Abstract
OBJECTIVE
Fetal alcohol syndrome (FAS) is the most common cause of nongenetic mental retardation. Oxidative stress is one of the purported mechanisms. Nicotinamide adenine dinucleotide phosphate oxidase (NOX) is an enzyme involved in the production of reactive oxygen species. Our objective was to evaluate NOX in the fetal brain of a well-validated mouse model of FAS.
STUDY DESIGN
Timed, pregnant C57BL/6J mice were injected intraperitoneally with 0.03 mL/g of either 25% ethyl alcohol or saline. Fetal brain, liver, and placenta were harvested on gestational day 18. The unit of analysis was the litter; tissue from 6–8 litters in the alcohol and control group was isolated. Evaluation of messenger ribonucleic acid (mRNA) expression of NOX subunits (DUOX1, DUOX2, NOX1, NOX2, NOX3, NOX4, NOXA1, NOXO1, RAC1, p22phox, and p67phox) was performed using quantitative real-time polymerase chain reaction; alcohol vs placebo groups were compared using a Student t test or a Mann-Whitney test (P < .05).
RESULTS
Alcohol exposed fetal brains showed significant up-regulation in subunits DUOX2 (1.61 ± 0.28 vs 0.84 ± 0.09; P = .03), NOXA1 (1.75 ± 0.27 vs 1.09 ± 0.06; P = .04), and NOXO1 (1.59 ± 0.10 vs 1.28 ± 0.05; P = .02). Differences in mRNA expression in the placenta were not significant; p67phox was significantly up-regulated in alcohol-exposed livers.
CONCLUSION
Various NOX subunits are up-regulated in fetal brains exposed to alcohol. This effect was not observed in the fetal liver or placenta. Given the available evidence, the NOX system may be involved in the causation of FAS through the generation of reactive oxygen species and may be a potential target for preventative treatment in FAS.
Keywords: fetal alcohol syndrome, nicotinamide adenine, dinucleotide phosphate oxidase, reactive oxygen species
Despite a 1981 Surgeon General report warning against the use of alcohol in pregnancy in the United States, up to 50% of child-bearing aged women drink alcohol and up to 20% continue to consume alcohol after finding out they are pregnant; 1 in 25 pregnant women admit to binge drinking.1,2 A study in 5 states in the United States from 1995 to 1997 revealed a fetal alcohol syndrome (FAS) prevalence rate from 0.3 to 1.5 cases per 1000 live births.3 FAS is the most common cause of mental retardation not caused by genetics.4
Alcohol crosses the placenta yet the exact quantity of alcohol that is deleterious to a growing fetus remains unknown. Binge drinking is associated with a more negative impact on pregnancy than small amounts of alcohol consumption.5,6 Moderate alcohol consumption in early gestation has not been shown to affect the intelligence quotient of offspring at 8 years of age.7 However, in a separate study, women who consumed more than 70 g of alcohol in a week’s time (or binge drank 1–2 times per week) during the first trimester had an increased risk of alcohol-related birth defects.8
The brain accounts for 20% of total body oxygen consumption.9 Oxygen consumption causes the generation of free radicals, and these may be increased in response to alcohol exposure. The antioxidative system functions to prevent cellular damage produced by free radicals. Superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) are members of the antioxidant system and are present in the cerebral cortex, cerebellum, and hypothalamus.10 We have previously shown that maternal alcohol exposure causes a decrease in messenger ribonucleic acid (mRNA) expression of SOD, GPx, and CAT in the fetal brain.11
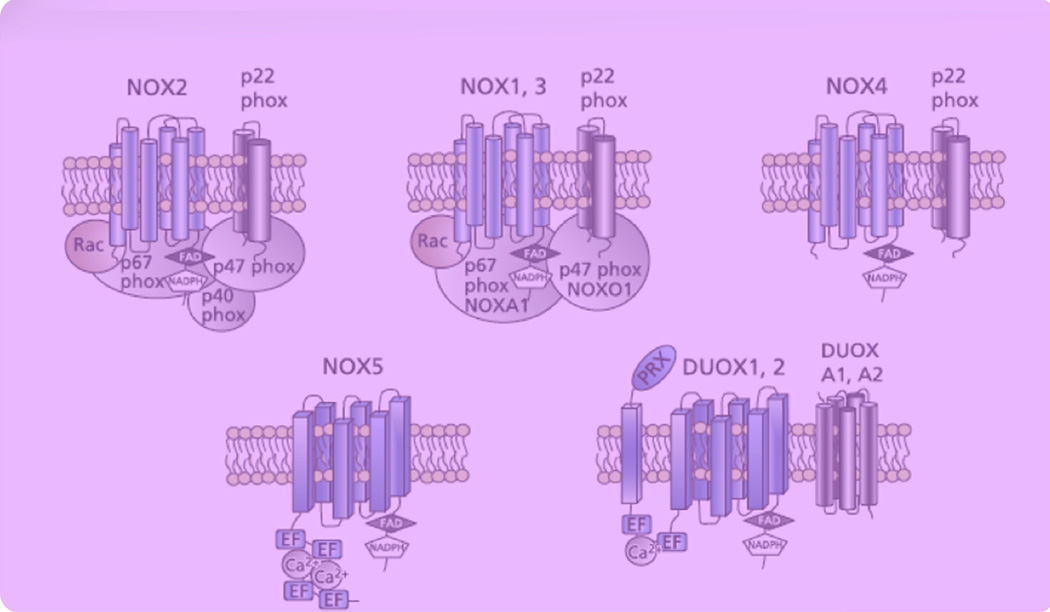
Nicotinamide adenine dinucleotide phosphate oxidase (NADPH) oxidase (NOX) has been found to play a significant role in ethanol induced oxidative stress and has been identified as a source of reactive oxidative species (ROS) in mouse embryos exposed to ethanol.12 The NOX system consists of an intricate web of mechanisms for activation that ensure the ROS created are regulated in quantity and duration of function.13 NOX1 through NOX5 each require different complexes and regulators to function14 including catalytic (DUOX1 and DUOX2) and regulatory (p22phox, p47phox, p67phox, NOXA1, and ½F1± NOXO1) subunits (Figure 1). For example, NOX1 through NOX4 require an intramembranous activator, or maturation factor, called p22phox.13 Our goal was to determine whether FAS is associated with the up-regulation of NOX in the fetal brain.
Figure 1. Illustrated diagrams of NADPH.
EF, EF-hands; FAD, flavin adenine dinucleotide; NADPH, nicotinamide adenine dinucleotide phosphate; PRX, peroxidase-like domain.14
Hill. NADPH oxidase and fetal alcohol syndrome. Am J Obstet Gynecol 2014.
Materials and Methods
Animal care and dosing
The study protocol and all related procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch (Galveston, TX). Pregnant C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were received, and vaginal plug detection was considered gestational day 0. The mice were maintained in the animal care facility at the University of Texas Medical Branch, housed separately in temperature- and humidity-controlled quarters with constant 12-hour light/12-hour dark cycles, and provided with food and water ad libitum. A well-characterized mouse model of FAS was used.15
On gestational day 8, pregnant mice in the alcohol group received an intraperitoneal injection of 25%ethyl alcohol (0.03 mL/g per body weight), whereas those in the control group received an equivalent weight-based dose of saline. On gestational day 18, the pregnant mice were euthanized with carbon dioxide. The placenta, fetal brain, and portion of the fetal liver were immediately flash frozen in liquid nitrogen and stored at −80±C. A total of 8 mice received alcohol and 7 received saline, yielding 53 alcohol- and 50 saline-exposed pups.
RNA isolation and reverse-transcriptase reaction
Tissue samples from each fetus (brain, placenta, and liver) were analyzed individually. Samples were homogenized using Bullet Blender from Next Advance (Averill Park, NY). To adequately represent each litter, 2–3 pup samples per litter were chosen at random to achieve a sample size of 20 for the alcohol group and 20 for the saline group. Total ribonucleic acid (RNA) was isolated using Trizol and a Zymo RNA isolation kit (Ambion, Austin, TX, and Zymo Research Corporation, Irvine, CA) according to the manufacturer’s instructions. Quantification of RNA was performed by measuring the absorbance of RNA sample solutions at 260 nm. For the reverse transcription reaction, we used a high-capacity complementary deoxyribonucleic acid reverse transcription kit (Applied Biosystems, Foster City, CA) with the manufacturer manual (25±C for 10minutes → 37±C for 120 minutes → 85±C for 5 minutes → 4±C for infinity).
Quantitative real-time polymerase chain reaction
SYBR green polymerase chain reaction (PCR) master mix (2 times) (Applied Biosystems, Warrington, UK) was used according to the manufacturer’s instructions for quantitative real-time PCR performed in a 7500 Fast real-time PCR system (Applied Biosystems). Mouse-specific TaqMan primers (Applied Biosystems) were used (Table 1). Gene expression was calculated as the mRNA of the targeted gene relative to the glyceraldehyde-3-phosphate dehydrogenase mRNA levels in each specific sample (relative unit). Each reaction was carried out in duplicate. The relative quantification was determined using 7500 software version 2.06 (Applied Biosystems).
TABLE 1.
Primers used in the experiments
| Gene | Forward (5′-3′) | Reverse (3′–5′) |
|---|---|---|
| NOX1 | AGGTCGTGATTACCAAGGTTGTC | AAGCCTCGCTTCCTCATCTG |
| NOX2 | AGCTATGAGGTGGTGATGTTAGTGG | CACAATATTTGTACCAGACAGACTTGAG |
| NOX3 | GCTGGCTGCACTTTCCAAAC | AAGGTGCGGACTGGATTGAG |
| NOX4 | CCCAAGTTCCAAGCTCATTTCC | TGGTGACAGGTTTGTTGCTCCT |
| DUOX1 | CCACCATGCTGTACATCTGTGA | AGGGAGGGCGACCAAAGT |
| DUOX2 | TCCAGAAGGCGCTGAACAG | GCGACCAAAGTGGGTGATG |
| p22phox | CGTGGCTACTGCTGGACGTT | GCACACCTGCAGCGATAGAG |
| p67phox | TGGACTTCGGATTCACCCTCAGTC | CACCTTGAGCATGTAAGGCATAGG |
| RAC1 | CCCCACCGTCTTTGACAACT | CATAGGCCCAGATTCACTGGTT |
| NOXA1 | ACTCTGCGCTGTGCTTCTTCT | ACAGCCCCTGTTAAAGTACATCCT |
| NOXO1 | GGCAGCCTTGACATTCATAGC | TCTGTCTCTTATGTCAAGAGTGTGGAA |
| GAPDH | AACGACCCCTTCATTGAC | TCCACGACATACTCAGCAC |
Hill. NADPH oxidase and fetal alcohol syndrome. Am J Obstet Gynecol 2014.
Data analysis
The unit of analysis was the litter. Statistical analysis was performed using GraphPad Software, Inc (version 5.04; La Jolla, CA). Data are expressed as mean ± SEM. A Shapiro-Wilk test to check for normality was performed, and then a Student t test or Mann-Whitney test was used accordingly. A 2-tailed value of P < .05 was considered statistically significant.
Results
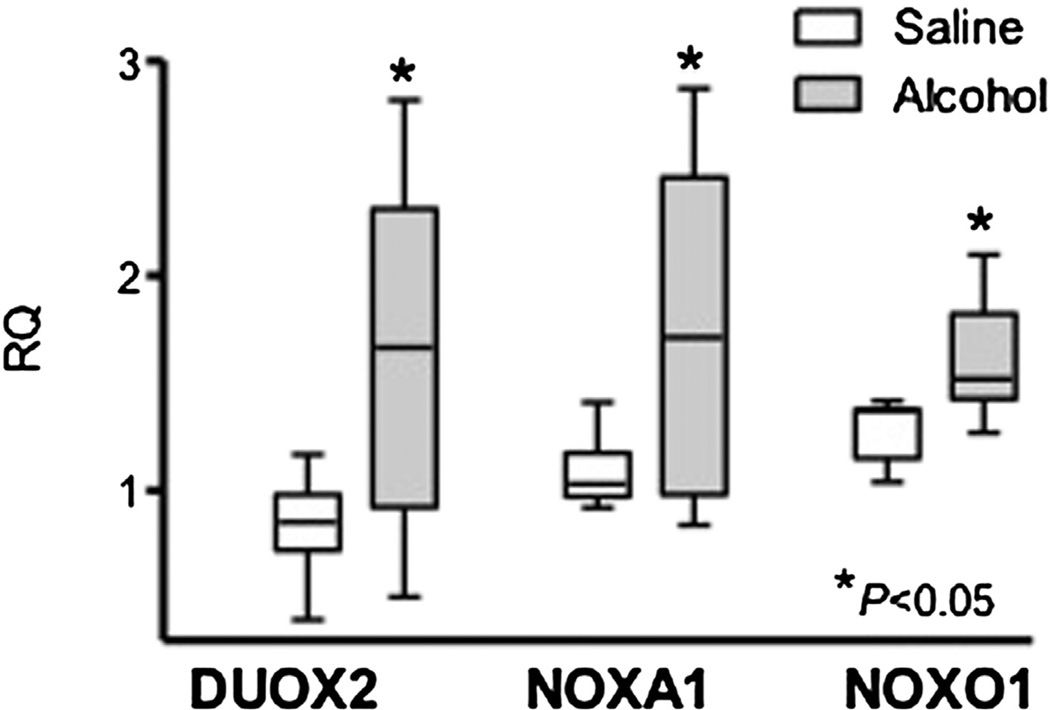
The mRNA expression of DUOX2 (1.61 ± 0.28 vs 0.84 ± 0.09; P =.03), NOXA1 (1.75 ± 0.27 vs 1.09 ± 0.06; P = .04), and NOXO1 (1.59± 0.10 vs 1.28 ± 0.05; P = .02) was found to be significantly increased in the brains of litters born to dams exposed to alcohol compared with the control group (Figure 2). Increased mRNA expression was noted in NOX1-NOX4, DUOX1, and p67phox in brains from the alcohol group but did not reach significance (Table 2). A significant decrease in NOXA1 was found in the control group (0.19 ± 0.07) compared with the alcohol group (0.59 ± 0.07; P = .003) in the placenta (Table 3). mRNA expression of p67phox was increased in livers of pups born to mice in the alcohol group (0.72 ± 0.13 vs 0.29 ± 0.08; P =.02) (Table 4).
Figure 2. Box plot of enzyme mRNA expression.
Box plot of enzyme mRNA expression (RQ) in the fetal brain in alcohol- (8 litters) and saline -(7 litters) exposed groups for DUOX2 (P = .03), NOXA1 (P = .04), and NOXO1 (P = .02). The box extends from the 25th percentile to the 75th percentile with a line at the median (the 50th percentile). Whiskers show the highest and lowest values. The asterisk denotes the statistically significant differences (P < .05) between the groups.
mRNA, messenger RNA; RQ, relative quantity.
Hill. NADPH oxidase and fetal alcohol syndrome. Am J Obstet Gynecol 2014.
TABLE 2.
mRNA expression in mean RQ ± SEM in the brain from pups exposed to alcohol (8 litters) and saline (7 litters)
| Enzyme | Alcohol (n = 8) | Control (n = 7) | P value |
|---|---|---|---|
| DUOX1 | 1.14 ± 0.13 | 1.12 ± 0.11 | .89 |
| DUOX2 | 1.61 ± 0.28a | 0.84 ± 0.09 | .03 |
| NOX1 | 0.95 ± 0.95 | 0.72 ± 0.72 | .23 |
| NOX2 | 1.46 ± 0.20 | 1.08 ± 0.06 | .11 |
| NOX3 | 1.48 ± 0.25 | 1.07 ± 0.07 | .15 |
| NOX4 | 1.66 ± 0.15 | 1.28 ± 0.25 | .21 |
| NOXA1 | 1.75 ± 0.27a | 1.09 ± 0.06 | .04 |
| NOXO1 | 1.59 ± 0.10a | 1.28 ± 0.05 | .02 |
| RAC1 | 1.10 ± 0.12 | 1.17 ± 0.11 | .66 |
| p22phox | 1.76 ± 0.22 | 1.82 ± 0.15 | .82 |
| p67phox | 1.55 ± 0.20 | 1.38 ± 0.09 | .51 |
RQ, relative quantity.
Denotes statistically significant differences (P < .05) between the groups.
Hill. NADPH oxidase and fetal alcohol syndrome. Am J Obstet Gynecol 2014.
TABLE 3.
mRNA expression in mean RQ ± SEM in the placenta from pups exposed to alcohol (6 litters) and saline (6 litters)
| Enzyme Alcohol | (n = 6) Control | (n = 6) | P value |
|---|---|---|---|
| DUOX1 | 0.64 ± 0.13 | 0.70 ± 0.11 | .73 |
| DUOX2 | 0.68 ± 0.20 | 1.58 ± 0.36 | .06 |
| NOX1 | 2.01 ± 0.56 | 1.46 ± 0.45 | .46 |
| NOX2 | 0.43 ± 0.09 | 0.50 ± 0.06 | .81 |
| NOX3 | 0.33 ± 0.13 | 0.60 ± 0.06 | .09 |
| NOX4 | 1.30 ± 0.30 | 0.89 ± 0.23 | .31 |
| NOXA1 | 0.19 ± 0.07a | 0.59 ± 0.07 | .003 |
| NOXO1 | 0.57 ± 0.22 | 0.57 ± 0.16 | .99 |
| RAC1 | 0.47 ± 0.05 | 0.53 ± 0.19 | .78 |
| p22phox | 0.69 ± 0.10 | 0.56 ± 0.07 | .31 |
| p67phox | 0.64 ± 0.04 | 0.87 ± 0.11 | .08 |
RQ, relative quantity.
Denotes statistically significant differences (P < .05) between the groups.
Hill. NADPH oxidase and fetal alcohol syndrome. Am J Obstet Gynecol 2014.
TABLE 4.
mRNA expression in mean RQ ± SEM in the fetal liver from pups exposed to alcohol (8 litters) and saline (7 litters)
| Enzyme | Alcohol (n = 8) | Control (n = 7) | P value |
|---|---|---|---|
| DUOX1 | 1.59 ± 0.26 | 1.09 ± 0.31 | .23 |
| DUOX2 | 0.62 ± 0.26 | 0.42 ± 0.07 | .87 |
| NOX1 | 0.22 ± 0.06 | 0.16 ± 0.04 | .39 |
| NOX2 | 0.33 ± 0.03 | 0.35 ± 0.09 | .86 |
| NOX3 | 0.52 ± 0.27 | 2.11 ± 1.21 | .41 |
| NOX4 | 0.64 ± 0.10 | 1.13 ± 0.32 | .15 |
| NOXA1 | 1.26 ± 0.20 | 0.78 ± 0.17 | .09 |
| NOXO1 | 0.49 ± 0.07 | 0.45 ± 0.07 | .67 |
| RAC1 | 1.11 ± 0.15 | 0.76 ± 0.14 | .12 |
| p22phox | 0.91 ± 0.16 | 0.58 ± 0.10 | .11 |
| p67phox | 0.72 ± 0.13a | 0.29 ± 0.08 | .02 |
RQ, relative quantity.
Denotes statistically significant differences (P < .05) between the groups.
Hill. NADPH oxidase and fetal alcohol syndrome. Am J Obstet Gynecol 2014.
Comment
Our findings demonstrate that exposure to alcohol in utero increased the mRNA expression of 3 components of the NOX family (DUOX2, NOXA1, and NOXO1) specifically in the fetal brain with no or little differences in the fetal liver or placenta. Because NOX has been identified as a source of ROS lending toward apoptosis and teratogenesis in neurons, glia, and cerebral blood vessels,16–18 our findings support NOX as an enzyme contributing to increased oxidative stress in the brains of mouse pups exposed to alcohol. Our results further contribute to reports that ROS play a major role in the development of FAS after in utero exposure to alcohol.11,12 Cognitive deficits in the alcohol-affected fetus such as learning new tasks19 and achieving developmental milestones20 have been shown; NOX appears to be involved in the causation of FAS through the generation of ROS.
Supportive data for the role of NOX in FAS have been shown previously. Dong et al,12 using a similar mouse model of FAS, found increased mRNA expression of DUOX1, p22phox, p6phox, NOXA1, and NOXO1 in pooled samples from whole mouse embryos harvested 12 hours after exposure to alcohol when compared with an equal volume of lactated ringers. The up-regulation of NOX in a male mouse model given intragastric alcohol for 10 days showed significantly increased expression of p91phox (another name for NOX2) in the brain but not the liver; p67phox was increased, yet not statistically significantly, in both brain and liver.21 Moreover, a chronic alcohol exposure model in pregnant rats administered various, increasing percentages of alcohol from gestational day 6 until delivery noted the up-regulation in NOX1 and NOX3 in alcohol-exposed pup cerebella.22
The p67phox was the single subunit up-regulated in tissue other than the brain. Increased levels of p67phox in liver is not surprising because it is an activator subunit similar in structure to NOXA1 with documented expression in hepatic stellate cells, kidney, endothelial cells, and glomerular meningeal cells.23
Although we demonstrate a significant increase in DUOX2 in the fetal brain of alcohol-exposed pregnancies, DUOX2 does not have peroxidase activity in the human; thus, the translational significance of this finding still needs to be determined.24 Although DUOX1 and DUOX2 differ structurally from other NOX members by having an extra N-terminal extracellular domain that has peroxidase homology (Figure 1), the C terminal has 50% similarity to NOX2,24 the most researched NOX member. Overlap in the function of these 2 members may be possible. DUOX2 has already been shown to be important in thyroid hormone synthesis in mice and humans,25–27 but much more information is needed before exact conclusions can be made about its relationship to FAS.
Studies have shown that tumor necrosis factor alpha up-regulates NOXO1.28 Vink et al,19 using the same animal model depicted in our study, showed that tumor necrosis factor alpha levels are increased in alcohol-treated pups; this increase is prevented via treatment with peptides NAPVSIPQ and SAL-LRSIPA. NAPVSIPQ and SALLRSIPA are known for their antioxidant properties; thus, it is plausible that NOXO1 could be a specific marker to determine the formation of ROS in the fetal brain exposed to alcohol in utero.
Mouse protein expression suggests that NOX1, NOXO1, and NOXA1 are constitutively active, although this may not correlate with data in humans. NOXA1 is expressed in more tissues than NOXO1, including the spleen, inner ear, stomach, colon, small intestine, uterus, lungs, thyroid, basilar arterial epithelial cells, and vascular smooth muscle cells. Further studies in vivo are still needed to better understand the role of NOXA1.23
We did not find any other differences in the subunits of NOX that could be explained by the lack of specificity in their function. The range of gene expression in the alcohol-exposed pup brains was notably wider than the control group. A larger sample size could decrease this variability, but the sample size chosen showed an equal representation of each litter. Finally, identifying specific enzymes linked to FAS will help expand on inhibitors of NOX such as diphenyleneiodonium, a nonselective flavoenzyme inhibitor, shown to prevent oxidative stress and apoptosis induced by ethanol exposure.12
Given that we found similar up-regulation of NOX in our study 10 days after a single intraperitoneal injection vs 12 hours in the study by Dong et al,12 it appears that the effect of alcohol on NOX is long lasting. However, this long-term effect did not apply to all NOX subunits. Because we analyzed 3 different fetal tissues, we were able to determine that the NOX system in the fetal brain is specifically affected by alcohol exposure.
In conclusion, we have shown a significant up-regulation in 3 NOX subunits in the fetal brain of a well-documented FAS mouse model; therefore, our findings identify the NOX system as a potential target for the preventative treatment in FAS.
Acknowledgments
E.B. is supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program) from the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Office of the Director, National Institutes of Health.
Footnotes
The authors report no conflict of interest.
Presented at the 33rd annual meeting of the Society for Maternal-Fetal Medicine, San Francisco, CA, Feb. 11–16, 2013.
REFERENCES
- 1.Floyd RI, O’Connor MJ, Sokol RJ, Bertrand J, Cordero JF. Recognition and prevention of fetal alcohol syndrome. Obstet Gynecol. 2005;106:1059–1064. doi: 10.1097/01.AOG.0000181822.91205.6f. [DOI] [PubMed] [Google Scholar]
- 2.Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Fetal alcohol syndrome—Alaska, Arizona, Colorado, and New York, 1995–1997. MMWR Morb Mortal Wkly Rep. 2001;51:433. [PubMed] [Google Scholar]
- 4.Spong CY. Protection against prenatal alcohol-induced damage. PLOS Med. 2006;3:e196. doi: 10.1371/journal.pmed.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayal K, Heron J, Golding J, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123:e289. doi: 10.1542/peds.2008-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly YJ, Sacker A, Gray R, et al. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Health. 2012;66:41. doi: 10.1136/jech.2009.103002. [DOI] [PubMed] [Google Scholar]
- 7.Alati R, Macleod J, Hickman M, et al. Intra-uterine exposure to alcohol and tobacco use and childhood IQ: findings from a parental-offspring comparison within the Avon Longitudinal Study of Parents and Children. Pediatr Res. 2008;64:659. doi: 10.1203/PDR.0b013e318187cc31. [DOI] [PubMed] [Google Scholar]
- 8.O’Leary CM, Nassar N, Kurinczuk JJ, et al. Prenatal alcohol exposure and risk of birth defects. Pediatrics. 2010;126:e843. doi: 10.1542/peds.2010-0256. [DOI] [PubMed] [Google Scholar]
- 9.Bailey DM, Bartsch P, Knauth M, Baumgartner RW. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: from the molecular to the morphological. Cell Mol Life Sci. 2009;66:3583–3594. doi: 10.1007/s00018-009-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustyniak A, Michalak K, Skrzydlewska E. The action of oxidative stress induced by ethanol on the central nervous system. Postepy Hig Med Dosw. 2005;59:464–471. [PubMed] [Google Scholar]
- 11.Drever N, Yin H, Kechichian T, et al. The expression of antioxidant enzymes in a mouse model of fetal alcohol syndrome. Am J Obstet Gynecol. 2012;206:358, e19–e22. doi: 10.1016/j.ajog.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong J, Sulik KK, Chen S. The role of NOX enzymes in ethanol-induced oxidative stress and apoptosis in mouse embryos. Toxicol Lett. 2010;193:94. doi: 10.1016/j.toxlet.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leto TL, Adams AG, de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to prolinerich targets. Proc Natl Acad Sci USA. 1994;91:10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsuyama M, Matsuno K, Yabe-Nishimura C. Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J Clin. Biochem. Nutr. 2012;50:9–22. doi: 10.3164/jcbn.11-06SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster WS, Walsh DA, Lipson AH, McEwen SE. Teratogenesis after acute alcohol exposure in inbred and outbred mice. Neurobehav Toxicol. 1980;2:227–234. [Google Scholar]
- 16.Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- 17.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 18.Miller AA, Drummond GR, Sobey CG. Novel isoforms of NADPH-oxidase in cerebral vascular control. Pharmacol Ther. 2006;111:928–948. doi: 10.1016/j.pharmthera.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Vink J, Auth J, Abebe DT, Brenneman DE, Spong CY. Novel peptides prevent alcohol-induced spatial learning deficits and proinflammatory cytokine release in a mouse model of fetal alcohol syndrome. Am J Obstet Gynecol. 2005;193(3 Pt 1):825–829. doi: 10.1016/j.ajog.2005.02.101. [DOI] [PubMed] [Google Scholar]
- 20.Endres M, Toso L, Roberson R, et al. Prevention of alcohol-induced developmental delays and learning abnormalities in a model of fetal alcohol syndrome. Am J Obstet Gynecol. 2005;193(3 Pt 2):1028–1034. doi: 10.1016/j.ajog.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 21.Qin L, He J, Hanes RN, Pluzarev O, Hong J, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflamm. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu J, Tong M, de la Monte SM. Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. Acta Neuropathol. 2007;113:659–673. doi: 10.1007/s00401-007-0199-4. [DOI] [PubMed] [Google Scholar]
- 23.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 24.Ameziane-El-Hassani R, Morand S, Boucher JL, et al. Dual oxidase-2 has an intrinsic Ca2+ dependent H2O2 generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 25.Johnson KR, Marden CC, Ward-Bailey P, Gagnon LH, Bronson RT, Donahue LR. Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol Endocrinol. 2007;21:1593–1602. doi: 10.1210/me.2007-0085. [DOI] [PubMed] [Google Scholar]
- 26.Moreno JC, Bikker H, Kempers MJ, et al. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med. 2002;347:95–102. doi: 10.1056/NEJMoa012752. [DOI] [PubMed] [Google Scholar]
- 27.Zamproni I, Grasberger H, Cortinovis F, et al. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab. 2008;93:605–610. doi: 10.1210/jc.2007-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuwano Y, Tominaga K, Kwahara T, et al. Tumor necrosis factor alpha activates transcription of the NADPH oxidase organizer 1 (NOXO1) gene and upregulates superoxide production in colon epithelial cells. Free Radic Biol Med. 2008;45:1642–1652. doi: 10.1016/j.freeradbiomed.2008.08.033. [DOI] [PubMed] [Google Scholar]