Abstract
OBJECTIVE
To determine, in a case-control study, whether pelvic organ prolapse (POP) is associated with overall lifetime physical activity (combined leisure, outdoor, household, occupational), and lifetime leisure, lifetime strenuous, and teen years strenuous activity.
STUDY DESIGN
191 POP cases (defined as maximal vaginal descent ≥1 cm below the hymen) and 191 age and recruitment-site matched controls (defined as maximal vaginal descent ≤1 cm above the hymen) between 39–65 years with no or mild urinary incontinence, were recruited chiefly from primary care clinics. Participants completed Lifetime Physical Activity (LPAQ) and Occupation (OQ) Questionnaires, recalling activities during 4 age epochs. We performed separate logistic regression models for physical activity measures.
RESULTS
Compared to controls, POP cases had greater BMI and parity. Median overall lifetime activity, expressed in MET-hours/week, did not differ significantly between cases and controls. In adjusted analyses, we observed no associations between odds of POP and overall lifetime physical activity, lifetime leisure activity, or lifetime strenuous activity. There was a marginally significant nonlinear relationship between teen strenuous activity and POP with an increase in the log-odds of POP for women reporting ≥ 21 hours/week of strenuous activity (p=0.046).
CONCLUSION
Lifetime physical activity does not increase the odds of anatomic POP in middle-aged women not seeking care for POP. Strenuous activity during teenage years may confer higher odds of POP. This relationship and the potential role of physical activity and POP incidence should be evaluated prospectively.
Keywords: exercise, leisure, pelvic organ prolapse, physical activity, strenuous activity
INTRODUCTION
Physical activity is crucial in maintaining health, but high intensity activity increases risk for injury.1 Understanding how physical activity impacts pelvic organ prolapse (POP) is important: in their lifetimes, up to one in five women have surgery for POP.2 Childbirth, in particular vaginal delivery, increases the risk of POP, but our understanding of other potentially modifiable risk factors is limited.3,4,5 Prevailing expert opinion holds that chronic repetitive straining, heavy lifting and high-impact activity can eventually produce changes in muscles, ligaments and connective tissue, leading to POP. To prevent POP, the American Urogynecologic Society recommends avoiding heavy lifting and repetitive strenuous activities (http://www.voicesforpfd.org/index.php?mo=cm&op=ld&fid=25; accessed 10/22/13).
Women with POP appear more likely to report strenuous jobs than women without.6–9 However, limitations of published studies include not considering confounders, poorly defining occupational and activity histories, using non-standardized POP outcomes, and excluding household activities, which represent a large portion of daily activitiy for many women. No study systematically assesses lifetime activity. Exploring the association between lifetime physical activity and POP cannot ethically be done in a randomized trial; a life-long cohort study, while possible, would be infeasible. Therefore, we conducted this case-control study to determine whether POP, defined by structured pelvic examination, is associated with a) overall lifetime activity (leisure, outdoor, household, and occupational), b) lifetime leisure activity, c) lifetime strenuous activity, and d) strenuous activity during the teen years. We analyzed strenuous activity during teen years as it is plausible that such activity, during this period of rapid changes in musculoskeletal structure, hormones and weight, could influence pelvic floor integrity.
METHODS
Institutional Review Boards of the University of Utah and Intermountain Healthcare approved this study. All participants completed an informed consent process. Detailed study methods have been published.10
Research nurses recruited women attending one of 17 primary care level gynecologic and family medicine clinics located across the Salt Lake Valley. Initially, we also recruited women from community advertising (flyers, brochures) but as relatively few women responded, relied primarily on in-person recruitment.
Women were initially excluded if they were pregnant or within six months postpartum, < 39 or > 65 years, had prior surgical treatment for POP or incontinence, were not able to walk independently, had medical conditions associated with pelvic floor disorders or low physical activity (uncontrolled diabetes, neurologic disorders such as multiple sclerosis, spinal cord injury, or stroke, rheumatoid arthritis, radical hysterectomy or pelvic irradiation), had urgency-predominant incontinence, were currently undergoing treatment for cancer, or were unable to complete questionnaires. Underweight women (BMI < 18.5 kg/m2) and women in obesity class III (BMI ≥ 40 kg/m2) were excluded as they are more likely to have functional and activity limitations. We chose the age range 39–65 years to reflect the population, included in the original validation of the physical activity instrument chosen for this study11, which is likely to have developed POP and is still of an age likely to engage in a variety of physical activities. Trained research nurses performed the Pelvic Organ Prolapse Quantification (POP-Q), a reproducible method for assessing vaginal support.12–14 We defined POP as present when any segment of the vagina descended at least 1 cm below the hymen (≥ +1 cm) and absent when all vaginal segments were at least 1 cm above the hymen (≤ −1 cm). We did not standardize the time of POP-Q exams, as others found no differences in POP-Q values between examinations done in the morning or afternoon.15 All participants voided immediately before the exam.
To assess lifetime physical activity, we used the self-administered, reliable and valid Lifetime Physical Activity Questionnaire (LPAQ) designed for use in women.11,16 The LPAQ assesses physical activity over four age periods, menarche to age 21, 22–34, 35–50, and 51–65 years, and includes leisure activity, outdoor work and housework. The LPAQ is scored using METs (metabolic equivalents) obtained from the Compendium of Physical Activities17 to calculate MET hours per week. METs provide a way to standardize absolute activity intensity that reflects multiples of the resting metabolic rate. (For examples, see Table 3 legend.) Because the LPAQ does not query occupational activity, we added the Occupation Questionnaire (OQ), a component of the Lifetime Overall Physical Activity Questionnaire (LTPAQ).18
Table 3.
Physical activity summary measures in study population
| Control | POP Case | ||
|---|---|---|---|
|
| |||
| Overall lifetime activity (average MET-hours/week)* | N | 191 | 191 |
| Mean (SD) | 154.64 (85.64) | 154.67 (74.31) | |
| Median (IQR) | 146.38 (92.24, 196.33) | 142.88 (104.23, 190.69) | |
|
| |||
| Lifetime leisure activity (average MET-hours/week) | N | 191 | 191 |
| Mean (SD) | 38.77 (37.34) | 32.83 (33.90) | |
| Median (IQR) | 29.27 (13.35, 49.70) | 22.34 (9.87, 46.40) | |
|
| |||
| Lifetime strenuous activity (average hours/week) | N | 191 | 191 |
| Mean (SD) | 9.30 (6.19) | 10.56 (7.63) | |
| Median (IQR) | 7.77 (4.54, 13.02) | 8.98 (5.13, 14.04) | |
|
| |||
| Lifetime moderate activity (average hours/week)** | N | 191 | 191 |
| Mean (SD) | 21.63 (16.06) | 23.01 (14.16) | |
| Median (IQR) | 16.97 (10.73, 29.40) | 19.82 (12.41, 29.96) | |
|
| |||
| Lifetime vigorous activity (average hours/week)** | N | 191 | 191 |
| Mean (SD) | 2.30 (2.82) | 1.75 (2.07) | |
| Median (IQR) | 1.42 (0.52, 3.10) | 1.05 (0.39, 2.33) | |
|
| |||
| Strenuous Activity (average hours/week) in 1st age epoch (12–21 years) | N | 191 | 191 |
| Mean (SD) | 5.08 (4.88) | 5.36 (6.21) | |
| Median (IQR) | 3.30 (1.29, 7.54) | 3.18 (1.42, 6.57) | |
IQR: Interquartile range
All variables, with the exception of lifetime leisure activity, include leisure, household, outdoor, and occupation related activity.
Moderate activity: activities with 3–6 METs; Vigorous activity: activities with >6 METs; based on ACSM’s Guidelines for Exercise Testing and Prescription (8th Ed.)19
We obtained overall lifetime physical activity by multiplying the MET score assigned to each activity by the reported number of hours per week, fraction of months in a year, and fraction of years lived in each age epoch, and added the average MET hours per week calculated on the Occupation Questionnaire. To calculate overall leisure physical activity, we restricted activities to those related to traditional exercise and recreation. While there is much overlap between vigorous activities (defined as >6 METs19) and activities that result in higher force on the pelvic floor (which we term strenuous activity), some vigorous activities are not strenuous (like fast swimming) and some strenuous activities are not vigorous (like carrying a toddler for extended periods). We classified activities associated with relatively higher intra-abdominal pressures or considered by pelvic floor experts to be potentially associated with the development or progression of POP20 as strenuous (Table 1) and reported average weighted strenuous hours per week.
Table 1.
Activities classified as strenuous.
| Aerial dance trapeze |
| Backpacking |
| Bailing hay |
| Basketball |
| Carrying large pails of water or feed |
| Carrying loads over 30 lb |
| Cheerleading |
| Chopping wood |
| Cleaning large animal pens/farm work |
| Climbing > 10 flights of stairs per day |
| European (team) handball |
| Field hockey |
| Football |
| Hangliding/windsurfing |
| Health club exercise, general |
| Heavy carpentry |
| Heavy garden work (shoveling, turning soil) |
| Heavy housecleaning |
| High jumping (track and field) |
| Jet ski |
| Jumping on trampoline |
| Jumping rope |
| Kickball |
| Kickboxing |
| Lacrosse |
| Lifting > 30 lb from floor |
| Lifting >30 lb from counter height |
| Lifting heavy weights (recreational/fitness) |
| Lifting or carrying children or dependent elder |
| Martial arts (all varieties) |
| Motorcycle racing (motor cross) |
| Moving heavy furniture without assistance |
| Mowing lawn with push mower |
| Other racquet sports |
| Rock climbing |
| Rugby |
| Skiing, downhill; snowboarding |
| Snow shoveling by hand |
| Soccer |
| Softball/baseball |
| Springboard diving |
| Sprinting |
| Tennis |
| Ultimate Frisbee |
| Volleyball |
| Wallyball |
| Water skiing |
We collected self-reported information about risk factors for pelvic floor disorders (Table 1). Because of the inaccuracy of recall of obstetric events, other than type of delivery, we did not ask more focused questions about childbirth history. 21 We used the validated Epidemiology of Prolapse and Incontinence Questionnaire (EPIQ) to collect pelvic floor symptoms.22 Participants completed questionnaires either on a paper or an electronic survey.23,24 Exercise science graduate students reviewed missing and improbable responses on each LPAQ and OQ with participants using an established protocol. The LPAQ+OQ was considered insufficient for analysis if: 1) No physical activity was recorded of any type for an entire age epoch, 2) No physical activity over the entire LPAQ was recorded for leisure time or household domains, 3) Overall physical activity was reported for more than 168 hours per week in any age epoch, or 4) Calculated physical activity exceeded 671 MET hours/week in any age epoch.20
From the initial pool of participants, we then applied additional exclusion criteria. Because urinary incontinence and POP may coexist but have different risk factors, we excluded women with moderate/severe urinary incontinence defined as a score of ≥ 3 on the reliable, validated Incontinence Severity Index25,26. Consistent with research by others, we excluded women with vaginal descent at the hymen to more clearly delineate POP vs no POP.27,28 Finally, we excluded those that did not return the activity questionnaires, or that returned them but their quality was insufficient for analysis.
Research nurses obtaining outcome measures were masked to LPAQ + OQ results and exercise science researchers were masked to group assignment.
The a priori calculated sample size, fully explained elsewhere10, of at least 175 cases and 175 controls was calculated to provide over 80% power at the 2-sided 5% significance level to detect a protective odds ratio of 0.295 for a 1 SD increase in actual physical activity, accounting for measurement error. 29
Analysis
We planned a priori to frequency match controls and cases for age, BMI and recruitment source (primary care clinics vs community advertising). However, before beginning data analysis, we elected not to frequency match or adjust for BMI, as two prospective cohort studies published after our study began showed that lifetime PA ‘causes’ BMI.30,31 Thus, BMI is on the direct pathway between lifetime activity and POP and is an effect of lifetime PA; adjusting could eliminate the association of activity with POP by over-adjustment. We frequency matched controls to cases 1:1 by recruitment source and age (39–49, 50–60, 61–65 years), and selected controls using a computerized random number generator when > 1 was eligible.
We grouped physical activity variables into quintiles based on their distribution in the selected control group. In light of recent literature highlighting the independent deleterious effect of sedentary activity32, we assigned the 2nd quintile as the reference group. We performed logistic regression with variable selection guided by an updated directed acyclic graph (DAG), in which BMI was depicted as an intermediate variable, developed using DAGitty version 2.0.33,34 Required adjustment variables were education and the age match variable. Cough and constipation were also suggested, but the cell sizes for these were too small to include. We further adjusted for number of vaginal deliveries and hysterectomy status, based on past literature, which was permissible per the DAG. Regression diagnostics were checked for multicollinearity and influential observations. The primary physical activity measures were analyzed in separate models. Plots of initial regression coefficients were inspected, and the Stata multivariable fractional polynomials (mfp) procedure was run to examine the functional relationship of physical activity variables with POP. Variables demonstrated a linear relationship on the logit scale, except for strenuous activity in the teen epoch which had a cubic relationship.
Missing values were addressed in the final models using multiple imputations in SAS 9.3 with fully conditional specification, predictive mean matching of continuous variables, and logistic regression prediction of categorical variables.35–38 As a sensitivity analysis, odds ratios were re-estimated using simulation-extrapolation (SIMEX),39 with bootstrapped standard errors to adjust for measurement error, using measurement error variances from our auxiliary reproducibility sub-study, in which test-retest and inter-method (web vs. paper administration) intraclass correlations (ICC) were 0.64–0.88.40
We used a 5% significance level for tests of effects, but considered p-values for individual quintiles versus the reference category to be significant if <0.01, to adjust for multiple comparisons. All statistical programming calculations were verified by a second independent research team member. Analysis was performed using SAS 9.3 and the multivariable fractional polynomial and simulation extrapolation procedures in Stata 11 and 12.
RESULTS
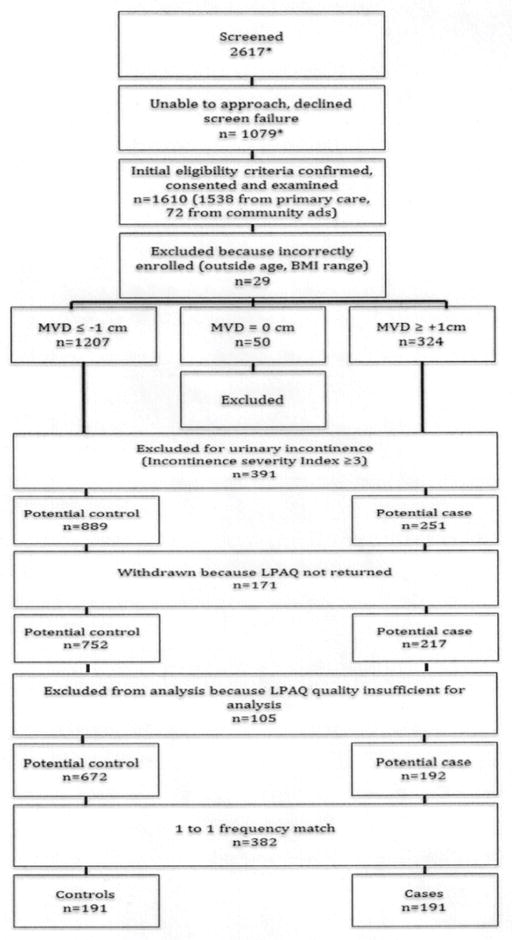
We enrolled 1610 women; 1538 (95.5%) from primary care clinics and 72 (4.5%) from community advertising. After applying exclusion criteria demonstrated in Figure 1, there were 251 potential cases and 889 potential controls. Of these, 969/1140 (85%) returned the study questionnaires. There were no differences in age, BMI, race, ethnicity or case/control status between those that did or did not return questionnaires. Of those that returned study questionnaires, LPAQ + OQ quality was sufficient for analysis in 864/969 (89.2%); there were no differences in these demographics between those with sufficient or insufficient questionnaire quality. All but one of the 192 potential cases could be matched 1:1 with a control. Participant characteristics are summarized in Table 2. The mean age (SD) of the population was 50.1 (7.1) years. There was a trend towards higher BMI in cases compared to controls (26.2 versus 25.2 kg/m2, respectively, p=0.051). POP cases had greater parity (2.83 (SD 1.59) versus 1.84 (1.57) in controls, p<0.0001) and more vaginal deliveries (2.66 (1.6) versus 1.53 (1.58), p<0.0001). Compared to women with 0 vaginal deliveries, those with 1, 2 and ≥ 3 had 3.50 (95% CI 1.62, 7.57), 5.64 (2.95, 10.79) and 7.37 (4.02, 13.53) times the odds of being POP cases. Other than the symptom of vaginal bulge, more common in the POP group (19.95% versus 4.2% in controls, p<0.001), there were no differences in other pelvic floor symptoms between cases and controls, respectively, in urinary frequency (29.1% versus 26.7%, p=0.60), urinary urgency (38.4% versus 30.0%, p=0.08), urge urinary incontinence (23.7% versus 22.3%, p=0.76), pelvic pain (12.0% versus 11.0%, p=0.75), or fecal incontinence (20.4% versus 18.3%, p=0.60).
Figure 1. Participant flow.
The number of women screened and screen failures/declines refer to women recruited from the primary care source. These numbers are not available for women that responded to advertisements (community source), however, this recruitment technique was stopped early in the progress of the study.
Table 2.
Participant characteristics
| Control | POP Case | P-Value | Univariate Odds Ratio (95% CI) | ||
|---|---|---|---|---|---|
| Age, (continuous) | N | 191 | 191 | NA | NA |
| Mean (SD) | 50.74 (7.09) | 51.31 (7.07) | |||
|
| |||||
| Age (categorical) | NA | NA | |||
| 39 to 50- | N | 81 | 81 | ||
| %* | 42.41 | 42.41 | |||
| 50 to 61- | N | 88 | 88 | ||
| % | 46.07 | 46.07 | |||
| 61 to 65 | N | 22 | 22 | ||
| % | 11.52 | 11.52 | |||
|
| |||||
| BMI (continuous, units = 5 for OR estimate) | N | 191 | 191 | 0.051 | 1.243 (0.999, 1.545) |
| Mean | 25.23 (4.60) | 26.17 (4.74) | |||
|
| |||||
| BMI (categorical) | 0.1288 | ||||
| 18.5 to 25- | N | 112 | 95 | Referent | |
| % | 58.64 | 49.74 | |||
| 25 to 30- | N | 49 | 52 | 1.251 (0.777, 2.015) | |
| % | 25.65 | 27.23 | |||
| 30 to 40- | N | 30 | 44 | 1.729 (1.009, 2.963) | |
| % | 15.71 | 23.04 | |||
|
| |||||
| Parity (continuous) | N | 190 | 189 | <0.0001 | 1.495 (1.299, 1.722) |
| Mean (SD) | 1.84 (1.57) | 2.83 (1.59) | |||
| Median (range) | 2.00 (0.00,7.00) | 3.00 (0.00, 8.00) | |||
|
| |||||
| Parity (categorical) | <0.0001 | ||||
| Missing | N | 1 | 2 | ||
| % | 0.52 | 1.05 | |||
| 0 | N | 54 | 15 | Referent | |
| % | 28.27 | 7.85 | |||
| 1 | N | 23 | 13 | 2.035 (0.837, 4.948) | |
| % | 12.04 | 6.81 | |||
| 2 | N | 57 | 58 | 3.663 (1.858, 7.222) | |
| % | 29.84 | 30.37 | |||
| 3+ | N | 56 | 103 | 6.621 (3.429, 12.787) | |
| % | 29.32 | 53.93 | |||
|
| |||||
| Number of vaginal deliveries | N | 190 | 189 | <0.0001 | 1.547 (1.346, 1.779) |
| Mean (SD) | 1.53 (1.58) | 2.66 (1.64) | |||
| Median (range) | 1 (0–7) | 2 (0–8) | |||
|
| |||||
| Vaginal Delivery (categorical) | <0.0001 | ||||
| Missing | N | 1 | 2 | ||
| % | 0.52 | 1.05 | |||
| 0 | N | 76 | 19 | Referent | |
| % | 39.79 | 9.95 | |||
| 1 | N | 24 | 21 | 3.500 (1.618, 7.573) | |
| % | 12.57 | 10.99 | |||
| 2 | N | 39 | 55 | 5.641 (2.948, 10.793) | |
| % | 20.42 | 28.80 | |||
| 3+ | N | 51 | 94 | 7.372 (4.016, 13.533) | |
| % | 26.70 | 49.21 | |||
|
| |||||
| Number of Cesarean deliveries | N | 189 | 189 | 0.0459 | 0.703 (0.497, 0.994) |
| Mean (SD) | 0.31 (0.77) | 0.17 (0.49) | |||
| Median (range) | 0(0,6) | 0(0, 3) | |||
|
| |||||
| Cesarean Delivery (categorical) | 0.0628 | ||||
| Missing | N | 2 | 2 | ||
| % | 1.05 | 1.05 | |||
| 0 | N | 154 | 163 | Referent | |
| % | 80.63 | 85.34 | |||
| 1 | N | 17 | 21 | 1.167 (0.593, 2.295) | |
| % | 8.90 | 10.99 | |||
| 2 | N | 15 | 3 | 0.189 (0.054, 0.666) | |
| % | 7.85 | 1.57 | |||
| 3+ | N | 3 | 2 | 0.630 (0.104, 3.821) | |
| % | 1.57 | 1.05 | |||
|
| |||||
| Hispanic | 0.5873 | ||||
| No | N | 183 | 185 | Referent | |
| % | 95.81 | 96.86 | |||
| Yes | N | 8 | 6 | 0.742 (0.252, 2.180) | |
| % | 4.19 | 3.14 | |||
|
| |||||
| Race (OR and p-value are based on non-white versus white) | 0.9909 | 0.995 (0.386, 2.563) | |||
| Missing | N | 1 | 0 | ||
| % | 0.52 | 0.00 | |||
| American Indian | N | 0 | 2 | ||
| % | 0.00 | 1.05 | |||
| Asian | N | 8 | 6 | ||
| % | 4.19 | 3.14 | |||
| Black | N | 1 | 1 | ||
| % | 0.52 | 0.52 | |||
| White | N | 181 | 182 | ||
| % | 94.76 | 95.29 | |||
|
| |||||
| Highest grade or year of school completed | 0.0805 | ||||
| Less Than High School | N | 0 | 1 | NA | |
| % | 0.00 | 0.52 | |||
| High School | N | 16 | 20 | 1.983 (0.935, 4.205) | |
| % | 8.38 | 10.47 | |||
| Some College/Associates | N | 48 | 54 | 1.700 (0.989, 2.921) | |
| % | 25.13 | 28.27 | |||
| Bachelors | N | 59 | 71 | 1.818 (1.091, 3.031) | |
| % | 30.89 | 37.17 | |||
| Graduate/Professional Degree | N | 68 | 45 | Referent | |
| % | 35.60 | 23.56 | |||
|
| |||||
| Current smoker | 0.7784 | ||||
| No | N | 184 | 185 | Referent | |
| % | 96.34 | 96.86 | |||
| Yes | N | 7 | 6 | 0.853 (0.281, 2.586) | |
| % | 3.66 | 3.14 | |||
|
| |||||
| Hysterectomy | 0.8841 | ||||
| No | N | 163 | 164 | Referent | |
| % | 85.34 | 85.86 | |||
| Yes | N | 28 | 27 | 0.958 (0.541, 1.697) | |
| % | 14.66 | 14.14 | |||
|
| |||||
| Post-menopausal | 0.2189 | ||||
| Missing | N | 2 | 0 | ||
| % | 1.05 | 0.00 | |||
| No | N | 103 | 87 | Referent | |
| % | 53.93 | 45.55 | |||
| Yes | N | 80 | 97 | 1.435 (0.952, 2.166) | |
| % | 41.88 | 50.79 | |||
| Don’t Know | N | 6 | 7 | 1.381 (0.447, 4.264) | |
| % | 3.14 | 3.66 | |||
|
| |||||
| Hypertension | 0.7655 | ||||
| No | N | 164 | 166 | ||
| % | 85.86 | 86.91 | |||
| Yes | N | 27 | 25 | 0.915 (0.510, 1.642) | |
| % | 14.14 | 13.09 | |||
|
| |||||
| Arthritis | 0.7815 | ||||
| No | N | 161 | 159 | Referent | |
| % | 84.29 | 83.25 | |||
| Yes | N | 30 | 32 | 1.080 (0.627, 1.861) | |
| % | 15.71 | 16.75 | |||
|
| |||||
| Diabetes | 0.4198 | ||||
| No | N | 187 | 189 | Referent | |
| % | 97.91 | 98.95 | |||
| Yes | N | 4 | 2 | 0.495 (0.090, 2.734) | |
| % | 2.09 | 1.05 | |||
|
| |||||
| Cancer | 0.1097 | ||||
| No | N | 182 | 174 | Referent | |
| % | 95.29 | 91.10 | |||
| Yes | N | 9 | 17 | 1.976 (0.858, 4.550) | |
| % | 4.71 | 8.90 | |||
|
| |||||
| Cough | 0.1383 | ||||
| No | N | 186 | 190 | Referent | |
| % | 97.38 | 99.48 | |||
| Yes | N | 5 | 1 | 0.196 (0.023, 1.692) | |
| % | 2.62 | 0.52 | |||
|
| |||||
| Heart attack or angina | NA | NA | |||
| No | N | 190 | 191 | ||
| % | 99.48 | 100.00 | |||
| Yes | N | 1 | 0 | ||
| % | 0.52 | 0 | |||
|
| |||||
| Major depression | 0.4819 | ||||
| No | N | 183 | 180 | Referent | |
| % | 95.81 | 94.24 | |||
| Yes | N | 8 | 11 | 1.398 (0.550, 3.556) | |
| % | 4.19 | 5.76 | |||
|
| |||||
| Allergies | 0.2729 | ||||
| No | N | 125 | 135 | Referent | |
| % | 65.45 | 70.68 | |||
| Yes | N | 66 | 56 | 0.786 (0.510, 1.209) | |
| % | 34.55 | 29.32 | |||
|
| |||||
| Sleep apnea | 0.0418 | ||||
| No | N | 188 | 180 | ||
| % | 98.43 | 92.24 | |||
| Yes | N | 3 | 11 | 3.830 (1.051, 13.952) | |
| % | 1.57 | 5.76 | |||
|
| |||||
| Chronic Constipation | 0.6624 | ||||
| No | N | 162 | 165 | ||
| % | 84.82 | 86.39 | |||
| Yes | N | 29 | 26 | 0.880 (0.497, 1.560) | |
| % | 15.18 | 13.61 | |||
|
| |||||
| Number of prescription medications, other than vitamins and hormones (continuous) | N | 189 | 190 | 0.7601 | 1.016 (0.915, 1.129) |
| Mean (SD) | 1.5 (1.74) | 1.6 (2.10) | |||
| Median (range) | 1 (0,9) | 1(0,12) | |||
|
| |||||
| Self-reported health status | 0.5401 | ||||
| Excellent | N | 62 | 56 | Referent | |
| % | 32.46 | 29.32 | |||
| Very Good | N | 91 | 97 | 1.180 (0.744, 1.871) | |
| % | 47.64 | 50.79 | |||
| Good | N | 37 | 34 | 1.017 (0.564, 1.834) | |
| % | 19.37 | 17.80 | |||
| Fair | N | 1 | 4 | 4.428 (0.481, 40.810) | |
| % | 0.52 | 2.09 | |||
|
| |||||
| Recruitment Type | NA | ||||
| Primary | N | 175 | 176 | NA | |
| % | 92.15 | 92.15 | |||
| Community | N | 15 | 15 | NA | |
| % | 7.85 | 7.85 | |||
Column percentage
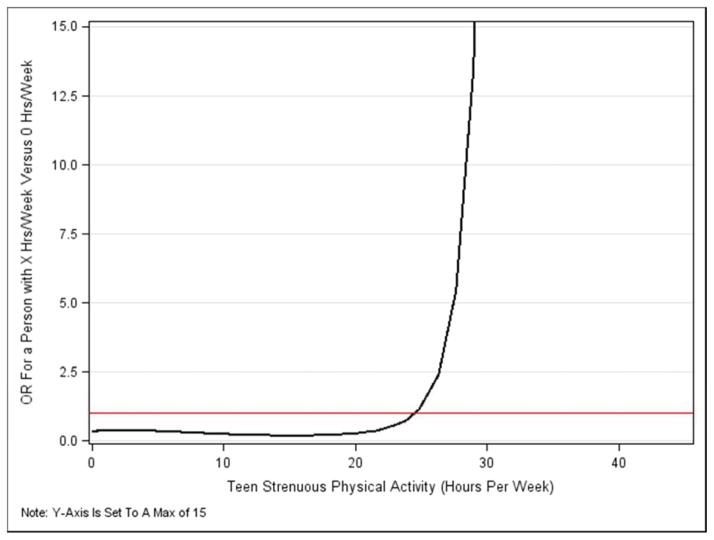
Summary measures for the primary physical activity variables by group are shown in Table 3. We observed no evidence that either lifetime overall, leisure or strenuous physical activity were associated with increased odds of POP in multivariable models (Table 4). However, strenuous physical activity in the teenage years exhibited a nonlinear (cubic polynomial) relationship with the log-odds of POP (p=0.046) and was a risk factor for women reporting ≥ 21 hours/week of teen strenuous physical activity. Because this is a nonlinear relationship, the odds ratio is not constant and is illustrated in Figure 2.
Table 4.
Logistic regression analyses modeling the probability of POP by physical activity measure
| Variable | Adjusted OR (95% CI) [adjusted for age and recruitment source] | Multivariable adjusted OR (95% CI) [adjusted for age, recruitment source, education, # vaginal deliveries and hysterectomy] |
|---|---|---|
| PRIMARY PHYSICAL ACTIVITY VARIABLES | ||
|
| ||
| Overall lifetime activity (quintiles) | ||
| Quintiles: | ||
| 1 vs 2 | 0.56 (0.29, 1.07) | 0.61 (0.29, 1.25) |
| 3 vs 2 | 0.77 (0.41, 1.43) | 0.79 (0.39, 1.57) |
| 4 vs 2 | 1.00 (0.54, 1.84) | 0.92 (0.47, 1.80) |
| 5 vs 2 | 0.73 (0.39, 1.37) | 0.63 (0.31, 1.26) |
| Overall lifetime activity (continuous) (units = 70*) | 1.00 (0.84, 1.20) | 0.95 (0.78, 1.16) |
|
| ||
| Lifetime leisure activity (quintiles) | ||
| 1 vs 2 | 1.58 (0.85, 2.93) | 1.36 (0.69, 2.67) |
| 3 vs 2 | 1.21 (0.63, 2.29) | 1.11 (0.55, 2.23) |
| 4 vs 2 | 0.90 (0.46 1.77) | 0.83 (0.40, 1.71) |
| 5 vs 2 | 0.89 (0.46, 1.72) | 1.16 (0.56, 2.43) |
| Lifetime leisure activity (continuous) (units = 35**) | 0.85 (0.69, 1.04) | 0.97 (0.77, 1.21) |
|
| ||
| Lifetime strenuous activity (quintiles) | ||
| 1 vs 2 | 0.99 (0.51, 1.93) | 1.19 (0.56, 2.51) |
| 3 vs 2 | 1.18 (0.62, 2.26) | 0.87 (0.43, 1.79) |
| 4 vs 2 | 1.53 (0.82, 2.88) | 1.01 (0.50, 2.05) |
| 5 vs 2 | 1.21 (0.64, 2.30) | 0.77 (0.38, 1.58) |
| Lifetime strenuous activity (continuous) (units = 7***) | 1.21 (0.98, 1.50) | 0.98 (0.78, 1.24) |
|
| ||
| Strenuous activity in teen epoch****(quintiles) | ||
| 1 vs 2 | 0.85 (0.45, 1.62) | 0.82 (0.40, 1.65) |
| 3 vs 2 | 1.03 (0.55, 1.92) | 0.98 (0.49, 1.95) |
| 4 vs 2 | 0.98 (0.52, 1.83) | 0.73 (0.37, 1.47) |
| 5 vs 2 | 0.90 (0.48, 1.70) | 0.77 (0.38, 1.54) |
| Strenuous activity in teen epoch (cubic polynomial) (units = 7) | nonlinear relationship: see Figure 2 odds ratios | |
|
| ||
| SECONDARY PHYSICAL ACTIVITY VARIABLES | ||
|
| ||
| Overall activity in teen epoch (quintiles) | ||
| 1 vs 2 | 0.84 (0.44, 1.57) | 0.70 (0.35, 1.40) |
| 3 vs 2 | 0.79 (0.42, 1.49) | 0.70 (0.35, 1.41) |
| 4 vs 2 | 0.91 (0.48, 1.71) | 0.74 (0.37, 1.50) |
| 5 vs 2 | 0.88 (0.47, 1.65) | 0.70 (0.35, 1.39) |
| Overall activity in teen epoch (continuous) (units =70*) | 1.01 (0.80, 1.26) | 1.01 (0.79, 1.29) |
|
| ||
| Overall activity between 21–35 years (quintiles) | ||
| 1 vs 2 | 0.56 (0.28, 1.11) | 0.76 (0.36, 1.61) |
| 3 vs 2 | 1.15 (0.62, 2.14) | 1.01 (0.51, 1.98) |
| 4 vs 2 | 0.84 (0.44, 1.61) | 0.70 (0.34, 1.43) |
| 5 vs 2 | 1.22 (0.65, 2.28) | 0.79 (0.40, 1.60) |
| Overall activity between 21–35 years (continuous) (units = 70*) | 1.14 (0.97, 1.33) | 0.95 (0.79, 1.15) |
|
| ||
| Lifetime vigorous activity (quintiles) | ||
| 1 vs 2 | 1.26 (0.69, 2.31) | 1.23 (0.63, 2.40) |
| 3 vs 2 | 1.05 (0.56, 1.95) | 1.00 (0.50, 1.97) |
| 4 vs 2 | 0.67 (0.35, 1.31) | 0.65 (0.31, 1.35) |
| 5 vs 2 | 0.64 (0.33, 1.24) | 0.67 (0.32, 1.40) |
| Lifetime vigorous activity (continuous) (units = 7***) | 0.50 (0.27, 0.95) | 0.59 (0.29, 1.20) |
70 units is equivalent to an increase of 10 MET-hrs per day for each day of the week (for example, running at 10 minutes per mile pace for one extra hour per day or doing child care for 3.5 extra hours per day each day of the week)
35 units is equivalent to an increase of 5 MET-hours per day for each day of the week (for example, playing doubles tennis for one extra hour per day)
7 units is equivalent to an increase of 1 strenuous hour per day for each day of the week (for example, running at 10 minutes per mile pace for one extra hour per day)
menarche to age 21 years
Figure 2.
Odds ratios for POP as a nonlinear function of hours per week of strenuous activity in the teenage years: the effect of x hours/week versus none. The horizontal line marks an odds ratio of 1.0: odds ratios below this are protective; and above this, indicate increased odds of POP.
Note: Y-Axis Is Set To A Max of 15
We noted no statistically significant differences in odds of POP associated with physical activity in age and recruitment site-adjusted analyses stratified by number of vaginal births (data not shown) All results were similar in sensitivity analyses adding BMI as a covariate to the fully adjusted models, as well as analyses restricted to women recruited only from primary care clinics. We repeated all analyses adjusting for measurement error using the SIMEX technique. No p value approached significance (additional data not shown) except teen strenuous activity (p=0.055). In a non-significant trend, strenuous lifetime activity appeared protective against POP but the confidence interval was wide (OR 0.18 per additional 7 hours per week (CI 0.01, 6.08).
DISCUSSION
In this population of relatively healthy middle-aged women, neither lifetime overall or strenuous activity increased the odds of POP. Only very high levels of teen strenuous activity increased the odds of POP, while lower levels appeared protective. The seven women with the highest reported hours per week of teen strenuous activity (21–39 hours/week) were all POP cases. However, the sensitivity analysis adjusting for measurement error was marginally nonsignificant and very sensitive to changes in the coefficients of the cubic polynomial model. Thus we recommend future studies which investigate teen strenuous activity and its relationship to vaginal support.
The literature addressing the relationship between physical activity and POP is sparse. Similar to our study, no published report assessing exercise and POP supported an association.9,41–43 In contrast, two studies reported that heavy work increased the odds of POP surgery, but neither adjusted for parity.7,8 Heavy lifting increased the odds of bulge symptoms in one study, while two others reported no association between job classification and bulge symptoms or prolapse assessed using a non-validated measure.42,44,9 In three other studies, heavy work was associated with POP based on the POP-Q system, as was military paratrooper training.28,43,45
Our study differs, in that we quantified lifetime physical activity inclusive of all domains. Rather than classifying jobs into categories, we collected data about each job to parse out whether a job considered strenuous, like “factory worker”, actually required operating heavy machines and lifting. To minimize differential misclassification, we studied women who were not seeking care for POP. Other than vaginal bulge, pelvic floor symptoms that might impact physical activity were similar between groups. Given that a minority of women with end-stage POP reported POP interfering substantially with physical activity46, it is unlikely that our cases with POP preferentially did less activity because of pelvic floor symptoms, which would have biased our results towards the null hypothesis.
Strengths of our study include minimizing bias by recruiting participants not seeking care for POP and masking research nurses conducting POP assessment to physical activity or symptom status. We used a validated objective instrument to assess POP and a reliable lifetime physical activity instrument developed for women. We also conducted a nested reproducibility study within this population to enable sensitivity analyses adjusting for measurement error, and found few differences in the results.
The primary limitation of our study is the cross-sectional nature of the data collection; therefore, we cannot establish causality. It is infeasible to directly measure activity prospectively over a lifetime and therefore, no a lifetime physical activity questionnaire will ever be completely validated. However, the LPAQ has acceptable validity over the past 1 year compared to activity measured by accelerometry, and a similar interviewer administered historical adulthood physical activity questionnaire demonstrated moderate correlations between the questionnaire and objectively measured activity collected 15 years earlier.11,47 Retrospective, self-reported physical activity is commonly over reported.48 However, it is unlikely that our results were affected by differential misclassification; participants were not patients seeking care and were not told the study hypothesis or examination findings before questionnaire completion. Our results are most applicable to Caucasian, well educated women with access to medical care. Further, these results may not apply to other populations or to women with both POP and SUI.
While we did not collect recalled BMI, another factor that increases intra-abdominal pressure, by life epoch it is unlikely that obesity as a teen influenced our results because most of our participants were teens prior to the observed significant increases in adolescent obesity.49 Physical activity done just prior to the exam was not standardized, which may have affected POP-Q values,50 but it is unlikely that different proportions of cases and controls performed recent activity.
While our study results challenge the conventional wisdom, they don’t refute a large body of rigorous evidence. Rather, they provide rigorous evidence that lifetime physical activities, including strenuous activities done by women in the course of their lives, do not increase the odds of POP in middle-aged women, except possibly very high levels of strenuous activity performed in the teenage years. It is possible that isolated extreme events, difficult to detect by traditional physical activity questionnaires, may increase the risk of POP, especially in women predisposed based on delivery or genetic risk.3,4,51 While a life-long prospective study is infeasible, studies targeting the shorter-term effects of physical activity on POP progression, recurrence, pelvic floor symptoms, and treatment-seeking in women with varying degrees of vaginal descent are feasible and important to undertake to fully understand the role physical activity plays.
Based on our results, we recommend that adult women be physically active over their lifespan and not restrict activity to prevent POP. The teenage years, as well as early postpartum and post pelvic surgery are potentially vulnerable time points and women with early POP or high genetic risk are potentially vulnerable populations. Our results should not be used to counsel such women. Further research is needed to understand whether physical activity during these times and/or in these populations impacts future pelvic floor function and end-stage POP.
Acknowledgments
Grant support acknowledgement: The project described was supported by Grant Number R01HD057895-01 from the Eunice Kennedy Schriver National Institute of Child Health and Human Development and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Reprints will not be available
Disclosure: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ingrid E. NYGAARD, Email: Ingrid.nygaard@hsc.utah.edu, Department of Obstetrics and Gynecology, University of Utah.
Janet M. SHAW, Email: Janet.Shaw@health.utah.edu, Department of Exercise and Sport Science, University of Utah.
Tyler BARDSLEY, Email: Tyler.bardsley@hsc.utah.edu, Department of Obstetrics and Gynecology, University of Utah.
Marlene J. EGGER, Email: Marlene.egger@hsc.utah.edu, Department of Family and Preventive Medicine, University of Utah.
References
- 1.Colbert LH, Hootman JM, Macera CA. Physical activity-related injuries in walkers and runners in the aerobics center longitudinal study. Clin J Sport Med. 2000;10(4):259–263. doi: 10.1097/00042752-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116(5):1096–1100. doi: 10.1097/AOG.0b013e3181f73729. [DOI] [PubMed] [Google Scholar]
- 3.Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM. Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol. 2006;107(6):1253–1260. doi: 10.1097/01.AOG.0000218096.54169.34. [DOI] [PubMed] [Google Scholar]
- 4.Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Munoz A. Pelvic floor disorders 5–10 years after vaginal or cesarean childbirth. Obstet Gynecol. 2011;118(4):777–784. doi: 10.1097/AOG.0b013e3182267f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risk factors for genital prolapse in non-hysterectomized women around menopause. Results from a large cross-sectional study in menopausal clinics in Italy. Progetto Menopausa Italia Study Group. Eur J Obstet Gynecol Reprod Biol. 2000;93(2):135–140. [PubMed] [Google Scholar]
- 6.Spernol R, Bernaschek G, Schaller A. Entstehungsursachen des deszensus (Factors promoting descensus, in German with English abstract) Geburtsh u Frauenheilk. 1983;43:33–36. doi: 10.1055/s-2008-1037054. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen S, Hein HO, Gyntelberg F. Heavy lifting at work and risk of genital prolapse and herniated lumbar disc in assistant nurses. Occup Med (Lond) 1994;44(1):47–49. doi: 10.1093/occmed/44.1.47. [DOI] [PubMed] [Google Scholar]
- 8.Chiaffarino F, Chatenoud L, Dindelli M, et al. Reproductive factors, family history, occupation and risk of urogenital prolapse. Eur J Obstet Gynecol Reprod Biol. 1999;82(1):63–67. doi: 10.1016/s0301-2115(98)00175-4. [DOI] [PubMed] [Google Scholar]
- 9.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186(6):1160–1166. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 10.Nygaard I, Shaw J, Egger MJ. Exploring the association between lifetime physical activity and pelvic floor disorders: study and design challenges. Contemp Clin Trials. 2012;33(4):819–827. doi: 10.1016/j.cct.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chasan-Taber L, Erickson JB, Nasca PC, Chasan-Taber S, Freedson PS. Validity and reproducibility of a physical activity questionnaire in women. Med Sci Sports Exerc. 2002;34(6):987–992. doi: 10.1097/00005768-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Kobak WH, Rosenberger K, Walters MD. Interobserver variation in the assessment of pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7(3):121–124. doi: 10.1007/BF01894199. [DOI] [PubMed] [Google Scholar]
- 13.Hall AF, Theofrastous JP, Cundiff GW, et al. Interobserver and intraobserver reliability of the proposed International Continence Society, Society of Gynecologic Surgeons, and American Urogynecologic Society pelvic organ prolapse classification system. Am J Obstet Gynecol. 1996;175(6):1467–1470. doi: 10.1016/s0002-9378(96)70091-1. discussion 1470–1461. [DOI] [PubMed] [Google Scholar]
- 14.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 15.Pearce M, Swift S, Goodnight W. Pelvic organ prolapse: is there a difference in POPQ exam results based on time of day, morning or afternoon? Am J Obstet Gynecol. 2008;199(2):200, e201–205. doi: 10.1016/j.ajog.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Chasan-Taber L, Erickson JB, McBride JW, Nasca PC, Chasan-Taber S, Freedson PS. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. 2002;155(3):282–289. doi: 10.1093/aje/155.3.282. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 18.Friedenreich CM, Courneya KS, Bryant HE. The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc. 1998;30(2):266–274. doi: 10.1097/00005768-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Medicine ACoS. ACSM’s Guidelines for Exercise Testing and Prescription. 8. Lippincott, Williams & Wilkins; 2010. [Google Scholar]
- 20.Nygaard IE. Exploring the association between lifetime physical activity and pelvic floor disorders: study and design challenges. 2012. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkadry E, Kenton K, White P, Creech S, Brubaker L. Do mothers remember key events during labor? Am J Obstet Gynecol. 2003;189(1):195–200. doi: 10.1067/mob.2003.371. [DOI] [PubMed] [Google Scholar]
- 22.Lukacz ES, Lawrence JM, Buckwalter JG, Burchette RJ, Nager CW, Luber KM. Epidemiology of prolapse and incontinence questionnaire: validation of a new epidemiologic survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(4):272–284. doi: 10.1007/s00192-005-1314-5. [DOI] [PubMed] [Google Scholar]
- 23.Egger MJ, Lukacz ES, Newhouse M, Wang J, Nygaard I. Web versus paper-based completion of the epidemiology of prolapse and incontinence questionnaire. Female Pelvic Med Reconstr Surg. 2013;19(1):17–22. doi: 10.1097/SPV.0b013e31827bfd93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan SS, Cheung RY, Yiu AK, et al. Prevalence of levator ani muscle injury in Chinese women after first delivery. Ultrasound Obstet Gynecol. 2012;39(6):704–709. doi: 10.1002/uog.10132. [DOI] [PubMed] [Google Scholar]
- 25.Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19(2):137–145. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Sandvik H, Espuna M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(5):520–524. doi: 10.1007/s00192-005-0060-z. [DOI] [PubMed] [Google Scholar]
- 27.DeLancey JO, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 28.Woodman PJ, Swift SE, O’Boyle AL, et al. Prevalence of severe pelvic organ prolapse in relation to job description and socioeconomic status: a multicenter cross-sectional study. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(4):340–345. doi: 10.1007/s00192-005-0009-2. [DOI] [PubMed] [Google Scholar]
- 29.Tosteson TD, Buzas JS, Demidenko E, Karagas M. Power and sample size calculations for generalized regression models with covariate measurement error. Stat Med. 2003;22(7):1069–1082. doi: 10.1002/sim.1388. [DOI] [PubMed] [Google Scholar]
- 30.Waller K, Kaprio J, Kujala UM. Associations between long-term physical activity, waist circumference and weight gain: a 30-year longitudinal twin study. Int J Obes (Lond) 2008;32(2):353–361. doi: 10.1038/sj.ijo.0803692. [DOI] [PubMed] [Google Scholar]
- 31.Hankinson AL, Daviglus ML, Bouchard C, et al. Maintaining a high physical activity level over 20 years and weight gain. JAMA. 2010;304(23):2603–2610. doi: 10.1001/jama.2010.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner A, Dallongeville J, Haas B, et al. Sedentary behaviour, physical activity and dietary patterns are independently associated with the metabolic syndrome. Diabetes Metab. 2012;38(5):428–435. doi: 10.1016/j.diabet.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Sung VW. Reducing bias in pelvic floor disorders research: using directed acyclic graphs as an aid. Neurourol Urodyn. 2012;31(1):115–120. doi: 10.1002/nau.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 35.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 36.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Brand J. Development, Implementation and Evaluation of Multiple Imputation Strategies for the Statistical Analysis of Incomplete Data Sets. Rotterdam: Erasmus University; 1999. [Google Scholar]
- 38.Yuan Y. Multiple Imputation Using SAS Software. Journal of Statistical Software. 2011;45(6) doi: 10.18637/jss.v045.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook J, Stefanski LA. A simulation extrapolation method for parametric measurement error models. J Amer Statistical Assoc. 1995;89:1314–1328. [Google Scholar]
- 40.Chung J, Shaw J, Nygaard I, Egger M. Test-retest reliability of paper and web versions of a lifetime physical activity questionnaire. 2013. Submitted. [Google Scholar]
- 41.Larsen WI, Yavorek TA. Pelvic organ prolapse and urinary incontinence in nulliparous women at the United States Military Academy. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(3):208–210. doi: 10.1007/s00192-005-1366-6. [DOI] [PubMed] [Google Scholar]
- 42.Miedel A, Tegerstedt G, Maehle-Schmidt M, Nyren O, Hammarstrom M. Nonobstetric risk factors for symptomatic pelvic organ prolapse. Obstet Gynecol. 2009;113(5):1089–1097. doi: 10.1097/AOG.0b013e3181a11a85. [DOI] [PubMed] [Google Scholar]
- 43.Braekken IH, Majida M, Ellstrom Engh M, Holme IM, Bo K. Pelvic floor function is independently associated with pelvic organ prolapse. BJOG. 2009;116(13):1706–1714. doi: 10.1111/j.1471-0528.2009.02379.x. [DOI] [PubMed] [Google Scholar]
- 44.Slieker-ten Hove MC, Pool-Goudzwaard AL, Eijkemans MJ, Steegers-Theunissen RP, Burger CW, Vierhout ME. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol. 2009;200(2):184, e181–187. doi: 10.1016/j.ajog.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 45.Larsen WI, Yavorek T. Pelvic prolapse and urinary incontinence in nulliparous college women in relation to paratrooper training. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):769–771. doi: 10.1007/s00192-006-0226-3. [DOI] [PubMed] [Google Scholar]
- 46.Nygaard I, Handa V, Brubaker L, et al. Physical activity in women planning sacrocolpopexy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(1):33–37. doi: 10.1007/s00192-006-0116-8. [DOI] [PubMed] [Google Scholar]
- 47.Besson H, Harwood CA, Ekelund U, et al. Validation of the historical adulthood physical activity questionnaire (HAPAQ) against objective measurements of physical activity. Int J Behav Nutr Phys Act. 2010;7:54. doi: 10.1186/1479-5868-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lissner L, Potischman N, Troiano R, Bengtsson C. Recall of physical activity in the distant past: the 32-year follow-up of the Prospective Population Study of Women in Göteborg, Sweden. Am J Epidemiol. 2004;159(3):304–307. doi: 10.1093/aje/kwh048. [DOI] [PubMed] [Google Scholar]
- 49.Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med. 1995;149(10):1085–1091. doi: 10.1001/archpedi.1995.02170230039005. [DOI] [PubMed] [Google Scholar]
- 50.Ali-Ross NS, Smith AR, Hosker G. The effect of physical activity on pelvic organ prolapse. BJOG. 2009;116(6):824–828. doi: 10.1111/j.1471-0528.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- 51.Lewicky-Gaupp C, Margulies RU, Larson K, Fenner DE, Morgan DM, DeLancey JO. Self-perceived natural history of pelvic organ prolapse described by women presenting for treatment. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(8):927–931. doi: 10.1007/s00192-009-0890-1. [DOI] [PMC free article] [PubMed] [Google Scholar]