Abstract
Background:
Deep brain stimulation has become a routine therapy for movement disorders, but it is relatively invasive and costly. Although stimulation intensity relates to battery longevity, less is known about how diagnosis and stimulation target contribute to this clinical outcome. Here we evaluate battery longevity in movement disorders patients who were treated at a tertiary referral center.
Objective:
To compare single channel pulse generator longevity in patients with movement disorders.
Methods:
With Institutional Review Board approval, we evaluated 470 consecutive Soletra implants for routine care. Battery longevity was estimated with Kaplan-Meier analyses, and group comparisons were performed with the log rank mean test. The frequency of clinic encounters for ongoing care was evaluated across diagnoses with analysis of variance (ANOVA).
Results:
The mean pulse generator longevity was 44.9±1.4 months. Pallidal DBS for dystonia was associated with shorter battery longevity than subthalamic and thalamic DBS for Parkinson's disease and essential tremor (28.1±2.1 versus 47.1±1.8 and 47.8±2.6 months, respectively, mean ± standard error, p<0.001), and dystonia patients required more frequent clinic visits for routine care (F=6.0, p=0.003). Pallidal DBS for Parkinson's disease and thalamic DBS for cerebellar outflow tremor were associated with shorter battery longevity, as well (35.3±4.6 and 26.4±4.3 months, respectively).
Conclusions:
Pallidal DBS for dystonia was associated with shorter battery longevity and more frequent stimulator adjustments versus DBS for Parkinson’s disease and essential tremor. Characteristics of the stimulation target and disease pathophysiology both likely contribute to battery longevity in patients with movement disorders.
Introduction
Deep brain stimulation (DBS) is remarkably effective for movement disorders such as Parkinson's disease (PD), essential tremor (ET), and dystonia when medications do not provide adequate symptomatic improvement (1-3). Despite this, replacement of implanted pulse generators (IPGs) for battery expiration contributes significantly to the cost and potential morbidity of this therapy over time. Although DBS has become a routine treatment for movement disorders such as PD, less is known about battery longevity in DBS patients with dystonia, ET, and other forms of tremor.
Globus pallidus interna (pallidal) DBS for PD is associated with higher average stimulator settings than subthalamic DBS in randomized clinical trials (4, 5). Similarly, pallidal DBS for dystonia is associated with shorter pulse generator longevity in case series, prompting consideration of new programming strategies and alternative surgical targets (6-8). Although the difference in stimulation intensities used at these targets has largely been attributed to the larger anatomical volume of the pallidum, other disease-specific factors may contribute to IPG longevity as well (9). In contrast to DBS for ET and PD, clinical improvement following pallidal DBS for dystonia typically occurs over hours, days, or even weeks or months, potentially encouraging increases in DBS stimulation parameters that result in little additional symptomatic benefit and/or adverse effects. Because of the considerable variation in battery longevity between individual patients with movement disorders, recent efforts have focused on the development of rechargeable devices and other potential strategies to decrease procedure-related morbidity and cost.
Here we evaluate how diagnosis and stimulation target relate to IPG longevity in a relatively large sample of patients with Parkinson’s disease, essential tremor, dystonia, and severe cerebellar outflow tremor (midbrain stroke or trauma, multiple sclerosis, and cerebellar ataxia) treated at a tertiary movement disorders center. Better understanding disease- and target-specific differences in IPG longevity can provide normative outcomes data upon which to base therapeutic decisions to motivate innovations in clinical management and device technology.
Methods
With Institutional Review Board approval, we retrospectively collected data from DBS patients between 1998 and 2011 at the University of Alabama at Birmingham. Informed consent was not obtained individually because these were deidentified, retrospective analyses. We collected data on 229 patients (143 PD, 70 ET, 10 generalized dystonia, 9 focal dystonia and 6 cerebellar outflow tremor patients) with 470 unique IPGs.Our clinical practice was to implant single channel, constant voltage devices (Soletra®, Medtronic, Inc., Minneapolis, MN), and all patients underwent routine postoperative MRI to confirm correct electrode placement. Our approach to programming is similar to published practice parameters, and our prior published work (10-14). Briefly, all patients receive a monopolar survey of the electrode contacts upon activation of a newly placed stimulator to evaluate the thresholds for side effects and symptomatic improvement. In all patients, the ultimate goal is to adjust the DBS system such that it provides maximal symptomatic benefit. In patients with PD, ET and cerebellar tremor, settings were adjusted to immediate clinical response and optimized in subsequent follow-up visits as indicated. In patients with dystonia, full symptomatic response is often delayed by hours, days, or even weeks or months after initial programming. In these cases, we activate the stimulation contact that either shows an immediate clinical response, or else is best positioned in the posterior, lateral part of the globus pallidus interna based upon post operative MRI. Regardless of diagnosis, initial settings employed at our center are typically monopolar or bipolar configuration at 3-3.5 volts, 60-90 microsecond pulse width with 160 Hertz frequency. Monopolar and bipolar settings were used in the vast majority of patients, except in instances where insufficient or partial symptomatic benefit prompted the use of more intense double monopolar or tripolar stimulation. We discourage deactivating the DBS system during sleep in patients with PD and dystonia, and we do not routinely recommend deactivating the device at night in ET patients. We collected demographic data and recorded the indication for surgery, brain hemisphere, stimulation target, and longevity for each IPG in a database. Additionally, we entered all programming adjustments (voltage, pulse width, stimulation frequency and bipolar/monopolar configuration) and calculated the average stimulator settings over time for each IPG. Devices that became infected or had hardware malfunctions were excluded from the analyses. Kaplan-Meier survival analyses estimated IPG longevity, using the log rank test for group comparisons by diagnosis and stimulation target. We censored the following data: (1) patients whose IPG had not yet expired and (2) patients lost to follow-up to their most recent clinical encounter date. Additionally, we compared the frequency of clinic encounters including visits for adjustment of stimulator settings among the three most common indications (PD, ET, and dystonia) using one-way analysis of variance (ANOVA). Secondary analyses evaluated both the average DBS settings across diagnoses and targets and the battery longevity for the smaller samples of patients with pallidal or thalamic DBS for PD and cerebellar outflow tremor. We defined p<0.05 as the significance threshold for all statistical tests.
Results
Pulse generator longevity across diagnoses and stimulation targets
We analyzed 3,440 individual programming adjustmentsfrom 470 IPGs and 248 unique patients, averaging 11.7±0.7 clinical encounters per IPG. The mean battery longevity was 44.9±1.4 months across all diagnoses and stimulation targets (median 39.7 months). Many patients underwent multiple battery replacements and/or had bilateral placement of single channel devices. Detailed demographic data are provided in Table 1.
Table 1.
Baseline demographic data across diseases
| Diagnosis | Target | Patients (n) |
IPGs (n) | Average age at implantation |
Gender | Side | ||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Left | Right | |||||
| PD | STN | 125 | 250 | 60.3 ± 0.6 | 182(0.73) | 68 (0.27) | 134(0.54) | 116(0.46) |
| PD | GPI | 11 | 16 | 63.5 ± 2.1 | 11 (0.69) | 5 (0.31) | 6 (0.37) | 10 (0.62) |
| PD | VIM | 7 | 14 | 74.4 ± 2.7 | 3 (0.21) | 11 (0.78) | 3 (0.21) | 11 (0.70) |
| ET | VIM | 70 | 129 | 66.5 ± 1.2 | 84 (0.65) | 45 (0.35) | 92 (0.71) | 37 (0.29) |
| Dystonia | GPI | 21 | 49 | 35.5 ± 2.3 | 10 (0.20) | 39 (0.79) | 29 (0.59) | 20 (0.41) |
| Cerebellar | VIM | 6 | 12 | 43.7 ± 2.4 | 7 (0.58) | 5 (0.42) | 9 (0.75) | 3 (0.25) |
| Total | 229 | 470 | 59.5 ± 0.7 | 297(0.63) | 173(0.37) | 273(0.58) | 197(0.42) | |
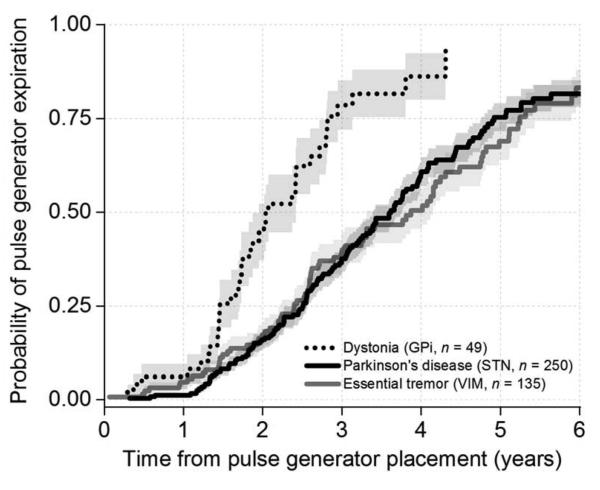
Pallidal DBS for dystonia was associated with shorter battery longevity than subthalamic and thalamic DBS for PD and ET (28.1±2.1 versus 47.1±1.8 and 47.8±2.6 months, respectively, mean ± standard error, p<0.001, log rank test, Figure 1). We performed an additional paired comparison between subthalamic DBS for PD and thalamic DBS for ET, and there was no statistically significant difference in IPG longevity at our level of power (p = 0.80). Pallidal DBS for PD and thalamic DBS for cerebellar outflow tremor were also associated with shorter average battery longevity versus subthalamic DBS for PD and thalamic DBS for ET, albeit with smaller samples of patients (35.3±4.6, and 26.4±4.3 months, respectively, mean ± standard error). Detailed longevity data and average DBS settings over time are provided in Table 2.
Figure 1.
Kaplan-Meier survival analysis curve for dystonia, Parkinson’s disease and essential tremor representing probability of pulse generator expiration over time.
Table 2.
Average DBS settings over time across stimulation targets and diseases
| Diagnosis | Target | IPGs (n) |
IPG longevity mean ± SE (months) |
IPG longevity quartiles and 95% confidence intervals (months) |
monopolar | bipolar | double monopolar |
tripolar | voltag e (Volts) |
pulse width (μs) |
frequency (Hertz) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25% | UCL | LCL | 50% | UCL | LCL | 75% | UCL | LCL | mean proportion ± SE | mean ± SE | |||||||||
| PD | STN | 250 | 47.2 ± 1.8 | 30.1 | 26.5 | 32.2 | 43.4 | 38.8 | 47.4 | 59.0 | 55.1 | 86.6 | 0.49 ± 0.03 | 0.38 ± 0.03 |
0.06 ± 0.01 | 0.07 ± 0.02 |
3.5 ± 0.0 |
98.9 ± 2.0 | 168.9 ± 1.1 |
| PD | GPI | 16 | 35.3 ± 4.6 | 24.8 | 23.2 | 29.4 | 29.4 | 24.8 | 38.5 | 38.5 | 29.4 | 38.5 | 0.69 ± 0.12 | 0.17 ± 0.09 |
0.06 ± 0.06 | 0.08 ± 0.06 |
3.9 ± 0.2 |
103.1 ± 4.7 |
170.7 ± 4.0 |
| PD | VIM | 14 | 42.0 ± 5.0 | 29.6 | 20.7 | 32.6 | 32.6 | 29.6 | 61.1 | 61.1 | 32.6 | 61.1 | 0.17 ± 0.10 | 0.47 ± 0.13 |
0.07 ± 0.07 | 0.29 ± 0.13 |
3.2 ± 0.1 |
101.3 ± 4.8 |
180.6 ± 3.0 |
| ET | VIM | 129 | 47.8 ± 2.6 | 28.8 | 24.3 | 31.3 | 46.5 | 36.5 | 51.7 | 62.9 | 57.3 | 75.0 | 0.41 ± 0.04 | 0.34 ± 0.04 |
0.15 ± 0.03 | 0.09 ± 0.02 |
3.4 ± 0.1 |
116.4 ± 3.6 |
180.1 ± 0.9 |
| Cerebellar | VIM | 12 | 26.5 ± 4.4 | 17.7 | 4.3 | 32.1 | 32.1 | 17.7 | 33.6 | 33.6 | 32.1 | 43.2 | 0.15 ± 0.10 | 0.04 ± 0.02 |
0.71 ± 0.12 | 0.09 ± 0.07 |
3.4 ± 0.2 |
106.4 ± 8.6 |
180.0 ± 2.7 |
| Dystonia | GPI | 49 | 28.1 ± 2.1 | 17.5 | 17.1 | 21.9 | 24.9 | 20.8 | 31.2 | 33.8 | 29.1 | 51.7 | 0.74 ± 0.06 | 0.10 ± 0.04 |
0.15 ± 0.05 | 0.00 ± 0.00 |
3.9 ± 0.1 |
142.9 ± 10.7 |
158.4 ± 3.4 |
| total | 470 | 45.0 ± 1.4 | 26.5 | 24.5 | 29.3 | 39.7 | 36.5 | 44.1 | 59.0 | 55.3 | 63.1 | 0.48 ± 0.02 |
0.33 ± 0.02 |
0.11 ± 0.01 |
0.08 ±
0.01 |
3.5 ±
0.0 |
108.7 ±
2.0 |
171.5 ± 0.8 | |
Frequency of clinic encounters across diagnoses and stimulation targets
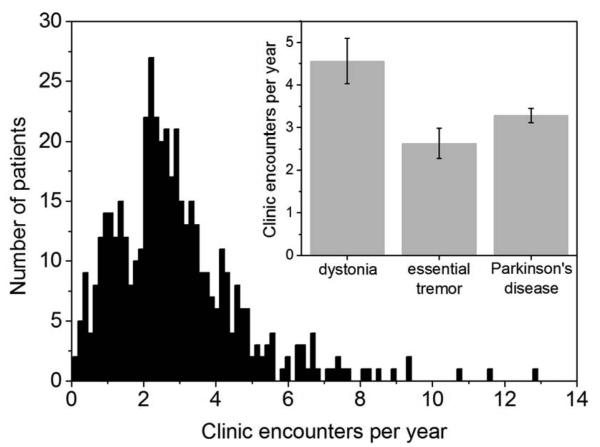
We evaluated the frequency of clinic encounters for DBS adjustment across the three most common surgical indications (PD, ET, and dystonia, Figure 2), regardless of stimulation target, and generated a histogram of encounter rates across all patients with a bin width of 0.14 encounters per year. One-way ANOVA compared the encounter frequency across diagnoses, and both the omnibus statistical test and the pair-wise comparisons between dystonia and the other two diagnoses were statistically significant (F = 6.04 and p = 0.003; dystonia versus ET and PD, p = 0.002 and p = 0.04, respectively), suggesting that dystonia patients underwent more frequent stimulator adjustments in routine care versus those with ET and PD.
Figure 2.
Histogram of frequency of clinic encounters for DBS encounter across all the patients. In the intercept, average number of clinic encounters per year for Parkinson, dystonia and essential tremor groups.
Discussion
In this large retrospective study, pallidal DBS for dystonia was associated with shorter single channel IPG longevity and more frequent adjustment of stimulator settings versus patients with ET and PD. Furthermore, patients with pallidal DBS for PD and thalamic DBS for cerebellar outflow tremor experienced shorter battery longevity, as well. Although characteristics of the stimulation target such as its anatomical volume, local impedance, and microanatomy contribute to pulse generator longevity, our collective findings suggest that disease pathophysiology (the timing and magnitude of the symptomatic response to DBS) likely influence battery longevity, as well. Our overall estimate of battery longevity across different movement disorders and stimulation targets is consistent with a prior study reporting a combined outcome from 122 IPGs placed for PD, ET, dystonia, and cerebellar outflow tremor (15).
Pallidal stimulation for dystonia
Our battery longevity estimate for pallidal DBS for dystonia is consistent with prior case series (16,17). Blahak et al. evaluated battery longevity of single channel devices in consecutive dystonia patients and found that 80% had experienced battery depletion by 16 to 30 months (mean 25.1 months). Additionally, Isaias et al. retrospectively analyzed dystonia patients with two different programming strategies (≥130 and 60 Hz), suggesting that a subset achieved both significant symptomatic benefit and improved battery longevity with lower frequency stimulation. Our data suggest that a typical dystonia patient with bilateral single channel Soletra devices placed at age 35 could expect approximately 36 pulse generator replacements for battery depletion over an average lifespan of 77 years, using conventional stimulation settings.
Dystonia symptoms often improve over a period of hours, days, or even weeks/months after stimulator adjustment, therefore the acute behavioral response to DBS often does not guide the initial programming strategy, potentially increasing the complexity of stimulator adjustment (3). Consistent with this, we found that dystonia patients returned to clinic more frequently both stimulator adjustment and battery replacements during routine care versus those with PD and ET. Other factors likely contribute to the decreased IPG longevity associated with pallidal DBS, as well. Importantly, the pallidum occupies a larger volume than either the subthalamic nucleus or the ventral intermediate thalamus, likely requiring greater energy expenditure to yield similar symptomatic benefit (4,5). Additionally, the close proximity of the subthalamic nucleus to the internal capsule and other structures is often associated with a lower threshold to stimulation-related side effects, potentially imposing a stricter ceiling on the maximum stimulator settings in a given patient (18). Regardless, our findings demonstrate that the cost and morbidity of DBS therapy varies substantially across these different disease states.
Ventral intermediate thalamic stimulation for tremor
There is very little published data on battery longevity in patients with thalamic DBS for ET and other forms of tremor. At our level of statistical power, we found no significant difference between battery longevity with thalamic DBS for ET versus subthalamic DBS for PD, in contrast to a prior report (19). Ondo et al developed a predictive model for IPG consumption and reported no significant difference in IPG longevity in patients with ET and PD, as well (15). Cerebellar outflow tremor is difficult to treat with DBS, although some patients experience tremor suppression and associated improvements in daily living activities. Despite sharing the same thalamic stimulation target, patients with cerebellar outflow tremor were stimulated at higher average stimulator settings than patients with ET, in an attempt to maximally improve residual disability from tremor (20). Although our sample is small for these heterogeneous, severe tremor disorders, the difference in battery longevity in cerebellar outflow tremor versus ET suggests that the pathophysiology of cerebellar outflow tremor and its response to DBS may independently influence battery longevity outcome.
Subthalamic and pallidal stimulation for Parkinson’s disease
Our data are largely consistent with prior reports on pulse generator longevity in patients with subthalamic DBS for PD. Although Anheim et al. reported that subthalamic DBS for PD with Itrel II® devices (Medtronic, Inc., Minneapolis, MN) was associated with 83±14 months of battery longevity, other groups found mean longevities of 44.3±11.6 and 45 months, which more closely parallel our findings (19, 21).
There is little published data on pulse generator longevity for PD patients who undergo pallidal DBS. Consistent with recent prospective, randomized trials that report lower average stimulator settings with subthalamic versus pallidal DBS for PD, we found greater average battery longevity with subthalamic stimulation in our series (4, 5). Although the relative merits of these targets can be debated in individual patients with various disease subtypes and comorbidities, recent randomized studies did not detect significant differences in cognitive/behavioral outcomes by target in patients with PD, suggesting that subthalamic stimulation should be considered in most cases because of greater battery longevity, motor improvement, and medication reduction (5, 22, 23).
Strengths and potential limitations
This study has several strengths. First, we evaluate a substantially larger sample of movement disorders patients versus prior studies. Second, we provide battery longevity outcomes for diagnoses and stimulation targets that are not well-characterized in the prior literature (essential tremor, cerebellar outflow tremor, pallidal DBS for PD). Third, this study evaluates only Soletra single channel devices, therefore we did not have to take into account stimulator settings for the second DBS electrode on the opposite side of the brain. Fourth, we went to considerable lengths to incorporate data from every stimulator adjustment to calculate the average stimulation parameters over time for a each device. Fifth, the Soletra device does not allow patient adjustment of stimulation parameters other than complete activation/inactivation; whereas the newer DBS systems are increasingly adjustable and will likely be associated with greater technical demands in terms of summarizing stimulator settings over time for a given IPG.
Although this study is potentially subject to limitations of its retrospective, non-randomized design, we report an important patient outcome from the context of real-world care, outside of the inclusion criteria, protocol constraints, and limited follow-up of a prospective clinical trial. Additionally, while the stimulation properties of the Soletra device are largely similar to newer devices, its energy consumption becomes less efficient at stimulation voltages above 3.6 volts, suggesting that we may underestimate IPG longevity in patients with newer devices that have been adjusted to higher average stimulation voltages. Soletra has been replaced by modern devices in many centers. Third, modern devices with greater patient control at home might not require as many clinic visits for stimulator adjustments versus the Soletra device, potentially decreasing the frequency of follow-up visits across a variety of diagnoses. Fourth, despite our relatively large overall sample size, there were fewer patients with cerebellar outflow tremor and pallidal/thalamic DBS for PD, potentially limiting the generalizability of those battery longevity estimates. Finally, although a single center study has the advantage of uniformity of providers and data capture, some aspects of our results might represent isolated practice patterns or specific patient characteristics in our region.
Conclusions
Single channel battery longevity varies significantly across movement disorders and stimulation targets. Specifically, pallidal DBS for dystonia was associated with shorter IPG longevity and more frequent clinical stimulator adjustments versus ET and PD, likely translating into disease-specific morbidity and cost associated with DBS. As DBS is used more broadly for neurologic and psychiatric diseases, future work should incorporate improvements in battery technology and identify predictive biological markers and programming strategies that yield sustained clinical improvement with minimal battery depletion (24-26).
Acknowledgement
This work was supported by funding from the United States National Institutes of Health / National Institutes of Neurological Disorders and Stroke K23NS067053 (HW) and from a Dystonia Center of Excellence Grant from the Bachmann Strauss Dystonia Coalition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Rawal reported no financial interests or potential conflicts of interest. Dr. Almeida reported no financial interests or potential conflicts of interest. Dr. Smelser reported no financial interests or potential conflicts of interest. Huang reported no financial interests or potential conflicts of interest. Dr. Guthrie reported no financial interests or potential conflicts of interest. Dr. Walker discloses receiving United States National Institutes of Health / National Institutes of Neurological Disorders and Stroke K23NS067053 (HW) and from a Dystonia Center of Excellence Grant from the Bachmann Strauss Dystonia Coalition.
References
- 1.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for parkinson's disease. N Engl J Med. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 2.Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. NEngl J Med. 2000;342(7):461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- 3.Kupsch A, Benecke R, Muller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355(19):1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 4.Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for parkinson's disease. N Engl J Med. 2010;362(22):2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 5.Odekerken VJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced parkinson's disease (NSTAPS study): A randomised controlled trial. Lancet Neurol. 2013;12(1):37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- 6.Alterman RL, Miravite J, Weisz D, Shils JL, Bressman SB, Tagliati M. Sixty hertz pallidal deep brain stimulation for primary torsion dystonia. Neurology. 2007;69(7):681–688. doi: 10.1212/01.wnl.0000267430.95106.ff. [DOI] [PubMed] [Google Scholar]
- 7.Kleiner-Fisman G, Liang GS, Moberg PJ, et al. Subthalamic nucleus deep brain stimulation for severe idiopathic dystonia: Impact on severity, neuropsychological status, and quality of life. J Neurosurg. 2007;107(1):29–36. doi: 10.3171/JNS-07/07/0029. [DOI] [PubMed] [Google Scholar]
- 8.Ostrem JL, Racine CA, Glass GA, et al. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology. 2011;76(10):870–878. doi: 10.1212/WNL.0b013e31820f2e4f. [DOI] [PubMed] [Google Scholar]
- 9.Okun MS, Foote KD. Subthalamic nucleus vs globus pallidus interna deep brain stimulation, the rematch: Will pallidal deep brain stimulation make a triumphant return? Arch Neurol. 2005;62(4):533–536. doi: 10.1001/archneur.62.4.533. [DOI] [PubMed] [Google Scholar]
- 10.Krack P, Fraix V, Mendes A, Benabid AL, Pollak P. Postoperative management of subthalamic nucleus stimulation for parkinson's disease. MovDisord. 2002;17(Suppl 3):S188–97. doi: 10.1002/mds.10163. [DOI] [PubMed] [Google Scholar]
- 11.Kupsch A, Tagliati M, Vidailhet M, et al. Early postoperative management of DBS in dystonia: Programming, response to stimulation, adverse events, medication changes, evaluations, and troubleshooting. MovDisord. 2011;26(Suppl 1):S37–53. doi: 10.1002/mds.23624. [DOI] [PubMed] [Google Scholar]
- 12.Walker HC, Lyerly M, Cutter G, et al. Weight changes associated with unilateral STN DBS and advanced PD. Parkinsonism RelatDisord. 2009 doi: 10.1016/j.parkreldis.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Walker HC, Watts RL, Guthrie S, Wang D, Guthrie BL. Bilateral effects of unilateral subthalamic deep brain stimulation on parkinson's disease at 1 year. Neurosurgery. 2009;65(2):302–9. doi: 10.1227/01.NEU.0000349764.34211.74. discussion 309-10. [DOI] [PubMed] [Google Scholar]
- 14.Amara AW, Standaert DG, Guthrie S, Cutter G, Watts RL, Walker HC. Unilateral subthalamic nucleus deep brain stimulation improves sleep quality in parkinson's disease. Parkinsonism RelatDisord. 2012;18(1):63–68. doi: 10.1016/j.parkreldis.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ondo WG, Meilak C, Vuong KD. Predictors of battery life for the activa soletra 7426 neurostimulator. Parkinsonism RelatDisord. 2007;13(4):240–242. doi: 10.1016/j.parkreldis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Alterman RL, Shils JL, Gudesblatt M, Tagliati M. Immediate and sustained relief of levodopa-induced dyskinesias after dorsal relocation of a deep brain stimulation lead. case report. Neurosurg Focus. 2004;17(1):E6. doi: 10.3171/foc.2004.17.1.6. [DOI] [PubMed] [Google Scholar]
- 17.Blahak C, Capelle HH, Baezner H, Kinfe TM, Hennerici MG, Krauss JK. Battery lifetime in pallidal deep brain stimulation for dystonia. Eur J Neurol. 2011;18(6):872–875. doi: 10.1111/j.1468-1331.2010.03290.x. [DOI] [PubMed] [Google Scholar]
- 18.Shenai MB, Walker H, Guthrie S, Watts R, Guthrie BL. Construction of relational topographies from the quantitative measurements of functional deep brain stimulation using a 'roving window' interpolation algorithm. StereotactFunctNeurosurg. 2010;88(1):16–23. doi: 10.1159/000260075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bin-Mahfoodh M, Hamani C, Sime E, Lozano AM. Longevity of batteries in internal pulse generators used for deep brain stimulation. StereotactFunctNeurosurg. 2003;80(1-4):56–60. doi: 10.1159/000075161. [DOI] [PubMed] [Google Scholar]
- 20.Wishart HA, Roberts DW, Roth RM, et al. Chronic deep brain stimulation for the treatment of tremor in multiple sclerosis: Review and case reports. J NeurolNeurosurg Psychiatry. 2003;74(10):1392–1397. doi: 10.1136/jnnp.74.10.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halpern CH, McGill KR, Baltuch GH, Jaggi JL. Longevity analysis of currently available deep brain stimulation devices. StereotactFunctNeurosurg. 2011;89(1):1–5. doi: 10.1159/000321710. [DOI] [PubMed] [Google Scholar]
- 22.Krack P, Hariz MI. Deep brain stimulation in parkinson's disease: Reconciliation of evidence-based medicine with clinical practice. Lancet Neurol. 2013;12(1):25–26. doi: 10.1016/S1474-4422(12)70270-3. [DOI] [PubMed] [Google Scholar]
- 23.Okun MS, Fernandez HH, Wu SS, et al. Cognition and mood in parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: The COMPARE trial. Ann Neurol. 2009;65(5):586–595. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker HC, Huang H, Gonzalez CL, et al. Short latency activation of cortex during clinically effective subthalamic deep brain stimulation for parkinson's disease. MovDisord. 2012;27(7):864–873. doi: 10.1002/mds.25025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker HC, Huang H, Gonzalez CL, et al. Short latency activation of cortex by clinically effective thalamic brain stimulation for tremor. MovDisord. 2012;27(11):1404–1412. doi: 10.1002/mds.25137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosin B, Slovik M, Mitelman R, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72(2):370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]