Abstract
In this study, a full factorial approach was employed to investigate the effects of poly(ethylene glycol) (PEG) molecular weight (10,000 vs. 35,000 nominal molecular weight), crosslinker-to-macromer carbon-carbon double bond ratio (40 vs. 60), crosslinker type (PEG-diacrylate (PEGDA) vs. N,N′–methylene bisacrylamide (MB)), crosslinking extent of incorporated gelatin microparticles (low vs. high), and incubation medium composition (with or without collagenase) on the swelling and degradation characteristics of oligo(poly(ethylene glycol) fumarate) (OPF) hydrogel composites as indicated by the swelling ratio and the % mass remaining, respectively. Each factor consisted of two levels, which were selected based on previous in vitro and in vivo studies utilizing these hydrogels for various tissue engineering applications. Fractional factorial analyses of the main effects indicated that the mean swelling ratio and the mean % mass remaining of OPF composite hydrogels were significantly affected by every factor. In particular, increasing the PEG chain MW of OPF macromers significantly increased the mean swelling ratio and decreased the mean % mass remaining by 5.7±0.3 and 17.2±0.6 %, respectively. However, changing the crosslinker from MB to PEGDA reduced the mean swelling ratio and increased the mean % mass remaining of OPF composite hydrogels by 4.9±0.2 and 9.4±0.9 %, respectively. Additionally, it was found that the swelling characteristics of hydrogels fabricated with higher PEG chain MW or with MB were more sensitive to increases in DBR. Collectively, the main and cross effects observed between factors enables informed tuning of the swelling and degradation properties of OPF-based hydrogels for various tissue engineering applications.
Keywords: hydrogels, swelling and degradation, factorial study, poly(ethylene glycol)-based materials, fabrication parameters
INTRODUCTION
Poly(ethylene glycol) (PEG)-based hydrogels are currently being investigated for a myriad of biomedical applications, including drug delivery, surface modifications, diagnostic devices, and tissue engineering due to their appeal as a hydrophilic and cytocompatible biomaterial as well as the range of physicochemical properties that can be achieved to meet the needs of the application 1. These materials are of particular interest for tissue engineering applications because of their ability to mimic the extracellular matrix of tissues and direct the growth of neo-tissue within a three-dimensional architecture 2,3. By varying parameters such as network morphology, crosslinking density, and polymer composition, PEG-based hydrogels can be easily tailored to meet the requirements for regeneration of a multitude of tissue types 4.
Many tissue engineering strategies involving hydrogel scaffolds have taken advantage of their high water content to deliver a variety of bioactive molecules and encapsulated cells to promote tissue regeneration 2,3. The high equilibrium swelling allows increased diffusion of nutrients in and cellular waste and bioactive molecules out of the hydrogels. Additionally, the extent of hydrogel swelling has been shown to be a critical factor in directing cell behavior and tuning the release kinetics of bioactive molecules 5–7. Also, just as important is the role of hydrogel degradation in affecting cell fate and facilitating neo-tissue formation 8–10. Indeed, finding the appropriate balance among different parameters that can affect swelling and degradation of PEG-based hydrogels remains a challenge for targeted tissue regeneration.
The present study examines the use of oligo(poly(ethylene glycol) fumarate) (OPF)-based hydrogels that have shown great tunability for a variety of tissue engineering applications 11–19. OPF is a water-soluble synthetic macromer with alternating PEG chains and fumarate groups that allow for crosslinking and degradation into products readily cleared by the body 20. It has been previously shown that modulating the PEG chain length 15 and changing the crosslinker-to-OPF carbon-carbon double bond ratio (DBR) 12 can affect the hydrogel mesh size and crosslinking density of the hydrogel. In addition, gelatin microparticles (GMP) encapsulated within the gel can provide digestible porogens to expedite OPF hydrogel composite degradation 11,21. However, possible interactions between various factors and their combined effects on key hydrogel properties remain to be elucidated through a systematic investigation.
Accordingly, the objective of this study is to investigate the effect of hydrogel construction parameters on the swelling ratio and degradation of OPF hydrogel composites and to evaluate the statistical interactions between various parameters in a full factorial design. Five hydrogel construction parameters are studied: PEG chain molecular weight (MW), DBR, type of crosslinker, crosslinking density of GMPs, and the incubation medium composition.
MATERIALS AND METHODS
Materials
For the synthesis of OPF, PEG of 10,000 g/moL and 35,000 g/moL nominal MW, calcium hydride, ammonium persulfate (APS), ethyl acetate, triethylamine, and N,N,N′,N′ –tetramethylethylenediamine (TEMED) were purchased from Sigma Aldrich (St. Louis, MO). Dichloromethane and ethyl ether were obtained from EMD Millipore Chemicals (Billerica, MA). Anhydrous methylene chloride was obtained by distillation after the reflux of dichloromethane for 2 hrs in the presence of calcium hydride. Fumaryl chloride was purchased from Fisher Scientific and distilled before use. Toluene was also purchased from Fisher Scientific (Pittsburgh, PA). All other solvents were of reagent grade and used as received.
For the fabrication of gelatin microparticles (GMP), acidic gelatin with an isoelectric point of 5.0 was obtained from Nitta Gelatin INC (Osaka, Japan). Tween 80, glutaraldehyde, Span 80, and glycine were purchased from Sigma Aldrich. For the fabrication of OPF/GMP composite hydrogels, poly(ethylene glycol)-diacrylate (PEGDA) (nominal MW of 3,400) was purchased from Laysan (Arab, AL). N,N′–methylene bisacrylamide (MB) was purchased from Sigma Aldrich.
OPF Synthesis and Characterization
OPF of two different formulations was synthesized using PEG of two different nominal MW (10,000 g/moL and 35,000 g/moL) according to established procedures 13,22 and will be referred to as OPF 10K and OPF 35K, respectively. Briefly, PEG (~50 g) was first dried via azeotropic distillation in 200 mL of toluene and then dissolved in ~300 mL of anhydrous methylene chloride for the synthesis reaction. After the dissolution of PEG, the reaction vessel was stirred in an ice bath and maintained under nitrogen, while distilled fumaryl chloride (0.9 mol fumaryl chloride/1 mol PEG) and triethylamine (2 mol triethylamine/1 mol fumaryl chloride) were added dropwise. Upon completing the addition of the reagents, the reaction flask was removed from the ice bath and stirred for an additional 48 hrs at room temperature (25 °C). OPF was then purified by rotary evaporation for the removal of methylene chloride, dissolved in ethyl acetate, vacuum-filtered to remove salt precipitates (from the reaction of chloride with triethylamine), and washed with ethyl ether. The PEG (PEG 10K: Mn of 8880±90 and Mw of 12110±40; PEG 35K: Mn of 40650±1140 and Mw of 54830±1560) as well as the resulting OPF were characterized via gel permeation chromatography where PEG standards were used to construct a calibration curve (OPF 10K: Mn of 24880±1680 and Mw of 152490±5800; OPF 35K: Mn of 44880±840 and Mw of 92120±8860). The OPF was stored at −20 °C until use.
Gelatin Microparticle Fabrication
GMPs were fabricated using acidic gelatin with an isoelectric point of 5.0 following previously established procedures 11. Briefly, 5 g of gelatin was dissolved in 45 mL of distilled, deionized water (ddH20) at 60 °C for ~20 minutes. The resulting aqueous gelatin solution was added dropwise to 250 mL of olive oil containing 0.5 wt.% Span 80 during mixing at 500 rpm for the formation of microspheres in the water/oil emulsion. The emulsion was then placed in an ice/water bath with continued stirring for 30 minutes, after which 100 mL of chilled acetone was added. After 1 hr, gelatin microspheres were collected from the emulsion via filtration and washing with acetone for the removal of residual olive oil. Collected gelatin microspheres were then crosslinked in 0.1 wt.% solution of Tween 80 in ddH20 containing either 10 mM (10 mM GMP) or 40 mM glutaraldehyde (40 mM GMP) for 20 hrs in an ice/water bath with stirring at 500 rpm. Glycine was then added to the reaction flask to a concentration of 25 mM and incubated for 1 hr to terminate the crosslinking reaction by blocking the residual aldehyde groups of unreacted glutaraldehyde. The crosslinked GMPs were vacuum-filtered, washed with ddH20, and lyophilized overnight. After lyophilization, GMPs with a diameter of 50–100 μm were selected by sieving and then stored at −20 °C until use.
Experimental Design
A full factorial design was utilized to investigate the effects of (1) PEG chain MW, (2) DBR, (3) crosslinker type, (4) crosslinking density of GMPs, and (5) incubation medium composition on the degradation characteristics and profiles of OPF composite hydrogels (Table 1). During fabrication of the OPF composite hydrogels, it was found that formulations containing OPF 35k and MB as a crosslinker could not be formed under similar conditions for other formulations. Thus, the full factorial design could not be completed as planned. Accordingly, two 24 fractional factorial studies were designed, as outlined in Table 2, to fully investigate the effects of all five aforementioned parameters. Each factor consisted of two levels, which were chosen based on previous in vitro and in vivo studies utilizing these hydrogels for various orthopedic applications 11,20,23–25. 10k and 35k PEG chain MWs were seen to facilitate the greatest chondrogenic differentiation among mesenchymal stem cells encapsulated in OPF-based hydrogels over smaller PEG chain MWs 15. Additionally, the DBR level of 60 was based on OPF:PEGDA weight ratios previously used 7,18,25 and the DBR level of 40 represents the threshold beyond which gels containing OPF 35k and PEGDA could not be formed. All formulations in Study 1 used PEGDA as the crosslinker and all formulations in Study 2 used 10k as the PEG MW for OPF hydrogel fabrication.
Table 1.
Two Levels of Five Parameters Tested in a Two-Level Full Factorial Design
| Parameters | Level 1 | Level 2 |
|---|---|---|
| PEG chain MW | 10K | 35K |
| DBR | 40 | 60 |
| Crosslinker Type | MB | PEGDA |
| GMP Crosslinking Extent | 10 mM | 40 mM |
| Incubation Medium Composition | 400 ng/mL collagenase in PBS | PBS |
Table 2.
Levels for the Factors Tested in Studies 1 and 2 Following a Two-Level Fractional Factorial Design
| Study 1 | GMP | Medium | DBR | PEG chain MW |
|---|---|---|---|---|
| Level 1 | 10 mM | Col-PBS | 40 | 10,000 |
| Level 2 | 40 mM | PBS | 60 | 35,000 |
|
| ||||
| Study 2 | GMP | Medium | DBR | Crosslinker Type |
|
| ||||
| Level 1 | 10 mM | Col-PBS | 40 | MB |
| Level 2 | 40 mM | PBS | 60 | PEGDA |
Composite Fabrication
OPF composite hydrogels were fabricated by encapsulating either 10 mM or 40 mM GMPs in OPF hydrogels according to previously established methods 26,27. Varying amounts of OPF macromer were combined with varying amounts of PEGDA or MB to give two different levels of DBR as seen in Supplementary Table 1. DBR was defined as the ratio of the total number of carbon-carbon double bonds in the crosslinker (PEGDA or MB) over the total number of carbon-carbon double bonds in OPF. The number of carbon-carbon double bonds in OPF was estimated by the following formula: of a fumarate unit (i.e., 72 g/mol) × x = Mn of OPF, where x is the average number of carbon-carbon double bonds in an OPF macromer. To outline the general fabrication procedure, the prescribed amounts of OPF and either PEGDA or MB were dissolved in 468 μL of phosphate-buffered saline (PBS) and thoroughly mixed with 22 mg of GMPs that were previously swollen overnight with 110 μL of PBS. Equal parts (48.6 μL) of the thermal radical initiators APS (0.3 M) and TEMED (0.3 M) were then added to initiate the crosslinking reaction. After mixing, the polymeric suspension was quickly injected into a cylindrical Teflon mold (6 mm in diameter and 2 mm in thickness) and further incubated at 37 °C for 10 minutes to achieve crosslinking.
OPF Swelling and Degradation Study
For the swelling and the degradation studies, OPF composite hydrogels were placed in either 3 mL of PBS or collagenase-conditioned PBS (Col-PBS; 400 ng collagenase 1A per mL PBS) in a 12-well plate and incubated at 37 °C for 28 days under shaking at 70 rpm. The culture medium was replaced every 3 days. At days 1, 4, 7, 14, 21, and 28, composite hydrogels (n=4) dedicated to each time point were retrieved to determine swelling ratio and % mass remaining using the following equations: and where Wi, Ws, and Wd are the weight of the dried composite immediately after fabrication prior to swelling, the weight of the composite after swelling at each time point, and the weight of the dried composite after swelling at each time point, respectively.
Statistical Analysis
Data for the measured properties were reported as mean ± standard deviation for samples (n=4) at each time point. Differences in the degradation characteristics between the various formulations were determined via one-way ANOVA followed by Tukey’s HSD post hoc analysis (p<0.05). Main and interaction effects were analyzed using a linear regression analysis methodology via the SAS JMP Pro 10 software according to previously established methods 28,29.
RESULTS
Swelling of OPF Composite Hydrogels
Equilibrium swelling ratios were first compared between groups with the same incubation medium composition as outlined in Tables 3 and 4. In standard PBS, all formulations containing 10 mM GMPs maintained generally constant swelling ratios within each respective group over 28 days (Supplementary Figure 1a). Composites from formulations 35kDA40:10 and 10kMB40:10 both exhibited the highest swelling ratios up until day 28. Composites fabricated with OPF 10K macromers and PEGDA as the crosslinker (10kDA60:10 and 10kDA40:10) showed the lowest mean swelling ratios over time. Among formulations containing 40 mM GMPs (Supplementary Figure 1b), 35kDA40:40 and 10kMB40:40 had the highest swelling ratios at the measured time points after day 1. Despite having similar mean swelling ratios, which refer to overall values over time, of 17.2±0.4 for 35kDA40:40 and 17.9±0.6 for 10kMB40:40, composites from 10kMB40:40 swelled more than those from 35kDA40:40 at each time point measured after day 14. The mean swelling ratios of formulations 35kDA60:40 (13.6±0.3) and 10kMB60:40 (14.1±0.4), while lower than those of their lower DBR counterparts, were greater than the mean swelling ratios of 10kDA60:40 (5.6±0.2) and 10kDA40:40 (6.0±0.2) as shown in Table 3.
Table 3.
Mean Degradation Characteristics of Composite Formulations in Standard PBS Over 28 Days. Mean values for the swelling ratio and the % mass remaining were calculated by averaging the measured values for each formulation over 28 days. For each property, values not connected by the same letters are significantly different.
| Formulation | PEG chain MW | GMP Crosslinking Extent | DBR | Crosslinker Type | Mean Swelling Ratio | Mean Mass Remaining (%) |
|---|---|---|---|---|---|---|
| 35kDA60:10 | 35,000 | 10 mM | 60 | PEGDA | 13.4±0.2b | 55.9±1.0c |
| 35kDA40:10 | 35,000 | 10 mM | 40 | PEGDA | 17.3±0.8a | 47.5±2.4d |
| 10kDA60:10 | 10,000 | 10 mM | 60 | PEGDA | 5.4±0.1c | 89.3±1.3a |
| 10kDA40:10 | 10,000 | 10 mM | 40 | PEGDA | 5.9±0.1c | 82.8±1.4a |
| 10kMB60:10 | 10,000 | 10 mM | 60 | MB | 13.2±0.4b | 67.3±2.0b |
| 10kMB40:10 | 10,000 | 10 mM | 40 | MB | 16.6±0.5a | 67.6±2.3b |
| 35kDA60:40 | 35,000 | 40 mM | 60 | PEGDA | 13.6±0.3b | 55.7±1.5c, d |
| 35kDA40:40 | 35,000 | 40 mM | 40 | PEGDA | 17.2±0.4a | 56.2±1.6c |
| 10kDA60:40 | 10,000 | 40 mM | 60 | PEGDA | 5.6±0.2c | 89.6±1.3a |
| 10kDA40:40 | 10,000 | 40 mM | 40 | PEGDA | 6.0±0.2c | 86.2±1.5a |
| 10kMB60:40 | 10,000 | 40 mM | 60 | MB | 14.1±0.4b | 70.2±2.1b |
| 10kMB40:40 | 10,000 | 40 mM | 40 | MB | 17.9±0.6a | 63.6±2.3b, c |
Table 4.
Mean Degradation Characteristics of Composite Formulations in Col-PBS Over 28 Days. Mean values for the swelling ratio and the % mass remaining were calculated by averaging the measured values for each formulation over 28 days. For each property, values not connected by the same letters are significantly different.
| Formulation | PEG chain MW | GMP Crosslinking Extent | DBR | Crosslinker Type | Mean Swelling Ratio | Mean Mass Remaining (%) |
|---|---|---|---|---|---|---|
| 35kDA60:10 | 35,000 | 10 mM | 60 | PEGDA | 18.1±0.8b | 43.1±2.3f, g |
| 35kDA40:10 | 35,000 | 10 mM | 40 | PEGDA | 26.7±1.5a | 37.4±2.7g |
| 10kDA60:10 | 10,000 | 10 mM | 60 | PEGDA | 6.4±0.2d | 82.1±1.4a |
| 10kDA40:10 | 10,000 | 10 mM | 40 | PEGDA | 6.9±0.2d | 75.9±1.4a, b |
| 10kMB60:10 | 10,000 | 10 mM | 60 | MB | 13.3±0.3c | 64.5±2.1c, d |
| 10kMB40:10 | 10,000 | 10 mM | 40 | MB | 19.2±0.6b | 56.5±2.2d, e |
| 35kDA60:40 | 35,000 | 40 mM | 60 | PEGDA | 13.9±0.7c | 47.3±2.4f |
| 35kDA40:40 | 35,000 | 40 mM | 40 | PEGDA | 19.6±0.5b | 50.4±1.5e, f |
| 10kDA60:40 | 10,000 | 40 mM | 60 | PEGDA | 5.9±0.2d | 84.3±1.2a |
| 10kDA40:40 | 10,000 | 40 mM | 40 | PEGDA | 6.6±0.3d | 79.4±1.4a |
| 10kMB60:40 | 10,000 | 40 mM | 60 | MB | 14.2±0.4c | 68.2±1.9b, c |
| 10kMB40:40 | 10,000 | 40 mM | 40 | MB | 18.6±0.7b | 61.3±1.8c, d |
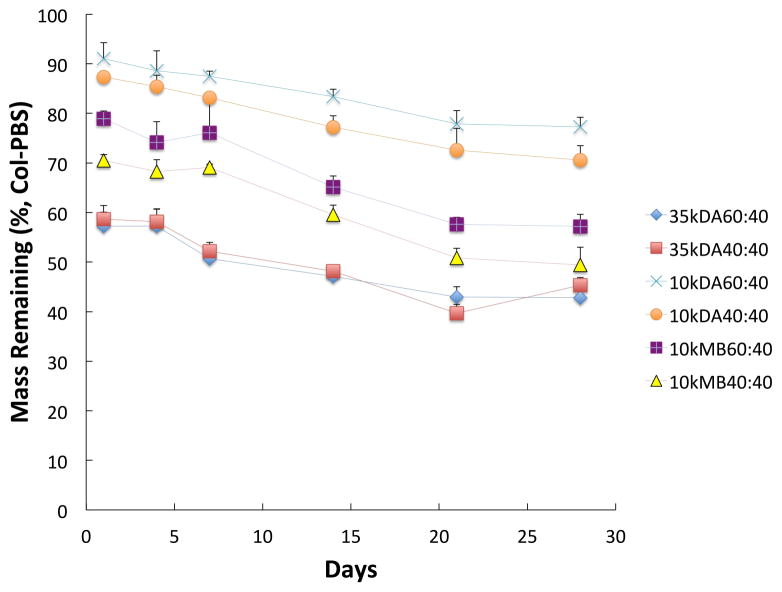
In Figure 1a, formulation 35kDA40:10, which maintained the highest mean swelling ratio of 26.7±1.5 in col-PBS over 28 days, exhibited greater increases in swelling following day 4 in the presence of collagenase as compared to in PBS alone. As outlined in Table 3, formulations 35kDA60:10 and 10kMB40:10 displayed the next highest swelling capacities with mean swelling ratios of 18.1±0.8 and 19.2±0.6, respectively. Indeed, formulations 10kDA60:10 and 10kDA40:10, both of which had similar respective mean swelling ratios of 6.4±0.2 and 6.9±0.2, displayed the lowest swelling ratios at all time points when compared to other formulations. For the formulations containing 40 mM GMPs (Figure 1b), the same differences observed between swelling profiles of the respective experimental groups in standard PBS were also observed in col-PBS.
Figure 1.
Swelling ratio profiles of (a) 10 mM GMP or (b) 40 mM GMP containing OPF composite hydrogels in col-PBS over 28 days (n=4). The mass remaining (%) profiles of corresponding (c) 10 mM GMP or (d) 40 mM GMP formulations in col-PBS over 28 days are also shown (n=4). Error bars represent the standard deviation.
Degradation of OPF Composite Hydrogels
In congruence with the hydrogel swelling ratios, the % mass remaining profiles of the various experimental groups were first compared within the same incubation medium composition in order to evaluate differences between formulations. In standard PBS, formulations with OPF 35K (35kDA60:10 and 35kDA40:10) had the lowest mean % mass remaining, which refer to overall values over time, and thereby the greatest overall polymer loss over 28 days (Supplementary Figure 1c). As shown in Table 4, 10kDA60:10 (89.3±1.3 %) and 10kDA40:10 (82.8±1.4 %) maintained the highest mean % mass remaining amongst formulations containing 10 mM GMPs. For the 40 mM GMP containing groups, similar differences in mean % mass remaining between corresponding formulations were also observed (Supplementary Figure 1d). For example, all formulations with OPF 10K and PEGDA displayed the least polymer loss overall regardless of the GMP crosslinking extent (Table 4). In standard PBS, the amount of polymer loss for all formulations (containing 10 mM GMPs or 40 mM GMPs) stabilized after day 7.
In col-PBS, the overall polymer loss amid the 10 mM GMP composite formulations was highest amongst formulations containing OPF 35K macromers. For instance, 35kDA60:10 and 35kDA40:10 had mean mass remaining percentages of 43.1±2.3 % and 37.4±2.7 %, respectively, which were at least 12% lower than other formulations (Table 4). With the exception of the formulations 10kDA60:10 and 10kDA40:10, which had the lowest amounts of overall polymer loss, all groups exhibited increasing polymer loss under enzymatic conditions until stabilization after day 14 (Figure 1c). Although the differences in mean % mass remaining between 10 mM GMP containing formulations were also observed between the 40 mM GMP containing groups, the latter maintained higher mean mass remaining percentages than their 10 mM GMP containing counterparts (Figure 1d).
In order to formally investigate the main effects of the construction parameters outlined in Table 1 and their various interactions on the swelling and degradation of OPF composite hydrogels, two 24 fractional factorial analyses were performed as detailed in Table 2. In Figures 2, 3, 4, and 5, the magnitude of the changes associated with the main effects and interactions are shown with standard error bars relative to the zero line, which is representative of the overall population mean at each effect. Main effects and interactions were considered significant (p<0.05) if their standard error did not cross the zero line.
Figure 2.
The main effects of PEG chain MW, GMP crosslinking extent, DBR, and incubation medium composition on the (a) swelling and (b) % mass remaining of OPF composite hydrogels in Study 1. A positive value indicates an increasing effect caused by a change in the corresponding parameter from level 1 to level 2. Error bars represent the standard error.
Figure 3.
Interaction effects between PEG chain MW, DBR, and the incubation medium composition on the mean swelling ratio of OPF composite hydrogels in Study 1. A negative slope line indicates that the swelling was decreased when the DBR was increased from 40 to 60.
Figure 4.
The main effects of crosslinker type, GMP crosslinking extent, DBR, and the incubation medium composition on the (a) swelling and (b) % mass remaining of OPF composite hydrogels in Study 2. A positive value indicates an increasing effect caused by a change in the corresponding parameter from level 1 to level 2. Error bars represent the standard error.
Figure 5.
Interaction effects between the crosslinker type and the DBR on the mean swelling ratio of OPF composite hydrogels in Study 2.
Fractional Factorial Analysis of Study 1
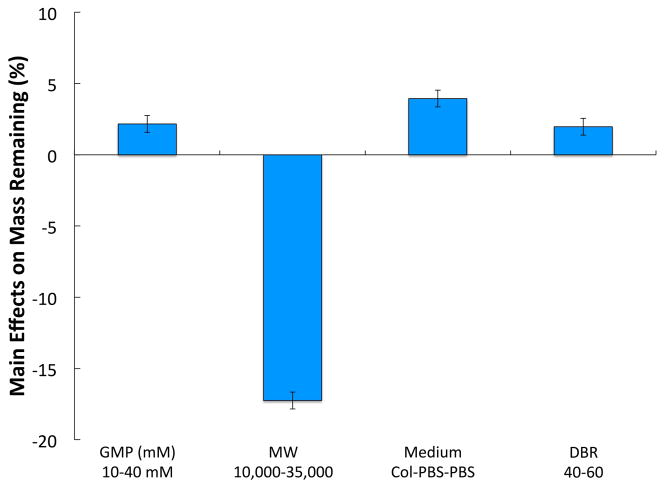
Study 1 investigated four main factors: GMP crosslinking extent, PEG chain MW, incubation medium composition, and DBR, while keeping the crosslinker type (PEGDA) constant. Main effects analysis of the OPF composite formulations over 28 days indicated that all four factors had an effect on the swelling ratio (Figure 2a). When OPF composites were fabricated with GMPs of the higher crosslinking density (40 mM) and the higher DBR (60), the mean swelling ratio decreased by an average of 0.7±0.3 and 1.5±0.3, respectively. Similarly, changing the incubation medium composition from col-PBS to standard PBS caused a decrease in the mean swelling ratio by an average of 1.2±0.3. An increase in the PEG chain MW from 10K to 35K resulted in a significant increase in the mean swelling ratio by an average of 5.7±0.3. Through the analysis of the main effects at each time point, it was observed that the OPF macromer MW and the DBR significantly affected the swelling ratio at all times (Supplementary Figure 2a). Although the incubation medium did not have an immediate effect on the swelling ratio, the presence of collagenase caused an increase in the swelling from day 4 onward. While increasing the GMP crosslinking extent (10 mM to 40 mM) also did not have an immediate effect on the swelling ratio, it significantly decreased the swelling ratio from day 4 until day 21.
The mean % mass remaining was also affected by all four factors as indicated by main effects analysis (Fig 3b). Particularly, an increase in the GMP crosslinking extent and the DBR resulted in an increase in the mean % mass remaining by an average of 2.2±0.6 % and 2.0±0.6 %, respectively. Removal of the enzymatic incubation condition also caused an increase in the mean % mass remaining by an average of 4.0±0.6 %. However, an increase in the PEG chain MW yielded a significant decrease in the mean % mass remaining by an average of 17.2±0.6 %. An analysis of the main effects at each time point showed that PEG chain MW and incubation medium composition had an effect on mean % mass remaining at all times (Supplementary Figure 2b). GMP crosslinking extent and DBR also had an effect on the mean % mass remaining at all measured time points except for day 1. An evaluation of the interactions between factors on the mean swelling ratio revealed an interdependence of factors upon one another in the form of several 2-factor and 3-factor interactions. In particular, significant 2-factor interactions (where A*B denotes a cross effect between factor A and factor B) included GMP*MW, GMP*Medium, MW*Medium, MW*DBR, and Medium*DBR, while 3-factor interactions included GMP*MW*Medium and MW*Medium*DBR (data not shown). For the mean % mass remaining, significant interactions only included GMP*MW and GMP*DBR. The cross effects between PEG chain MW, DBR, and the incubation medium composition, which had the largest impact on swelling, are further illustrated in Figure 3.
Fractional Factorial Analysis of Study 2
Main effects analysis of the formulations from Study 2 over 28 days, which corroborated the effects of incubation medium composition and DBR on swelling, also demonstrated that the crosslinker type significantly impacted the mean swelling ratio (Figure 4a). Employing PEGDA as the crosslinker instead of MB caused a significant decrease in the mean swelling ratio by an average of 4.9±0.2. Amongst formulations incorporating only OPF 10K macromers, increasing the DBR resulted in a decrease in the mean swelling ratio by an average of 1.2±0.2. However, GMP crosslinking extent was not a significant factor affecting the mean swelling ratio of these formulations. Changing the GMP crosslinking extent, the incubation medium composition, and the DBR from the respective level 1 to level 2 values raised the mean % mass remaining by an average of 1.0±0.9 %, 2.8±0.9 %, and 2.7±0.9 %, respectively (Figure 4b). Most notably, changing the crosslinker type from MB to PEGDA increased the mean % mass remaining by an average of 9.4±0.9 %. An analysis of the main effects on mean swelling ratio over time showed that the crosslinker type and the DBR had an effect at all times, while the GMP crosslinking extent and the incubation medium composition both had an effect at day 28 (Supplementary Figure 3a). Similar to the main effects on the mean swelling ratio, the crosslinker type and DBR significantly affected the mean % mass remaining at all measured time points (Supplementary Figure 3b). However, GMP crosslinking extent and the incubation medium composition only had effects on the mean % mass remaining at select time points and did not display any noticeable effects pattern. From the evaluation of the interactions present in Study 2, notable cross effects between factors included only three 2-factor interactions that affected the mean swelling ratio: GMP*Crosslinker, Crosslinker*DBR, and Medium*DBR (data not shown). The interaction effects between crosslinker and DBR, which proved to have the greatest impact on swelling, are further described in Figure 5.
DISCUSSION
This study utilized a full factorial design with fractional factorial analyses to investigate the effects of PEG chain MW, DBR, crosslinker type, GMP crosslinking extent, and the incubation medium composition on the swelling and degradation characteristics of OPF composite hydrogels. With each factor comprising two-levels, it was hypothesized that the swelling and degradation characteristics of OPF composite hydrogels could be precisely controlled by individually or collectively modulating the levels of the aforementioned parameters. Furthermore, it was envisioned that the use of a factorial design would allow for the evaluation of how each individual factor and their various interactions with each other would affect the swelling and degradation of these composites. Indeed, the fractional factorial analyses of the main effects showed that the mean swelling ratio and the mean % mass remaining of OPF composite hydrogels were significantly impacted by every parameter investigated. Additionally, an analysis of the interactions between parameters yielded important insights to guide the tuning of the swelling and degradation properties of OPF-based hydrogels.
Previous studies have briefly evaluated the individual effects of PEG chain MW 15, GMP crosslinking extent 11, and DBR 20 on the degradation of OPF composite hydrogels. Additionally, previous research conducted by our laboratory has investigated the effect of PEG chain MW on the swelling and mechanical properties of OPF-based hydrogels and found that with an increase in PEG molecular weight and hence, the hydrogel swelling, the tensile strength of the OPF-based hydrogels decreased 16. However, the combined effects of these factors, along with the crosslinker type and the incubation medium composition, as well as their various interactions on swelling and degradation had not been investigated. While the mechanical properties of hydrogel scaffolds are important, particularly for dynamic tissues, the main focus of the current study is on how different hydrogel construction parameters affect swelling and degradation for applications in cell and drug delivery. Hence, a factorial experiment combining all of these factors was designed. Out of the composite formulations that were generated from this factorial design, all formulations containing OPF 35K macromers and the crosslinker MB failed to yield any viable hydrogels due to a low level of crosslinking. This could be attributed to the limited mobility of the second double bond of the MB crosslinking agent after the first one has reacted during the crosslinking reaction with the larger sized OPF 35K macromers. However, crosslinking with PEG-DA is possible because the mobility of the second double bond is greater due to the presence of a PEG spacer, which is not the case for MB. This could also be due to the reduced number of fumarate groups (which carry the unsaturated carbon-carbon double bonds) per OPF macromer for OPF 35K when compared to OPF 10K as a result of the steric hindrance of higher MW PEG molecules inhibiting the addition of fumarate groups to the ends of the PEG molecules during synthesis 22. Accordingly, two 24 fractional factorial analyses were performed with the remaining composite formulations in order to fully evaluate the main and interaction effects of all construction parameters on swelling and degradation.
First, the mean swelling ratios between groups within the same incubation medium composition were compared. Although composites fabricated with OPF 35K macromers were expected to achieve the highest swelling ratios, this was not the case as formulations 35kDA40:10, 10kMB40:10, 35kDA40:40, and 10kMB40:40 exhibited similarly high mean swelling ratios over 28 days. Additionally, 10kMB60:10, 10kMB60:40, 10kMB40:10, and 10kMB40:40 achieved swelling ratios similar to or greater than that of 35kDA60:10 or 35kDA60:40. From the swelling data alone, this result could be mainly due to the crosslinker type. Assuming that all OPF macromers were incorporated during crosslinking to form a polymer network, the theoretical swelling capacity of the resulting hydrogel would be directly related to the size of the PEG chain component, as larger PEG components equal greater swelling30. Indeed, 35kDA60:10/35kDA60:40 and 35kDA40:10/35kDA40:40 exhibited greater swelling ratios than 10kDA60:10/10kDA60:40 and 10kDA40:10/10kDA40:40 at all measured times. However, a comparison of 10kDA60:10/10kDA60:40 and 10kDA40:10/10kDA40:40 with 10kMB60:10/10kMB60:40 and 10kMB40:10/10kMB40:40 indicated that formulations containing MB swelled more than the corresponding PEGDA-containing formulations despite MB being the smaller crosslinker (154.2 nominal MW compared to 3,400 nominal MW for PEGDA). This phenomenon could potentially be explained by the relatively higher reactivity of acrylates (PEGDA) or amides (MB) when compared to the carbon-carbon double bonds of the fumarate groups along the OPF backbone 16,31,32. As a result, the acrylates from the PEGDA crosslinkers may have more readily reacted with each other than with the fumarate double bonds, leading to the formation of regional PEGDA networks within the overall OPF macromolecular structure. This would act to not only lower the swelling capacity of the OPF composites since the crosslinker PEGDA has a lower nominal MW compared to that of the OPF macromers, but also to generally result in higher mass remaining percentages. Indeed, main effects of increasing DBR on the swelling ratio and the % mass remaining as shown in Figure 2 for Study 1 further indicate that the crosslinking density of such hydrogels is directly correlated with the amount of acrylates present from the PEGDA crosslinker. Although similar reactions may have occurred amongst the amides of the MB crosslinkers, the smaller size of MB would have less of an effect on the overall OPF network33 as suggested by the lack of differences in mean mass remaining percentages between 10kMB groups (incorporating GMPs of similar crosslinking extent) with changes in DBR (Tables 3 and 4).
While the size difference between MB and PEGDA may act to confound their differences in reactivity as crosslinkers for OPF hydrogel systems, the selection of MB and PEGDA was informed by previous studies from our laboratory and others investigating the efficacy of materials utilizing these common crosslinkers for various in vitro and in vivo biomedical applications 17,18,20,21,23,25. In particular, our laboratory has previously implanted OPF hydrogels crosslinked with MB as controlled growth factor delivery vehicles for osteochondral tissue repair 17,21. Although such acellular composites conferred some therapeutic effects in vivo, the inclusion of stem cells to improve results may have been difficult given reports of potential cytotoxicity with using MB 34,35. Hence, PEGDA was employed as the crosslinker for the development of cell-laden OPF constructs. However, when these constructs were used to deliver cells to an osteochondral defect site, fragments of non-degraded hydrogel still present within the defect after 12 weeks obstructed tissue repair 18. Given that OPF hydrogels crosslinked with MB displayed near complete degradation in vivo by this time point 36, the presence of such non-degraded hydrogel fragments was attributed to the use of PEGDA (via the formation of regional PEGDA networks). As a result, both MB-crosslinked and PEGDA-crosslinked OPF systems were compared in the present study in order to guide and optimize future strategies for combining growth factor and cell delivery for in vivo tissue regeneration.
The absence of differences between corresponding formulations carrying either 10 mM GMPs or 40 mM GMPs in standard PBS was expected given the lack of enzyme activity to degrade GMPs in composite hydrogels. In the presence of collagenase, which mimics the enzymatic conditions of an in vivo environment 26, various formulations including 35kDA60:10, 35kDA40:10, and 35kDA40:40 exhibited an increase in swelling capacity when compared to their non-enzymatically treated counterparts. Indeed, encapsulated GMPs swell and undergo enzymatic digestion over time, leading to the disruption of the OPF network and the introduction of large pores into the bulk hydrogel that together enhance hydrogel swelling 26. Additionally, higher swelling ratios were observed in 10 mM GMP containing formulations in the collagenase medium condition when compared with corresponding 40 mM GMP containing formulations, since GMPs of a higher crosslinking extent can better resist enzymatic attack by collagenase. Previous results have shown that the presence of collagenase failed to elicit enhanced swelling of OPF composites fabricated with OPF 10K macromers that only contain 40 mM GMPs 26. However, in the current study, the mean swelling ratio of 35kDA40:40 in col-PBS is significantly greater than its corresponding value in standard PBS, suggesting an interaction between size of the OPF macromer, the GMP crosslinking extent, and the enzyme.
Differences in the mass remaining percentages between formulations at day 1 are reflective of the initial % sol fractions of each formulation. The % sol fraction, which is defined as 100 % minus the % mass remaining at day 1, reflects the amount of polymer that was not initially incorporated into the crosslinked network during composite fabrication. Although formulations containing OPF 10K macromers and MB achieved similar swelling ratios with formulations containing OPF 35K macromers and PEGDA, the latter displayed higher sol fractions and lower subsequent polymer % mass remaining when compared to the former. Indeed, given the increasing difficulty of attaching fumarate groups to the ends of larger PEG molecules during OPF synthesis 13,22, hydrogels fabricated from OPF macromers with larger PEG chains would contain many unreacted PEG molecules entangled within the greater hydrogel network. Aside from the hydrolytic degradation of a less densely crosslinked network, the release of these PEG molecules during swelling of the hydrogel network and hydrolysis of degradable ester bonds could be the reason why formulations with OPF 35K macromers exhibited greater polymer loss when compared to their 10K counterparts.
For Study 1, the most significant factor affecting the mean swelling ratio and % mass remaining was the PEG chain MW (Figure 2). Increasing the PEG chain MW caused a 2-to-3-fold increase in the swelling capacity of the composite hydrogels. This was expected, as larger PEG chains would allow for the formation of polymer networks with larger theoretical mesh sizes. While not as influential as PEG chain MW, the DBR, GMP crosslinking extent and the incubation medium composition also significantly impacted the swelling properties of OPF composite hydrogels. Increasing the GMP crosslinking extent decreased the overall swelling capacity of OPF composite hydrogels, most likely due to the diminished swelling of more crosslinked GMPs 37. An increase in the DBR, which correlates to an increased hydrogel crosslinking density, expectedly led to a decrease in hydrogel swelling as well.
Interestingly, an analysis of the factor cross effects on swelling revealed that the effects of DBR were more prominent for hydrogels formed from OPF 35K macromers. In standard PBS, increasing the DBR from 40 to 60 decreased the mean swelling ratio of OPF 35K composites by an average of 5.3 while the same increase in DBR for OPF 10K composites decreased the mean swelling ratio by 0.5 (Figure 3). The interaction between these two factors was even more apparent in enzymatic conditions. This is likely a consequence of the difference in available fumarate groups for gelation between OPF 10K and OPF 35K macromers. Compared to OPF 35K macromers, OPF 10K macromers have more fumarate groups per macromer and thereby an increased probability of crosslinking during hydrogel formation. In addition, with less fumarate groups per macromer, gelation of OPF 35K hydrogels may be more dependent on the proportion of crosslinkers present in the reaction mixture 33, which aligns with the Flory-Stockmayer theory for gelation. The presence of collagenase predictably enhanced the interdependence of these two factors. Other interactions involving the enzyme could also be explained by this same reason.
The Flory-Stockmayer theory would also explain the effects of DBR on the mass loss. Increased branching during the gelation of OPF composites as a result of increased crosslinker present in the reaction mixture yielded more crosslinked networks with decreased overall hydrogel swelling capacity. The decrease in mass loss with increasing DBR as seen in both fractional factorial studies further confirms this hypothesis. As mentioned previously, the main effect of the PEG chain MW on the mean % mass remaining could be explained by the faster degradation of OPF 35K composites (due to the hydrolysis of a more loosely crosslinked network) and/or the release of entangled unreacted PEG molecules during swelling. The effect of GMP crosslinking extent could be explained by two reasons. First, the decreased swelling of more crosslinked GMPs caused less hydrogel network disruption and as a result, slowed the degradation of the composites. Second, more crosslinked GMPs provided greater diffusional barriers against the release of OPF fragments during degradation, thereby slowing overall polymer loss. Despite their individual effects, the cross effects of PEG chain MW and DBR did not significantly influence the mean % mass remaining and, hence the polymer loss. While there were several interactions between factors, the cross effects on the mean % mass remaining were mainly peripheral.
For Study 2, the fractional factorial design was employed to primarily evaluate the impact of crosslinker type and its interaction with other construction parameters on the swelling and degradation of OPF composite hydrogels. As a result, the experimental groups involved in this analysis only include composites fabricated using OPF 10K macromers. Among these formulations, the most significant factor affecting the mean swelling ratio and % mass remaining was the type of crosslinker used for gelation. Intriguingly, the utilization of the larger crosslinker, PEGDA, instead of the smaller crosslinker, MB, led to a 20% to 50% decrease in composite swelling capacity and a 30% to 60% decrease in composite degradation. As mentioned previously, the use of PEGDA as the crosslinker, which has a lower MW when compared to the OPF macromer, possibly resulted in the incorporation of regional PEGDA networks within the overall hydrogel network that act to decrease the overall hydrogel mesh size. With a decreased average mesh size, OPF composites incorporating PEGDA would have decreased mean swelling ratios. Indeed, the cross effects between crosslinker type and the DBR corroborates this explanation. The swelling properties of OPF composite hydrogels fabricated with PEGDA were less sensitive to changes in the DBR when compared to composites fabricated using MB. When coupled with the limited swelling extent of the former, these results suggest that regional PEGDA networks could potentially make up a portion of the overall polymer network of OPF composite hydrogels. With regard to the other factors, the observed effects of GMP crosslinking extent, DBR, and incubation medium composition on the swelling and degradation of composites in Study 2 mainly corroborated those observed in Study 1.
The formulations evaluated in this study were based on various OPF systems that were previously utilized as implants for in vivo tissue repair 17,18,27,36. As previously mentioned, OPF systems incorporating MB were mainly used for growth factor delivery, while OPF systems incorporating PEGDA were employed for the development of cell-laden constructs. In cases where the beneficial effects of both growth factors and stem cells need to be combined, the selection of the appropriate hydrogel formulation can be guided by the findings of the current study. For instance, several of the formulations incorporating PEGDA shared similar swelling and degradation properties with some formulations incorporating MB, which may allow for the development of cell/growth factor-laden hydrogel constructs that can swell and degrade as desired in vivo. Overall, the present study demonstrated that the PEG chain MW and the crosslinker type had the greatest impact on the swelling and degradation of OPF hydrogels. Additionally, the significant interaction effects between these two factors and the DBR showcased the high tunability that can be achieved with such hydrogel systems, and have broad implications informing the design of similar PEG-based hydrogel systems for cell encapsulation and growth factor delivery applications.
CONCLUSIONS
In conclusion, the main and cross effects of (1) PEG chain MW, (2) DBR, (3) crosslinker type, (4) GMP crosslinking extent, and (5) incubation medium composition on the swelling and degradation characteristics of OPF composite hydrogels were investigated. While the mean swelling ratio and mean % mass remaining of these hydrogels were impacted by every factor, modifying the PEG chain MW and the crosslinker type produced the largest effects. In particular, increasing the PEG chain MW from 10K to 35K increased both swelling and polymer loss whereas using PEGDA instead of MB resulted in the opposite effect. Moreover, it was found that interactions involving the DBR had the greatest cross effects on swelling properties. The OPF hydrogels fabricated with PEGDA had lower increases in swelling when the DBR was reduced when compared to hydrogels fabricated with MB. Additionally, a cross effect was seen with 10mM GMP containing formulations in Col-PBS exhibiting greater swelling than 40mM GMP containing formulations in the same condition. Together, these results indicate that the swelling and degradation properties of OPF hydrogels can be readily controlled via the collective modulation of key construction parameters and underscore the versatility of these hydrogels as well as other PEG-based hydrogels for drug and cell delivery in tissue engineering applications.
Supplementary Material
Supplementary Figure 1: Swelling ratio profiles of (a) 10 mM GMP or (b) 40 mM GMP containing OPF composite hydrogels in standard PBS over 28 days (n=4). The mass remaining (%) profiles of corresponding (c) 10 mM GMP or (d) 40 mM GMP formulations in standard PBS over 28 days is also shown (n=4). Error bars represent the standard deviation.
Supplementary Figure 2: The main effects of PEG chain MW, GMP crosslinking extent, DBR, and incubation medium composition on the (a) swelling and (b) % mass remaining of OPF composite hydrogels in Study 1 at each time point over 28 days. Error bars represent the standard error.
Supplementary Figure 3: The main effects of crosslinker type, GMP crosslinking extent, DBR, and incubation medium composition on the (a) swelling and (b) % mass remaining of OPF composite hydrogels in Study 2 at each time point over 28 days. Error bars represent the standard error.
Acknowledgments
This work was supported by the National Institutes of Health (R01-AR048756).
Abbreviations
- APS
Ammonium persulfate
- Col-PBS
PBS containing 400 ng/mL collagenase 1A
- DBR
Double bond ratio
- GMP
Gelatin microparticles
- MB
N,N′-methylene bisacrylmide
- MW
Molecular weight
- OPF
Oligo(poly(ethylene glycol) fumarate)
- PEG
Poly(ethylene glycol)
- PEGDA
PEG-diacrylate
- TEMED
N,N,N′,N′-tetramethylethylenediamine
Contributor Information
Johnny Lam, Email: johnny.lam@rice.edu.
Kyobum Kim, Email: sallidal@gmail.com.
Steven Lu, Email: steven.lu@rice.edu.
Yasuhiko Tabata, Email: yasuhiko@frontier.kyoto-u.ac.jp.
David W. Scott, Email: scottdw@rice.edu.
Antonios G. Mikos, Email: mikos@rice.edu.
F. Kurtis Kasper, Email: kasper@rice.edu.
References
- 1.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Advanced Materials. 2006;18(11):1345–1360. [Google Scholar]
- 2.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 3.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–79. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 4.Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev. 2011;17(4):281–99. doi: 10.1089/ten.teb.2011.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandl F, Kastner F, Gschwind RM, Blunk T, Tessmar J, Gopferich A. Hydrogel-based drug delivery systems: comparison of drug diffusivity and release kinetics. J Control Release. 2010;142(2):221–8. doi: 10.1016/j.jconrel.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59(1):63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 7.Park H, Temenoff JS, Tabata Y, Caplan AI, Raphael RM, Jansen JA, Mikos AG. Effect of dual growth factor delivery on chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in injectable hydrogel composites. J Biomed Mater Res A. 2009;88(4):889–97. doi: 10.1002/jbm.a.31948. [DOI] [PubMed] [Google Scholar]
- 8.Benoit DS, Durney AR, Anseth KS. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006;12(6):1663–73. doi: 10.1089/ten.2006.12.1663. [DOI] [PubMed] [Google Scholar]
- 9.Bryant SJ, Anseth KS. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J Biomed Mater Res A. 2003;64(1):70–9. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 10.Patterson J, Siew R, Herring SW, Lin AS, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31(26):6772–81. doi: 10.1016/j.biomaterials.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91(3):299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 12.Jo S, Shin H, Mikos AG. Modification of oligo(poly(ethylene glycol) fumarate) macromer with a GRGD peptide for the preparation of functionalized polymer networks. Biomacromolecules. 2001;2(1):255–61. doi: 10.1021/bm000107e. [DOI] [PubMed] [Google Scholar]
- 13.Jo S, Shin H, Shung AK, Fisher JP, Mikos AG. Synthesis and characterization of oligo(poly(ethylene glycol) fumarate) macromer. Macromolecules. 2001;34(9):2839–2844. [Google Scholar]
- 14.Kinard LA, Kasper FK, Mikos AG. Synthesis of oligo(poly(ethylene glycol) fumarate) Nature Protocols. 2012;7(6):1219–1227. doi: 10.1038/nprot.2012.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park H, Guo X, Temenoff JS, Tabata Y, Caplan AI, Kasper FK, Mikos AG. Effect of swelling ratio of injectable hydrogel composites on chondrogenic differentiation of encapsulated rabbit marrow mesenchymal stem cells in vitro. Biomacromolecules. 2009;10(3):541–6. doi: 10.1021/bm801197m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Temenoff JS, Athanasiou KA, LeBaron RG, Mikos AG. Effect of poly(ethylene glycol) molecular weight on tensile and swelling properties of oligo(poly(ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J Biomed Mater Res. 2002;59(3):429–37. doi: 10.1002/jbm.1259. [DOI] [PubMed] [Google Scholar]
- 17.Holland TA, Bodde EW, Cuijpers VM, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage. 2007;15(2):187–97. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Guo X, Park H, Young S, Kretlow JD, van den Beucken JJ, Baggett LS, Tabata Y, Kasper FK, Mikos AG, Jansen JA. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6(1):39–47. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim CT, Ren X, Afizah MH, Tarigan-Panjaitan S, Yang Z, Wu Y, Chian KS, Mikos AG, Hui JH. Repair of Osteochondral Defects with Rehydrated Freeze-Dried Oligo[Poly(Ethylene Glycol) Fumarate] Hydrogels Seeded with Bone Marrow Mesenchymal Stem Cells in a Porcine Model. Tissue Eng Part A. 2013 doi: 10.1089/ten.TEA.2012.0621. [DOI] [PubMed] [Google Scholar]
- 20.Shin H, Quinten Ruhe P, Mikos AG, Jansen JA. In vivo bone and soft tissue response to injectable, biodegradable oligo(poly(ethylene glycol) fumarate) hydrogels. Biomaterials. 2003;24(19):3201–11. doi: 10.1016/s0142-9612(03)00168-6. [DOI] [PubMed] [Google Scholar]
- 21.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101(1–3):111–25. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Kinard LA, Kasper FK, Mikos AG. Synthesis of oligo(poly(ethylene glycol) fumarate) Nat Protoc. 2012;7(6):1219–27. doi: 10.1038/nprot.2012.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temenoff JS, Park H, Jabbari E, Sheffield TL, LeBaron RG, Ambrose CG, Mikos AG. In vitro osteogenic differentiation of marrow stromal cells encapsulated in biodegradable hydrogels. J Biomed Mater Res A. 2004;70(2):235–44. doi: 10.1002/jbm.a.30064. [DOI] [PubMed] [Google Scholar]
- 24.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28(21):3217–27. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo X, Park H, Liu G, Liu W, Cao Y, Tabata Y, Kasper FK, Mikos AG. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials. 2009;30(14):2741–52. doi: 10.1016/j.biomaterials.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland TA, Tessmar JK, Tabata Y, Mikos AG. Transforming growth factor-beta 1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Release. 2004;94(1):101–14. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Kim K, Lam J, Lu S, Spicer PP, Lueckgen A, Tabata Y, Wong ME, Jansen JA, Mikos AG, Kasper FK. Osteochondral tissue regeneration using a bilayered composite hydrogel with modulating dual growth factor release kinetics in a rabbit model. J Control Release. 2013;168(2):166–78. doi: 10.1016/j.jconrel.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henslee AM, Gwak DH, Mikos AG, Kasper FK. Development of a biodegradable bone cement for craniofacial applications. J Biomed Mater Res A. 2012;100(9):2252–9. doi: 10.1002/jbm.a.34157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekenseair AK, Boere KWM, Tzouanas SN, Vo TN, Kasper FK, Mikos AG. Structure-Property Evaluation of Thermally and Chemically Gelling Injectable Hydrogels for Tissue Engineering. Biomacromolecules. 2012;13(9):2821–2830. doi: 10.1021/bm300797m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Temenoff JS, Park H, Jabbari E, Conway DE, Sheffield TL, Ambrose CG, Mikos AG. Thermally cross-linked oligo(poly(ethylene glycol) fumarate) hydrogels support osteogenic differentiation of encapsulated marrow stromal cells in vitro. Biomacromolecules. 2004;5(1):5–10. doi: 10.1021/bm030067p. [DOI] [PubMed] [Google Scholar]
- 31.Otsu T, Matsumoto A, Shiraishi K, Amaya N, Koinuma Y. Effect of the Substituents on Radical Copolymerization of Dialkyl Fumarates with Some Vinyl Monomers. Journal of Polymer Science Part a-Polymer Chemistry. 1992;30(8):1559–1565. [Google Scholar]
- 32.Shin H, Jo S, Mikos AG. Modulation of marrow stromal osteoblast adhesion on biomimetic oligo[poly(ethylene glycol) fumarate] hydrogels modified with Arg-Gly-Asp peptides and a poly(ethyleneglycol) spacer. J Biomed Mater Res. 2002;61(2):169–79. doi: 10.1002/jbm.10193. [DOI] [PubMed] [Google Scholar]
- 33.Bansil R, Gupta MK. Effect of Varying Crosslinking Density on Polyacrylamide Gels. Ferroelectrics. 1980;30(1–4):63–71. [Google Scholar]
- 34.Hayashi M, Tanii H, Horiguchi M, Hashimoto K. Cytotoxic effects of acrylamide and its related compounds assessed by protein content, LDH activity and cumulative glucose consumption of neuron-rich cultures in a chemically defined medium. Arch Toxicol. 1989;63(4):308–13. doi: 10.1007/BF00278644. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto K, Sakamoto J, Tanii H. Neurotoxicity of acrylamide and related compounds and their effects on male gonads in mice. Arch Toxicol. 1981;47(3):179–89. doi: 10.1007/BF00368678. [DOI] [PubMed] [Google Scholar]
- 36.Holland TA, Bodde EW, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A. 2005;75(1):156–67. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 37.Tabata Y, Yamada K, Miyamoto S, Nagata I, Kikuchi H, Aoyama I, Tamura M, Ikada Y. Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials. 1998;19(7–9):807–15. doi: 10.1016/s0142-9612(98)00233-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Swelling ratio profiles of (a) 10 mM GMP or (b) 40 mM GMP containing OPF composite hydrogels in standard PBS over 28 days (n=4). The mass remaining (%) profiles of corresponding (c) 10 mM GMP or (d) 40 mM GMP formulations in standard PBS over 28 days is also shown (n=4). Error bars represent the standard deviation.
Supplementary Figure 2: The main effects of PEG chain MW, GMP crosslinking extent, DBR, and incubation medium composition on the (a) swelling and (b) % mass remaining of OPF composite hydrogels in Study 1 at each time point over 28 days. Error bars represent the standard error.
Supplementary Figure 3: The main effects of crosslinker type, GMP crosslinking extent, DBR, and incubation medium composition on the (a) swelling and (b) % mass remaining of OPF composite hydrogels in Study 2 at each time point over 28 days. Error bars represent the standard error.