henceforth referred to as HIV) enters the central nervous system (CNS) during the acute phase of infection (1), via infected monocytes, which upon entry into the CNS differentiate into macrophages, thus allowing viral infection to persist primarily in perivascular macrophages and microglia (reviewed in (2)). In certain cases, HIV infection in the brain can develop into a neuropathology known as HIV-associated encephalitis (HIV-E), which is comprised of microglial nodules, activated resident microglia, multinucleated giant cells, infiltration predominantly by monocytoid cells including blood-derived macrophages, and distinct neuronal loss (3). Clinically, HIV infection in the CNS can manifest as a spectrum of disorders collectively known as HIV-associated neurocognitive disorders (HAND). In the post-ART (anti retroviral treatment) era, the incidence of the most severe form of HAND, HIV-associated dementia (HAD) has dramatically decreased, however, over 50% of HIV positive patients continue to suffer from milder versions of HAND (4).
Since neurons are not productively infected with HIV, other pathological mechanisms have been implicated in neuronal damage and death that occurs during HIV-E. Indeed, changes in the brain microenvironment are thought to occur as a consequence of an overwhelming inflammatory response, involving overstimulation and excitotoxicity, induced either by viral proteins such as Tat, gp120, and Vpr, or pro-inflammatory host factors secreted from microglia and macrophages, such as tumor necrosis factor-α (TNFβ), monocyte chemoattractant protein-1 (MCP-1), and platelet activating factor (PAF) (5, 6). Interestingly, clinical signs of HAND are more closely associated with increased numbers of activated microglia and infiltrating monocytes, rather than the viral load within the CNS (7, 8).
Human monocytes are classified into two subtypes based on the expression of CD16. Classical monocytes are CD14+CD16− and comprise 80-90% of all circulating monocytes, while CD14+CD16+ monocytes represent 5-10% of the monocyte population under normal conditions, and are considered to be a more mature and pro-inflammatory subset (reviewed in (9)). Consistently, CD16+ monocytes are expanded up to 40% during HIV infection (10), and are more susceptible to infection than CD16− monocytes (11). These cells are also the most abundant subtype of monocyte found within the CNS during HIV infection (12) and their expansion in the blood might be predictive of HIV-associated neuroinflammation (13, 14). CD16+ monocytes also exhibit an enhanced capacity for migration across endothelial barriers, attributable to increased expression of integrins such as α4β1 and αMβ2 (15), that mediate adherence to endothelial cells, and chemokine receptors such as CX3CR1, which increase responsiveness to fractalkine, respectively (16).
Previous reports have shown that the CD16+ phenotype is also induced by the interaction of monocytes with activated platelets. Activated platelets form transient complexes with monocytes in circulation, termed platelet-monocyte complexes (PMC), and these complexes are elevated in diseases involving inflammation such as cardiovascular disease (CVD) (17) and type-2 diabetes (reviewed in (18)). Interestingly, PMCs were found to be a more sensitive marker of platelet activation and predictor of myocardial infarction as compared to levels of P-selectin, also known as CD62P, which is the traditional hallmark of platelet activation (19). Moreover, the extent to which PMCs develop is predominantly dependent on the platelet activation status (20, 21), and only to a very limited extent on monocyte activation (22).
Previous studies from our group (21), and others (23), have demonstrated that HIV infection induces an increase in platelet activation, despite thrombocytopenia. Infection, as well as cognitive impairment during infection, is associated with an increase in plasma levels of soluble CD40L (sCD40L) (24), for which platelets are the major source (25). Consistent with this notion, recent reports from our group indicate that excess sCD40L contributes to blood brain barrier (BBB) permeability in the context of HIV (26), and that PMCs are elevated in individuals with HIV infection in spite of suppressive combined antiretroviral therapy (cART) (21). Collectively, these findings underscore the importance of PMCs in the pathogenesis of HIV-associated illnesses, and led us to the underlying hypothesis that during HIV infection, the increase in platelet activation and subsequent release of sCD40L promote the formation of PMCs, which in turn have a higher propensity to cross the blood brain barrier (BBB), thereby exacerbating HIV-associated neuroinflammation.
The findings reported herein indicate that HIV infected individuals exhibit increased levels of PMCs as compared to uninfected individuals, and that sCD40L activates platelets in a manner that promotes the formation of complexes with monocytes via engagement of P-selectin and P-selectin glycoprotein ligand-1 (PSGL-1) on platelets and monocytes, respectively. Consistently, mice treated with CD40L also exhibit a significant increase in Gr-1hi (the functional equivalent of CD16+ monocytes in humans) inflammatory monocyte complexes as compared to saline treated mice. Using an in vitro co-culture system, as well as brain specimens derived from individuals with HIV-E, we now reveal that monocyte extravasation into the CNS is accentuated by the increased adherence of PMCs to brain microvascular endothelial cells. Importantly, we also show that these complexes can be detected as marginalized to the vessel lumen and in perivascular cuffs in brain tissue derived from individuals with HIV-E. Taken together, these results shed light on the underlying mechanisms that may contribute to the pathogenesis of neuroinflammation and HIV-E, thus revealing novel therapeutic targets. Currently, there remains a critical lack of adjunctive therapies for the management of HIV-associated neurocognitive disorders; however, without addressing the underlying neuroinflammation, no realistic control of these complications can be achieved.
Materials and Methods
Ethics statement
The Research Subjects Review Board at the University of Rochester Medical Center approved studies involving human samples. All the study participants were adults and blood samples were obtained after written informed consent, in accordance with the Declaration of Helsinki. Mouse experiments were carried out in accordance with the Animal Welfare Act and the National Institute of Health (NIH) guidelines, and the University Committee on Animal Resources of the University of Rochester Medical Center approved the animal protocol (protocol # 2005-161). The facilities and programs of the Vivarium and Division of Laboratory Animal Medicine of the School of Medicine and Dentistry are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Patient Samples
Persons with (n=36) and without (n=37) HIV infection (without any occurrence of cardiovascular disease for at least one preceding year) were enrolled in the study, and blood samples were drawn into ACD (acid citrate dextrose) buffered vacutainers (BD Biosciences, CA, USA). All persons with HIV infection were on antiretroviral therapy (ART) at the time of the draw. Six of the HIV infected individuals were co-infected with Hepatitis C, one was infected with TB, and one was infected with HPV. Patient demographics are as outlined in Table I.
Table I.
Demographic and clinical characteristics of study participants.
| Characteristics | HIV− | HIV+ |
|---|---|---|
| Mean age ± SD, years | 34±13 | 48±13 |
| Race, N (%) | ||
| Black | 3 (7) | 24 (51) |
| White | 38 (86) | 15 (32) |
| Hispanic | 1 (2) | 3 (6) |
| Unspecified | 2 (5) | 5 (11) |
| Gender, N (%) | ||
| Male | 24 (55) | 36 (78) |
| Female | 20 (45) | 11 (22) |
| Drug Use, N (%) | ||
| No | 43 (98) | 21 (45) |
| Cocaine | 1 (2) | 11 (24) |
| Marijuana | 0 (0) | 12 (26) |
| Heroin | 0 (0) | 2 (4) |
| Nicotine | 0 (0) | 0 (0) |
| Alcohol | 1 (2) | 8 (17) |
| Mean CD4 count ±SD, cells/mm3 | NA | 585 ± 240 |
| Viral Load | ||
| Undetectable, N (%) | NA | 26 (57) |
| Detectable, Mean± SD, RNA copies/ml, | NA | 128 ±132 |
Individuals with (n=36) and without (n=37) HIV infection were enrolled in the study. All the HIV infected individuals were on ART. Six HIV+ individuals were co-infected with Hepatitis C, one with TB and one with HPV. Unless otherwise stated the values indicate numbers with the percentage of total study population in parentheses
Flow Cytometry and Imagestream Analysis
PMCs were detected in whole blood, using previously described method (21), within one hour of the blood draw. In brief, 100 μL blood was fixed, RBCs were lysed and then stained with 10 μL anti-CD14 PE, 3 μL anti-CD16 PE Cy7, 10 μL anti-CD62P FITC (all obtained from BD Biosciences, CA, USA) and 3 μL anti-CD61 AF647 (from AbD Serotec, UK). Following the staining, samples were acquired using a flow cytometer (Accuri C6, Accuri Cytometers, MI, USA). Whole blood leukocytes and monocytes were gated based on forward and side scatter. Further monocytes (CD14+ cells) were divided into two subtypes based on CD16 expression. PMCs were defined as monocytes that were positive for CD61, a platelet specific marker. The same tubes were also analyzed for platelet activation using CD62P (also known as P-selectin) expression. FMO (fluorescence minus one) controls were used to define various gates, and sizing beads (Mega Mix, Biocytex, Marseille, France) were used to delineate the platelet gate (0.9-3 μm).
Representative samples from HIV positive (n=5) and HIV negative (n=6) donors were also acquired and analyzed using Image Stream (Amnis Corporation, WA, USA). For these studies, 10 μL of anti-CD16 Pac B (from BD Biosciences, CA, USA) was used in place of anti-CD16 PE Cy7. Ideas software (Amnis Corporation, WA, USA) was used to analyze the Image Stream data. The Gating strategy used was as described above. Initial analysis of the data indicated two types of PMCs: one in which platelets were attached to the surface of monocytes (henceforth called type 1 complexes), and another in which there was no visible platelet attached, but the monocyte itself expressed the platelet marker CD61 (henceforth called type 2 complexes). The internalization feature of the Ideas software, which measures and plots the distance between fluorochromes of interest, i.e. CD14 for monocytes and CD61 for platelets, was used to differentiate between these two types of complexes, and to measure the relative percentage of these two types among the total PMC population. In addition, the spot count feature was used to measure the number of platelets per monocyte in a complex. This feature measures pixel intensity of a particular fluorochrome after subtracting the background (in this case CD61 expression on monocytes) and examines whether this connects to a particular spot on the image (i.e. a platelet).
Cell Culture
Monocytes were isolated using MACS Pan Monocyte Isolation Kit (Miltenyi Biotec, CA, USA) as per the manufacturer's instructions with minor modifications. In all experiments except for electron microscopy, biotin labeled antibody against CD41 (250 ng per 25 million PBMCs; Abcam, MA, USA) was added to the antibody cocktail provided with the kit, to remove contaminating platelets and existing PMCs. The resulting monocytes contained less than 10% residual PMCs (data not shown). Monocytes used for electron microscopy contained 35-40% PMCs (data not shown). Monocytes were cultured in RPMI supplemented with 10% FBS and 2% PSG (penicillin, streptomycin and glutamine).
Whole blood from HIV negative samples was centrifuged at 250 × g for 15 minutes, and platelet rich plasma (PRP) was collected. Following addition of prostaglandin I2 (PGI2; 1 uL per mL of PRP), to maintain platelet quiescence, PRP was centrifuged at 1000 × g for 10 minutes to pellet the platelets. The platelet pellet was then washed and resuspended using Tyrode's salt solution (Sigma-Aldrich, MO, USA) supplemented with acid-citrate-dextrose (ACD) anticoagulant and PGI2. Subsequently, washed platelets were centrifuged once again at 1000 × g for 10 minutes, and the remaining purified platelet pellet was resuspended in Tyrode's salt solution without supplements. The purity of isolated platelets was determined using Sysmex KX 21N hematology analyzer and was found to be 99% pure population.
Primary human brain microvascular endothelial cells (BMVECs; ACBRI, WA, USA) were cultured in DMEM/F12 supplemented with 10% FBS, 2% PSG and 100 ng/mL ECGS (endothelial cell growth supplement, BD biosciences, CA, USA). Cells up to passage 10 were used for performing experiments.
Scanning Electron Microscopy (SEM)
Monocytes isolated from the whole blood of HIV negative donors were allowed to adhere onto poly-L-lysine coated coverslips for 2 hours and were subsequently placed into 0.1M sodium cacodylate buffered 2.5% glutaraldehyde at 4 °C for overnight fixation. The cells on the cover glasses were post-fixed using the same buffer in 1.0% osmium tetroxide, and then transitioned through a graded series of ethanol to 100% (x3). The last change was allowed to evaporate off of the cover glasses overnight in a fume hood. The cover glasses were then mounted onto aluminum stubs and sputter coated with gold. Imaging was performed using a Zeiss Auriga field emission SEM with an attached Gatan digital camera system.
Transmission Electron Microscopy (TEM)
Monocytes were isolated, as described above, from whole blood obtained from HIV negative donors and were allowed to adhere to a two chamber slide for 2 hours, following which, media was removed and immediately replaced with room temperature fixative composed of 0.1M sodium cacodylate buffered 2.5% glutaraldehyde. The slides were fixed for 1 hour at room temperature, and then at 4°C overnight. The plastic chambers were removed, and slides were rinsed in the same buffer, post-fixed in 1.0% Osmium tetroxide for 30 minutes, and then dehydrated through a graded series of ethanol to 100% (x3) concentration. The slides were then placed into a 1:1 ratio of 100% ethanol and Spurr epoxy resin for one hour, and transferred to Spurr epoxy resin overnight. The next day, size 3 BEEM capsules were filled with Spurr resin, inverted, and placed on top of the area where cells were present on the slides, and then placed into a 60°C oven to allow for overnight polymerization. The following day, glass slides/capsules were dipped 3-4 times in liquid nitrogen and polymerized BEEM capsules were wiggled and “popped off” the glass (27). The epoxy blocks with the entrapped cells were trimmed of excess plastic resin, placed into an ultramicrotome, thin-sectioned at 70 nm onto carbon coated nickel grids and stained with uranyl acetate and lead citrate. Imaging was done using a Hitachi 7650 TEM with an attached Gatan Erlangshen 11 megapixel digital camera.
Detection of PMCs in sCD40L-Treated Mice
C57BL/6J mice were purchased from The Jackson Laboratory, Bar Harbor, ME. Ten to twelve-week old WT mice (n=6 for each group) were injected retro-orbitally with recombinant mouse sCD40L (rmsCD40L; 0.2 μg/g body weight) that had been resuspended in saline, as previously described (26). Two hours post-injection, whole blood was obtained via cardiac exsanguination and used for detection of PMCs. 2 μL anti-CD115 APC (eBiosciences, CA, USA), 2.5 μL CD61 AF647 (AbD Serotec, UK), 2.5 μL Gr-1 PE Cy7, and 1 μL CD62P FITC (BD Biosciences, CA USA) were used to stain 50 μL whole blood. Samples were acquired using an Accuri C6. 15,000 leukocytes per sample were collected and analyzed using Flow Jo. Monocytes were gated based on CD115 expression and then classified into two subtypes using Gr-1 expression as Gr-1hi and Gr-1lo, as murine monocytes expressing high levels of Gr-1 have been shown to be representative of the “inflammatory” monocyte population that corresponds to the CD16+ monocyte subset within humans (9, 26). Monocytes expressing CD61 were defined as PMCs and CD62P expression was used as a marker of platelet activation. FMO controls were used for analysis, as described above.
In Vitro Whole Blood Treatment with Recombinant Human sCD40L (rhsCD40L)
Blood from HIV uninfected donors (n=4) was treated with either adenosine diphosphate (ADP) at 10 uM for 30 minutes, or with recombinant human sCD40L (rhsCD40L; R & D Systems, MN, USA) at 1 μg/mL for 2 hours at 37°C. When indicated, blood was pre-treated with either 8 μg/mL MK13 (neutralizing antibody against CD40L) (24) or 5 μg/mL 9E1 (neutralizing antibody against P-selectin; R & D Systems, MN, USA) for 15 minutes, followed by the addition of rhsCD40L. After the treatments blood was processed and analyzed as described above for the detection of CD14+ PMCs.
Platelet-Monocyte Co-Culture
Monocytes were isolated from HIV uninfected donors as described above and incubated overnight at 37°C. The next day, platelets were isolated from the same donor and treated with 1 μg/mL rhsCD40L, or were left untreated, for 20 minutes at 37°C. The platelets were washed with Tyrode's buffer and mixed with monocytes at a ratio of 10:1. Monocytes with or without rhsCD40L treatment were used as controls. Mixed cultures were incubated for 1 hour at 37°C, following which, half of the cells were fixed with 4% PFA and stained with antibodies against CD14, CD16, CD61 and CD62P. The remaining cells were incubated for 20 hours and subsequently stained with antibodies against PSGL-1-FITC, CCR2-FITC, or CD40-FITC in separate tubes. 5,000 monocytes were acquired on an Accuri C6 flow cytometer and were analyzed for detection of CD14+ PMCs (after 1 hour of mixed cultures) or various cell surface markers (after 20 hours of mixed cultures).
Monocyte Adhesion Assay
This experiment was performed as described previously (28). Briefly, 2 × 104 BMVECs per well were plated onto black-walled, transparent-bottom 96-well plates (Corning Inc., USA) that had been coated with rat-tail collagen type I (BD Biosciences, CA, USA). After the formation of confluent monolayers, the BMVECs were cultured without growth factors for 4-6 hours and were then either treated with recombinant human TNFα (10 ng/mL, R&D systems, MN, USA) to activate the endothelial cells, or left untreated for 4 hours. Monocytes and platelets were isolated separately from whole blood obtained from the same HIV uninfected donor (n=3). Monocytes were labeled with Calcein AM green (5μM/1 × 106 cells for 45 minutes, Invitrogen Life Technologies, USA). A co-culture of monocytes and platelets that had been activated by rhsCD40L (1 μg/mL) was established, as described above. Monocytes treated with or without rhsCD40L, as well as monocytes treated with LPS (10 ng/mL), were used as controls. 24 hours post-treatment, 2.5 × 104 monocytes per well were added onto the BMVECs and were allowed to adhere for 30 minutes at 37°C. The plates were subsequently washed three times with PBS to rinse away any unattached cells, and the relative fluorescence (RFU) was measured on a Spectramax M3 fluorescence plate reader (Molecular Devices, CA, USA). RFU values obtained using only the monolayer of BMVECs was subtracted from all readings as background and results are represented as the fold difference between the number of monocytes that attached to BMVECs under the different experimental conditions and the number of non-treated monocytes that attached to the untreated endothelial cells.
Transendothelial Migration Assay
This assay was performed as described previously (28) with some modifications. BMVECs were plated on rat-tail collagen coated FluoroBlok tinted tissue culture inserts (with 3μm pores, BD Biosciences, CA, USA) at the density of 2.5 × 104 cells/insert. Platelet and monocyte co-cultures were established as described before (n=3). Platelets were activated using 1μg/ml rhsCD40L in presence or absence of 5μg/ml 9E1 (neutralizing antibody against P-selectin, R and D Systems, MN, USA) for 20 minutes. The monocytes were either left un-treated or were treated with 1μg/ml rhsCD40L or with 5μg/ml 9E1. The monocytes were alternatively mixed with activated platelets with or without 9E1 at the ratio of 1:10. One hour post-treatment, 5 ×105 monocytes/treatment were stained with antibodies against CD14-PE, CD16-PE Cy7 and CD61-AF647 and were acquired on Accuri C6 flow cytometer to enumerate the percentage of CD14+ PMCs. 2 hours post-treatment as well as 24 hours post-treatment, 5 ×105 monocytes/treatment were added to the upper chamber of FluoroBlok inserts in presence or absence of monocyte chemotactive protein (MCP)-1 (30ng/ml, R and D Systems, MN, USA) in the lower chamber, that is, the monocytes were allowed to migrate with or without a chemokine gradient. The migration was allowed to continue up to 7 hours at 37°C. After 7 hours, the cells in the lower chamber were collected and stained with antibodies against CD14-PE and CD16-PE Cy7, as described previously. After staining, the cells were resuspended in 250μl 1X PBS. 20 μl/sample was acquired on Accuri C6 flow cytometer at slow speed and at the same instrument settings for each experiment and the number of CD14+ monocytes/20μl/sample was obtained. Final number of migrated cells was estimated by subtracting the number of CD14+ monocytes that had migrated in absence of MCP-1 (passive migration) from the number of CD14+ monocytes that had migrated in presence of MCP-1 (active migration). A fold change in monocyte migration was then calculated by dividing the final number of migrated monocytes/treatment by the final number of migrated cells in un-treated monocytes.
PMC Detection by Immunohistochemical Staining of Human Brain Sections
Immunohistochemistry was performed on serial sections from postmortem paraffin-embedded brain tissue of cases obtain from the National NeuroAIDS Tissue Consortium (Washington, DC). The area under observation included the basal ganglia and cortical regions from three HIV-1 encephalitic (HIV-E) cases and from two seronegative age-matched controls (Case #1, 2, 3 and Cases #8 and 10) (29). The immunofluorescence was performed using standard methodology. Briefly, serial sections (5 micron in thickness) were baked at 65°C for 20 min followed by de-paraffinization and rehydration. The tissue was then subjected to antigen retrieval by incubating the sections at 100°C in 10 mM sodium citrate buffer (pH 6.0) for 20 min. To minimize autofluorescence, the sections were placed for ~8hrs 15 inches apart from a fluorescent lamp using a 13-watt compact fluorescent bulb. To block nonspecific antibody binding, the slides were incubated with 1% goat serum in 1xPBS and 0.1% Triton X-100 (Sigma-Aldrich). For immunolabeling, the following primary antibodies were used: monoclonal antibodies to human CD68 (diluted 1:300, Abcam), polyclonal antibodies to human CD61 (1:200, Cell Signaling Technology). Tissue sections were then rinsed, and secondary antibodies donkey anti-mouse conjugated to Alexa-488 (diluted 1:500, Invitrogen) or donkey anti-Rabbit conjugated to Alexa-594 (diluted 1:500, Invitrogen) were added for 1 h. The slides were mounted with Prolong antifade reagent containing DAPI (for detection of nucleus, Invitrogen). Images (at 40X or 100x objective magnification) were acquired using a Coolsnap-EZ CCD camera (Photometrics) coupled to a Nikon i80 Eclipse (Nikon). Both the acquisition and pseudo-color conversion was performed using the NIS-Elements software from Nikon.
Statistical Analysis
Graph Pad Prism (version 4, GraphPad Software Inc, CA, USA) was used to perform all statistical analyses. Unpaired t tests were used to compare data obtained from HIV infected and uninfected samples. For multiple groups statistical significance was determined using one-way ANNOVA followed by Bonferroni's test. Pearson correlation test was used to correlate PMCs and platelet activation. Statistical significance is indicated in the figures as *p<0.05, **p<0.01, and ***p<0.001.
Results
HIV infection leads to elevated levels of CD16+ PMCs, despite cART
Previous studies of cardiovascular diseases have shown that complexes between activated platelets and monocytes are inflammatory in nature and induce monocyte maturation (reviewed in (17)). Since HIV infection is a chronic inflammatory disease and is known to cause platelet activation, we conducted a pilot study to assess the levels of PMCs in HIV infected individuals (21). Since we found that there was a significant increase in PMCs in HIV infected individuals, we recruited more participants. Whole blood samples obtained from HIV+ (n=36) and HIV− (n=37) individuals were stained and subsequently analyzed using flow cytometry. HIV infected individuals showed significantly increased levels of CD16+ PMCs as compared to HIV uninfected individuals (p=0.013, R2=0.08369; Figure 1A). In contrast, CD16− PMCs and platelet-granulocyte complexes did not differ between the two groups (Figures 1B and 1C). Platelets obtained from HIV infected donors appeared activated as compared to uninfected samples, and these platelets exhibited increased expression of CD62P (P-selectin) on their surface (p=0.036; Figure 1D). Consistently, the % CD62P+ platelets in both HIV+ and HIV− population demonstrated a positive correlation with the percentage of CD16+ PMCs (p=0.008, R2=0.0888; Figure 1E). We also observed a significant increase in the percentage of CD16+ monocytes in HIV infected individuals, which correlated positively with the CD16+ PMCs, while there was no difference in CD16− monocytes (data not shown). Additional representative samples from HIV+ (n=5) and HIV− (n=6) individuals were also stained and analyzed using Imagestream (Amnis Corporation, WA, USA) and demonstrated a similar increase in CD16+ PMCs (data not shown).
Figure 1. Detection of PMCs in HIV infected and uninfected individuals.
100 μL whole blood obtained from HIV+ (n=36) and HIV− (n=37) was fixed, stained with antibodies against CD14, CD16, CD61 and CD62P (P-selectin) and subsequently acquired on an Accuri C6 flow cytometer. A. CD16+ PMCs were significantly increased in HIV infected individuals. B. There was no difference in CD16− PMCs between the two groups. C. Platelet-granulocyte complexes did not differ between the groups. D. Platelets from HIV infected individuals expressed more CD62P (P-selectin) than platelets obtained from HIV negative donors. E. Platelet CD62P expression correlated positively with CD16+ PMC percentages. In panels (A) – (D) samples were compared using an unpaired t-test which indicated significance as *p<0.05.
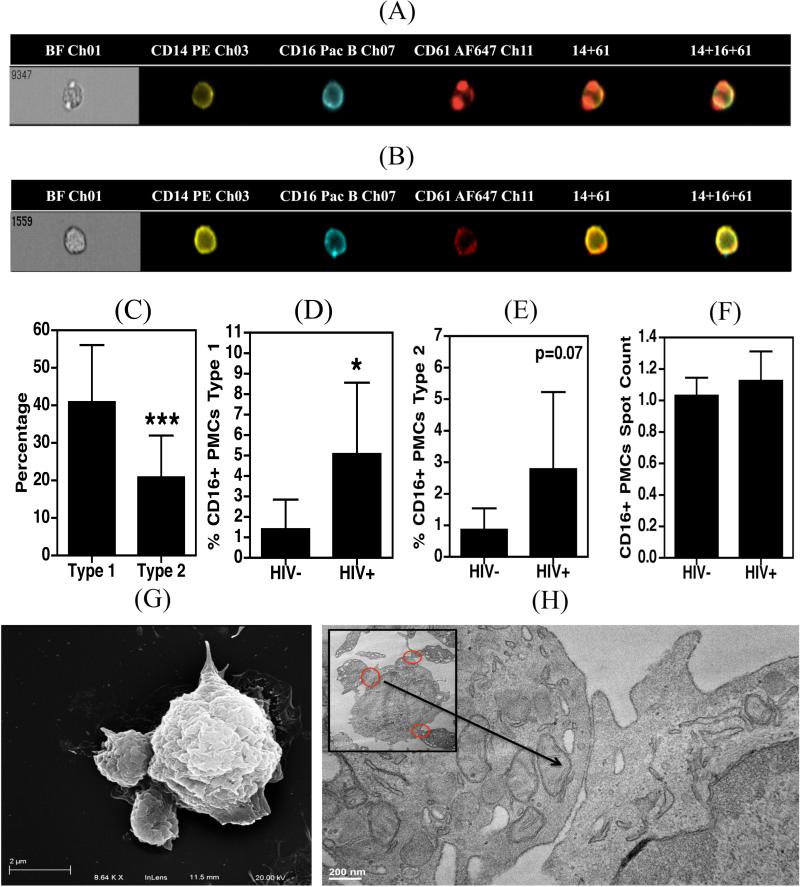
Morphological characteristics of PMCs
Corresponding images generated from the Imagestream analysis of the PMCs indicated the presence of two types of complexes; those with one or more platelets attached to the surface of the monocyte (type 1; Figure 2A), and another in which the monocytes express CD61 internally or on its surface (type 2; Figure 2B). In all samples (HIV+ and HIV−) the percentage of type 1 complexes was significantly higher than type 2 complexes (p=0.0008; Figure 2C), and the percentage of CD16+ type 1 complexes was increased in HIV infected individuals (p<0.05; Figure 2D) and that of type 2 complexes showed a similarly elevated trend towards increase (p=0.07, Figure 2E). The type I complexes were primarily observed with one or two platelets per monocyte, however, several cells demonstrated more than two platelets that had attached (range 1-4). The average number of platelets attached did not differ significantly between HIV infected and uninfected samples (Figure 2F). Consistently, images obtained using scanning and transmission electron microscopy (SEM and TEM, respectively) also indicated that there were 1-3 platelets per monocyte (Figures 2G and 2H).
Figure 2. Morphological characterization of PMCs.
A-F. 100 μL whole blood obtained from HIV+ (n=5) and HIV− (n=6) donors was fixed, stained with antibodies against CD14, CD16 and CD61 and acquired on an Amnis Imagestream flow cytometer. A and B. Representative images of type 1 and type 2 CD16+ PMCs, respectively. C. Type 1 complexes were more prevalent in HIV infected and uninfected samples. D and E. HIV infected individuals contain significantly higher percentages of type-1, and -2 PMCs. F. The average number of platelets per monocyte in a PMC did not differ between the two study groups. G and H. Monocytes were isolated from PBMCs derived from HIV seronegative subjects (n=3) and were allowed to adhere to the culture dishes for 2 hrs. Cells were further processed for SEM (G) and TEM (H). In panels (A) – (F) samples were compared using an unpaired t-test which indicated significance as *p<0.05 and ***p<0.001.
The presence of type 2 complexes is very intriguing as there were no platelets physically attached to these monocytes. Monocyte may express some level of CD61 on their surface, as a subunit of the vitronectin receptor (VNR). The exact level of expression of this receptor on primary monocytes is not well understood. A study by Weerasinghe et al (30) detected low levels of VNR on monocytes. Another report by Lefrenie et al (31) demonstrated total absence of VNR in primary monocytes unless these cells were further cultured in presence of MCSF for 3-5 days. It is noteworthy that the monocytes in our experiments are unexposed to such external stimuli. Further, our TEM images of the type 1 complexes illustrate that the platelet and monocyte membranes remain in close proximity when complexed (Figure 2H), which may facilitate the transfer of platelet microparticles (which often expresses CD61 (32-34)) to the monocyte. This seems to be a more plausible explanation for the presence of type 2 complexes as we did not observe fusion between the membranes of the two cell types.
sCD40L induces PMC formation via the interaction of platelet P-selectin with monocyte PSGL-1
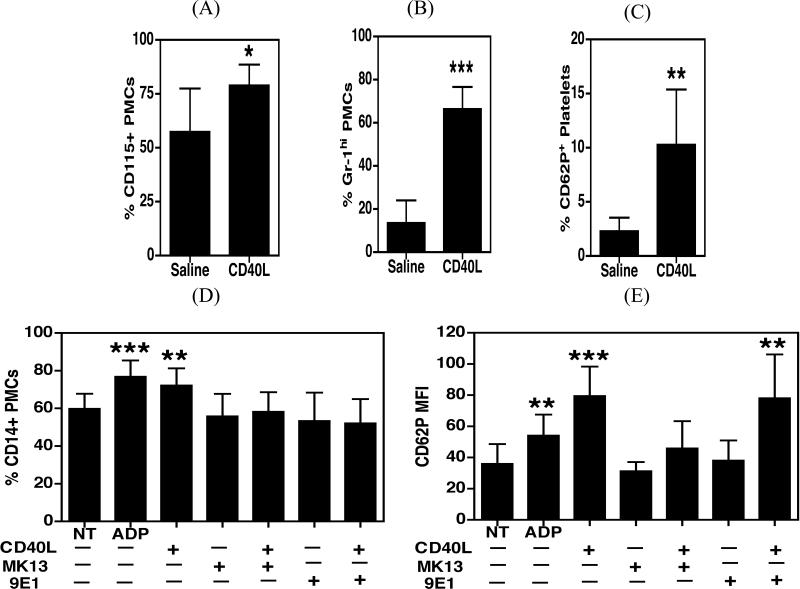
Previously we reported that HIV infected individuals with cognitive impairment have increased levels of sCD40L in their plasma and cerebrospinal fluid (CSF) as compared to infected individuals without cognitive impairment (24). To investigate the role of sCD40L in the platelet monocyte interaction, wild-type C57BL/6J mice were injected with either saline or recombinant mouse sCD40L (rmsCD40L; 0.2 μg/g body weight), and PMC formation was subsequently analyzed in whole blood. CD40L treated animals demonstrated a significant increase in total PMCs (p=0.023, Figure 3A), which was more pronounced in GR-1hi inflammatory complexes (p<0.0001, Figure 3B). As expected, sCD40L treatment induced a significant increase in platelet activation (p=0.008, Figure 3C) as compared with saline-treated animals.
Figure 3. CD40L induced platelet activation leads to PMC formation.
A-C. C57BL/6J mice were injected retro-orbitally with either recombinant mouse sCD40L or saline (n=6). Two hours post-injection, blood was drawn, stained with antibodies against CD115, Gr-1, CD61 and CD62P (P-selectin) and acquired on an Accuri C6 flow cytometer. A and B. Injection of rmsCD40L lead to a significant increase in PMCs, with a more pronounced difference in the inflammatory Gr-1hi monocyte subset. C. rmsCD40L also caused an increase in platelet CD62P expression. D and E. Whole blood obtained from HIV negative study participants (n=4) was treated with ADP or rhsCD40L in the presence or absence of either MK13 (neutralizing antibody against CD40L) or 9E1 (a blocking antibody against P-selectin). The blood was then processed as described previously for the detection of PMCs using an Accuri C6 flow cytometer. D. CD40L induced a significant increase in percentage for CD14+ PMCs, which was abrogated by both MK13 and 9E1. E. CD40L treatment leads to an increase in CD62P (P-selectin) expression on platelets, which was reversed by MK13 but not by 9E1. Samples in panels (A) – (C) were compared using an unpaired t-test and samples in panels (D)-(E) were compared using one-way ANNOVA followed by Bonferroni's test which indicated significance as *p<0.05, **p<0.01, and ***p<0.001.
In line with this, whole blood treatments using samples from HIV negative donors also showed that treatment with recombinant human sCD40L (rhsCD40L) increases the degree of CD14+ PMC formation (p=0.006, Figure 3D) and platelet activation (p<0.0001, Figure 3E), to levels comparable to ADP treatment (positive control, p=0.006 for platelet activation and p=0.0009 for PMCs). This effect was reduced by the addition of MK13, a neutralizing antibody raised against CD40L, indicating that the increase in PMCs was specifically due, in part, to CD40L. Similarly, 9E1, a blocking antibody raised against P-selectin, also ameliorated PMC formation implying the involvement of the P-selectin-PSGL-1 interaction in PMC formation. In addition, while MK13 treatment abrogated P-selectin (CD62P) expression by platelets, 9E1 had no effect on this marker (p=0.001, Figures 3D and 3E).
Monocytes in complex with activated platelets exhibit an enhanced pro-inflammatory and pro-migratory phenotype
To further investigate the effect of the interaction of monocytes with platelets on monocyte activation, we developed a co-culture system using platelets and monocytes isolated from HIV uninfected donors. Platelets were either treated using rhsCD40L, or left untreated, and analyzed for P-selectin (CD62P) expression to verify activation status (Figure 4A). The platelets were subsequently mixed with monocytes, and the co-cultures were then used for the detection of CD14+ PMCs, as well analysis of cell surface expression of CCR2, PSGL-1, and CD40 on the complexed monocytes. Single cell type cultures were also treated in parallel, and used as controls. Platelets that were pre-treated with rhsCD40L induced significantly higher levels of PMC formation upon mixture with monocytes, as compared to platelets that had been left untreated (p=0.029, Figure 4B). This effect of platelets on PMC formation was not due to direct action of rhsCD40L on monocytes because such treatment failed to increase CD16 expression on monocytes (data not shown). Monocytes in complex with activated platelets showed elevated expression of CCR2 (p=0.07, Figure 4C), PSGL-1 (p=0.048, Figure 4D), and CD40 (p=0.017, Figure 4E). In addition, in some experiments, the platelet-monocyte co-cultures were allowed to adhere to BMVECs that were either non-activated or were activated using TNF-α for 30 minutes. Monocytes that had been co-cultured with activated platelets demonstrated enhanced adhesion to endothelial cells, regardless of endothelial cell activation status (p<0.01, Figure 5A).
Figure 4. Interaction with activated platelets increases the expression of functionally important molecules on monocytes.
Monocytes and platelets were isolated separately from whole blood obtained from HIV negative donors (n=4). Platelets were either treated with rhsCD40L or were left untreated, and were subsequently mixed with monocytes at the ratio of 10:1. M indicates non-treated monocytes, M+C indicated monocytes treated with CD40L, M+pl indicates monocytes treated with non-activated platelets and M+Acpl indicates monocytes treated with CD40L-activated platelets. A and B. One hour post-co-culture, cells were used to enumerate PMCs as described before. Platelets activated using rhsCD40L lead to an increase in percentage of CD14+ PMCs as compared with unactivated platelets. C-E. 20 hours post-co-culture, monocytes were stained with antibodies against CCR2, CD40 and PSGL-1 in addition to antibodies for the detection of PMCs. There was a significant increase in the percentage of monocytes expressing of CCR2, CD40, and PSGL-1 up on interaction with activated platelets as compared to monocytes in complex with unactivated platelets. Samples in panels (A) were compared using an unpaired t-test and samples in panel (B)-(E) were compared using one-way ANNOVA followed by Bonferroni's test which indicated significance as *p<0.05, and **p<0.01.
Figure 5. Monocytes in PMCs display increased adherence to endothelium and enhanced transendothelial migration.
A. Monocytes isolated from HIV uninfected blood samples (n=3) were labeled with Calcein AM green and were either co-cultured with activated platelets or, alternatively, treated with rhsCD40L, LPS, or left untreated. 24 hours post-treatment cells were allowed to adhere to BMVECs that had been treated with TNFα or that were left untreated. Fluorescence from adhered cells was measured using a fluorescent plate reader. Results are represented as the fold difference between the number of monocytes that attached to BMVECs under the different experimental conditions and the number of non-treated monocytes that attached to the untreated endothelial cells. Monocytes in complex with activated platelets showed increased adherence to BMVECs. Samples were compared using one-way ANNOVA followed by Bonferroni's test which indicated significance as **p<0.01 and ## p<0.01as compared to non-treated monocytes that adhered to not activated and activated BMVEC respectively. B. Monocytes isolated from HIV uninfected blood samples (n=3) were were either co-cultured with platelets activated using rhsCD40L in presence or absence of 9E1, a p-selectin blocking antibody, or, alternatively, treated with rhsCD40L, 9E1, or left untreated. One hour post-treatment the cells were used to detect PMCs and 24 hours post treatment, the cells were allowed to migrate across BMVEC monolayer plated on FluoroBlok tissue culture inserts with 3μm pore size and the number of migrated CD14+ monocytes was calculated for each treatment. Results of the migration experiment are represented as the fold difference between the numbers of migrated CD14+ monocytes among various treatments and un-treated monocytes. M indicates non-treated monocytes, M+C indicated monocytes treated with CD40L, M+9E1 indicates monocytes treated with 9E1 antibody, M+ Acpl indicates monocytes treated with CD40L-activated platelets and M+AcPl+9E1 indicates monocytes mixed with platelets that were activated with CD40L in presence of 9E1 antibody. Monocytes in complex with activated platelets showed significantly increased migration across BMVECs as compared to un-treated monocytes as well as cultures where PMC formation was blocked through use of 9E1 antibody. Samples were compared using one-way ANNOVA followed by Bonferroni's test which indicated significance as ***p<0.001, **p<0.01 as compared to un-treated monocytes and #p<0.05 as compared with monocytes that were treated with platelets activated in presence of 9E1 antibody.
Interaction of monocytes with activated platelets promotes transendthelial migration of monocytes
Since monocytes in complex with activated platelets showed increased adherence to BMVECs, we sought to investigate if these cells also migrate more efficiently through the trans-endothelial barrier. We established platelet-monocyte co-cultures as described previously. In addition, this time we used 9E1, which is a p-selectin blocking antibody, to prevent p-selectin dependent formation of PMCs. One hour post-treatment, cells were stained and acquired to enumerate the percentages of CD14+ PMCs. Consistent to the data shown in Figure 3D, Co-culturing of rhsCD40L-exposed platelets with monocytes resulted significantly higher levels of PMC formation (p<0.001 as compared to un-treated monocytes), while activated platelets that were treated with 9E1 showed significantly reduced PMCs formation (p<0.05 as compared to monocytes treated with activated platelets, data not shown). Twenty-four hours later the cells were allowed to migrate through a monolayer of BMVECs. It was our speculation that the incubation of PMCs for 24 hrs will lead to enhanced expression of CCR2, PSGL-1 and CD40, followed by increased adhesion to BMVECs (as shown in Figures 4C-E and 5A), As expected, higher number of CD14+ monocytes that were treated with activated platelets migrated through the BMVEC monolayer (P<0.01 as compared to un-treated monocytes). Interestingly, this population of monocytes appeared to be predominantly CD16+ (over 65%; data not shown). On the other hand, the migration of monocytes was significantly reduced when PMC formation was blocked with the use of 9E1 antibody (p<0.05 as compared to monocytes treated with activated platelets, Figure 5B).
Immunohistochemical evidence of PMCs in brain tissue from cases of HIV-associated encephalitis (HIV-E)
We next sought to evaluate whether PMCs could be detected in the CNS of individuals that had prominent neuropathology due to HIV infection. Post-mortem tissues derived from basal ganglia and cortical region of three patients with HIV-E and two HIV seronegative patients were double immunolabeled for CD68 (monocytic/macrophage marker) and CD61. Clinical, pathologic and demographic details of these cases have been published before (29, 35). As shown in Figure 6, the brain specimens obtained from patients with HIV-E contained evidence of PMCs, both in the lumen (panels, C, D and F; only basal ganglia regions are shown) and marginalized (attached to the endothelium) to the brain microvasculature (panels E and G). Of note, there was an increased presence of type 1 PMCs, featuring multiple platelets on a single monocyte/macrophage (Figure 6 panels, C-G). In contrast, no PMCs were observed in control brain samples derived from HIV-1 seronegative subjects ( panels A and B). Although CD68 positive cells in the perivascular space (PS) were seen in perivascular cuffs and disseminated throughout the brain parenchyma, no platelets were detected alone or in complex with monocytes within the perivascular space or in the parenchyma. As previously noted these tissues have extensive neuroinflammation, showing microgliosis (as revealed by Iba-1 antibodies) and infiltrating CD68 positive cells that are also immunoreactive for the HIV-1 p24 core antigen (35). These results suggest that the increased presence of PMCs is associated with HIV-induced neuroinflammation, thus offering the possibility that PMCs contribute to immune cell migration and breach of the BBB during infection.
Figure 6. Immunohistochemical analysis showed PMCs marginalized to the endothelial wall in brain tissue samples obtained from individuals with HIVE.
Post-mortem brain tissue sections from HIV-1 seronegative individuals (n=2, Panels A and B) and individuals with HIV-E (n=3, Panels C-H) were immunostained using indirect immunofluorescence with antibodies against CD68 (monocytic marker -green) and CD61 (platelet marker -red). Sections were also counterstained with DAPI (blue) to aid in the identification of nucleated cells and platelet clusters. Panels A and B, show tissue sections from uninfected controls lacking PMCs which only display uncoupled CD61+ platelets and CD68+ monocytes. Panels C, D and F, exemplify traveling PMCs present in vessel lumens of HIVE cases. Panel E and G, indicates PMCs appearing as lined or marginalized to the endothelial wall in areas where perivascular cuffing was also evident. Panel H, shows a high magnification image of a traveling PMC inside a cerebral capillary vessel. Asterisk (*), denotes the perivascular space (PS) and vessel lumens in relation to the brain parenchyma. Images were taken at either 40x or 100x (Objective magnifications), scale bars are 50 microns and 10 microns for the 40x and 100x, respectively.
Discussion
HIV-associated neuroinflammation and HIV-E are associated with excess immune activation, which outweighs the amount of virus present within the CNS (7). The inflammatory response from resident, as well as infiltrating, monocytes and macrophages is known to fuel the neuropathogenesis of these disorders (12). In order to transmigrate into the CNS, monocytes must traverse the BBB, formed by endothelial cells that line the cerebral vasculature. Immune trans-endothelial migration can alter the normal function of the neurovascular unit, which consists of endothelial cells, astrocytes, pericytes, and neurons. We have previously indicated that sCD40L secreted by aberrantly activated platelets is implicated in the impairment of the BBB during HIV infection (26), thereby providing monocytes with an increased chance of thwarting this barrier. Furthermore, almost all cells of the monocytic lineage found within the CNS of HIV infected individuals are CD16+ pro-inflammatory monocytes (12). Importantly CD16+ monocytes are well known for their invasiveness across endothelial barriers. Interestingly, the findings reported here now serve to bridge these reports; wherein, we demonstrate that during HIV infection, activated platelets interact more frequently with monocytes that consequently promote a pro-inflammatory and pro-migratory phenotype and cause an increase in the migration of these monocytes across transendothelial barrier. Thus these monocytes, with an enhanced capacity to transmigrate, are better equipped to extravasate through a compromised BBB and consequently, contribute to neuroinflammation.
Consistent with this notion, we now demonstrate that HIV infected individuals enrolled in our study showed the occurrence of increased circulating PMCs, especially in the CD16+ inflammatory monocyte subset, as well as increased platelet activation, in spite of successful combined antiretroviral therapy (cART). Studies have shown an increased incidence of milder forms of HAND in patients treated with cART (36). Thus, these studies highlight the need for the development of adjunctive therapies to supplement cART that could limit the neurological complications associated with infection. The data reported here, as well as previous studies from our group, indicate that modulation of platelet activation, and more specifically the release of sCD40L, may be a novel therapeutic target for these disorders. We previously demonstrated that the formation of PMCs was an event subsequent to platelet activation, and not monocyte activation (21), while we now report that PMCs have an enhanced migratory and invasive phenotype. Soluble CD40L is almost entirely platelet derived (25, 26), and in the current report, the role of CD40L signaling in the generation of PMCs was corroborated via in vitro co-culture of monocytes with CD40L-activated or non-activated platelets. These experiments demonstrated that monocytes in complex with CD40L-activated platelets express higher levels of CCR2, a chemokine receptor important for MCP-1 driven chemotaxis of monocytes into the CNS. PSGL-1, which is necessary for the interaction of monocytes with not only platelets, but also with endothelial cells, and CD40, which is a co-stimulatory molecule and the receptor for CD40L, were also elevated in monocytes incubated with CD40L-activated platelets. Monocytes that were exposed to CD40L-activated platelets also demonstrated an increased propensity to adhere to endothelial cells and migrate across BMVEC monolayer as compared to non-activated monocytes. Corroborating the in vitro results, immunohistochemical analysis of brain tissue samples from patients with HIV-E showed an increased presence of PMCs lining the brain endothelium (lumen) within the basal ganglia and cortical regions, as compared to tissue from uninfected controls.
These data are consistent with the notion that excess levels of sCD40L, and thus platelet activation, found in the plasma of HIV patients (24, 26, 37, 38) contributes to the pathogenesis of neurologic disorders presumably via formation of PMCs, leading to invasion of the CNS by activated monocytes/macrophages. Indeed, our recent report also highlighted the ability of brain microvascular endothelial cells (BMVECs) to respond to CD40L in a manner that promotes monocyte attachment and migration, as exposure of primary human BMVECs to sCD40L in vitro lead to increased expression of adhesion molecules, ICAM-1 and VCAM-1. The same study also showed that the receptor for CD40L, CD40, was found to be highly expressed on endothelial cells in brain tissue collected from HIV-E patients (29). Consistently, other studies speculated that the interaction between platelets and monocytes increases the expression and activity of α4β1 and αMβ2 integrins on monocytes (15, 39) thereby exhibiting increased ability of primary and secondary tethering to endothelial cells and other inflammatory cells (40, 41).
Interestingly, short-term treatment of low dose aspirin, a known antiplatelet agent, was able to attenuate platelet and immune activation in virologically suppressed HIV infected individuals (42). Furthermore, Gremmel et al have shown that prasugrel, an ADP receptor antagonist, reduces agonist induced platelet activation and platelet-leukocyte interactions (43). Similarly, the clinically used mood stabilizer valproic acid (VPA) exerts antiplatelet activity in a manner that attenuates sCD40L levels in HIV infected individuals (37). It noteworthy that the VPA treatment improves cognitive performance, as well as indices of brain metabolism, when tested in a controlled pilot study of HIV infected individuals (44). Thus, dampening platelet activation, and in turn PMC formation, with antiplatelet agents such as aspirin, prasugrel, or VPA may prove a worthy avenue of pursuit in developing adjunctive therapies for use in combination with cART in an effort to reduce HIV-associated inflammatory illnesses such as HAND and cardiovascular disease. Indeed, PMCs are a known risk factor for cardiovascular disease, and HIV infected individuals are at an increased risk for developing these secondary disorders (45, 46). Therefore, the use of antiplatelet agents as an adjunctive therapeutic strategy for HIV infection may have benefits that are multi-factorial.
Formation of PMCs appears to be a very rapid phenomenon, as injection of rmsCD40L caused an increase in total (CD115+), as well as Gr-1hi (inflammatory murine monocyte subset) PMCs in mice as early as 2 hours post-treatment. Gr-1hi monocytes are the functional counterpart in mice for CD16+ monocytes in human, as these cells initiate inflammatory activity and accumulate at the sites of injury more abundantly than Gr-1lo monocytes (47). Consistently, ex vivo treatments using rhsCD40L in whole blood samples obtained from HIV seronegative individuals also induced the formation of PMCs within one hour, an effect that was nullified by the use of a neutralizing antibody raised against CD40L (MK13). Blockade of P-selectin on platelets using the blocking antibody 9E1 did not affect platelet activation in response to rhsCD40L, but significantly inhibited PMC formation, indicating the involvement of the P-selectin-PSGL-1 interaction during PMC formation. Additional molecules known to be involved in platelet monocyte interactions include EMMPRIN (48), CX3CR1 (49), and monocytic CD115 with platelet P-selectin. However, the association of PSGL-1 with platelet P-selectin is considered to be the most critical interface, while other ligands play an additive role (17). Collectively these results indicate that inhibition of sCD40L signaling with antiplatelet agents would be capable of attenuating the formation of PMCs, further highlighting the potential benefits of antiplatelet therapies for the management of neuroinflammatory consequences of HIV-1 infection in patients.
Presence of CD68+ monocytes/macrophages in the perivascular space and adjoining parenchymal regions within HIV-E brain is clearly evident; however, we were unable to detect PMCs and/or platelets that had extravasated into the brain of these patients. Platelets are occasionally shown to possess the ability to extravasate into tissues. For example, a study by Laidlaw et al reported the presence of platelet-leukocyte complexes in inflamed respiratory tissue of subjects with aspirin-exacerbated respiratory disease (50), while another report by Pitchford et al indicated that platelets migrate extra-vascularly in response to a sensitizing allergen and can participate directly in allergic tissue inflammation in a mouse model (51). In contrast to these observations, a recent report by van Gils et al suggests that platelets relocate with monocytic PSGL-1 to the rear of the monocyte, following adherence to the endothelium, subsequently detach from monocytes, and remain at the endothelial cell surface (52). Our data is consistent with that of van Gils et al and suggests that the platelets may not leave the circulation, however, association with activated platelets in the periphery could prime monocytes to migrate into the CNS, with the release of the platelet behind in the vessel lumen.
Taken together, our findings substantiate the role of PMCs in HIV-associated neuroinflammation. In the post-cART era, the severity of neurological diseases has been reduced, however, with the increased life span of HIV infected individuals, mild and asymptomatic forms of neurocognitive impairment are increasing in prevalence (reviewed in (36)). While this means that the morbidity associated with neurologic complications has been lessened, milder forms of the disease can still interfere with the day-to-day functions of an individual, and can often be predictive of more severe forms of these disorders. Given the current absence of effective therapeutic options to address this aspect of the disease, the results presented herein shed light on the underlying mechanisms that may drive the pathogenesis of HIV-associated neuroinflammation, thereby identifying novel targets for the development of adjunctive therapies and highlighting the potential utility of anti-platelet agents as such.
Acknowledgements
We would like to thank the University of Rochester Infectious Disease unit and Rochester Victory alliance, specifically; Carol Greisberger, Catherine Bunce, Emily Cosimano, Mary Adams, Chris Foote and Ann Casey for their help in recruiting study subjects. We would like to thank Karen Bentley at the Electron Microscopy core, Linda Callahan, Maria Jepson and Paivi Jordan at the Multiphoton core. We are also thankful to the University of Rochester Center for AIDS Research (NIH P30 AI078498) for their excellent institutional support
Footnotes
Financial Disclosure
This publication was supported by the NIH grants RO1 NS054578 and RO1 NS066801 (to SBM), T32 AI049815 (to JWJ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 2.Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petito CK, Cho ES, Lemann W, Navia BA, Price RW. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol Exp Neurol. 1986;45:635–646. doi: 10.1097/00005072-198611000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 6.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 8.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ 12 Suppl. 2005;1:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 9.Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- 10.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner- Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 11.Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 12.Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14:318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L'Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 14.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 15.da Costa Martins PA, van Gils JM, Mol A, Hordijk PL, Zwaginga JJ. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol. 2006;79:499–507. doi: 10.1189/jlb.0605318. [DOI] [PubMed] [Google Scholar]
- 16.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85:195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 18.Elalamy I, Chakroun T, Gerotziafas GT, Petropoulou A, Robert F, Karroum A, Elgrably F, Samama MM, Hatmi M. Circulating plateletleukocyte aggregates: a marker of microvascular injury in diabetic patients. Thromb Res. 2008;121:843–848. doi: 10.1016/j.thromres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, Lessard DM, Marchese P, Frelinger AL, 3rd, Goldberg RJ, Michelson AD. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38:1002–1006. doi: 10.1016/s0735-1097(01)01485-1. [DOI] [PubMed] [Google Scholar]
- 20.Rinder HM, Bonan JL, Rinder CS, Ault KA, Smith BR. Dynamics of leukocyte-platelet adhesion in whole blood. Blood. 1991;78:1730–1737. [PubMed] [Google Scholar]
- 21.Singh MV, Davidson DC, Kiebala M, Maggirwar SB. Detection of circulating platelet-monocyte complexes in persons infected with human immunodeficiency virus type-1. J Virol Methods. 2012;181:170–176. doi: 10.1016/j.jviromet.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Hu H, Lindqvist M, Wikstrom-Jonsson E, Goodall AH, Hjemdahl P. Platelet-leukocyte cross talk in whole blood. Arterioscler Thromb Vasc Biol. 2000;20:2702–2708. doi: 10.1161/01.atv.20.12.2702. [DOI] [PubMed] [Google Scholar]
- 23.Holme PA, Muller F, Solum NO, Brosstad F, Froland SS, Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. Faseb J. 1998;12:79–89. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Sui Z, Sniderhan LF, Schifitto G, Phipps RP, Gelbard HA, Dewhurst S, Maggirwar SB. Functional synergy between CD40 ligand and HIV-1 Tat contributes to inflammation: implications in HIV type 1 dementia. J Immunol. 2007;178:3226–3236. doi: 10.4049/jimmunol.178.5.3226. [DOI] [PubMed] [Google Scholar]
- 25.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Plateletderived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 26.Davidson DC, Hirschman MP, Sun A, Singh MV, Kasischke K, Maggirwar SB. Excess soluble CD40L contributes to blood brain barrier permeability in vivo: implications for HIV-associated neurocognitive disorders. PLoS One. 2012;7:e51793. doi: 10.1371/journal.pone.0051793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Mesy Jensen K. Pop-off technique for FNA smears for diagnostic electron microscopy. American Society of Clinical Pathologists Conference Abstract. 1987 [Google Scholar]
- 28.Ramirez SH, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. J Immunol. 2008;180:1854–1865. doi: 10.4049/jimmunol.180.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez SH, Fan S, Dykstra H, Reichenbach N, Del Valle L, Potula R, Phipps RP, Maggirwar SB, Persidsky Y. Dyad of CD40/CD40 ligand fosters neuroinflammation at the blood-brain barrier and is regulated via JNK signaling: implications for HIV-1 encephalitis. J Neurosci. 2010;30:9454–9464. doi: 10.1523/JNEUROSCI.5796-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weerasinghe D, McHugh KP, Ross FP, Brown EJ, Gisler RH, Imhof BA. A role for the alphavbeta3 integrin in the transmigration of monocytes. J Cell Biol. 1998;142:595–607. doi: 10.1083/jcb.142.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lafrenie RM, Lee SF, Hewlett IK, Yamada KM, Dhawan S. Involvement of integrin alphavbeta3 in the pathogenesis of human immunodeficiency virus type 1 infection in monocytes. Virology. 2002;297:31–38. doi: 10.1006/viro.2002.1399. [DOI] [PubMed] [Google Scholar]
- 32.Rank A, Nieuwland R, Delker R, Pihusch V, Wilkowski R, Toth B, Kolb HJ, Pihusch R. Surveillance of megakaryocytic function by measurement of CD61-exposing microparticles in allogeneic hematopoietic stem cell recipients. Clin Transplant. 2011;25:E233–242. doi: 10.1111/j.1399-0012.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 33.George JN, Thoi LL, McManus LM, Reimann TA. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982;60:834–840. [PubMed] [Google Scholar]
- 34.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 35.Ramirez SH, Reichenbach NL, Fan S, Rom S, Merkel SF, Wang X, Ho WZ, Persidsky Y. Attenuation of HIV-1 replication in macrophages by cannabinoid receptor 2 agonists. J Leukoc Biol. 2013;93:801–810. doi: 10.1189/jlb.1012523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 37.Davidson DC, Hirschman MP, Spinelli SL, Morrell CN, Schifitto G, Phipps RP, Maggirwar SB. Antiplatelet activity of valproic acid contributes to decreased soluble CD40 ligand production in HIV type 1-infected individuals. J Immunol. 2011;186:584–591. doi: 10.4049/jimmunol.1001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson DC, Schifitto G, Maggirwar SB. Valproic acid inhibits the release of soluble CD40L induced by non-nucleoside reverse 35 transcriptase inhibitors in human immunodeficiency virus infected individuals. PLoS One. 2013;8:e59950. doi: 10.1371/journal.pone.0059950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yago T, Tsukuda M, Minami M. P-selectin binding promotes the adhesion of monocytes to VCAM-1 under flow conditions. J Immunol. 1999;163:367–373. [PubMed] [Google Scholar]
- 40.da Costa Martins P, van den Berk N, Ulfman LH, Koenderman L, Hordijk PL, Zwaginga JJ. Platelet-monocyte complexes support monocyte adhesion to endothelium by enhancing secondary tethering and cluster formation. Arterioscler Thromb Vasc Biol. 2004;24:193–199. doi: 10.1161/01.ATV.0000106320.40933.E5. [DOI] [PubMed] [Google Scholar]
- 41.Theilmeier G, Lenaerts T, Remacle C, Collen D, Vermylen J, Hoylaerts MF. Circulating activated platelets assist THP-1 monocytoid/endothelial cell interaction under shear stress. Blood. 1999;94:2725– 2734. [PubMed] [Google Scholar]
- 42.O'Brien M, Montenont E, Hu L, Nardi MA, Valdes V, Merolla M, Gettenberg G, Cavanagh K, Aberg JA, Bhardwaj N, Berger JS. Aspirin attenuates platelet activation and immune activation in HIV-infected subjects on antiretroviral therapy: A Pilot Study. J Acquir Immune Defic Syndr. 2013;63:280–288. doi: 10.1097/QAI.0b013e31828a292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gremmel T, Eslam RB, Koppensteiner R, Lang IM, Panzer S. Prasugrel reduces agonists’ inducible platelet activation and leukocyteplatelet interaction more efficiently than clopidogrel. Cardiovasc Ther. 2013;5:e40– 45. doi: 10.1111/1755-5922.12021. [DOI] [PubMed] [Google Scholar]
- 44.Schifitto G, Peterson DR, Zhong J, Ni H, Cruttenden K, Gaugh M, Gendelman HE, Boska M, Gelbard H. Valproic acid adjunctive therapy for HIV-associated cognitive impairment: a first report. Neurology. 2006;66:919– 921. doi: 10.1212/01.wnl.0000204294.28189.03. [DOI] [PubMed] [Google Scholar]
- 45.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13:453–468. doi: 10.1111/j.1468-1293.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 46.Palella FJ, Jr., Phair JP. Cardiovascular disease in HIV infection. Curr Opin HIV AIDS. 2011;6:266–271. doi: 10.1097/COH.0b013e328347876c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schulz C, von Bruhl ML, Barocke V, Cullen P, Mayer K, Okrojek R, Steinhart A, Ahmad Z, Kremmer E, Nieswandt B, Frampton J, Massberg S, Schmidt R. EMMPRIN (CD147/basigin) mediates platelet-monocyte interactions in vivo and augments monocyte recruitment to the vascular wall. J Thromb Haemost. 2011;9:1007–1019. doi: 10.1111/j.1538-7836.2011.04235.x. [DOI] [PubMed] [Google Scholar]
- 49.Postea O, Vasina EM, Cauwenberghs S, Projahn D, Liehn EA, Lievens D, Theelen W, Kramp BK, Butoi ED, Soehnlein O, Heemskerk JW, Ludwig A, Weber C, Koenen RR. Contribution of platelet CX(3)CR1 to platelet-monocyte complex formation and vascular recruitment during hyperlipidemia. Arterioscler Thromb Vasc Biol. 2012;32:1186–1193. doi: 10.1161/ATVBAHA.111.243485. [DOI] [PubMed] [Google Scholar]
- 50.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, Castells MC, Chhay H, Boyce JA. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119:3790–3798. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitchford SC, Momi S, Baglioni S, Casali L, Giannini S, Rossi R, Page CP, Gresele P. Allergen induces the migration of platelets to lung tissue in allergic asthma. Am J Respir Crit Care Med. 2008;177:604–612. doi: 10.1164/rccm.200702-214OC. [DOI] [PubMed] [Google Scholar]
- 52.van Gils JM, da Costa Martins PA, Mol A, Hordijk PL, Zwaginga JJ. Transendothelial migration drives dissociation of plateletmonocyte complexes. Thromb Haemost. 2008;100:271–279. [PubMed] [Google Scholar]