Abstract
Purpose
Treatment for ocular surface squamous neoplasia (OSSN) has historically been surgery, but non-surgical interventions are increasingly employed. Treatment with interferon is efficacious, but evidence is needed regarding recurrence and complication rates in comparison to surgery. The objective of this study is to compare the recurrence and complication rates of surgical versus interferon treatment for OSSN.
Design
A matched, case-control study.
Participants
Ninety eight patients with OSSN, 49 of whom were treated with interferon alpha 2b (IFNα2b) therapy and 49 of whom were treated with surgical intervention.
Methods
Patients with OSSN were treated with surgery versus IFNα2b therapy, either in topical or injection form. Median follow up after lesion resolution for the IFNα2b group was 21 months (range 0–173 months) and for the surgery group was 24 months (range 0.9–108 months).
Main outcome measure
The primary outcome measure for the study was the rate of recurrence of OSSN in each of the treatment groups. Recurrence rates were evaluated using Kaplan-Meier survival analysis.
Results
Mean patient age and gender were similar between the groups. There was a trend toward higher clinical American Joint Committee on Cancer tumor grade in the IFNα2b group. Despite this, the number of recurrences was equal at 3 per group. The one year recurrence rate was 5% in the surgery group versus 3% in the IFNα2b group (p=0.80). There was no statistically significant difference in the recurrence rate between the surgically and medically treated groups. Non-limbal location was a risk factor for recurrence (hazard ratio 8.96), in the entire study population. In patients treated successfully, the side effects of the two treatments were similar, with mild discomfort seen in the majority of patients in both groups. There was no limbal stem cell deficiency, symblephara, or diplopia noted in either group. Two patients were excluded from the IFNα2b group due to intolerance to the medication.
Conclusion
No difference in the recurrence rate of OSSN was found between surgical versus IFNα2b therapy.
Ocular surface squamous neoplasia (OSSN) is a term that encompasses a spectrum of epithelial squamous malignancies, ranging from dysplasia to invasive carcinoma. It represents the most common non-pigmented tumor of the ocular surface. Risk factors for this disease include human immunodeficiency virus (HIV)1, 2, ultraviolet light exposure3, 4, exposure to petroleum products5, heavy cigarette smoking5, age6, and male gender6. Human papilloma virus (HPV) has also been implicated in the pathogenesis of OSSN, although its role remains controversial.7–11
Traditional treatment for OSSN involves excision alone with a no-touch technique.12 However, there is likely microscopic disease beyond the edge of the clinically-identified lesion, and the frequency of recurrence with excision alone has been reported to be as high as 56%.13 Even with clear margins on pathology specimens, recurrences of up to 33% have been reported.13 As a result, adjuvant therapies are often performed with excision, including cryotherapy or topical chemotherapy, with reduction in the rates of recurrence.14–16
Tumor excision does, however, carry risks of limbal stem cell deficiency and symblephara formation. In order to potentially avoid these risks and treat the entire ocular surface, medical treatment alone has increased in popularity.17 Chemotherapeutic agents used for treatment of OSSN include mitomycin-C, 5-fluorouracil, and interferon-alpha-2b (IFNα2b), all of which have been shown to be effective.16–24 In particular, topical IFNα2b has gained appeal for OSSN treatment because of its minimal toxicity.25, 26
Interferons are naturally occurring glycoproteins that are released by various types of immune cells and activate effector proteins by binding to the cell surface of their targets. Interferons have antiviral, antimicrobial, and antineoplastic activities.27 Their role as antineoplastic agents is thought to be secondary to a combination of anti-proliferative, anti-angiogenic, and cytotoxic effects, as well as through a possible enhancement of the host antitumor surveillance mechanism.28 Systemic interferon-alpha has been used in treatment for hairy cell leukemia, follicular lymphoma, Kaposi’s sarcoma, renal cell carcinoma, and other malignancies.29, 30 With respect to OSSN, interferon has been used in a recombinant form, IFNα2b, both topically as a drop and as a subconjunctival/perilesional injection.20, 21, 31
Although several studies have been performed to evaluate recurrences after medical treatment, there is limited direct and long term comparison in the literature of recurrence rates between surgical and medical therapy. Therefore, when choosing treatment for patients, physicians are limited to theoretical advantages, personal experience, and patient preferences. An understanding of the recurrence rates and complications of treatment with surgery or medical therapy is important in determining which treatment should be recommended to individual patients. Such information is also valuable in the discussion of the specific risks of each treatment. We therefore performed a case-control study of patients with OSSN treated with surgery or IFNα2b in order to compare the recurrence rates and complications of these treatments.
Methods
This study was approved by the institutional review board of the University of Miami, and the methods adhered to the tenets of the Declaration of Helsinki and were compliant with the Health Insurance Portability and Accountability Act. The design was a retrospective, matched, case-control study. The medical records of 98 patients, who presented with OSSN to the Bascom Palmer Eye Institute between 01/1997 to 11/2011 and who received either surgery or IFNα2b treatment with complete resolution of the tumor, were reviewed. Twenty-seven of the medically treated and all of the surgically treated patients were subgroups of previously reported cases.20, 21, 25,32 These patients were included in the current study in order to obtain the most comprehensive follow up times for evaluation of recurrence rates.
Patients treated with IFNα2b were selected by a screening of the Bascom Palmer Eye Institute (BPEI) pharmacy database. Patients were included if they had OSSN and were treated with IFNα2b as the primary treatment for their lesion. Patients were excluded from the analysis if their OSSN did not successfully resolve with medical therapy or excisional biopsy, as the primary outcome of this study was to evaluate recurrence rates between the two modalities. Of the 61 patients identified, 49 met the criteria for inclusion. Of the 12 excluded patients, 6 failed treatment with IFNα2b or were lost to follow up prior to possible resolution, 3 used IFNα2b for treatment of positive surgical margins, 2 were unable to tolerate IFNα2b and preferred excision, and 1 had been started on IFNα2b by an referring ophthalmologist but with a unclear diagnosis. In order to select the matched cases for the surgical arm of the study, the Florida Lions Eye Bank pathology database, which contains over 500 patients with excisional surgery as the primary treatment for OSSN from 01/1997 to 11/2011, was screened. Matches were created based on patient age (within 10 years) and date of surgical excision (within 10 years). Matched patients were treated with surgery only; patients treated with adjuvant medical therapy after their surgeries were not eligible for the matching process. The treatment modality used was based on patient preference after a discussion of the options between the patient and physician. Patients were treated with IFNα2b in the form of drops (n =40), subconjunctival/ perilesional injections (n=1) or combination drop and injection therapy (n=8). A dose of 1 million IU/mL (n=35) or 3 million IU/mL (n=11; n=2 for combination of doses) was used for topical therapy. A dose of 3 million international units (in 0.5 ml) was used for subconjunctival injections. Eye drops were administered with an initial dose of 4 times daily (with one exception of 3 times daily) until clinical resolution, after which the frequency was tapered. Patients were initially seen on a monthly basis to assess treatment response, with a gradual lengthening of follow up time to every 2 to 3 months. Duration of IFNα2b therapy was based on treatment response.
Surgical treatment consisted of lesion excision with up to 4 mm of tumor-free conjunctival margins (mean 2.7mm for those with known margin width, n=34). Cryotherapy was applied to the limbus and conjunctival edges in a double freeze-thaw method in 41 of the excisions, intra-operative mitomycin C was applied in 1 case, and sclerectomy was performed in 6 cases. Amniotic membrane was used to cover the area of excision in 14 cases, conjunctival autograft in one case, primary closure in ten cases, and the rest were left open to bare sclera. Pathology to identify the lesion as an OSSN was performed by one of two experienced ocular pathologists at the Bascom Palmer Eye Institute.
Patient records were reviewed for demographic information (age, race, ethnicity), OSSN risk factors (skin cancer, HPV, HIV, smoking), and prior history of OSSN as well as previous treatment. Characteristics of the current lesion were also documented including the involved eye, tumor location, tumor size and involved ocular structures (conjunctiva, cornea, limbus, orbit), uni-vs. multi-focality, and appearance (leukoplakic, gelatinous, papillomatous, flat/nodular) based on descriptions and photographs. The tumor size and location provided the basis for American Joint Committee on Cancer (AJCC) clinical stage of the tumor.33 Pathologic grading of the tumor (mild, moderate, severe, carcinoma in situ, or invasive squamous cell carcinoma) and margin positivity were also recorded for all surgical lesions and for any other lesions that underwent biopsy. Treatment information documented included modality of treatment, and for medical therapy included the dose, frequency, and length of treatment.
Response information was recorded in terms of complete resolution of the lesion (defined clinically) and time to lesion resolution. Recurrence was defined as a reappearance of a lesion, in the same or similar location as the original tumor, after complete resolution of the original tumor. A new lesion was defined to be in a distant location (180 degrees away from the original lesion). Follow up was carried out from the time of clinical resolution of the lesion until the last visit. Documented complications included those volunteered by the patient as well as those elicited by the examiner during the clinic visit; this information was not obtained by a questionnaire. Complications recorded included redness, pain, irritation, itching, flu-like symptoms, infection, hyphema, limbal stem cell deficiency, symblepharon, and diplopia.
The main outcome measure was recurrence rate after IFNα2b treatment or surgical excision. A secondary outcome of the study was the frequency of complications associated with each therapy during the follow-up period. Statistical analyses were performed using the SPSS 20.0 (SPSS Inc, Chicago, IL) statistical package. Frequencies of demographic and clinical variables were calculated for each group. Categorical variables were compared using a Chi square analysis; continuous variables were compared using the student t-test. Uni- and multivariable Cox proportional hazards analyses were employed to evaluate factors associated with disease recurrence. A forward step-wise approach was used for the multi-variable analysis. Time-to-event curves were generated using the Kaplan-Meier method. Kaplan-Meier times included the time from lesion resolution to censorship, defined as loss to follow-up, recurrence, or presence of a new lesion.
Results
There was no statistically significant difference in age, gender, race, area of the tumor, clinical AJCC stage, or history of prior OSSN between the IFNα2b (n=49) and surgical (n=49) groups (Table 1). Mean patient age at the time of treatment was 62, with a range from 29 to 87. The majority of the patients were male (53%) and Caucasian (83%). There was a statistically significantly higher proportion of Hispanic patients in the surgical group (54% vs. 33%, p=0.03). Five patients had a known history of HIV, 4 of whom were diagnosed with OSSN prior to age 50. Eight percent of patients in the surgery group and 20% of those in the IFNα2b group had a history of prior OSSN. There was a trend, although not statistically significant, toward a higher clinical AJCC stage overall in patients treated with IFNα2b (p=0.07). Patients treated with IFNα2b were more likely to have corneal (82% vs. 61%, p=0.03) and limbal (94% vs. 74%, p=0.001) involvement of their lesions compared to the surgical group. Median follow up after lesion resolution for the IFNα2b group was 21 months (range 0–173 months) and for the surgery group was 24 months (range 0.9–108 months).
Table 1.
Demographic and clinical information in patients with ocular surface squamous neoplasia treated by excisional removal or IFNα2b therapy
| Surgical | Medical | P-value | ||
|---|---|---|---|---|
|
| ||||
| Number of eyes/patients | 49 | 49 | ||
|
| ||||
| Pt information | Age (years), mean [SD] | 64 [14] | 64 [15] | 0.97 |
|
| ||||
| Gender, male n [%] | 26 [53%] | 26 [53%] | 1.00 | |
|
| ||||
| Race | ||||
| white n [%] | 41 [84%] | 33 [82.5%] | 0.33 | |
| black n [%] | 5 [10%] | 2 [5%] | ||
| other n [%] | 3 [6%] | 5 [12.5%] | ||
|
| ||||
| Ethnicity, Hispanic n [%] | 26 [54%] | 16 [33%] | 0.03 | |
|
| ||||
| Clinical information | Involved eye, right n [%] | 25 [51%] | 20 [41%] | 0.31 |
|
| ||||
| History of OSSN, n [%] | 4 [8%] | 10 [20%] | 0.08 | |
|
| ||||
| Area, mm2 | 24 [30] | 34 [36] | 0.14 | |
|
| ||||
| Location | ||||
| nasal* n [%] | 25 [51%] | 24 [49%] | 0.84 | |
| temporal | 23 [47%] | 16 [33%] | 0.15 | |
| superior | 2 [4%] | 10 [20%] | 0.01 | |
| inferior | 12 [25%] | 12 [25%] | 0.99 | |
|
| ||||
| Corneal involvement, n [%] | 30 [61%] | 40 [82%] | 0.03 | |
|
| ||||
| Tarsal involvement, n [%] | 0 [0%] | 1 [2%] | 0.32 | |
|
| ||||
| Limbal involvement, n [%] | 36 [74%] | 46 [94%] | 0.001 | |
|
| ||||
| Clinical AJCC stage | ||||
| T1 n [%] | 13 [27%] | 5 [10%] | 0.07 | |
| T2 | 6 [12%] | 4 [8%] | ||
| T3 | 30 [61%] | 40 [82%] | ||
|
| ||||
| Multifocal tumor, n [%] | 1 [2%] | 3 6%] | 0.31 | |
|
| ||||
| Appearance† | ||||
| leukoplakia, n [%] | 21 [51%] | 14 [29%] | 0.03 | |
| papillomatous | 9 [18%] | 10 [22%] | 0.64 | |
| nodular | 28 [57%] | 15 [31%] | 0.008 | |
| gelatinous | 22 [58%] | 16 [33%] | 0.02 | |
|
| ||||
| Pathology | Biopsy performed, n [%] | 49 [100%] | 27 [55%] | |
|
| ||||
| Pathologic grade | ||||
| mild dysplasia [%] | 6 [12%] | 0 [0%] | 0.05** | |
| moderate dysplasia | 8 [16%] | 5 [28%] | ||
| severe dysplasia | 9 [18%] | 0 [0%] | ||
| CIS | 23 [47%] | 12 [67%] | ||
| SCC | 3 [6%] | 0 [0%] | ||
|
| ||||
| Positive margins, n [%] | 9 [18%] | |||
|
| ||||
| Pathologic multifocality, n [%] | 1 [2%] | 2 [4%] | 0.56 | |
n=number of individuals in group; IFNα2b = Interferon-alpha-2b; OSSN= ocular surface squamous neoplasia; SD=standard deviation; CIS= carcinoma in situ; SCC=squamous cell carcinoma; AJCC=American Joint Committee on Cancer clinical stage (Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17(6):1471–4); TNM = Tumor, node, metastasis.
Tumors could involve more than one quadrant, e.g. a tumor involving the temporal and superior bulbar conjunctivae would appear both in the temporal and the superior location categories.
Tumors could have more than 1 descriptor for appearance
Pathologic grade not available for 10 specimens in the incisional biopsy group and not all patients in the medical group had a biopsy performed.
Patients treated with any form of IFNα2b received drops for a median of 3.9 months, with treatment continued for a median of 1 month past the time of lesion resolution (range two months prior to resolution to seven months after resolution). Although treatment was never stopped by the physician prior to resolution, there were 5 patients who self-discontinued their drops 1 week to 2 months prior to the visit at which resolution was noted. For those in whom only topical IFNα2b was used (no IFNα2b injections), median time to resolution was 2.3 months (range 0.7–9.4 months) and median total treatment time was 4.0 months. For those treated with subconjunctival injections, a median of 6 injections were performed (range 1–10) with a frequency between 1 and 3 times per week. The median time to resolution for patients treated with injections as part of their IFNα2b therapy was 1.5 months (range 1–3 months).
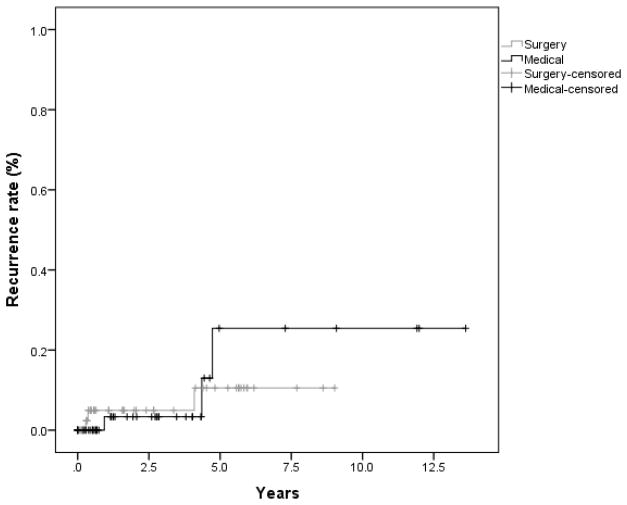
The number of recurrences was equal in both the surgically and medicallytreated groups. A total of 3 recurrences were seen in each cohort. Using Kaplan-Meier survival analysis, the 1 and 5 year recurrence rates in the surgical group were 5% and 11% versus 3% and 25% in the IFNα2b group (Figure 1). There was no significant difference between these rates (p=.80). In addition, a multivariable analysis including treatment, and patient and lesion characteristics that were unequal between the 2 groups (p<0.1), showed that type of treatment (surgical or IFNα2b) was not a significant predictor of recurrence (Table 2). No increased risk of recurrence (Table 2) was noted with respect to area, tumor characteristics (nodular vs. flat and presence of leukoplakia), history of OSSN, or AJCC classification of the tumor. There was a trend toward recurrence, though not statistically significant, in patients with a higher pathologic grade of the initial tumor (hazard ratio (HR) 6.65, p=0.07). Non-limbal location was predictive of recurrence (HR 8.96, p=0.02). All recurrences in the surgical group occurred in patients with negative margins, and 2 of the 3 recurrences occurred in patients who had cryotherapy at the time of the excision.
Figure 1.
Kaplan-Meier survival curve depicting time to recurrence in patients treated with surgical excision versus interferon therapy for ocular surface squamous neoplasia (p=0.80).
Table 2.
Cox Proportional Hazards analysis of factors predictive of ocular surface squamous neoplasia recurrence in the entire patient population.
| Univariable | |||
|---|---|---|---|
|
| |||
| HR | 95% CI | p-value | |
|
| |||
| Treatment group: IFNα2b versus surgical | 1.23 | 0.24–6.16 | 0.80 |
|
| |||
| Stage: AJCCc T3 versus T1 | 1.19 | 0.14–10.20 | 0.88 |
|
| |||
| Grade: As continuous, from mild CIN to SCC | 6.65 | 0.88–49.78 | 0.07 |
|
| |||
| Characteristics: | |||
| Nodular versus flat | 0.93 | 0.17–5.28 | 0.93 |
| Leukoplakia versus no leukoplakia | 0.02 | 0.00–76.3 | 0.35 |
|
| |||
| Location: Conjunctival versus limbal | 8.96 | 1.49–53.8 | 0.02 |
|
| |||
| Area: As continuous variable | 0.96 | 0.91–1.03 | 0.25 |
|
| |||
| History: h/o OSSN vs no history | 3.43 | 0.62–18.9 | 0.16 |
HR = hazard ratio; HR greater than 1 signifies increased risk of tumor recurrence; CI=confidence interval; IFNα2b=Interferon-alpha-2b; CIN=conjunctival intraepithelial neoplasia; SCC=squamous cell carcinoma; AJCC=American Joint Committee on Cancer clinical stage (Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17(6):1471–4); TNM=tumor, node, metastasis
One new lesion (180 degrees away from the original lesion) was seen in a patient previously treated with IFNα2b. There were no new lesions seen during follow up in patients treated with surgical excision. Even if this new lesion was classified as a recurrence, there was no statistically difference in recurrence rates between the medical and surgical groups.
Treatment complications (Table 3) were similar between the 2 groups with respect to mild adverse effects of redness and discomfort. More patients in the surgical group complained of pain during their treatment course. There was 1post-operative hyphema in the surgical group. Flu-like illness was seen more often in the IFNα2b group (10% vs. 0%, p=0.03), although this reaction was seen only in patients who received the injected form of the drug and is a known side effect of IFNα2b injections. No evidence of limbal stem cell deficiency, symblephara, or diplopia was seen as a result of treatment in either group. No other systemic adverse effects were noted.
Table 3.
Treatment complications in patients with ocular surface squamous neoplasia treated by excisional removal (surgical) or IFNα2b therapy (medical)
| Surgical | Medical | p-value | |
|---|---|---|---|
| Any discomfort (pain, irritation, itching), n [%] | 29 [59%] | 31 [63%] | 0.68 |
| Pain | 20 [41%] | 13 [27%] | 0.14 |
| Irritation | 23 [47%] | 26 [53%] | 0.54 |
| Itching | 9 [18%] | 6 [12%] | 0.40 |
| Redness | 19 [39%] | 14 [29%] | 0.29 |
| Flu like symptoms | 0 [0%] | 5 [10%] | 0.003 |
| Hyphema | 1 [2%] | 0 [0%] | 0.32 |
| Limbal stem cell deficiency | 0 [0%] | 0 [0%] | |
| Symblephara | 0 [0%] | 0 [0%] | |
| Diplopia | 0 [0%] | 0 [0%] |
Discussion
Previous reports have found treatment with either IFNα2b or surgery to be effective as therapy for OSSN.26 However, our paper is the first to directly compare long term recurrence rates and complications between these two treatment modalities. In our study, with a mean of almost three years of follow-up, there was no significant difference in recurrence rates between these two therapies.
Patients in this study treated with topical IFNα2b had a similar time to lesion resolution (median 2.3 months) and total time of treatment (median 4.0 months) as has been found in many previous studies.20, 25, 34 The majority of patients in the current study used topical IFNα2b at a dose of 1 million IU/mL although some were treated with a dose of 3 million IU/mL; a previous report by Galor et al. showed no difference in recurrence rates between these two dosing regimens.20 It is notable, however, that there was a large range in our study in time to resolution in patients treated with IFNα2b. Several recent reports have documented a longer mean time to resolution, of 7–8 months, compared with earlier studies,35, 36 indicating that even tumors that do not resolve within the first few months of IFNα2b treatment might respond completely if treated for a longer period.
Recurrences after surgical excision have been found to be consistently higher if the lesion is not removed in its entirety13, but are much lower with adjunctive therapies such as cryotherapy and chemotherapy. Long-term follow up in studies evaluating excision with cryotherapy, as was performed for the majority of the patients in our study, have found a recurrence of 12%37; the recurrence rate of OSSN in the surgical group in the current study was 5% at 1 year and a comparable 11% at 5 years. In this series, all of the cases of recurrence in the surgical arm had negative margins, and 2 of the 3 had received cryotherapy. Recurrence rates of OSSN with IFNα2b treatment in its topical form, as evaluated in the recent literature, have ranged from 0–17% over a range of average follow up from 7 to 42 months.20, 25, 26, 34, 35, 38, 39 Karp et al.21 showed a frequency of recurrence of 7.7% using perilesional injections with a median follow up of 55 months. The recurrence rate of OSSN in patients treated with IFNα2b in the current study was 3% at 1 year and 25% at 5 years. Regarding the 5-year data, it is important to note that only patients with at least five years of follow-up (n=6) were included in this analysis. All recurrences occurred in patients who received topical drops (as opposed to perilesional injections) of IFNα2b. Most patients treated with IFNα2b therapy received only topical drop treatment, so we are unable to make any significant conclusions regarding recurrence rates after topical versus injection therapy.
Risk of recurrence in this study was higher in patients with only conjunctival involvement of the lesion (and with no corneal or limbal involvement) and there was a trend toward increased recurrence with higher pathologic grade of tumors. The latter of these findings is consistent with previous work.32, 40 In the current study, the surgical group was more likely to have lesions involving the conjunctiva only. Of note, although 18% of the cases in the surgical group had positive margins on pathology, all recurrences occurred in patients with negative pathologic margins. Patients with OSSN are at risk of recurrent disease regardless of the initial treatment and pathologic findings.
The excellent side effect profile of IFNα2b allows for significantly improved patient acceptance over other medical therapies with proven efficacy such as mitomycin C. Previous reports have described side effects with IFNα2b of irritation, conjunctival hyperemia, and follicular conjunctivitis that resolves with treatment cessation.25, 26, 35, 36 Rarely, corneal epithelial defects develop during treatment.35, 41 With use of perilesional injections of IFNα2b, transient flu-like symptoms may occur in up to 100% of patients, usually lasting a few hours after the injection.21, 41 In the current study, patients in both treatment groups were likely to experience discomfort, although this was more likely to be milder in the interferon group than in the surgical group. Febrile episodes in this study, as in previous work, were limited to patients receiving the injected form of IFNα2b. One caveat with regard to evaluation of complications within this study, however, is that patients were only included if they were able to be treated to resolution of the disease; patients who were unable to tolerate IFNα2b prior to resolution were excluded from evaluation. In this study, two such patients were excluded, one who developed corneal epithelial defects during IFNα2b use and the other who developed dizziness one day after beginning topical treatment.
The findings of this study must be interpreted within its design. The purpose of the study was to evaluate whether recurrence rates were different between medically and surgically treated patients. As a result, only patients who experienced successful lesion resolution with either modality were included. Rates of OSSN resolution with IFNα2b therapy have been reported to range between 80–100% across different studies.20, 25, 26, 34–36, 39 In the current study, 6 of the 55 patients (11%) who were treated with and tolerated IFNα2b as their primary treatment for OSSN, did not resolve or were lost to follow-up prior to lesion resolution. Two of the total of 61 patients (3%) who were treated with IFNα2b for any reason did not tolerate the medication. Clinicians opting to use IFNα2b must therefore keep in mind that although the recurrence rate between the two treatment modalities (IFNα2b and surgical excision) was not different in this study, not all patients will experience resolution of their disease with IFNα2b and some may not tolerate the medication.
The conclusions must also be evaluated within the study’s limitations. As a retrospective study, the patients differed on several baseline characteristics, including, likely, unmeasured ones. Additionally, patients with both primary and recurrent disease were evaluated and there was a trend toward more patients with recurrent disease in the IFNα2b group; these patients with previous episodes of recurrence may also have been more likely to get additional recurrences subsequently. The retrospective nature of the study also allowed for possible bias in the treatment algorithm, with patients with more extensive disease or multifocal disease more likely to be treated with medical treatment to avoid limbal stem cell deficiency (of note, this difference was not detected in the analysis of baseline characteristics). There may have also been a bias toward medical treatment in patients with recurrent disease and previous surgery, in order to avoid subjecting the patients twice to surgical risks. Side effect data was obtained from comments in the charts, which included those volunteered by the patient as well as those elicited by the examiner during the clinic visit; some side effects may therefore be under-reported. Additionally, the size of our sample was not large enough to make comparisons regarding recurrence with respect to length of continued IFNα2b treatment after lesion resolution, size of surgical margins, or other treatment characteristics.
In conclusion, we found that there was no statistically significant difference in recurrence rates in those patients successfully treated with IFNα2b and those treated with surgery for OSSN in this case-control study. Additionally, side effects were mild in both groups. A large, multi-centered, randomized trial would be needed to compare surgical and medical modalities for OSSN, in order to provide the most evidence-based therapeutic approach for these patients long term.
Acknowledgments
Financial Support:
Supported by NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, Department of Defense (DOD-Grant#W81XWH-09-1-0675). The Ronald and Alicia Lepke Grant, The Lee and Claire Hager Grant, The Jimmy and Gaye Bryan Grant.
Footnotes
Poster presentation for the American Academy of Ophthalmology Annual Meeting, New Orleans, November 2013
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guech-Ongey M, Engels EA, Goedert JJ, et al. Elevated risk for squamous cell carcinoma of the conjunctiva among adults with AIDS in the United States. Int J Cancer. 2008;122:2590–3. doi: 10.1002/ijc.23384. [DOI] [PubMed] [Google Scholar]
- 2.Nagaiah G, Stotler C, Orem J, et al. Ocular surface squamous neoplasia in patients with HIV infection in sub-Saharan Africa. Curr Opin Oncol. 2010;22:437–42. doi: 10.1097/CCO.0b013e32833cfcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee GA, Hirst LW. Incidence of ocular surface epithelial dysplasia in metropolitan Brisbane. A 10-year survey. Arch Ophthalmol. 1992;110:525–7. doi: 10.1001/archopht.1992.01080160103042. [DOI] [PubMed] [Google Scholar]
- 4.Waddell K, Kwehangana J, Johnston WT, et al. A case-control study of ocular surface squamous neoplasia (OSSN) in Uganda. Int J Cancer. 2010;127:427–32. doi: 10.1002/ijc.25040. [DOI] [PubMed] [Google Scholar]
- 5.Napora C, Cohen EJ, Genvert GI, et al. Factors associated with conjunctival intraepithelial neoplasia: a case control study. Ophthalmic Surg. 1990;21:27–30. [PubMed] [Google Scholar]
- 6.Lee GA, Hirst LW. Retrospective study of ocular surface squamous neoplasia. Aust N Z J Ophthalmol. 1997;25:269–76. doi: 10.1111/j.1442-9071.1997.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 7.Scott IU, Karp CL, Nuovo GJ. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology. 2002;109:542–7. doi: 10.1016/s0161-6420(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 8.Eng HL, Lin TM, Chen SY, et al. Failure to detect human papillomavirus DNA in malignant epithelial neoplasms of conjunctiva by polymerase chain reaction. Am J Clin Pathol. 2002;117:429–36. doi: 10.1309/RVUP-QMU3-5X6W-3CQ1. [DOI] [PubMed] [Google Scholar]
- 9.Tulvatana W, Bhattarakosol P, Sansopha L, et al. Risk factors for conjunctival squamous cell neoplasia: a matched case-control study. Br J Ophthalmol. 2003;87:396–8. doi: 10.1136/bjo.87.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guthoff R, Marx A, Stroebel P. No evidence for a pathogenic role of human papillomavirus infection in ocular surface squamous neoplasia in Germany. Curr Eye Res. 2009;34:666–71. doi: 10.1080/02713680903007162. [DOI] [PubMed] [Google Scholar]
- 11.Manderwad GP, Kannabiran C, Honavar SG, Vemuganti GK. Lack of association of high-risk human papillomavirus in ocular surface squamous neoplasia in India. Arch Pathol Lab Med. 2009;133:1246–50. doi: 10.5858/133.8.1246. [DOI] [PubMed] [Google Scholar]
- 12.Shields JA, Shields CL, De Potter P. Surgical management of conjunctival tumors. The 1994 Lynn B. McMahan Lecture. Arch Ophthalmol. 1997;115:808–15. doi: 10.1001/archopht.1997.01100150810025. [DOI] [PubMed] [Google Scholar]
- 13.Tabin G, Levin S, Snibson G, et al. Late recurrences and the necessity for long-term follow-up in corneal and conjunctival intraepithelial neoplasia. Ophthalmology. 1997;104:485–92. doi: 10.1016/s0161-6420(97)30287-5. [DOI] [PubMed] [Google Scholar]
- 14.Peksayar G, Altan-Yaycioglu R, Onal S. Excision and cryosurgery in the treatment of conjunctival malignant epithelial tumours. Eye (Lond) 2003;17:228–32. doi: 10.1038/sj.eye.6700331. [DOI] [PubMed] [Google Scholar]
- 15.Siganos CS, Kozobolis VP, Christodoulakis EV. The intraoperative use of mitomycin-C in excision of ocular surface neoplasia with or without limbal autograft transplantation. Cornea. 2002;21:12–6. doi: 10.1097/00003226-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Midena E, Angeli CD, Valenti M, et al. Treatment of conjunctival squamous cell carcinoma with topical 5-fluorouracil. Br J Ophthalmol. 2000;84:268–72. doi: 10.1136/bjo.84.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone DU, Butt AL, Chodosh J. Ocular surface squamous neoplasia: a standard of care survey. Cornea. 2005;24:297–300. doi: 10.1097/01.ico.0000138834.42489.ba. [DOI] [PubMed] [Google Scholar]
- 18.Ballalai PL, Erwenne CM, Martins MC, et al. Long-term results of topical mitomycin C 0. 02% for primary and recurrent conjunctival-corneal intraepithelial neoplasia. Ophthal Plast Reconstr Surg. 2009;25:296–9. doi: 10.1097/IOP.0b013e3181ac4c39. [DOI] [PubMed] [Google Scholar]
- 19.Frucht-Pery J, Sugar J, Baum J, et al. Mitomycin C treatment for conjunctival-corneal intraepithelial neoplasia: a multicenter experience. Ophthalmology. 1997;104:2085–93. doi: 10.1016/s0161-6420(97)30055-4. [DOI] [PubMed] [Google Scholar]
- 20.Galor A, Karp CL, Chhabra S, et al. Topical interferon alpha 2b eye-drops for treatment of ocular surface squamous neoplasia: a dose comparison study. Br J Ophthalmol. 2010;94:551–4. doi: 10.1136/bjo.2008.153197. [DOI] [PubMed] [Google Scholar]
- 21.Karp CL, Galor A, Chhabra S, et al. Subconjunctival/perilesional recombinant interferon alpha2b for ocular surface squamous neoplasia: a 10-year review. Ophthalmology. 2010;117:2241–6. doi: 10.1016/j.ophtha.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 22.Karp CL, Galor A, Lee Y, Yoo SH. Pegylated interferon alpha 2b for treatment of ocular surface squamous neoplasia: a pilot study. Ocul Immunol Inflamm. 2010;18:254–60. doi: 10.3109/09273948.2010.486687. [DOI] [PubMed] [Google Scholar]
- 23.Yeatts RP, Engelbrecht NE, Curry CD, et al. 5-Fluorouracil for the treatment of intraepithelial neoplasia of the conjunctiva and cornea. Ophthalmology. 2000;107:2190–5. doi: 10.1016/s0161-6420(00)00389-4. [DOI] [PubMed] [Google Scholar]
- 24.Yeatts RP, Ford JG, Stanton CA, Reed JW. Topical 5-fluorouracil in treating epithelial neoplasia of the conjunctiva and cornea. Ophthalmology. 1995;102:1338–44. doi: 10.1016/s0161-6420(95)30866-4. [DOI] [PubMed] [Google Scholar]
- 25.Schechter BA, Koreishi AF, Karp CL, Feuer W. Long-term follow-up of conjunctival and corneal intraepithelial neoplasia treated with topical interferon alfa-2b. Ophthalmology. 2008;115:1291–6. doi: 10.1016/j.ophtha.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Sturges A, Butt AL, Lai JE, Chodosh J. Topical interferon or surgical excision for the management of primary ocular surface squamous neoplasia. Ophthalmology. 2008;115:1297–302. doi: 10.1016/j.ophtha.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Baron S, Tyring SK, Fleischmann WR, Jr, et al. The interferons: mechanisms of action and clinical applications. JAMA. 1991;266:1375–83. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 28.Bracarda S, Eggermont AM, Samuelsson J. Redefining the role of interferon in the treatment of malignant diseases. Eur J Cancer. 2010;46:284–97. doi: 10.1016/j.ejca.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Decatris M, Santhanam S, O’Byrne K. Potential of interferon-alpha in solid tumours: part 1. BioDrugs. 2002;16:261–81. doi: 10.2165/00063030-200216040-00003. [DOI] [PubMed] [Google Scholar]
- 30.Santhanam S, Decatris M, O’Byrne K. Potential of interferon-alpha in solid tumours: part 2. BioDrugs. 2002;16:349–72. doi: 10.2165/00063030-200216050-00004. [DOI] [PubMed] [Google Scholar]
- 31.Vann RR, Karp CL. Perilesional and topical interferon alfa-2b for conjunctival and corneal neoplasia. Ophthalmology. 1999;106:91–7. doi: 10.1016/S0161-6420(99)90009-X. [DOI] [PubMed] [Google Scholar]
- 32.Galor A, Karp CL, Oellers P, et al. Predictors of ocular surface squamous neoplasia recurrence after excisional surgery. Ophthalmology. 2012;119:1974–81. doi: 10.1016/j.ophtha.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 34.Boehm MD, Huang AJ. Treatment of recurrent corneal and conjunctival intraepithelial neoplasia with topical interferon alfa 2b. Ophthalmology. 2004;111:1755–61. doi: 10.1016/j.ophtha.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 35.Shah SU, Kaliki S, Kim HJ, et al. Topical interferon alfa-2b for management of ocular surface squamous neoplasia in 23 cases: outcomes based on American Joint Committee on Cancer classification. Arch Ophthalmol. 2012;130:159–64. doi: 10.1001/archophthalmol.2011.385. [DOI] [PubMed] [Google Scholar]
- 36.Shields CL, Kaliki S, Kim HJ, et al. Interferon for ocular surface squamous neoplasia in 81 cases: outcomes based on the American Joint Committee on Cancer classification. Cornea. 2013;32:248–56. doi: 10.1097/ICO.0b013e3182523f61. [DOI] [PubMed] [Google Scholar]
- 37.Basti S, Macsai MS. Ocular surface squamous neoplasia: a review. Cornea. 2003;22:687–704. doi: 10.1097/00003226-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Schechter BA, Schrier A, Nagler RS, et al. Regression of presumed primary conjunctival and corneal intraepithelial neoplasia with topical interferon alpha-2b. Cornea. 2002;21:6–11. doi: 10.1097/00003226-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Holcombe DJ, Lee GA. Topical interferon alfa-2b for the treatment of recalcitrant ocular surface squamous neoplasia. Am J Ophthalmol. 2006;142:568–71. doi: 10.1016/j.ajo.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 40.Yousef YA, Finger PT. Squamous carcinoma and dysplasia of the conjunctiva and cornea: an analysis of 101 cases. Ophthalmology. 2012;119:233–40. doi: 10.1016/j.ophtha.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Kim HJ, Shields CL, Shah SU, et al. Giant ocular surface squamous neoplasia managed with interferon alpha-2b as immunotherapy or immunoreduction. Ophthalmology. 2012;119:938–44. doi: 10.1016/j.ophtha.2011.11.035. [DOI] [PubMed] [Google Scholar]