Abstract
Background
Hypovitaminosis D is common in obesity and insulin resistant states. Increased fat mass in patients with non-alcoholic fatty liver disease (NAFLD) may contribute to hypovitaminosis D.
Aims
To determine the relation between plasma vitamin D concentration, severity of disease and body composition in NAFLD.
Methods
Plasma vitamin D concentration was quantified in 148 consecutive biopsy proven patients with NAFLD (non alcoholic steatohepatitis-NASH: n=81; and hepatic steatosis n=67) and healthy controls (n=39). NAFLD was scored using the NASH CRN criteria. Body composition was quantified by bioelectrical impedance analysis and abdominal CT image analysis.
Results
Plasma vitamin D concentration was significantly lower in NAFLD (21.2±10.4 ng/ml) compared to healthy controls (35.7±6.0 ng/ml). Higher NAFLD activity scores were associated with lower plasma concentration of vitamin D (r2=0.29; p<0.001). Subgroup analysis among patients with NAFLD showed that patients with NASH had significantly lower (p<0.01) vitamin D levels than those with steatosis alone (18.1±8.4 vs. 25.0±11.3 ng/ml). Low concentrations of vitamin D were associated with greater severity of steatosis, hepatocyte ballooning and fibrosis (p<0.05). On multivariate regression analysis, only severity of hepatocyte ballooning was independently associated (p=0.02) with low vitamin D concentrations. Plasma vitamin D (p=0.004) and insulin concentrations (p=0.03) were independent predictors of the NAFLD activity score on biopsy. Patients with NAFLD had higher fat mass that correlated with low vitamin D (r2=0.26; p=0.008).
Conclusions
Low plasma vitamin D concentration is an independent predictor of the severity of NAFLD. Further prospective studies demonstrating the impact of vitamin D replacement in NAFLD patients are required.
Keywords: vitamin D, non-alcoholic fatty liver disease, body composition, fat mass, metabolic syndrome
Introduction
The high and increasing prevalence of hypovitaminosis D in the US population [1, 2] is of particular concern given the increasingly recognized immunomodulatory, anti-inflammatory and anti fibrotic effects of Vitamin D [3, 4]. Hypovitaminosis D is also more severe and frequent in insulin resistant states [5, 6]. Non alcoholic fatty liver disease (NAFLD) is the hepatic component of the metabolic syndrome and its associated insulin resistance [7]. Two human studies from Europe suggest that hypovitaminosis D is associated with increasing severity of non alcoholic fatty liver disease independent of other components of the metabolic syndrome [8, 9]. In the larger study, NAFLD was not diagnosed by histology [8]. In the other study in 60 subjects, patients with NAFLD had hypovitaminosis D and lower vitamin D concentrations were reported with more severe pathological features of NAFLD [9].
Body composition also impacts vitamin D levels [10, 11]. Increasing body fat mass has been identified to be an independent predictor of hypovitaminosis D with approximately 1.3 nmol/l reduction per 1kg/m2 increase in body mass index [12] even though body mass index is a relatively crude index of body composition since it consists of bone mass, skeletal muscle and fat mass. Increased body fat correlates with lower plasma vitamin D levels [13, 14]. Higher body fat mass is also associated with worsening insulin resistance [15] and potentially more severe hepatic consequences and histological measures of NAFLD. Vitamin D concentrations are lower with worsening insulin resistance and hypovitaminosis D may predispose to development of diabetes mellitus [16, 17]. Insulin resistance has been reported to result in reduction in skeletal muscle mass, and reduced muscle mass has also been reported in patients with diabetes mellitus [18]. Sarcopenia or loss of skeletal muscle mass and relative sarcopenia, or the ratio of muscle to fat mass, co-exist with obesity, and have additive effects on insulin resistance [19, 20]. However, skeletal muscle loss and relative sarcopenia have not been convincingly shown to be directly related to vitamin D concentrations [21, 22]. There are few reports on the effect of vitamin D on skeletal muscle mass and strength [21, 23] with weakness and reduced muscle mass in subjects with hypovitaminosis D [24]. These data show that increased fat mass, relative sarcopenia, insulin resistance and hypovitaminosis D co-exist. NAFLD is a disorder of insulin resistance and obesity, the relation between changes in body composition and vitamin D concentrations in patients with NAFLD are not known and relation between body composition and vitamin D concentrations in this population is not known.
Since NAFLD has been reported in 20–30% of the Western population [25], the present prospective study was conducted to determine the prevalence of hypovitaminosis D and its relation to body composition in patients with NAFLD compared with controls. Plasma concentration of vitamin D was evaluated in relation to both liver histology scored using the NASH Clinical Research Network histological criteria [26] and whole body total fat mass and fat free mass using bioelectrical impedance analysis (BIA). We also used CT image analysis that is used as a method to precisely quantify body composition based on skeletal muscle and fat areas [27–29].
Subjects and Methods
Design
We included 148 consecutive outpatients with non-alcoholic fatty liver disease being followed in the metabolic liver clinic after exclusion of those with recent acute illness, clinical evidence of cancer, renal disease and those with medications known to affect vitamin D metabolism. Subjects underwent detailed history and physical examination, alcohol intake was quantified and use of other confounding medications recorded. Laboratory investigations including amino transferases, blood urea nitrogen, serum creatinine and vitamin D concentration were performed in the clinical core laboratory. The diagnosis of NAFLD was confirmed in all patients by liver biopsy and exclusion of other causes of chronic liver disease (alcohol intake >20g/d, viral hepatitis B or C, autoimmune hepatitis, and the use of hepatotoxic drugs). During the same period, 39 healthy subjects with no known chronic diseases, normal transaminases and hepatic ultrasound were included as controls.
Vitamin D concentration
For biochemical measurements including vitamin D concentrations, venous blood was drawn after an overnight fast and assays done in the clinical core laboratory using standard automatic colorimetric methods. Since vitamin D concentrations depend on geography, seasonal variation and exposure to sunlight, all subjects were residents within the greater Cleveland area and vitamin D concentrations were obtained between October and February when the duration of exposure to sunlight for both controls and patients with NAFLD was similar.
Hepatic histology
An experienced hepatopathologist (AK) masked to the patients’ clinical details scored the biopsy using the NASH CRN criteria for NAFLD severity [26]. Briefly, severity was scored for the degree of steatosis from 0–3, fibrosis from 0–4, inflammation from 0–3 and hepatocyte ballooning degeneration from 0–2. The diagnosis of NASH vs. no NASH was based on the pathologist’s overall evaluation of the histology and not the NAS number.
Metabolic components
Components of metabolic syndrome [30] including waist circumference, type 2 diabetes mellitus and systemic hypertension were documented and plasma triglyceride and high density cholesterol were determined in patients with NAFLD. Young healthy controls (n=32) were enrolled from the family members of the patients in the outpatient family medicine clinic and subjects who came for routine physical examination, in whom illnesses were excluded on clinical, biochemical and imaging studies and were on no medications for acute or chronic diseases. Since body composition was one of the measures in patients with NAFLD, the healthy controls were not matched for age or body weight.
Body composition measures
Body composition was quantified using an RJL Quantum X tetrapolar bioelectrical impedance analyzer (RJL Industries, Clinton Twn, MI). Fat mass and fat free mass were determined using the manufacturer recommended software. Body mass index was measured using the height (measured with a fixed stadiometer) and weight. Blood pressure was measured with a standard manometer prior to the office visit. Information on daily alcohol consumption and other characteristics were obtained from all participants as part of clinical care using the AUDIT questionnaire. In 61 patients with NAFLD, CT of the abdomen was done as part of clinical care. This subset of patients did not have Vitamin D levels measured. Specific measures of skeletal muscle and abdominal fat area were quantified using the Image J software [29, 31]. Total adipose tissue, subcutaneous visceral adipose tissue and skeletal muscle cross-sectional areas (psoas, paraspinal, abdominal wall) were measured at the level of L4 (Figure 1) using standard Hounsfield unit ranges (adipose tissue: −190 to −30; skeletal muscle: −29 to +150)[32]. In an additional separate control group of 46 healthy subjects who had CT abdomen for unspecified abdominal pain, muscle and fat areas were quantified as described above. Inter-observer agreement was determined by intraclass correlation (ICC) for muscle and adipose tissue area measured independently by two investigators (SD, VB). The ICC was 0.975 or higher for each of the areas measured by the 2 investigators. The Bland Altman plot was used to analyze the agreement between body composition measured using BIA (fat free and fat mass) and CT image analysis (total muscle and fat area) and overall agreement was defined by the mean of differences or bias. Two standard deviations representing 95% confidence intervals were used to show the limits of agreement (Figure 2).
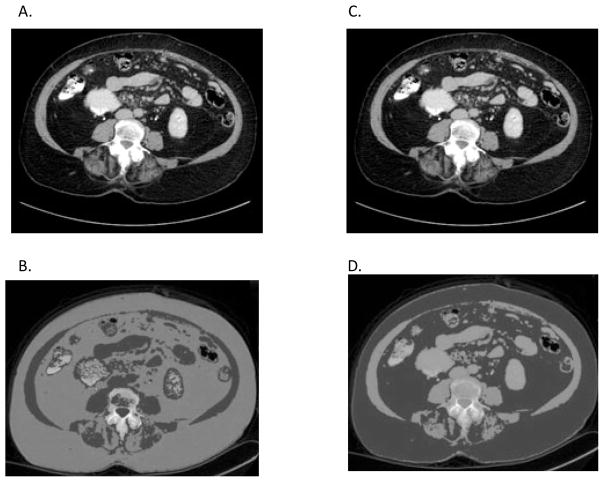
Figure 1. CT image analysis using NIH ImageJ software.
(a) and (c) Original CT image in digital imaging and communications in medicine (DICOM) format with (b) skeletal muscle and (d) adipose tissue thresholds applied with the subcutaneous and visceral adipose tissue identified shown in red.
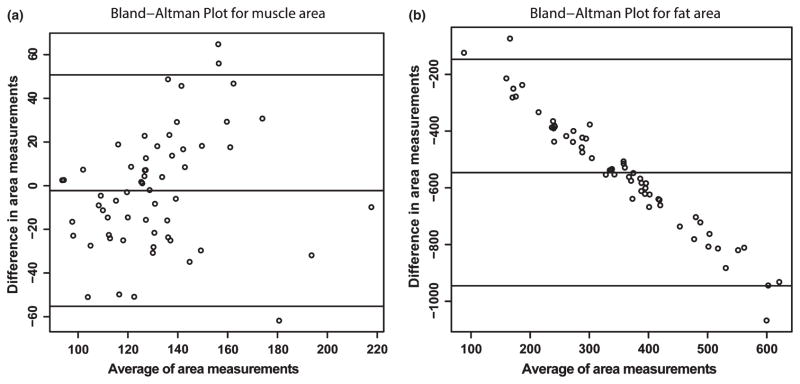
Figure 2.
Bland Altman plots comparing body composition using bioelectrical impedance analysis and CT image analysis. These showed a high degree of agreement between the two methods. The X axis shows the average of the two methods and the Y axis shows the difference between the two methods. Limits of agreement are shown by the confidence intervals shown within the graph. (a) Muscle area measured by CT image analysis and lean body mass by bioelectrical impedance analysis. (b) Fat area measured by CT image analysis and whole body fat mass quantified by bioelectrical impedance analysis.
All data were collected prospectively and studies were approved by the Institutional Review Boards at MetroHealth Medical Center and at the Cleveland Clinic, Cleveland, Ohio.
Statistical Analyses
All data are presented as mean±SD unless specified. Qualitative variables were compared using the chi square test. Quantitative and rating variables were compared using ANOVA with Bonferroni’s correction or the Student’s ‘t’ test for data that was normally distributed and the Kruskall Wallis test or the Mann Whitney test for skewed data. The Spearman correlation coefficient was calculated for associations of quantitative variables. Since there were no gender differences in plasma vitamin D concentrations, they were pooled for analyses. Multivariable logistical regression analysis was performed to identity predictors for the presence and severity of NAFLD. The co-variates included vitamin D concentration in plasma, age, gender, body mass index, whole body fat and fat free mass, plasma insulin and glucose. Separate regression models were tested with the individual components of the metabolic syndrome. P values <0.05 were considered statistically significant. Statistical analyses were performed using the SPSS v20.0 (IBM, Armonk, NY).
Results
Clinical characteristics
The baseline clinical, biochemical and body composition characteristics are shown in table 1. Young healthy controls were chosen to obtain concurrent normal body composition values. The gender ratio and height of the healthy control subjects was similar to that in patients with NAFLD. Patients with NAFLD were older, weighed more and had higher blood glucose, and serum transaminases (p<0.01). The characteristics of patients with NAFLD are shown in table 2. Among patients with NAFLD, 67 had steatosis alone and 81 had NASH. There were 17 patients with cirrhosis concurrent with NASH. Patients with NASH were more insulin resistant (higher plasma glucose, insulin and HOMA scores), had more severe injury on histology (steatosis, inflammation, ballooning, and fibrosis), greater NAS, higher amino transferases and lower vitamin D concentrations.
Table 1.
Clinical, body composition and biochemical characteristics of study subjects
| Factor | Controls | NAFLD |
|---|---|---|
| Number | 39 | 148 |
| Age | 37.5 ± 10.6 | 49.9 ± 12.3** |
| Gender (M:F) | 9:30 | 42:106 |
| Height(cm) | 167.0 ± 9.3 | 165.4 ±9.8 |
| Weight(lbs) | 149.0 ± 28.2 | 217.0 ± 53.6*** |
| Body mass index (kg/m2) | 25.5 ± 3.1 | 35.7 ± 7.0*** |
| Fat free mass (lb) | 52.1 ± 10.1 | 137.6 ± 37.3*** |
| Fat free mass/body weight | 70.0 ± 10.0% | 63.9 ± 9.5% |
| Fat mass (lb) | 23.2 ± 8.4 | 79.2 ± 31.2*** |
| Fat mass/body weight | 30.0 ± 10.0% | 36.1 ± 9.5% |
| Fat mass/fat free mass | 0.46 ± 0.17 | 0.61 ± 0.24** |
| Vitamin D (ng/ml) | 35.7 ± 6.0 | 21.2 ± 10.4*** |
| Albumin (g/dl) | 4.0 ± 0.6 | 4. 1± 0.4 |
| Bilirubin (mg/dl) | 0.7 ± 0.4 | 0.7 ± 0.4 |
| Glucose (mg/dl) | 87.7 ± 5.7 | 117.3 ± 39.1*** |
| Serum AST (IU/dl) | 19.6 ± 6.4 | 38.7 ± 24.6*** |
| Serum ALT (IU/dl) | 18.9 ± 12.0 | 45.9 ± 30.0*** |
| Serum calcium (mg/dl) | 9.4 ± 0.4 | 9.4 ± 0.4 |
| Serum phosphate (mg/dl) | 3.8 ± 0.6 | 3.6 ± 0.6 |
| Blood urea nitrogen (mg/dl) | 10.2 ± 3.8 | 12.1 ± 5.1 |
| Serum creatinine (mg/dl) | 0.75 ± 0.20 | 0.83 ± 0.22 |
Values presented as Mean ± SD
p<0.01;
p<0.001
Table 2.
Patient Characteristics by disease group
| Factor | Steatosis (n=67) | NASH(n=81) |
|---|---|---|
| Age | 47.9 ± 12.3 | 51.4 ± 12.2 |
| Gender (M:F) | 18:49 | 24:57 |
| Height(cm) | 165.3 ± 9.0 | 165.5 ± 10.5 |
| Weight(lbs) | 214.3 ± 60.1 | 219.2 ± 48.0 |
| Body mass index (kg/m2) | 35.2 ± 7.8 | 36.1 ± 6.2 |
| Glucose (mg/dl) | 109.3 ± 35.5 | 123.8 ± 40.8* |
| Insulin (mU/L) | 18.1 ± 11.1 | 27.5 ± 21.6** |
| HOMA IR | 5.0 ± 3.9 | 9.3 ±10.4** |
| HbA1c gm% | 6.2± 1.0 | 6.6 ± 1.6 |
| Steatosis | 1.2±0.8 | 2.3 ± 0.7*** |
| Inflammation | 0.9z ± 0.4 | 1.5 ± 0.7*** |
| Fibrosis | 1.0 ± 0.3 | 2.1 ± 1.2*** |
| Ballooning | 0.12 ± 0.03 | 1.4 ± 0.5*** |
| NAS | 2.3 ± 1.0 | 5.3 ± 1.1*** |
| AST | 33.6 ± 18.5 | 45.5 ± 26.9** |
| ALT | 38.3 ± 29.9 | 52.2 ± 28.6** |
| Bilirubin | 0.6 ± 0.3 | 0.8 ±0.4 |
| Vitamin D (ng/ml) | 25.0 ± 11.3 | 18.1 ± 8.4*** |
| Fat wt (lb) | 79.9 ± 33.0 | 78.7 ± 29.8 |
| FFM wt(lb) | 134.2 ± 37.4 | 140.5 ± 37.3 |
| Fat mass/FFM | 0.64 ± 0.24 | 0.59 ± 0.24 |
| Fat % of wt | 36.6 ± 8.7 | 35.7 ± 10.1 |
| FFM % of wt | 63.4 ± 8.7 | 64.3 ± 10.1 |
Values presented as Mean ± SD.
p<0.05
p<0.01;
p<0.001
Body composition
Body mass index and absolute fat mass, fat free mass and fat mass to fat free ratio measured by BIA were higher (p<0.001) in patients with NAFLD compared to controls (Table 1). In contrast, BIA measured fat mass, fat free mass, fat mass to whole body weight or fat free mass were not different between patients with and without NASH (Table 2).
On image analysis, patients with NAFLD had significantly lower psoas, paraspinal, abdominal wall and total muscle area and higher visceral and total fat area but not subcutaneous fat as compared to controls (Table 3). These differences persisted even when normalized for height of the subjects. In addition, all muscle areas were significantly lower in NASH and NASH cirrhosis compared to those with steatosis alone (Table 3). Only muscle area correlated inversely (r2=0.482; p<0.001) with the NAS. Measures of fat mass including whole body fat mass, fat/fat free mass ratio, visceral and subcutaneous fat area on CT did not correlate with the NAS.
Table 3.
Muscle and fat area on CT image analysis
| Control | Steatosis | NASH | NASH Cirrhosis | |
|---|---|---|---|---|
| Number | 46 | 25 | 26 | 10 |
| Psoas area (cm2) | 29.3 ± 4.1 | 26.4 ± 4.6a | 20.3 ± 2.5 b | 18.0 ± 3.0 d |
| Paraspinal area (cm2) | 60.1± 9.5 | 51.4 ± 10.2 a | 43.4 ± 4.4 b | 39.7 ± 4.1 d |
| Abdominal Wall area (cm2) | 78.9 ± 19.9 | 70.1 ± 17.4 a | 62.1 ± 10.2 b | 57.8 ± 13.0 d |
| Total muscle area (cm2) | 167.5 ± 30.3 | 147.9±29.2 a | 125.2 ±11.7 b | 115.5 ± 16.9 d |
| Visceral Fat (cm2) | 189.0 ± 58.6 | 413.5 ± 39.7 a | 428.3 ± 25.5 a | 336.4 ± 68.1 d |
| Subcutaneous Fat (cm2) | 200.2 ± 52.1 | 204.3 ± 19.7 | 255.5 ± 90.3 | 200.7 ± 32.6 e |
| Total Fat (cm2) | 389.3 ± 95.3 | 617.8 ± 46.8 c | 683.8 ± 163.4 c | 537.1 ± 95.7 d |
| Fat/Muscle area Ratio | 2.4 ± 0.56 | 4.2 ± 1.5 a | 5.4 ± 1.2 b | 4.5 ± 2.2 d |
p<0.01 control vs. steatosis
p<0.001 control vs. NASH and p<0.05 steatosis vs. NASH
p< 0.01 control vs. steatosis, NASH
p<0.01 vs. controls and steatosis
p<0.05 vs. NASH alone
Vitamin D concentrations
Plasma vitamin D concentrations were significantly (p<0.001) lower in patients with NAFLD (21.2±10.4 ng/ml) compared with healthy controls (35.7±6.0 ng/ml). In patients with NAFLD, vitamin D concentrations were significantly lower in NASH patients compared to those with hepatic steatosis alone (table 2). Plasma vitamin D concentrations were similar in patients with NASH alone (17.8±8.1 ng/ml) and in those with NASH cirrhosis (19.4±10.2 ng/ml). Using a normal lower limit of plasma vitamin D concentration 30 ng/ml, 70.1% steatosis, 89.7% NASH and 84.6% of cirrhotic patients had hypovitaminosis D while none of the controls had hypovitaminosis D. Amongst the study subjects, vitamin D concentration correlated inversely with body weight (r2=0.15; p=0.001), body mass index (r2=0.20; p=0.001), whole body fat mass on BIA (r2=0.26; p<0.001) and visceral fat area (r2=0.24; p<0.001) (Figure 3). On the other hand, vitamin D concentration did not correlate (p>0.1) with absolute fat free mass or the fat free mass expressed as a percentage of whole body weight or fat mass.
Figure 3.
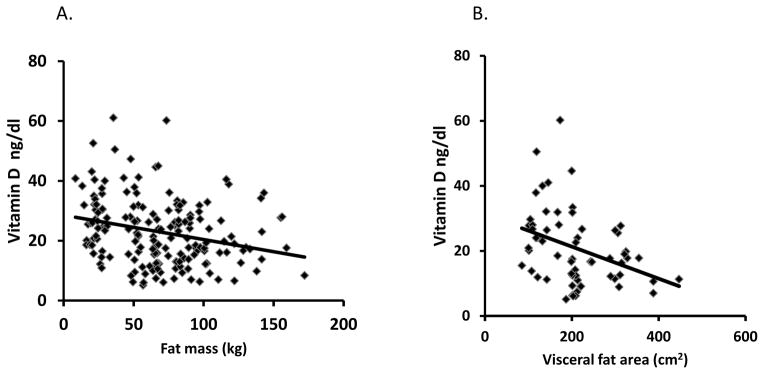
In patients with non alcoholic fatty liver disease (NAFLD) vitamin D concentration correlated inversely with whole body fat mass (r2=0.27; p<0.05) measured by BIA and visceral fat area (r2=0.41; p<0.01 ) on CT image analysis.
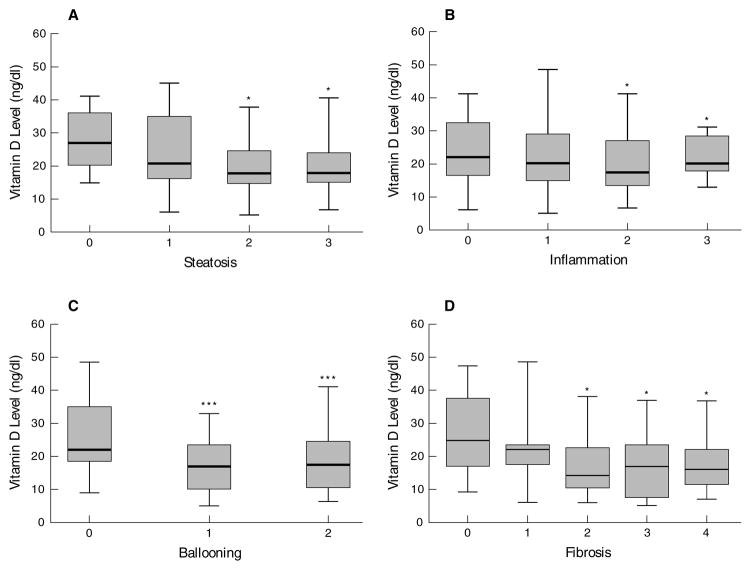
Vitamin D concentrations had a significant (p<0.001) inverse correlation (r2=0.29) with NAS score. Amongst the NAS components, vitamin D concentrations correlated inversely with the degree of steatosis (r2=0.26; p=0.001), ballooning (r2=0.17; p-0.04) and NAS score (r2=0.22; p=0.007). As shown in figure 4, plasma concentration of vitamin D was significantly lower (p<0.05) in patients with steatosis grade 2 or 3 compared to those with grade 1. Similarly, patients with higher grades (2 or 3) lobular inflammation had lower vitamin D. Higher grades of hepatocyte ballooning and fibrosis were also accompanied by lower vitamin D concentrations. On multivariate regression analysis, the severity of ballooning was the only NAS characteristic that was significantly associated (p=0.02) with low plasma vitamin D. Of the non histological variables, only vitamin D (p=0.004) and plasma insulin (p=0.03) independently predicted the NAS.
Figure 4.
Box and whisker plots of vitamin D concentrations related to the steatosis, inflammation, ballooning and fibrosis score on liver biopsy in patients with NAFLD. * p<0.05 *** p<0.001.
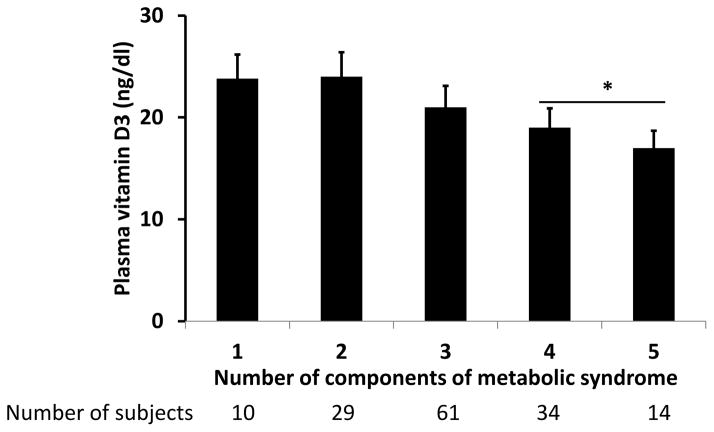
Among patients with NAFLD, metabolic syndrome was significantly more frequent (p<0.02) in patients with NASH (81.5%) compared to those with steatosis alone (65.6%). Patients with NAFLD with metabolic syndrome had lower (p<0.05) vitamin D concentration (20.4±8.2 ng/dl) compared to those without the metabolic syndrome (23.5±7.2 ng/dl). Components of metabolic syndrome were evaluated for their relation to the vitamin D concentration in patients with NAFLD (Figure 5). Patients with more than 3 components of metabolic syndrome had significantly lower vitamin D than those with 2 or fewer components. Of these components, patients with diabetes mellitus had significantly lower vitamin D. Patients with NASH had consistently lower vitamin D concentration than steatosis alone, whether or not they had metabolic syndrome. Multivariate analysis showed that presence of NASH and diabetes mellitus were independent predictors of low vitamin D concentration (Table 4).
Figure 5.
Histograms showing the plasma vitamin D concentration in patients with differing numbers of metabolic syndrome. Patients with more than 3 components had significantly lower (plasma vitamin D compared to those with 3 or fewer metabolic components. p<0.05
Table 4.
Multivariate analysis of components of metabolic syndrome
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | |
|---|---|---|---|---|---|
|
| |||||
| B | Std. Error | Beta | |||
|
| |||||
| NASH | −5.443 | 1.692 | −.262 | −3.217 | .002 |
| Diabetes mellitus | 5.064 | 1.744 | .244 | 2.903 | .004 |
| Hypertension | −.912 | 1.673 | −.044 | −.545 | .586 |
| High triglyceride | 2.074 | 1.749 | .097 | 1.186 | .238 |
| Low HDL | −.566 | 1.655 | −.027 | −.342 | .733 |
| Waist circumference | −4.171 | 2.675 | −.122 | −1.559 | .121 |
Dependent Variable: Vitamin D Level
Discussion
The present study shows, in our large cohort of clinically and histologically well characterized patients with NAFLD, significantly lower plasma concentrations of vitamin D than in healthy controls. Plasma vitamin D was also significantly lower in patients with NASH compared with those with steatosis alone. Presence of NASH and increasing scores of steatosis, fibrosis and ballooning were accompanied by lower plasma vitamin D concentration. The presence of metabolic syndrome was associated with hypovitaminosis D and patients with 3 or more components had significantly lower plasma D concentrations. In addition, only NASH and the presence of diabetes mellitus independently predicted lower vitamin D levels. These data along with those from others [33] emphasize the importance of insulin resistance on vitamin D levels and that low vitamin D levels can accelerate advanced stages of liver disease. The liver injury associated with low vitamin D concentration may be explained, in part, by a recent study that reported a TGFβ1/Smad3 mediated increase in hepatic profibrotic genes [34]. In addition, hypovitaminosis D in humans and animals contributes to increased oxidative stress and increased inflammation [35]. Vitamin D inhibits the production of inflammatory cytokines and hypovitaminosis with a high fat diet increases the expression of toll like receptors 2, 4 and 9 expression in rat livers [36].
Obesity as defined by body mass index is a risk factor for both NAFLD and hypovitaminosis D [37]. In our patients, using BIA, whole body fat mass and fat free mass were significantly higher in patients with NAFLD than controls. Patients with NAFLD also had significantly higher visceral and adipose tissue areas compared to that in controls quantified by CT image analysis. These data on fat mass are consistent with previous data that the metabolic adverse consequences of obesity are due to increased fat mass [38, 39] and adipose tissue mass is higher in patients with NASH [40, 41].
Our observations are consistent with previous reports that obesity and insulin resistance were inversely correlated with plasma vitamin D concentrations [6, 10, 11, 13]. Our data, however, extend these observations by providing a direct inverse relation between vitamin D and fat mass measured either by BIA and CT image analysis. It has been suggested that visceral fat is metabolically more active [39, 42] and consistently, our data show that visceral rather than whole body fat that correlated the best with low plasma vitamin D concentration. Although obesity, as quantified by waist circumference, forms a component of the metabolic syndrome, on multivariate analysis, we observed that NASH and diabetes mellitus- but not obesity- were independent predictors of hypovitaminosis D suggesting that it was the metabolic and hepatic consequences of insulin resistance that contributed to the hypovitaminosis D. These findings are consistent with previous reports that the severity of insulin resistance was accompanied by low vitamin D concentrations and that on follow up, patients with hypovitaminosis D were at risk of glucose intolerance [5, 43, 44].
On the other hand, there have been no data that systematically evaluated skeletal muscle mass in fatty liver. Since insulin resistance is associated with sarcopenia or loss of muscle mass [45], our observation of higher fat free mass in NAFLD was surprising but similar to the data reported in obese subjects [46]. However, the ratio of fat to fat free mass showed that patients with NAFLD had a higher relative fat and a lower relative fat free mass. This was similar to previous data that the relative muscle mass correlated with insulin sensitivity [19, 20]. Our data therefore suggest that even though absolute fat free mass is higher in patients with NAFLD, as a relative proportion to their fat mass, they were sarcopenic. Consistent with this interpretation, direct measurement of skeletal muscle area on CT showed that patients with NAFLD had significantly lower psoas and paraspinal muscle area compared to that in healthy controls. Furthermore, the total fat to muscle area was also higher in NASH than steatosis suggesting that patients with NASH have more severe sarcopenic obesity. These data are of significance given the recognition that relative sarcopenia identified by the ratio of fat and muscle mass is associated with insulin resistance and metabolic syndrome [47–49]. Our data on the altered fat to fat free mass in patients with NAFLD suggests that the liver-adipose tissue-skeletal muscle axis may be dysregulated in these patients. Recent data on the regulatory role of skeletal muscle secreted protein, irisin on adipose tissue function [50] [51] as well as TGFβ superfamily member, myostatin on insulin resistance [52, 53] suggest that these molecular abnormalities may play a contributory role in the development and progression of NAFLD. These exciting and potential novel molecular abnormalities can not only explain the body composition abnormalities in these patients, but also can be novel therapeutic targets in NAFLD.
Since vitamin D is a major regulator of bone mass, we expected that fat free mass would be lower in NAFLD patients who have low plasma concentrations of vitamin D. Previous data in adults have, however, shown that bone density is not significantly altered in NAFLD [54]. Even though our patients with NAFLD had lower muscle mass, plasma vitamin D was not significantly correlated with the muscle mass on CT image analysis. Others have also reported that plasma vitamin D did not correlate with muscle mass [22]. Consistently, we observed in patients with NAFLD that muscle and fat free mass did not correlate with low plasma vitamin D concentration. We thus, demonstrate for the first time that body composition, specifically, fat mass rather than BMI or fat free mass is an independent predictor of hypovitaminosis D in patients with NAFLD.
A number of mechanisms could contribute to the low plasma vitamin D in NAFLD. Increased fat mass in NAFLD may allow a greater volume of distribution of vitamin D in the adipose tissue compartment with lower plasma concentration [55, 56]. It has also been suggested that obese subjects are sedentary and have reduced sunlight along with consumption of high caloric foods low in mineral and vitamin content that may also contribute to the low vitamin D [9, 57]. Although we did not specifically evaluate the duration of exposure to sunlight, by measuring plasma vitamin D in both controls and patients with NAFLD in a similar low daytime period, we reduced the effect of differential exposure to sunlight. The active metabolite of vitamin D is formed in the liver and it is possible that low vitamin D is a consequence of hepatocellular dysfunction in NAFLD. This is, however unlikely, because vitamin D supplementation normalizes plasma vitamin D in patients with cirrhosis demonstrating that hepatic 25 hydroxylation is preserved even in the presence of advanced liver disease [58].
Our study has limitations but the strengths of the study significantly outweigh these limitations. The control group was small relative to the NAFLD group and was not matched for age or body weight. The primary goal of the control group was to demonstrate that in a young healthy population, plasma vitamin D fell within the normal range of our laboratory and this was shown in this group. Obesity is associated with a number of metabolic consequences that have a negative impact on plasma vitamin D and hence non obese, healthy subjects were included. A disease control group of patients with non NAFLD liver diseases was not included since hypovitaminosis D has been reported in a number of other liver diseases including hepatitis C where vitamin D status influences response to therapy [33, 59].
Our data in combination with previously published studies provide compelling evidence for routine screening for hypovitaminosis D in patients with NAFLD and treatment with supplementation when needed. Longitudinal human data on the therapeutic benefit of vitamin D in prevention of progression of disease are necessary.
Acknowledgments
Jaividhya Dasarathy, Carol Hawkins, Patricia Brandt, Arthur J McCullough and Srinivasan Dasarathy were funded in part by the NIH UO1 DK 061732 and SD was partially supported by NIH RO1 DK 083414 in the conduct of these studies.
Reference List
- 1.Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012 Mar;142(3):498–507. doi: 10.3945/jn.111.151977. [DOI] [PubMed] [Google Scholar]
- 2.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(4 Suppl 5):S5–101. [PubMed] [Google Scholar]
- 3.Marques CD, Dantas AT, Fragoso TS, Duarte AL. The importance of vitamin D levels in autoimmune diseases. Rev Bras Reumatol. 2010 Jan;50(1):67–80. [PubMed] [Google Scholar]
- 4.Khoo AL, Chai L, Koenen H, Joosten I, Netea M, van dV. Translating the role of vitamin D3 in infectious diseases. Crit Rev Microbiol. 2012 May;38(2):122–135. doi: 10.3109/1040841X.2011.622716. [DOI] [PubMed] [Google Scholar]
- 5.Rajakumar K, de las HJ, Lee S, Holick MF, Arslanian SA. 25-hydroxyvitamin D concentrations and in vivo insulin sensitivity and beta-cell function relative to insulin sensitivity in black and white youth. Diabetes Care. 2012 Mar;35(3):627–633. doi: 10.2337/dc11-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamendola CA, Ariel D, Feldman D, Reaven GM. Relations between obesity, insulin resistance, and 25-hydroxyvitamin D. Am J Clin Nutr. 2012 May;95(5):1055–1059. doi: 10.3945/ajcn.111.032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne CD. Non-alcoholic fatty liver disease, insulin resistance and ectopic fat: a new problem in diabetes management. Diabet Med. 2012 Jun 4; doi: 10.1111/j.1464-5491.2012.03732.x. [DOI] [PubMed] [Google Scholar]
- 8.Barchetta I, Angelico F, Del BM, Baroni MG, Pozzilli P, Morini S, et al. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85. doi: 10.1186/1741-7015-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007 Sep;17(7):517–524. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Seo JA, Cho H, Eun CR, Yoo HJ, Kim SG, Choi KM, et al. Association between visceral obesity and sarcopenia and vitamin D deficiency in older Koreans: the Ansan Geriatric Study. J Am Geriatr Soc. 2012 Apr;60(4):700–706. doi: 10.1111/j.1532-5415.2012.03887.x. [DOI] [PubMed] [Google Scholar]
- 11.Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004 Mar;89(3):1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 12.Stein EM, Strain G, Sinha N, Ortiz D, Pomp A, Dakin G, et al. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol (Oxf) 2009 Aug;71(2):176–183. doi: 10.1111/j.1365-2265.2008.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003 Jan;88(1):157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 14.Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, et al. The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr. 2007 Oct;86(4):959–964. doi: 10.1093/ajcn/86.4.959. [DOI] [PubMed] [Google Scholar]
- 15.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000 Aug;106(4):473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sung CC, Liao MT, Lu KC, Wu CC. Role of vitamin D in insulin resistance. J Biomed Biotechnol. 2012;2012:634195. doi: 10.1155/2012/634195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabadi SM, Lee BK, Liu L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes: results from the NHANES 2001–2006. Diabetes Care. 2012 Oct;35(10):2048–2054. doi: 10.2337/dc12-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Z, Liu L, Liu N, Liu Y. Muscular response and adaptation to diabetes mellitus. Front Biosci. 2008;13:4765–4794. doi: 10.2741/3038. [DOI] [PubMed] [Google Scholar]
- 19.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011 Sep;96(9):2898–2903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 20.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010 May 26;5(5):e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010 Apr;95(4):1595–1601. doi: 10.1210/jc.2009-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marantes I, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ, III, Amin S. Is vitamin D a determinant of muscle mass and strength? J Bone Miner Res. 2011 Dec;26(12):2860–2871. doi: 10.1002/jbmr.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005 Nov;16(11):1425–1431. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 24.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013 Feb;34(1):33–83. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 25.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 26.Kleiner DE, Brunt EM, Van NM, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005 Jun;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 27.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004 Dec;97(6):2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 28.Irving BA, Weltman JY, Brock DW, Davis CK, Gaesser GA, Weltman A. NIH ImageJ and Slice-O-Matic computed tomography imaging software to quantify soft tissue. Obesity (Silver Spring) 2007 Feb;15(2):370–376. doi: 10.1038/oby.2007.573. [DOI] [PubMed] [Google Scholar]
- 29.Richards CH, Roxburgh CS, MacMillan MT, Isswiasi S, Robertson EG, Guthrie GK, et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One. 2012;7(8):e41883. doi: 10.1371/journal.pone.0041883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 31.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012 Jul;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998 Jul;85(1):115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 33.Venu M, Martin E, Saeian K, Gawrieh S. High prevalence of vitamin A deficiency and vitamin D deficiency in patients evaluated for liver transplantation. Liver Transpl. 2013 Jun;19(6):627–633. doi: 10.1002/lt.23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013 Apr 25;153(3):601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gradinaru D, Borsa C, Ionescu C, Margina D, Prada GI, Jansen E. Vitamin D Status and Oxidative Stress Markers in Elderly with Impaired Fasting Glucose and Type 2 Diabetes Mellitus. Aging Clin Exp Res. 2012 Sep 10; doi: 10.3275/8591. [DOI] [PubMed] [Google Scholar]
- 36.Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology. 2012 Apr;55(4):1103–1111. doi: 10.1002/hep.24737. [DOI] [PubMed] [Google Scholar]
- 37.Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005 Jul;90(7):4119–4123. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 38.Pascot A, Lemieux S, Lemieux I, Prud’homme D, Tremblay A, Bouchard C, et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 1999 Sep;22(9):1471–1478. doi: 10.2337/diacare.22.9.1471. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010 Dec;95(12):5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008 Aug;48(2):449–457. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 41.Mehta SR, Godsland IF, Thomas EL, Pavitt DV, Morin SX, Bell JD, et al. Intrahepatic insulin exposure, intrahepatocellular lipid and regional body fat in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2012 Jun;97(6):2151–2159. doi: 10.1210/jc.2011-2430. [DOI] [PubMed] [Google Scholar]
- 42.Park BJ, Kim YJ, Kim DH, Kim W, Jung YJ, Yoon JH, et al. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol. 2008 Jun;23(6):900–907. doi: 10.1111/j.1440-1746.2007.05212.x. [DOI] [PubMed] [Google Scholar]
- 43.Husemoen LL, Skaaby T, Thuesen BH, Jorgensen T, Fenger RV, Linneberg A. Serum 25(OH)D and incident type 2 diabetes: a cohort study. Eur J Clin Nutr. 2012 Dec;66(12):1309–1314. doi: 10.1038/ejcn.2012.134. [DOI] [PubMed] [Google Scholar]
- 44.Husemoen LL, Thuesen BH, Fenger M, Jorgensen T, Glumer C, Svensson J, et al. Serum 25(OH)D and type 2 diabetes association in a general population: a prospective study. Diabetes Care. 2012 Aug;35(8):1695–1700. doi: 10.2337/dc11-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de RN, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009 Nov;32(11):1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bredella MA, Ghomi RH, Thomas BJ, Torriani M, Brick DJ, Gerweck AV, et al. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity (Silver Spring) 2010 Nov;18(11):2227–2233. doi: 10.1038/oby.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim TN, Park MS, Lim KI, Choi HY, Yang SJ, Yoo HJ, et al. Relationships between Sarcopenic Obesity and Insulin Resistance, Inflammation, and Vitamin D Status:The Korean Sarcopenic Obesity Study (KSOS) Clin Endocrinol (Oxf) 2012 May 7; doi: 10.1111/j.1365-2265.2012.04433.x. [DOI] [PubMed] [Google Scholar]
- 48.Kim CS, Nam JY, Park JS, Kim DM, Yoon SJ, Ahn CW, et al. The correlation between insulin resistance and the visceral fat to skeletal muscle ratio in middle-aged women. Yonsei Med J. 2004 Jun 30;45(3):469–478. doi: 10.3349/ymj.2004.45.3.469. [DOI] [PubMed] [Google Scholar]
- 49.Kim TN, Park MS, Lim KI, Yang SJ, Yoo HJ, Kang HJ, et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: The Korean Sarcopenic Obesity Study (KSOS) Diabetes Res Clin Pract. 2011 Aug;93(2):285–291. doi: 10.1016/j.diabres.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012 Jan 26;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belen CA, et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8(4):e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang C, McFarlane C, Lokireddy S, Bonala S, Ge X, Masuda S, et al. Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetologia. 2011 Jun;54(6):1491–1501. doi: 10.1007/s00125-011-2079-7. [DOI] [PubMed] [Google Scholar]
- 53.Zhao B, Wall RJ, Yang J. Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem Biophys Res Commun. 2005 Nov 11;337(1):248–255. doi: 10.1016/j.bbrc.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 54.Purnak T, Beyazit Y, Ozaslan E, Efe C, Hayretci M. The evaluation of bone mineral density in patients with nonalcoholic fatty liver disease. Wien Klin Wochenschr. 2012 Aug;124(15–16):526–531. doi: 10.1007/s00508-012-0211-4. [DOI] [PubMed] [Google Scholar]
- 55.Lenders CM, Feldman HA, Von SE, Merewood A, Sweeney C, Wilson DM, et al. Relation of body fat indexes to vitamin D status and deficiency among obese adolescents. Am J Clin Nutr. 2009 Sep;90(3):459–467. doi: 10.3945/ajcn.2008.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000 Sep;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 57.Bradlee ML, Singer MR, Qureshi MM, Moore LL. Food group intake and central obesity among children and adolescents in the Third National Health and Nutrition Examination Survey (NHANES III) Public Health Nutr. 2010 Jun;13(6):797–805. doi: 10.1017/S1368980009991546. [DOI] [PubMed] [Google Scholar]
- 58.Compston JE. Hepatic osteodystrophy: vitamin D metabolism in patients with liver disease. Gut. 1986 Sep;27(9):1073–1090. doi: 10.1136/gut.27.9.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petta S, Camma C, Scazzone C, Tripodo C, Di MV, Bono A, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010 Apr;51(4):1158–1167. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]