Abstract
Purpose
Although neuro-active peptides are highly potent as central nervous system (CNS) therapeutics, their systemic delivery across the blood-brain barrier (BBB) is limited due to lack of permeability in the brain and rapid systemic metabolism. In this study, we aimed at enhancing the brain delivery and stability of chemically modified [D-Arg2, Lys4]-dermorphin-(1-4)-amide)] (DALDA) peptide to achieve prolonged analgesic effects.
Methods
The C8-DALDA peptide analog was encapsulated in an oil-in-water nanoemulsion formulation made specifically with oils rich in omega-3 rich polyunsaturated fatty acid (PUFA) to enhance CNS availability. The nanoemulsion formulation was administered systemically in CD-1 mice and qualitative and quantitative biodistribution was evaluated. We have also examined the effect of curcumin, which is known to down-regulate efflux transporters and inhibit systemic metabolism, on the pharmacokinetic properties of the peptide.
Results
Qualitative and quantitative biodistribution and pharmacokinetic studies in mice clearly demonstrated improved plasma and brain exposure of modified DALDA when administered in nanoemulsion, thereby providing an exciting opportunity towards improved efficacy and/or lowered dose of the peptide. The various dosing regimens tested for modified DALDA solution and curcumin nanoemulsion directed towards a novel combination strategy for improved systemic delivery of peptides across the BBB.
Conclusions
Encapsulation of the drug in PUFA nanoemulsions is an effective strategy for delivery of peptides. This work provides a novel combination strategy for improved delivery of peptides to the brain.
Keywords: nanoemulsion, CNS delivery, pharmacokinetics, DALDA, biodistribution, curcumin
1. Introduction
Biological molecules, like peptides and proteins, are highly potent as central nervous system (CNS) therapeutic agents based on their specificity to the target site and potential for disease-modifying effects [1]. However, due to their hydrophilicity, ionization properties, and large molecular weight, peptides and proteins do not cross the blood-brain barrier (BBB) upon systemic administration. Additionally, these molecules are susceptible to rapid metabolism and clearance in the systemic circulation. While there are some invasive approaches, such as convection-enhanced delivery, can be used for systemic delivery of peptides and proteins across the BBB, the potential for serious CNS toxicity, especially upon chronic administration is a concern [2]. As such, there is a significant medical need for safe and efficient delivery of bio-molecules, like opioid peptide analgesics across the BBB, for effective pain therapy.
DALDA ([D-Arg2, Lys4]-dermorphin-(1-4)-amide), a potent and selective μ opioid receptor agonist, is an analogue of the N-terminal tetrapeptide segment of dermorphin [3]. It is relatively metabolically stable: the placement of a D-amino acid at the 2-position makes the peptide resistant to aminopeptidases, and the carboxyl terminal amidation reduces susceptibility of the peptide to carboxypeptidase [4]. DALDA is eliminated from plasma presumably by glomerular filtration. The metabolic stability of DALDA limits the systemic clearance of this peptide, and accordingly the plasma exposure is relatively high. Since DALDA slowly traverses the BBB via lipid-mediated free diffusion, the brain exposure is directly proportional to the plasma exposure. High plasma exposure for DALDA allows the brain delivery to reach a value of 0.02% injected dose/g in Sprague-Dawley rats, which was allowed to induce analgesia only after a high 5 mg/kg dose being administered intravenously [5].
In this study, we aimed at enhancing systemic stability and brain delivery of chemically-modified DALDA, with the aim to achieve better and prolonged analgesic effect with decreased dose and dose-related side-effects. Two strategies were considered to reach this goal. First, an omega-3 rich polyunsaturated fatty acid (PUFA)-based nanoemulsion formulation was developed for effective encapsulation of this peptide. PUFA are known to be critical modulators of neuronal function and play a key role in regulation of neuroinflammatory and oxidative stress-mediated mechanisms in the normal CNS during aging and chronic neurological diseases [6]. One of the models for fatty acid diffusion across the BBB rules out the involvement of transporters; i.e. it proposes that fatty acids cross the luminal and the transluminal leaflets of brain endothelium and plasma membrane by reversible flip-flop [7]. Once fatty acids reach the neurons, acetyl-CoA synthetase traps them by forming acetyl-CoA, which no longer can diffuse out of the cell. Another theory indicates that transport of fatty acids across the BBB is derived from fatty acid-albumin complexes, where the fatty acid undergoes translocation from extracellular to intracellular leaflet of the membrane via transporters [7]. Also, a recent article describes the role of PUFA as effective absorption enhancers at the BBB where they cause membrane fluidification and impact the tight junctions thereby increasing the paracellular and transcellular transport [8].
Second, curcumin was incorporated in the nanoemulsion and tested in vivo, as there is evidence that curcumin has the ability to down-regulate drug efflux transporters such as P-glycoprotein [9]. Using the nanoemulsion strategy, we carried out whole body imaging in anesthetized mice which were administered modified peptide tagged to a near infra-red (NIR) probe to study the qualitative biodistribution of the peptide. We also performed the quantitative biodistribution and pharmacokinetic studies of modified peptide when administered as a solution or nanoemulsion formulation with and without curcumin.
2. Materials and Methods
2.1. Materials
Extra pure omega-3 rich fish oil and Pluronic F68® were kindly provided as a gift by Jedwards International (Quincy, MA) and BASF Corporation (Florham Park, NJ), respectively. Lipoid E80® and DSPE-PEG2000 were purchased from Lipoid GMBH (Ludwigshafen, Germany). Tween 80® and Curcumin were purchased from Sigma Chemicals, Inc. (St. Louis, MO). [D-Arg2, Lys4]-dermorphin-(1-4)-amide (DALDA) and modified DALDA were purchased from Bachem, Inc. (Torrance, CA). Strata®-X-CW SPE columns were purchased from Phenomenex Inc. (Torrance, CA). All other chemicals and solvents were purchased from Fisher Scientific (Fair Lawn, NJ). LC/MS/MS analyses were carried out at using API5000 AB Sciex system at PhenoLogix, division of Phenomenex Inc. (Torrance, CA). Imaging experiments were conducted using a Lumina II In Vivo Imaging System (IVIS) (Caliper Life Sciences, Hopkinton, MA).
Male CD-1® mice (4–6 weeks old) were purchased from Charles River Laboratories (Cambridge, MA). All the animal procedures were approved by the Northeastern University’s Institutional Animal Care and Use Committee.
2.2. Synthesis of Lipid-Modified DALDA Analog
To improve the encapsulation of hydrophilic DALDA peptide in the oil phase of the nanoemulsion, the epsilon-amino group of the lysine residue was structurally modified with octanoic acid to increase its lipophilicity. Briefly, the side-chain fatty acid modified DALDA was prepared by 9-fluorenylmethyloxycarbonyl (Fmoc/tBu) solid phase peptide synthesis (SPPS) at Bachem Inc. (Torrance, CA). At the conclusion of chain assembly, epsilon-amino group of the lysine residue (Dde) group was deprotected, to allow for side chain acylation with octanoic acid. This was followed by global deprotection and cleavage from the resin, with trifluoroacetic acid (TFA). Crude products from the above chemistry were precipitated and purified by reversed-phase HPLC (RP-HPLC), and finally lyophilized. Several different fatty acids with varying carbon chain lengths were used for chemical modification and the C8 modified peptide showed high encapsulation efficiency in the nanoemulsion formulations. The peptide analog is referred as C8-DALDA in this paper.
For qualitative biodistribution study of C8-DALDA, NIR dye 800CW (Li-Cor, Lincoln, NE) was coupled via N-hydroxysuccinimide (NHS) ester chemistry to C8-DALDAconjugate at Bachem Inc. Briefly, the DALDA sequence was prepared by Fmoc/tBu SPPS as above, except for the 8-aminooctanoic acid residue was coupled to the lysine side chain. The product was then cleaved from the peptide resin with the N-terminal amine protected. IRDye800-NHS was coupled to the terminal amine of the 8-aminooctanoic acid residue under standard neutral pH buffered conditions. The N-terminal amine protecting group was then removed. The product, IRDye800-DALDA-C8, was purified by RP-HPLC and lyophilized.
2.3. Preparation and Characterization of Multifunctional Nanoemulsion Formulations
Preparation of the Nanoemulsion Formulations
Oil-in-water nanoemulsion was prepared by the sonication method as described previously [10]. Briefly, pre-warmed oil phase (1 ml) consisting of fish oil and the peptide alone (or with curcumin) was gradually added to the pre-warmed water phase (4 ml) containing egg phosphatidylcholine (Lipoid E80®) (120 mg), polysorbate 80 (Tween80®) (0.05 ml), DSPE-PEG2000 (1, 2-distearoyl- Sn- glycero- 3- phosphoethanolamine- N- [amino (polyethylene glycol)-2000]) (15 mg). The resultant mixture was stirred for 2 minutes using a silverson homogenizer (Model: L4RT-A, Silverson Machines, MA) at 6000 rpm and ultrasonicated for 10 minutes using the Vibra Cell VC 505 probe sonicator (Sonics and Material Inc., Newtown, CT) at 22% amplitude and 50% duty cycle.
Characterization of the Nanoemulsion Formulations
The nanoemulsions were characterized for particle size and surface charge using the Brookhaven Instrument’s 90Plus ZetaPALS particle size analyzer (Holtsville, NY), at a 90º fixed angle, and at 25ºC up on greater than 10,000-fold dilution with deionized distilled water. The morphology of oil droplets in the nanoemulsion formulations was visualized with transmission electron microscopy (TEM). Nanoemulsion sample was placed on formwar-coated copper grids (EM Sciences, Hatfield, PA), and negatively-stained for 10 minutes with 50 μL of 1.5% phosphotungstic dye at room temperature. Excess liquid was drained off with a filter paper, and the grid containing dry film of nanoemulsion sample was observed with a JEOL 100-X transmission electron microscope (Peabody, MA). The formulation was analyzed for extent of drug loading (i.e., amount of peptide incorporated in the nanoemulsion) and encapsulation efficiency (i.e. amount of peptide present in the internal or oil phase of the nanoemulsion) by using HPLC. For drug loading, the nanoemulsion was sufficiently diluted with organic (ethanol: acetonitrile), and a 50 μL aliquot was injected into the HPLC. For encapsulation efficiency, ultra-filtration method was used. The nanoemulsion sample (0.5 mL) was placed in the upper donor chamber of a centrifugal filter device (molecular weight cut-off 3,000 Daltons; Millipore, Bedford, MA), and the unit was centrifuged at 10,000 rpm for 30 minutes at 4ºC. The sample, along with encapsulated drug, remains in the donor chamber and the aqueous phase moves through the filter into the sample recovery chamber. The concentration of the peptide in the aqueous phase was estimated with use of HPLC and the encapsulation efficiency was calculated by mass balance and the drug loading measurements.
2.4. Quantitation of C8-DALDA Peptide in Plasma and Tissue Samples
Sample Preparation
Owing to the complexity of in vivo sample matrix and possibility of interference of plasma proteins with analytical techniques, sample preparation was performed using solid phase extraction (SPE). This is a well-known technique which is based on the principle of selective partitioning of analyte between a solid (sorbent) phase and a liquid (solvent) phase [11, 12]. SPE for C8-DALDA was developed based on the ion exchange principle. Due to the presence of predominantly basic groups on the peptide, cationic polymer-based columns, Strata®-X-CW, with affinity for basic groups were used for purification and extraction of peptide from in vivo sample matrix. Table I describes the SPE process utilized. C8-DALDA from in vivo samples was extracted using methanol. Briefly, 75 μl of plasma was mixed with 225 μl of methanol and centrifuged at 4,500 rpm for 20 minutes. Frozen tissue samples, including brain, were weighed and an equal amount of saline was added and the tissue homogenized at 6,000 rpm for 2 minutes. An excess of 4-fold by weight of methanol was added to the tissue homogenate and following vortexing, it was centrifuged at 4,500 rpm for 20 minutes. The clear supernatant was collected and loaded to the SPE columns. The % recovery of C8-DALDA after SPE was also determined by spiking plasma and tissue samples with known amounts of peptide and subjecting the spiked samples to the same procedure of SPE as the in vivo samples.
Table I.
Solid phase extraction for C8-DALDA
| Description | |
|---|---|
| Sorbent (Column) | Strata®-X-CW- cationic (33μm polymeric weak cation, 30 mg/1mL) |
| Condition step | Methanol -1ml |
| Equilibration step | Water- 1ml |
| Sample load step | Methanol extract of tissue or plasma sample- 300μl |
| Wash 1 | Water- 1ml |
| Wash 2 | Methanol- 1ml |
| Elute | 5% formic acid in methanol (freshly prepared) (2 x 150μl) |
LC/MS/MS Analysis
LC/MS/MS is a widely recognized technique for sensitive and accurate detection of nanogram levels of peptide analytes biological matrix. API5000 AB Sciex system at PhenoLogix was used for quantification of the SPE elute of the in vivo samples. Described in Table II is the LC/MS/MS method and conditions used for assaying the samples. A standard curve was obtained on LC/MS/MS using this method by running known amounts of C8-DALDA (solution in 0.1% formic acid in methanol) spiked in blank plasma sample matrix and eluted through SPE. Appropriate QC solutions with known amounts of C8-DALDA spiked plasma or tissue samples were run with every sample set.
Table II.
LC/MS/MS method for C8-DALDA analysis
| Condition | Description | |
|---|---|---|
| Mobile phase |
|
|
| Column |
|
|
| Flow rate | 0.5 ml/min | |
| Injection volume | 10μl | |
| Detector | API5000 | |
| Temperature | Ambient | |
| Pressure | 312 bars, 5500 psi | |
| Gradient run | ||
| Time (min) | A (%) | B (%) |
| 0.00 | 80 | 20 |
| 3.00 | 10 | 90 |
| 4.50 | 10 | 90 |
| 4.51 | 80 | 20 |
| 6.00 | 80 | 20 |
| Mass transitions | Q1, Da | Q3, Da |
| 369.9 | 484.5 | |
| 369.9 | 612.4 | |
2.5. Qualitative Biodistribution Studies with NIR-Conjugated C8-DALDA Peptide
Male CD-1® mice were dosed intravenously with IRDye800-C8-DALDA solution or nanoemulsion via tail-vein injection at a dose of 30 nmol equivalent of dye. Animals were imaged under anesthesia at pre-determined time points of 15, 60 and 180 min in the Caliper IVIS with high lamp power, excitation filter centered at 745 nm, emission filter at 820 nm, and a 1 second exposure. Image analysis was performed using Living Image® 4 software (Caliper Life Sciences)
2.6. In Vivo Biodistribution and Pharmacokinetic Analyses of C8-DALDA Peptide
C8-DALDA was dosed intravenously as solution or nanoemulsion formulation to male CD-1® mice, via tail vein injection at 3 mg/kg dose (i.e., 75 μg for 25 g weighing mice). Blood was collected in EDTA-treated tubes at various time intervals: 10, 20, 30, 60, 120 and 180 minutes post dosing and kept on ice. The samples were centrifuged at 10,000 rpm for 20 minutes at 4ºC to separate plasma. The plasma was stored at −20ºC or used immediately for analyses. For biodistribution study, the blood was completely withdrawn by cardiac puncture. Mice were sacrificed via cervical dislocation and the whole brain, liver, lungs, kidneys, spleen and heart were collected, weighed, and frozen at −80ºC until the time of analyses.
2.7. Effects of Curcumin on In Vivo Biodistribution and Pharmacokinetics
To compare and contrast the in vivo pharmacokinetics of C8-DALDA solution and nanoemulsion when administered with curcumin, different dosing options (co-dosing or pre-dosing) for curcumin were evaluated. Curcumin nanoemulsion at 2 mg/mL drug loading was prepared by ultrasonication, using the procedure described above. Curcumin nanoemulsion was administered intravenously to male CD-1® mice at 10 mg/kg dose 24 hours or 3 hours prior to C8-DALDA injection, or simultaneously. C8-DALDA was injected in solution or nanoemulsion at 3 mg/kg, except when curcumin nanoemulsion was co-administered with C8-DALDA nanoemulsion. In that case, the C8-DALDA nanoemulsion dose had to be reduced to half (1.5 mg/kg dose) due to viscosity and volume restriction. For the 3 hour and 24 hour curcumin pre-treatment groups and C8-DALDA solution dosing, blood was collected in EDTA-treated tubes at 10, 20 and 60 minutes and brain was collected as mentioned in the previous section. All samples were stored at −80ºC until the time of analyses.
2.8. Analgesic Efficacy Evaluations in Hot-Water Tail Withdrawal Assay
Male CD-1® mice were randomized into 3 groups of 6 animals per group. These mice were acclimatized to the test conditions prior to dosing the formulations. Temperature controlled, Isotemp® water bath (Fisher Scientific, Fair Lawn, NJ) was set at 52ºC. CD-1 mice were given an intravenous dose of 3 mg/kg DALDA solution in phosphate buffered saline (PBS) or DALDA-C8 solution in PBS or DALDA-C8 nanoemulsion (prepared as described in section 2.2.3). The animal’s tail tip (1 inch) was gently lowered in the water bath at set time points of 0 min (before injection) and at every 30 min interval up to 180 min post- dosing. Time taken for the mouse to withdraw its tail out of the hot water (i.e. the latency period) was recorded. A cut off time of 8 seconds was set to avoid burn to the tail due to prolonged exposure to hot water.
2.9. Data Analysis
Pharmacokinetic parameters were calculated taking into account individual data using Phoenix® WinNonlin® v. 1.3 software (Pharsight, Cary, NC). A non-compartmental analysis (NCA) was performed for plasma and brain, using an INTRAVENOUS bolus administration model for sparse data. The intravenous bolus model was chosen for brain based on the shape of the concentrations versus time curve, which suggested that distribution to brain could be assumed to be instantaneous. The slope of the terminal log-linear part of the concentration versus time curve (λz) was calculated using the best-fit method, with uniform weighting. Graph Pad Prism® software (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. The two-tailed unpaired homoscedastic t-test was used to compare mean values ± standard errors obtained for the solution and the nanoemulsion: p < 0.05 was considered statistically significant.
3. Results
3.1. Lipid Modification of DALDA for Encapsulation in Nanoemulsions
C8-DALDA and IRDye800-C8-DALDA analogues could be successfully prepared by 9-fluorenylmethyloxycarbonyl (Fmoc/tBu) solid phase peptide synthesis and purified by RP-HPLC. The purity was greater than 90% by RP-HPLC and the identity was confirmed by MALDI mass spectrometry.
3.2. Preparation and Characterization of Multifunctional Nanoemulsion Formulations
Ultrasonication led to uniform nanoemulsion formulation. Particle size and polydispersity index (PDI) were determined for peptide loaded nanoemulsion formulation. The formulation size was 177 ± 3 nm and PDI was 0.25. The charge was −40.6 ± 0.4 mV. TEM of nanoemulsion formulation confirmed its spherical shape and the size range of 180–220 nm as shown in Figure 1.
Figure 1. Transmission electron microscopy (TEM) of the nanoemulsion.
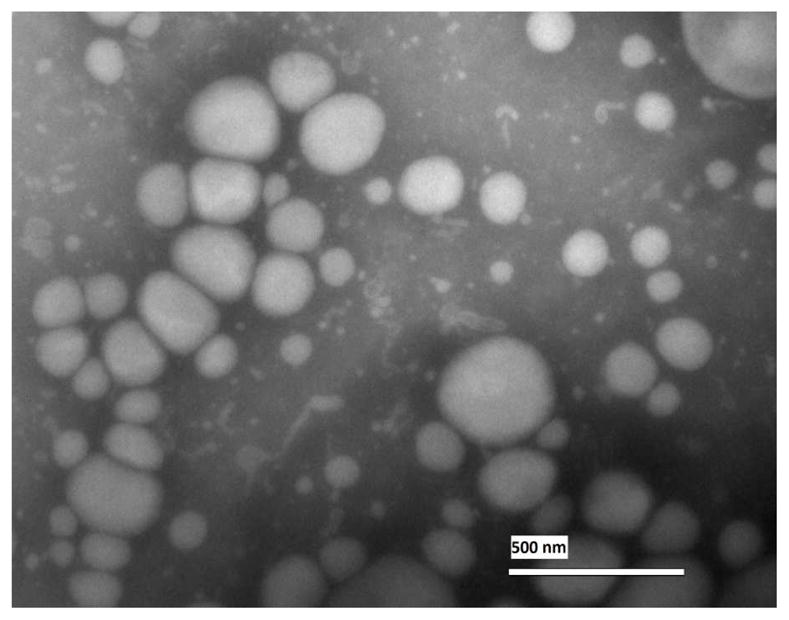
The oil droplets of the nanoemulsion sample were spherical and their size was in the range of 180–220 nm. Scale bar is 500 nm...
C8-DALDA had a drug loading of 0.7 mg/ml. C8-DALDA achieved encapsulation efficiency of 87% (versus unmodified DALDA which had less than 10% encapsulation), suggesting that C8 chain length modification applied to the DALDA peptide provided sufficient solubility in the internal oil phase.
3.3. Quantitation of C8-DALDA Peptide in Plasma and Tissue Samples
For solid phase extraction, the working hypothesis was that C8-DALDA peptide will form relatively strong ionic bonds with the carboxylic acid moieties on the SPE sorbent under neutral pH conditions, allowing the use of a strong wash with organic solvent to eliminate hydrophobic matrix components. An initial wash with water removes polar matrix components and salts. Adding 5% formic acid to the organic elution solvent (methanol) neutralizes the carboxylic acid groups on the SPE sorbent, allowing the plasma C8-DALDA to elute. A recovery of > 98% was obtained using this method for the in vivo plasma and tissue samples. The LC/MS/MS method gave a standard curve with R2= 0.9977 and concentration range of 1–2000 ng/ml.
3.4. Qualitative Biodistribution Studies with NIR-Conjugated C8-DALDA Peptide
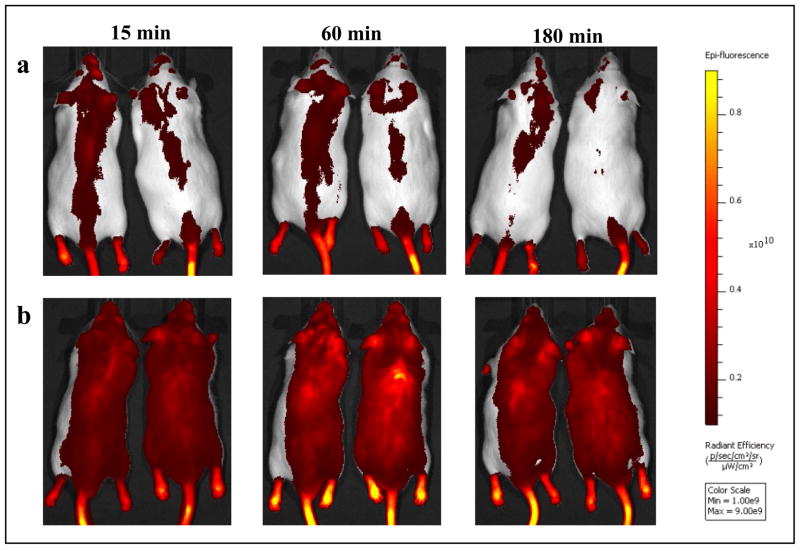
The IRDye800-C8-DALDA nanoemulsion induced a massive and diffuse signal, which persisted over 180 min, as shown in Figure 2. Contrarily, the solution formulation induced a low signal, which was concentrated on the backbone axis, and almost disappeared in 180 minutes.
Figure 2. Qualitative biodistribution of near infra-red labeled C8-DALDA as nanoemulsion and solution following intravenous dosing at 30 nmol equivalent of dye in CD-1 male mice under anesthesia.
n=2.
Panel A: In vivo image of mice at 15, 60 and 180 min post-administration of nanoemulsion
Panel B: In vivo image of mice at 15, 60 and 180 min post-administration of solution formulation The bar to the right is the epi-fluorescence scale with a range of 0.1x1010 – 0.9x1010 radiant frequency.
3.5. Quantitative Pharmacokinetics and Biodistribution of C8-DALDA Peptide in Solution and Nanoemulsion
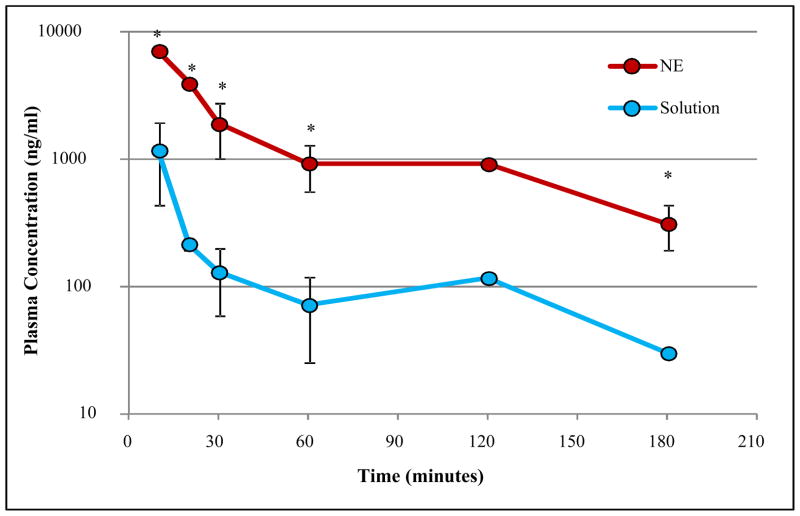
Figure 3 displays the plasma concentration (Cp) - time data for C8-DALDA solution and nanoemulsion on a log-linear scale, after intravenous administration at a 3 mg/kg dose. A biphasic decrease was observed for both formulations. Pharmacokinetic parameters calculated using Phoenix® WinNonlin® NCA are shown in Table III. Nanoemulsion induced significantly higher peptide levels in plasma when compared to the solution formulation, leading to an increased exposure, with a 5.5 fold higher AUC0-∞. This increased exposure was associated to an accordingly 5.5 fold decreased clearance Steady-state volume of distribution (Vss) was also decreased (3.3 fold) by nanoemulsion formulation, while the slope of the log-linear terminal part of the curve (λz) and corresponding half-lives (t1/2) were similar for both formulations, consistently with the parallel decrease observed in Figure 3. MRT was 1.6 fold higher for nanoemulsion, presumably due to a lower distribution rate in the first phase, as can be seen in Figure 3. This distribution rate could not be calculated because there were not enough data to perform a bi-compartmental analysis with sparse data.
Figure 3. Plasma concentration (ng/ml) versus time (minutes) for C8-DALDA solution and nanoemulsion (NE) following intravenous dosing at 3mg/kg to CD-1 male mice.
n=3 for 10, 20, 30, 60 and 180 minutes, n=1. NE= nanoemulsion; S= solution (* p < 0.05, two-tailed unpaired homoscedastic t-test)
Table III.
Pharmacokinetic parameters in plasma following intravenous administration of 3 mg/kg C8-DALDAsolution or nanoemulsion in mice
| PK Parameter | Solution Dosing* | Nanoemulsion Dosing* | Units |
|---|---|---|---|
| AUC 0-∞ | 62803 | 347244 | ng.min/ml |
| Cl | 1.194 | 0.216 | ml/min |
| Vss | 45.2 | 13.4 | ml |
| λz | 0.00915 | 0.0104 | 1/min |
| t1/2 λz | 75.7 | 66.4 | min |
| MRT | 37.8 | 62.1 | min |
n= 3, two-tailed unpaired homoscedastic t-test, p< 0.05
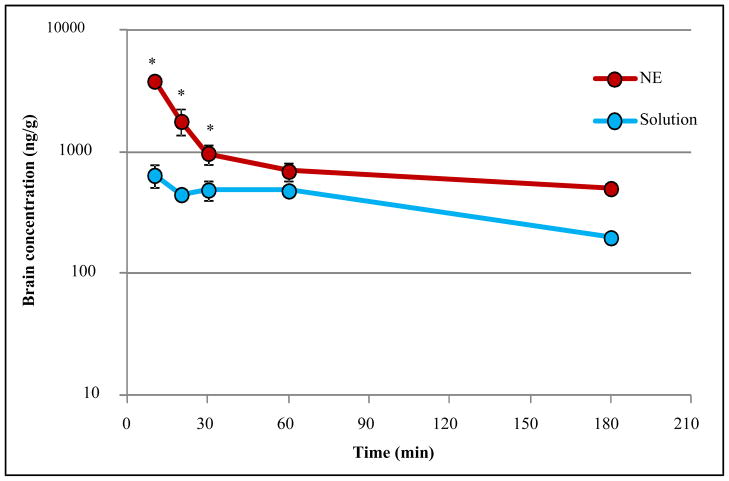
Brain concentration-time data (Figure 4) shows a similar trend. A biphasic decay was observed in brain, and nanoemulsion allowed significantly higher brain concentration of peptide when compared to the solution formulation, leading to an increased exposure, with a 3.3 fold higher AUC0-∞ (Table IV). MRT was also higher in brain for nanoemulsion, suggesting that the nanoemulsion would allow a longer residence of the peptide in the brain. Accordingly, λz was lower in brain for nanoemulsion.
Figure 4. Brain concentration (ng/g) versus time (minutes) for C8-DALDA solution and nanoemulsion (NE) following intravenous dosing at 3mg/kg to CD-1 male mice.
n=3 for 10, 20, 30, 60 minutes. NE= nanoemulsion; S= solution (* p < 0.05, two-tailed unpaired homoscedastic t-test)
Table IV.
Pharmacokinetic parameters in brain following intravenous administration of 3 mg/kg C8-DALDA solution or nanoemulsion in mice
| PK Parameter | Solution Dosing* | Nanoemulsion Dosing* | units |
|---|---|---|---|
| AUC 0-∞ | 104,634 | 325,851 | ng.min/g |
| MRT | 145.4 | 198.4 | min |
| λz | 0.00640 | 0.00396 | 1/min |
| t1/2 λz | 108.3 | 174.9 | min |
n= 3, two-tailed unpaired homoscedastic t-test, p< 0.05
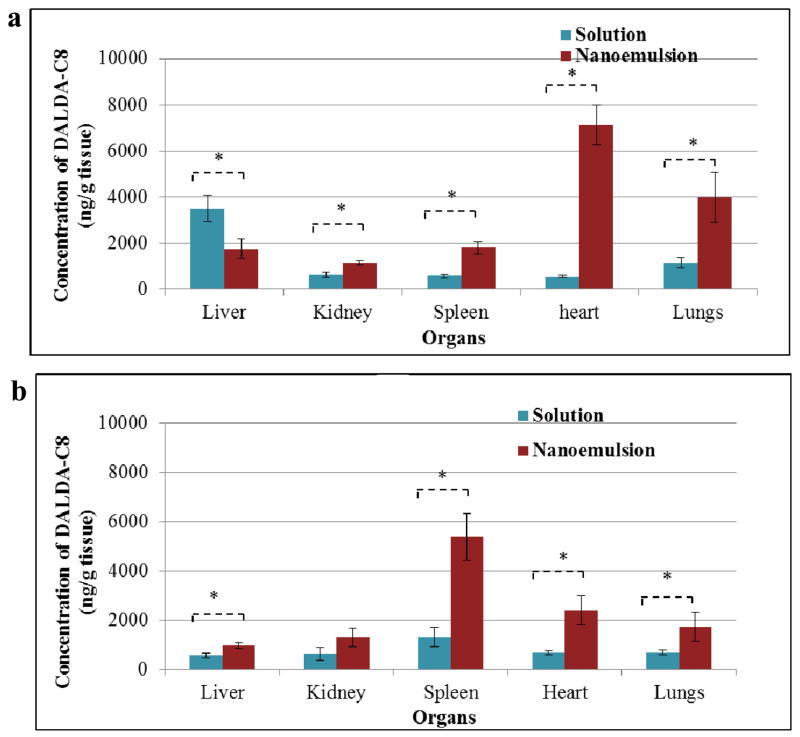
Tables V and VI and Figure 5 shows the biodistribution of C8-DALDA in highly perfused tissues (i.e., heart, lungs, spleen, kidneys and liver) at 20 min (A) and 180 min (B) following dosing of solution or nanoemulsion formulation. At 20 min, C8-DALDA concentrations are significantly twice lower in liver after nanoemulsion administration when compared to solution, but significantly higher in all other organs. The most striking difference is observed in heart, where concentration is 12.6 fold higher after nanoemulsion administration, reaching a level even higher than in plasma, while for other kidney, lungs and spleen, nanoemulsion induces a more modest 1.81 to 3.43 fold increase of concentration. At 180 min, concentrations have decreased in liver and lungs, but increased in spleen for both formulations. In heart, concentrations have increased for the solution and decreased for the nanoemulsion, leading to an only 3.54 fold higher concentration for nanoemulsion. In kidney, concentrations appear stable in time for both formulations. Comparing nanoemulsion to solution at 180 min, all organs display 1.70 to 4.11 fold higher concentrations after nanoemulsion administration.
Table V.
Biodistribution of DALDA-C8 solution or nanoemulsion in tissues at 20 minutes following intravenous dosing at 3 mg/kg
| Tissue | Concentration from Solution (ng/g) ± S.D.* | Concentration from Nanoemulsion (ng/g) ± S.D.* | p value |
|---|---|---|---|
| Brain | 449 ± 31 | 1804 ± 434 | 0.006 |
| Heart | 567 ± 30 | 7144 ± 865 | 0.0002 |
| Spleen | 590 ± 70 | 1804 ± 279 | 0.002 |
| Lungs | 1166 ± 230 | 4002 ± 1080 | 0.01 |
| Liver | 3517 ± 568 | 1754 ± 423 | 0.012 |
| Kidney | 633 ± 107 | 1148 ± 84 | 0.003 |
n= 3, two-tailed unpaired homoscedastic t-test, p< 0.05
Table VI.
Biodistribution of DALDA-C8 solution or nanoemulsion in tissues at 180 minutes following intravenous dosing at 3 mg/kg
| Tissue | Concentration from Solution (ng/g) ± S.D.* | Concentration from Nanoemulsion (ng/g) ± S.D.* | p value |
|---|---|---|---|
| Brain | 199 ± 8 | 502 ± 16 | 0.001 |
| Heart | 675 ± 72 | 2387 ± 587 | 0.007 |
| Spleen | 1309 ± 385 | 5375 ± 960 | 0.002 |
| Lungs | 683 ± 90 | 1721 ± 591 | 0.04 |
| Liver | 563 ± 107 | 955 ± 115 | 0.012 |
| Kidney | 631 ± 258 | 1297 ± 370 | 0.06 |
n= 3, two-tailed unpaired homoscedastic t-test, p< 0.05
Figure 5. C8-DALDA concentration in various organs for solution and nanoemulsion (NE) following intravenous dosing at 3 mg/kg to CD-1 male mice.
(* p < 0.05, two-tailed unpaired homoscedastic t-test)
A: Biodistribution at 20 minutes post-dosing, n=3
B: Biodistribution at 180 minutes post- dosing, n=3
3.6. Quantitative Pharmacokinetics of C8-DALDA Peptide in Solution and Nanoemulsion in the Presence of Curcumin
Figure 6 shows the plasma and brain concentrations for C8-DALDA after solution and nanoemulsion administration when curcumin was added following various schedules. For solution formulation of C8-DALDA peptide, addition of curcumin was able to induce an increase in plasma concentration at 20 minutes for all schedules tested, the co-dosing option being the most efficient with a 9.30-fold increase (p= 0.005). However, this increase in plasma concentrations did not translate into an increase of brain concentration. For nanoemulsion formulation, addition of curcumin led to a slightly lowered (0.7- fold) plasma concentration of C8-DALDA peptide upon 3 hour curcumin pre-treatment, but no significant difference when co-dosing curcumin, when compared to nanoemulsion alone at 20 minutes. For co-dosing of curcumin with C8-DALDA nanoemulsion, a half-dose has been given (see methods) and the concentrations have been dose-normalized.
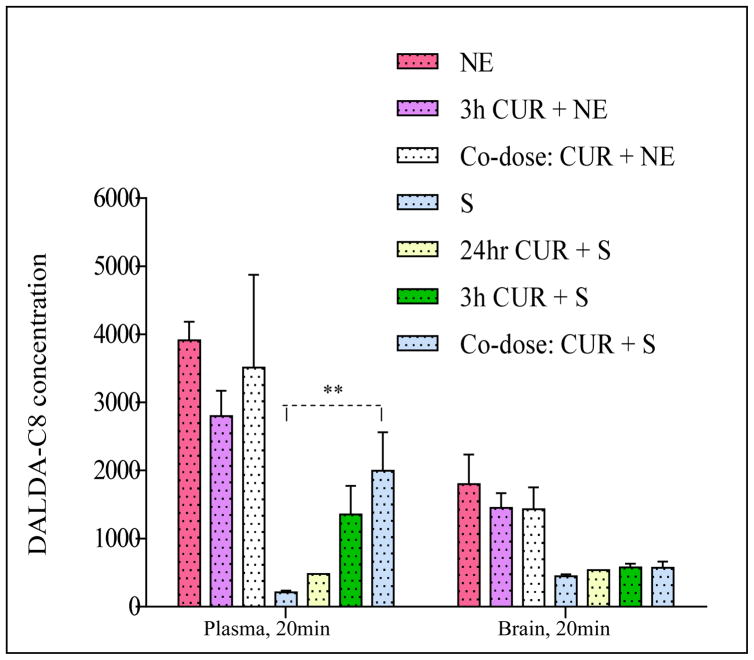
Figure 6. Mean plasma concentration (ng/ml) or brain concentration (ng/g) at 20 minutes after intravenous injection of C8-DALDA solution (S) or nanoemulsion (NE) dosing at 3 mg/kg (except for co-dose (corrected arm) of 1.5 mg/kg) with or without curcumin (CUR) nanoemulsion administered intravenously at 10 mg/kg.
Nanoemulsion formulation for C8-DALDA dosed alone (NE) or dosed after 3-hour of CUR NE dosing (3h CUR+ NE) or upon co-dosing with CUR NE (Co-dose: CUR + NE) shows higher plasma and brain concentration when compared to solution formulation of C8-DALDA(S) dosed alone or after 3-hour of CUR NE dosing (3h CUR + S) or upon co-dosing with CUR NE (Co-dose: CUR+S). ** p < 0.05, two-tailed unpaired homoscedastic t-test, n=3.
3.8. Analgesic Efficacy Evaluation using Hot-Water Tail Withdrwal Assay
Unmodified DALDA solution, DALDA-C8 solution and DALDA-C8 nanoemulsion, dosed at 3 mg/kg, via tail vein injection produced analgesia, as shown in Figure 7. The latency time increased 2–3.5- fold over baseline at 60–120 minutes post-dose for DALDA and DALDA-C8 solution. The response returned to baseline for solution formulations after 120 minutes. For the DALDA-C8 nanoemulsion, a 2–3.5- fold increase in latency time at 60–90 minutes and an extended duration of analgesia, for up to 180 minutes was observed. This highlights the potential for improved duration of action with the nanoemulsion formulation for the modified peptide. The experiment also suggests that the C8- linker i.e. modification introduced on the DALDA peptide did not adversely impact its efficacy.
Figure 7. Hot-water tail withdrawal analgesia assay.
Fold change in latency time for CD-1® male mice dosed with DALDA solution (green curve) or DALDA-C8 solution (blue curve) or DALDA-C8 nanoemulsion (red curve) at 3 mg/kg dose. The latency time does not change for DALDA-C8 when compared to unmodified DALDA peptide in solution. The DALDA-C8 nanoemulsion showed an extended duration of analgesic action as depicted by the prolonged latency time compared to solution, n= 6 mice
4. Discussion
Qualitative and quantitative data consistently showed that nanoemulsion induced an increase in C8-DALDA levels throughout the body, maintaining high levels 180 min after administration, when compared to solution. A biphasic decay profile was observed in plasma after C8-DALDA solution administration, in line with results obtained with DALDA in Sprague-Dawley rats by Samii et al.[13], with some minor differences in the values of PK parameters, likely due to difference in animal species (mouse versus rat) and modification of the lysine residue. Brain delivery of C8-DALDAin solution was comprised between 648 ng/g (or 0.864 %ID/g) at 10 min and 199 ng/g (or 0.265 %ID/g) at 180 min, which meant that brain delivery was higher than reported by Samii et al. for DALDA in rat (0.019 %ID/g at 30 min). A biphasic decay profile was also observed in plasma and brain with nanoemulsion of DALDA-C8. The two phases could not be characterized due to the sparseness of the data, which allowed only for non-compartmental analysis. Nanoemulsion formulation of C8-DALDA induced a higher brain exposure than solution, with values between 3831 ng/g (ie 5.11 %ID/g) at 10 min and 502 ng/g (ie 0.669 %ID/g) at 180 min post-administration. This level of brain exposure constitutes a major improvement not only over the solution formulation of DALDA-C8, but also over DALDA peptide[5] and even DALDA-[Dmt1] peptide, which display similar pharmacokinetic properties as DALDA [14] and does not allow for high brain delivery in male CD-1 mice[15]. Nanoemulsion seems to increase brain exposure through an increase in plasma level, in line with the observation by Samii et al, that the brain delivery is directly proportional to the plasma exposure. Since DALDA clearance is supposed to be mainly due to glomerular filtration [13], nanoemulsion might protect C8-DALDA from kidney elimination. With particle size of ~200 nm, nanoemulsion entrapping C8-DALDA is indeed too big to be filtrated by the glomerule [16], and will likely be eliminated by other routes, such as reticulo-endothelial system. For both solution and nanoemulsion formulations, exposure in brain (as quantitated by AUC) was similar to exposure in plasma, suggesting a free diffusion process, which is in line with published results for DALDA[5]. Interestingly, concentrations in brain got higher at later time points when compared to plasma, causing a higher MRT in brain than in plasma. This can be due to the affinity of the peptide to the target receptors, leading to a slight accumulation in the brain.
Curcumin had no effect on peptide kinetics, when peptide analog was administered as nanoemulsion. When C8-DALDA was administered as solution, curcumin enhanced plasma concentrations, but had no significant effect on brain concentration, despite down-regulation of BCRP, MDR1 and MRP1, and enhanced permeability and reduced efflux ratio, as demonstrated in Caco-2 cells (in vitro studies, data not shown here). This result suggests that the effect of curcumin on peptide pharmacokinetics might be associated to a decreased clearance of the peptide, without any effect on BBB permeability. The low brain exposure of the peptide might accordingly not be due to efflux associated to BCRP, MDR1 or MRP. Since DALDA is eliminated mainly by glomerular filtration, the increase of DALDA plasma concentration induced by curcumin should be associated to an effect of curcumin on glomerular filtration. To our knowledge, the effect of curcumin on glomerular filtration in physiological conditions has never been studied. Another hypothesis would be that, though considered metabolically stable, C8-DALDA could still be metabolized to some extent. Curcumin could decrease peptide degradation rate by reducing proteolysis, resulting in lower clearance of the peptide. Although never assessed in a pharmacokinetics context, a few studies suggest that curcumin could display such antiproteolytic activity. Siddiqui et al., [17] showed that curcumin had a dose-dependent effect on protein degradation as assayed by tyrosine release during serum starvation. Proteasome activity was inhibited in HeLa cells [18] and less increased in the muscle tissues of tumour-bearing mice [19] when treated with curcumin. Interestingly, in a transcriptomic study of nuclear factor E2-related factor 2 (Nfr2)-dependent genes, curcumin decreased expression of peptidases, such as kallikrein 26 and carboxypeptidase A4 [20]. The effect of curcumin on drug glomerular filtration and on protein drugs proteolysis deserves further investigation.
5. Conclusions
The pharmacokinetics and biodistribution studies clearly demonstrated the improved plasma and brain exposure of C8-DALDA peptide from omega-3 rich fish oil- based nanoemulsion formulation, thereby providing an exciting opportunity towards improved efficacy and/or lowered dose of peptide. The various dosing regimen options tested for C8-DALDA solution and curcumin nanoemulsion formulations directs recommending the co-dosing scenario based on the plasma and brain concentrations observed for C8-DALDA at the 20 min time point. This provides a novel combination strategy for improved delivery of such peptide drugs from solution. The precedence of much higher brain exposures from C8-DALDA nanoemulsion formulation as compared to any of the curcumin- based options and the negligible impact of curcumin on this nanoemulsion formulation, shows that encapsulation of the drug in polyunsaturated fatty acid (PUFA) based nanoemulsions should be retained as an effective delivery strategy for the DALDA peptide.
Acknowledgments
This study was partially supported by a grant (R21-NS066984) from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. We would like to thank Dr. Kevin Cash and Dr. Heather Clark for their assistance with the IVIS imaging studies. We appreciate the help from Seyed Sadjadi and Jenny Wei at Phenomenex with LC/MS/MS analyses. We also thank Ms. Purva Pandya for her assistance with the in vivo studies.
References
- 1.Prokai L. Peptide drug delivery into the central nervous system. Progress in Drug Research. 1998;51:95–131. doi: 10.1007/978-3-0348-8845-5_3. [DOI] [PubMed] [Google Scholar]
- 2.Shah L, Yadav S, Amiji M. Nanotechnology for CNS delivery of bio-therapeutic agents. Drug Delivery and Translational Research. 2013;3:1. doi: 10.1007/s13346-013-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiller PW, Nguyen TM, Chung NN, Lemieux C. Dermorphin analogues carrying an increased positive net charge in their “message” domain display extremely high mu opioid receptor selectivity. Journal of medicinal chemistry. 1989;32:698–703. doi: 10.1021/jm00123a035. [DOI] [PubMed] [Google Scholar]
- 4.Schiller PW, Weltrowska G, Berezowska I, Nguyen TM, Wilkes BC, Lemieux C, et al. The TIPP opioid peptide family: development of delta antagonists, delta agonists, and mixed mu agonist/delta antagonists. Biopolymers. 1999;51:411–25. doi: 10.1002/(SICI)1097-0282(1999)51:6<411::AID-BIP4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Samii A, Bickel U, Stroth U, Pardridge WM. Blood-brain barrier transport of neuropeptides: analysis with a metabolically stable dermorphin analogue. American Journal of Physiology. 1994;267:E124–E31. doi: 10.1152/ajpendo.1994.267.1.E124. [DOI] [PubMed] [Google Scholar]
- 6.Farooqui AA. n-3 fatty acid-derived lipid mediators in the brain: new weapons against oxidative stress and inflammation. Current Medicinal Chemistry. 2012;19:532–43. doi: 10.2174/092986712798918851. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton JA, Brunaldi K. A model for fatty acid transport into the brain. Journal of Molecular Neuroscience. 2007;33:12–7. doi: 10.1007/s12031-007-0050-3. [DOI] [PubMed] [Google Scholar]
- 8.Navarro C, Gonzalez-Alvarez I, Gonzalez-Alvarez M, Manku M, Merino V, Casabo VG, et al. Influence of polyunsaturated fatty acids on Cortisol transport through MDCK and MDCK-MDR1 cells as blood-brain barrier in vitro model. European Journal of Pharmaceutical Science. 2011;42:290–9. doi: 10.1016/j.ejps.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Dudhatra GB, Mody SK, Awale MM, Patel HB, Modi CM, Kumar A, et al. A comprehensive review on pharmacotherapeutics of herbal bioenhancers. Scientific World Journal. 2012;2012:637953. doi: 10.1100/2012/637953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Molecular Pharmaceutics. 2009;6:928–39. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- 11.Vuckovic D, Zhang X, Cudjoe E, Pawliszyn J. Solid-phase microextraction in bioanalysis: New devices and directions. Journal of Chromatography A. 2010;1217:4041–60. doi: 10.1016/j.chroma.2009.11.061. [DOI] [PubMed] [Google Scholar]
- 12.Zwir-Ferenc A, Biziuk A. Solid phase extraction technique- trends, opportunities and applications. Polish Journal of Environmental Studies. 2006;15:677–90. [Google Scholar]
- 13.Samii A, Bickel U, Stroth U, Pardridge WM. Blood-brain barrier transport of neuropeptides: analysis with a metabolically stable dermorphin analogue. American Journal of Physiology. 1994;267:E124–31. doi: 10.1152/ajpendo.1994.267.1.E124. [DOI] [PubMed] [Google Scholar]
- 14.Szeto HH, Lovelace JL, Fridland G, Soong Y, Fasolo J, Wu D, et al. In vivo pharmacokinetics of selective mu-opioid peptide agonists. Journal of Pharmacology Experimental Therapeutics. 2001;298:57–61. [PubMed] [Google Scholar]
- 15.Novoa A, Van Dorpe S, Wynendaele E, Spetea M, Bracke N, Stalmans S, et al. Variation of the net charge, lipophilicity, and side chain flexibility in Dmt(1)-DALDA: Effect on Opioid Activity and Biodistribution. Journal of medicinal chemistry. 2012;55:9549–61. doi: 10.1021/jm3008079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiology Reviews. 2008;88:451–87. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui RA, Hassan S, Harvey KA, Rasool T, Das T, Mukerji P, et al. Attenuation of proteolysis and muscle wasting by curcumin c3 complex in MAC16 colon tumour-bearing mice. British Journal of Nutrition. 2009;102:967–75. doi: 10.1017/S0007114509345250. [DOI] [PubMed] [Google Scholar]
- 18.Jana NR, Dikshit P, Goswami A, Nukina N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. Journal of Biological Chemistry. 2004;279:11680–5. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 19.Wyke SM, Russell ST, Tisdale MJ. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation. British journal of cancer. 2004;91:1742–50. doi: 10.1038/sj.bjc.6602165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen G, Xu C, Hu R, Jain MR, Gopalkrishnan A, Nair S, et al. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Molecular cancer therapeutics. 2006;5:39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]