Abstract
Background
Although the negative consequences on health of being obese are well known, most adults gain weight across the life span. The general increase in body mass index (BMI) is mainly considered to originate from behavioral and environmental changes, but few studies have evaluated the influence of these factors on change in BMI in the presence of genetic risk. We aimed to study the influence of multifactorial causes of change in BMI, over 65 years.
Methods and Findings
Totally, 6,130 participants from TwinGene, who had up to 5 assessments, and 536 from the Swedish Adoption/Twin Study of Aging, who had up to 12 assessments, ranging over 65 years were included. The influence of lifestyle factors, birth cohort, cardiometabolic diseases, and an individual obesity genetic risk score based on 32 single nucleotide polymorphisms on change in BMI was evaluated with a growth model. For both sexes, BMI increased from early adulthood to age 65 years, after which the increase leveled off; BMI declined after age 80 years. A higher obesity genetic risk score, birth after 1925, and cardiometabolic diseases were associated with higher average BMI and a steeper increase in BMI prior to age 65 years. Among men, few factors were identified that influence BMI trajectories in late life, while for women, type 2 diabetes mellitus and dementia were associated with a steeper decrease in BMI after the age of 65 years.
Conclusions
There are two turning points in BMI in late adulthood, one at age 65 years and one at age 80 years. Factors associated with an increase in BMI in midlife, were not associated with an increase in BMI after the age of 65 years. These findings indicate that the causes and consequences of change in BMI differ across the life span. Current health recommendations need to be adjusted accordingly.
Keywords: Aged, Body Mass Index, Cardiovascular disease, Genotypes, Life course, Obesity, Trajectories
Introduction
Although there is a general awareness about the negative consequences on health of being obese, the obesity rate (body mass index (BMI) ≥30 kg/m2) continues to increase. The increase in BMI is mainly considered to originate from behavioral and environmental changes, such as high caloric intake and a sedentary lifestyle.1 Nevertheless, a large proportion of the variation in BMI is still estimated to be influenced by genetic variants, as witnessed by a reported heritability of ~70%.2 Recent large-scale meta-analyses of genome-wide association studies have identify numerous genetic variants associated with BMI. Since the identified loci are estimated to account for only a small part of the variance in BMI, adding these small effects together into an obesity genetic risk score (OGRS) has been proposed to better capture these genetic effects.3 Studies evaluating both environmental and genetic influences on BMI trajectory across adulthood are rare, and to our knowledge no previous study has jointly studied these influences on BMI trajectory in midlife and late life.
Further, in late life, both low weight (often including normal weight) and weight decline is often associated with negative health outcomes.4, 5 This change, referred to as the obesity paradox, may be driven by weight loss primarily caused by disease processes occurring in late life. Hence, BMI and changes in BMI may be indicative of various processes across the life course, and thus may have different implications for health at different ages.
The aims of this study were to characterize the adult BMI trajectory and to analyze the influence of assessed genetic risk, lifestyle factors, and cardiometabolic diseases on the BMI trajectory at various stages of life. Data capturing up to 65 years of the adult lifespan were derived from two substudies of the Swedish Twin Registry (STR)6: TwinGene6 and the Swedish Adoption/Twin Study of Aging (SATSA).7
Methods
Design/participants
Both TwinGene and SATSA originate from the STR.6 The STR conducted mailed questionnaires in 1961, 1963, 1967, and 1970 for all like-sexed twins born between 1886 and 1925, and in 1973 for all like-sexed pairs born in 1926–1958 (Figure 1). A more recent STR wave contacted adult twins via the Screening Across the Lifespan Twin study (1998–2002), which targeted all twins born in 1958 or earlier.
Figure 1.
Flowchart of the Collection of BMI Values
For TwinGene, which took place between 2004 and 2008, 12,614 twins who had previously taken part in the Screening Across the Lifespan Twin study donated a blood sample during in-person testing (IPT).6 Of the TwinGene participants, 6,130 persons were associated with a BMI value and information on all covariates.
SATSA, which has been described elsewhere,7 is a longitudinal study with repeated IPTs. For the current analyses, seven IPTs (from 1986 to 2007) were available. A total of 859 persons had participated in at least one IPT. Of these, 644 individuals (those participating in IPT3 or IPT5) had available genotyping, and 536 had information on all covariates.
Ethic statement
TwinGene and SATSA have been approved by the ethics review board at Karolinska Institutet, Stockholm, Sweden. Informed consent was obtained from all participants.
BMI measurements
BMI scores were calculated as kg/m2. In addition to height and weight measurements taken in person, or through the STR (self-reported), the 1886–1925 cohort also retrospectively self-reported their height and weight at 25 years and 40 years of age (Figure 1). Through the STR alone, information on BMI is available for one-four occasions, depending on whether the twin belonged to the 1886–1925 cohort or to the 1926–1958 cohort. Thus, up to seven total assessments of height and weight were available for the TwinGene participants. SATSA participants had their height and weight assessed at least once, for a maximum of seven times through IPT. Combining the STR and SATSA data led to the availability of up to 12 BMI assessments in total. Mode of assessment was included as a covariate: (0=assessed in person, 1=self-reported, 2=retrospectively self-reported).
OGRS and genotyping
SATSA participants were genotyped with the Human Cardio-Metabo beadchip, and TwinGene participants were genotyped with the Illumina HumanOmniExpress 700K BeadChip. For 23 of 32 SNPs, the SNP was directly genotyped on the Metabochip; 28 of the 32 SNPs were directly genotyped on the OmniExpress. For the remaining loci, genotype information was imputed using a) a proxy SNP in high linkage disequilibrium with the reported SNP that was selected from a SNAP search8 (nine SNPs in SATSA); b) using IMPUTE2.0 (one SNP in TwinGene); or c) using the reported population frequency from a reference (three SNPs in TwinGene).9 The directly genotyped SNPs had a genotyping success of ≥99.4%. Exact Hardy-Weinberg equilibrium P-values were calculated using PLINK10, and all 32 SNPs had P-values >0.005. In order to estimate the cumulative effect of the 32 BMI loci, we calculated individual non-weighted OGRS (i.e. count of increasing alleles) and weighted OGRS, using beta coefficients from Speliotes et al.9
Demographic factors, cardiometabolic risk factors, and diseases
Educational level was coded as elementary school (0) or greater (1), and cohort was dichotomized as born between 1900–1925 (0) or 1926–1948 (1). Number of children was self-reported and entered as a mean-centered variable, at 2.1 in TwinGene and at 2.0 in SATSA. Based on self-reporting in the STR assessments (1967 or 1973), exercise was dichotomized as moderate to heavy exercise (0) and no or little exercise (1), and consumption of fruit and vegetables was divided into high (0) or low fruit consumption (1). Smoking status and alcohol consumption were based on self-reports from the STR and on first responses in SATSA IPT or TwinGene; these factors were dichotomized as never smoked (0) and had ever smoked (1), and as abstainers (never reported drinking alcohol, 0) and drinkers (1), respectively.
Data on cardiovascular diseases (CVD; stroke, myocardial infarction, heart failure, and angina pectoris) were extracted from the Swedish National Patient Register. Information on CVD and dementia was extracted from the National Patient Registry and the Cause of Death Register using the twins’ personal identification numbers; the information was based on the International Classification of Disease (ICD). Only main diagnoses were considered for cardiovascular outcomes. For stroke, the following ICD codes were used: ICD-8 codes 430–436, ICD-9 codes 430–436, and ICD-10 codes I60–I64 and G45. For coronary heart disease (myocardial infarction and angina pectoris), we used ICD-8 codes 410 and 411, ICD-9 codes 410 and 411B, and ICD-10 codes I20.0, I21, and I22. For heart failure, we considered ICD-8 codes 427.00 and 427.10, ICD-9 code 428, and ICD-10 code I50. The ICD codes used to detect Alzheimer’s disease were codes 304 and 305 (ICD-7), 290 (ICD-8), 290.0, 290.1, and 331.0 (ICD-9), and F00 and G30 (ICD-10). The ICD codes used to detect vascular dementia were codes 306 (ICD-7), 293.0 and 293.1 (ICD-8), 290.4 (ICD-9), and F01 (ICD-10). Additional ICD codes used to detect dementia were codes 294.1, 290.8, 290.9, 331.1, 331.2, and 331.9 (ICD-9), and F02, F03, G31.1, G31.8A, and F05.1 (ICD-10).
Hypertension was defined as resting systolic blood pressure above 140 mmHg and/or diastolic blood pressure above 90 mmHg in TwinGene or at least two stable measurements during the study period in SATSA, and/or self-reported use of antihypertensive medication. Presence of any of these disorders was summed into a CVD score (range, 0–5). Type 2 diabetes mellitus (T2DM) was based on self-reports of T2DM and/or diabetic agents and coded as absence (0) or presence (1) of T2DM across the study period.
Cancer diagnoses were extracted from the cancer registry and the cause of death registry, based on the following ICD-codes: 153, 154, 170, and 177 (ICD-7 and ICD-8), 153, 154, 159, 174, and 185 (ICD-9), and C180–C189, C199, C209–C211, C260, C500–C509, and C619 (ICD-10). Cancer was coded as absence (0) and presence (1). Information about asthma, bronchitis, and tuberculosis were self-reported in SALT (for TwinGene) and across the study in SATSA and coded as absence (0) or presence (1) of respiratory disease across the study period. Participants were continuously screened for dementia throughout SATSA11 and were diagnosed according to the relevant Diagnostic and Statistical Manual of Mental Disorders (Appendix 2). Dementia status was updated from the National Patient Register for subjects who were lost to follow-up in SATSA, and for all participants in TwinGene.
Statistical analysis
Baseline characteristics were analyzed in IBM SPSS statistics v. 21. Characterization of the BMI trajectory across the adult lifespan was performed first with SATSA data which includes up to twelve measurement points of BMI, and then confirmed with TwinGene data which includes fewer measurement points. This was done using SAS 9.3 (PROC MIXED, NLMIXED) and MPLUS12 using full-maximum likelihood estimation. Initial inspection of raw BMI trajectories (figure 2) and model-fitting analyses in PROC MIXED suggested nonlinear curves fitted the data best. Follow-up analyses, in PROC NLMIXED estimated a cubic model with age, age-squared and age-cubed terms where the centering of age was estimated as a parameter, and represented the first turning point in the BMI curve. Results suggested the first turning point was at ages 65.4 years and 65.8 years in men and women, respectively. Next, in MPLUS we fitted a series of nested linear and piecewise (spline) growth models that captured up to two turning points and directly compared models between men and women.13, 12 Age predictors were centered at the first turning point of 65 years. The best-fitting model chosen for further analysis was a piecewise model with three linear age-based trends: slope A (25 years to <65 years), slope B (≥65 years to <80 years), and slope C (≥80 years). Given that there were fewer assessments of BMI in late life in TwinGene, a two-part linear piecewise model was chosen, with a single change point at 65 years of age.
Figure 2.
Raw trajectories of BMI.
Note. Each line represents a single individuals actual BMI trajectory.
All subsequent growth models were run separately for men and women. Models were adjusted to account for within and between twin pair effects. Assessment type effects on the intercept and slopes were evaluated (i.e., retrospective self-report, concurrent self-report, or assessed weights), and found to be nonsignificant for the slopes, but not for the intercept. Hence, all models controlled for assessment type for the intercept. Previous analyses on SATSA have shown that retrospectively self-reported weight captures the population mean,14 and the accuracy of self-reported height and weight does not change substantially over time.15 This strengthens the assumption that potential errors due to report bias mainly affects the mean level and less so the trajectory shape.
Separately for TwinGene and SATSA, and separately for men and women, a stepwise procedure was adopted to evaluate the influence of various factors based on occurrence in time: OGRS, birth cohort, education, number of children, lifestyle factors, and diseases. For each factor, the effect was first evaluated on the intercept and then on the A and B (and C, for SATSA data) slopes. The summed OGRS was chosen for further analysis as it received a better fit than the weighted OGRS, based on the −2Log Likelihood test. Predictors with a significant effect (P≤0.05), when the main effect and interaction effects were entered, on the intercept or on any of the trajectories were carried on to the next step. In the final model, birth cohort and education were included as well as those factors that exerted an effect on the average BMI or on a specific linear term within sex, in either of the two studies.
Non-independency within and between twin pairs were controlled for in all models. BMI assessment mode was evaluated and found to be significant for the intercept, but not for the slopes. Hence, all models controlled for assessment mode on the intercept. Latent growth model analyses were performed in MPLUS12 and SAS 9.3,16 using full maximum-likelihood estimation.17
Results
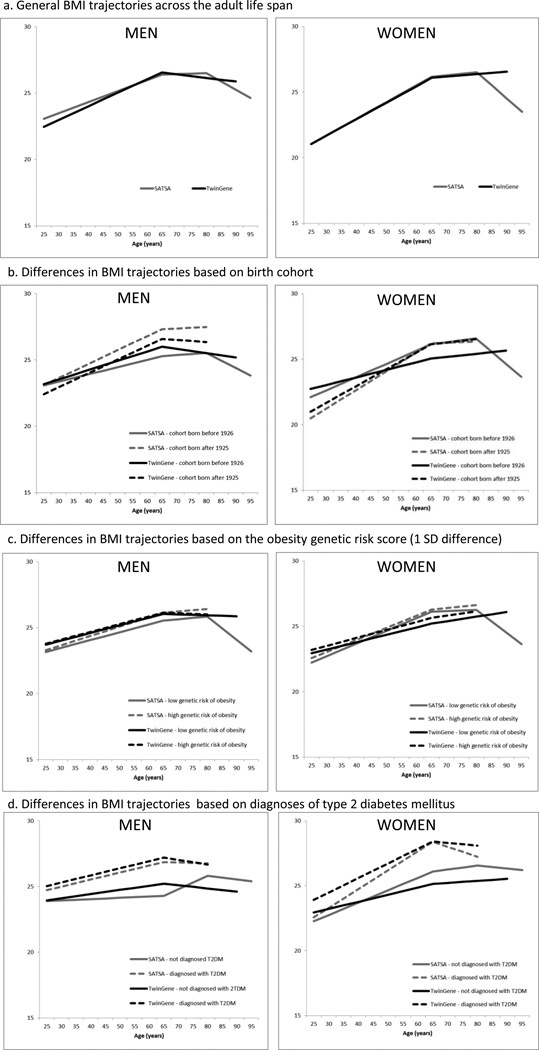
Participant characteristics are presented in Table 1. The best-fitting model, which was based on SATSA, was a three-spline model with an increase in BMI from age 25 years to age 65 years. After age 65 years, the increase leveled out and started to decline after the age of 80 years. The number of persons that had at least one assessment of BMI before and after the breakpoints was 49.1% (n = 263) before and after 65 years and 20.9% (n = 112) before and after 80 years. General BMI trajectories for men and women and study are presented in Figure 3a.
Table 1.
Sample Characteristics, Total Sample and by Sex.
| TwinGene | SATSA | |||||
|---|---|---|---|---|---|---|
| Total | Men | Women | Total | Men | Women | |
| n=6130 | n=2675 | n=3287 | n=536 | n=227 | n=309 | |
| Age in years at IPTentry,a mean (SD) | 64.8 (8.1) | 64.8 (8.1) | 65.5 (8.0) | 61.4 (8.1) | 60 (6.6) | 62.0 (8.7) |
| BMI kg/m2 at IPTentry, mean (SD) | 25.9 (3.9) | 25.9 (3.9) | 26.1 (3.4) | 25.7 (3.9) | 25.9 (3.3) | 25.5 (4.3) |
| Older cohort, n (%) | 297 (4.8) | 138 (4.9) | 159 (4.8) | 239 (44.6) | 100 (44.1) | 139 (45.0) |
| Education ≤6 years, n (%) | 1194 (19.5) | 609 (21.4) | 585 (17.8) | 317 (59.1) | 126 (55.5) | 191 (61.8) |
| Genetic risk score, mean (SD) | 28.6 (3.4) | 28.6 (3.4) | 28.6 (3.4) | 28.9 (3.7) | 29.0 (3.6) | 28.9 (3.8) |
| Number of children, mean (SD) | 2.1 (1.0) | 1.9 (1.1) | 2.3 (0.9) | 2.0 (1.4) | 1.9 (1.5) | 2.1 (1.4) |
| Midlife lifestyle factors | ||||||
| No/light exercise, n (%) | 1200 (19.6) | 469 (16.5) | 731 (17.8) | 153 (28.5) | 56 (24.7) | 97 (31.4) |
| Low intake fruit and veg., n (%) | 1589 (25.9) | 1004 (35.5) | 585 (17.8) | 277 (51.7) | 140 (61.7) | 137 (44.3) |
| Never smoked, n (%) | 2744 (44.8) | 1187 (41.8) | 1557 (47.4) | 263 (49.1) | 67 (29.5) | 196 (44.3) |
| Alcohol abstainers, n (%) | 356 (5.8) | 122 (4.3) | 234 (7.1) | 80 (14.9) | 20 (8.8) | 60 (31.4) |
| Diseases (across the study period) | ||||||
| T2DM, n (%) | 561 (9.2) | 342 (12.0) | 219 (6.7) | 92 (17.2) | 41 (18.1) | 51 (16.1) |
| CVD, n (%)b | 3380 (55.1) | 1350 (60.1) | 1470 (50.8) | 209 (39.0) | 104 (45.8) | 105 (34.0) |
| Cancer, n (%) | 434 (7.0) | 234 (8.7) | 200 (6.1) | 60 (11.2) | 32 (14.1) | 28 (9.1) |
| Lung disease, n (%)c | 550 (9.0) | 218 (8.1) | 332 (10.1) | 120 (22.1) | 47 (20.7) | 73 (23.7) |
| All-cause dementia, n (%) | 93 (1.5) | 45 (1.6) | 48 (1.5) | 62 (11.6) | 18 (7.9) | 44 (14.2) |
Age at the first in-person testing of BMI
Prevalence of one or more CVD (stroke, angina pectoris, heart failure, myocardial infarction, and/or hypertension)
Prevalence of one lung disease (asthma, bronchitis, and/or tuberculosis)
Figure 3.
Note. TwinGene contained scarce data after the age of 80 years. It was therefore not possible to evaluate whether there was a second turning point in the BMI trajectory for TwinGene.
The final model is presented separately for men (Table 2) and for women (Table 3). Unless otherwise indicated, the results were consistent across the studies. BMI trajectories based on birth cohort, genetic risk of obesity, and prevalence of T2DM are presented in Figure 3b–d.
Table 2.
Factors Associated with the BMI Trajectory from Age 25 Years to Late Life in Men: Final Model Results
| TwinGene | SATSA | |||||
|---|---|---|---|---|---|---|
| Estimate | Standard Error |
P-value | Estimate | Standard Error |
P-Value | |
| Intercept (age 65 years) | 24.904 | 0.457 | <0.001 | 24.177 | 0.882 | <0.001 |
| Slope A (age 25–65 years) | 0.029 | 0.011 | 0.011 | 0.017 | 0.021 | 0.410 |
| Slope B (age >65–80 years) | −0.007 | 0.021 | 0.727 | 0.046 | 0.026 | 0.084 |
| Slope C (age >80 years) | - | - | - | −0.178 | 0.055 | 0.001 |
| Demographic | ||||||
| Assessment bias | −0.206 | 0.039 | <0.001 | −0.298 | 0.138 | 0.031 |
| Cohort | 0.615 | 0.358 | 0.086 | 2.303 | 0.490 | <0.001 |
| Cohort*Slope A | 0.026 | 0.009 | 0.004 | 0.052 | 0.012 | <0.001 |
| Cohort*Slope B | 0.027 | 0.020 | 0.193 | −0.009 | 0.036 | 0.792 |
| Cohort*Slope C | - | - | - | - | - | - |
| Education | −0.037 | 0.156 | 0.814 | −0.503 | 0.411 | 0.221 |
| Education*Slope A | 0.020 | 0.004 | <0.001 | −0.010 | 0.011 | 0.345 |
| Education*Slope B | −0.038 | 0.017 | 0.022 | −0.023 | 0.035 | 0.497 |
| Education*Slope C | - | - | - | 0.231 | 0.108 | 0.033 |
| Genetic | ||||||
| OGRS | 0.085 | 0.019 | <0.001 | 0.148 | 0.055 | 0.007 |
| OGRS*Slope A | <0.001 | <0.001 | 0.251 | 0.003 | 0.001 | 0.038 |
| Midlife lifestyle factors | ||||||
| Exercise | −0.072 | 0.101 | 0.473 | −0.356 | 0.333 | 0.285 |
| Smoking | 0.270 | 0.125 | 0.031 | −0.152 | 0.432 | 0.724 |
| Smoking*Slope A | 0.010 | 0.003 | 0.002 | −0.008 | 0.012 | 0.528 |
| Alcohol | 0.601 | 0.287 | 0.037 | 0.574 | 0.687 | 0.403 |
| Alcohol*Slope A | 0.024 | 0.008 | 0.002 | 0.043 | 0.019 | 0.025 |
| Diseases (across the study period) | ||||||
| T2DM | 1.966 | 0.184 | <0.001 | 2.380 | 0.510 | <0.001 |
| T2DM*Slope A | 0.023 | 0.005 | <0.001 | 0.041 | 0.013 | 0.002 |
| T2DM*Slope B | −0.008 | 0.022 | 0.727 | −0.107 | 0.041 | 0.009 |
| CVD | 0.164 | 0.042 | <0.001 | 0.301 | 0.219 | 0.170 |
| Cancer | −0.358 | 0.210 | 0.089 | 0.877 | 0.508 | 0.085 |
| Cancer*Slope A | −0.013 | 0.006 | 0.015 | −0.006 | 0.014 | 0.669 |
| Respiratory diseases | 0.570 | 0.209 | 0.006 | −0.031 | 0.461 | 0.947 |
| Respiratory diseases*Slope A | 0.017 | 0.005 | 0.001 | −0.008 | 0.012 | 0.497 |
Slope A (25 years to <65 years), slope B (≥65 years to <80 years), and slope C (≥80 years)
Table 3.
Factors Associated with the BMI Trajectory from Age 25 Years to Late Life in Women: Final Model Results
| TwinGene | SATSA | |||||
|---|---|---|---|---|---|---|
| Estimate | Standard Error |
P-value | Estimate | Standard Error |
P-Value | |
| Intercept (age 65 years) | 23.9314 | 0.494 | <0.001 | 24.614 | 0.795 | <0.001 |
| Slope A (age 25–65 years) | 0.038 | 0.012 | 0.001 | 0.065 | 0.020 | 0.001 |
| Slope B (age >65–80 years) | 0.018 | 0.027 | 0.503 | 0.052 | 0.031 | 0.086 |
| Slope C (age >80 years) | - | - | - | −0.030 | 0.091 | 0.746 |
| Demographic | ||||||
| Assessment bias | −0.453 | 0.046 | <0.001 | −0.142 | 0.149 | 0.339 |
| Cohort | 1.756 | 0.467 | <0.001 | 1.052 | 0.679 | 0.123 |
| Cohort*Slope A | 0.074 | 0.011 | <0.001 | 0.056 | 0.015 | <0.001 |
| Cohort*Slope B | −0.030 | 0.032 | 0.362 | −0.124 | 0.044 | 0.005 |
| Cohort*Slope C | - | - | - | - | - | - |
| Education | −0.014 | 0.210 | 0.947 | 0.216 | 0.548 | 0.694 |
| Education*Slope A | 0.009 | 0.005 | 0.083 | 0.021 | 0.014 | 0.117 |
| Education*Slope B | −0.037 | 0.023 | 0.114 | 0.045 | 0.035 | 0.193 |
| Education*Slope C | - | - | - | −0.071 | 0.125 | 0.569 |
| Children | 0.391 | 0.077 | <0.001 | 0.097 | 0.172 | 0.571 |
| Children*Slope A | 0.005 | 0.001 | 0.006 | 0.003 | 0.004 | 0.495 |
| Genetic | ||||||
| OGRS | 0.128 | 0.024 | <0.001 | 0.064 | 0.068 | 0.346 |
| OGRS*Slope A | 0.001 | 0.0005 | 0.024 | <−0.001 | 0.002 | 0.833 |
| Midlife lifestyle factors | ||||||
| Fruit and Veg.*Slope B | 0.058 | 0.029 | 0.046 | 0.058 | 0.037 | 0.117 |
| Smoking | −0.405 | 0.156 | 0.010 | −1.499 | 0.508 | 0.003 |
| Smoking*Slope A | −0.007 | 0.004 | 0.072 | −0.025 | 0.013 | 0.051 |
| Smoking*Slope B | 0.014 | 0.023 | 0.534 | 0.083 | 0.034 | 0.015 |
| Alcohol*Slope C | - | - | - | −0.236 | 0.110 | 0.031 |
| Diseases (across the study period) | ||||||
| T2DM | 3.006 | 0.299 | <0.001 | 1.913 | 0.699 | 0.006 |
| T2DM*Slope A | 0.054 | 0.007 | <0.001 | 0.045 | 0.017 | 0.009 |
| T2DM*Slope B | −0.022 | 0.036 | 0.542 | −0.103 | 0.040 | 0.009 |
| CVD | 0.659 | 0.104 | <0.001 | 0.894 | 0.424 | 0.035 |
| CVD*Slope A | 0.010 | 0.002 | <0.001 | 0.020 | 0.011 | 0.066 |
| Respiratory disease | 0.803 | 0.238 | 0.001 | 0.663 | 0.534 | 0.215 |
| Respiratory disease*Slope A | 0.016 | 0.238 | 0.005 | −0.002 | 0.014 | 0.883 |
| Dementia | −1.540 | 0.675 | 0.022 | 0.348 | 0.742 | 0.639 |
| Dementia*Slope A | −0.041 | 0.019 | 0.029 | −0.007 | 0.019 | 0.712 |
| Dementia*Slope B | 0.057 | 0.047 | 0.225 | −0.119 | 0.043 | 0.006 |
Slope A (25 years to <65 years), slope B (≥65 years to <80 years), and slope C (≥80 years)
Men
For men, being born after 1925, having a higher OGRS, having T2DM, and having CVD were independently associated with higher average BMI. All of these factors (except CVD) were also associated with a steeper increase in BMI until the age of 65 years. Furthermore, higher intake of alcohol (compared to being an abstainer) was associated with a steeper increase in BMI until the age of 65 years. After age 65, no consistent patterns were found across the studies for men.
Some differences between the two studies were noticed. In TwinGene, men who smoked had a higher BMI and a steeper increase in BMI until the age of 65 years. Men with a higher educational level had a steeper increase in BMI until the age of 65 years, and a steeper decrease in BMI after the age of 65 years, compared to persons with lower education. In SATSA, after the age of 80 years, men with higher educational levels had a smaller decrease in BMI than those with lower educational levels. Further, men with cancer had a less steep increase in BMI until the age of 65 years, while men with respiratory disease had a higher BMI at the age of 65 years and until the age of 65 years. This was not replicated in SATSA.. Exercise was not significantly associated with the BMI trajectory when all other factors were considered.
Women
Across both studies, T2DM and CVD were independently associated with a higher average BMI and with a steeper increase in BMI until the age of 65 years (Table 3). Women who smoked had a lower average BMI and a less steep increase in BMI until the age of 65 years than women who did not smoke. A higher OGRS was associated with higher mean BMI and a steeper BMI increase up to age 65 years in TwinGene, but not in SATSA. In TwinGene, the cohort born after 1925 had a higher mean BMI, but in SATSA this difference did not achieve statistical significance. However, in both studies women born after 1925 had a steeper increase in BMI until the age of 65 years. Further, women who had given birth to a higher number of children had a higher mean BMI at the age of 65 years, and steeper increase in BMI until the age of 65 years.
Between ages 65 and 80 years, women who smoked had a steeper increase in BMI and women with T2DM had a steeper decline in BMI. Dementia was associated with lower mean BMI in TwinGene and a less steep increase in BMI until the age of 65 years, and in SATSA dementia was associated with a steeper decline in BMI between the ages of 65 and 80 years. Education was not significantly associated with BMI in either study after entering all other factors.
Discussion
Although it has previously been suggested that a turning point in BMI may occur late in life,18 our study covering the adult life course demonstrates that there are two turning points in BMI in late life, one at 65 years and another at 80 years. BMI increases across midlife, the increase levels off after the age of 65 years and BMI then declines around the age of 80 years. While genetic factors, birth cohort, and cardiometabolic diseases generally predicted higher average BMI and a steeper increase in BMI in midlife among both men and women, decrease in BMI after the age of 65 years was predicted by T2DM and dementia, among women.
Turning points in BMI in late life
Weight decline in late life is proposed to be driven by disease processes4 and mortality.19, 20 We found that diseases with increasing incidence in late life such as T2DM and dementia were associated with decline in BMI in late life, especially among women. Among the 80 years old and older, more than 50% have multimorbidity.21 Pathophysiological processes, for example those causing metabolic changes or behavioral changes, and/or drug side effects probably contribute to weight decline seen in late life, and subsequent death. Nonetheless, studies controlling for multimorbidity20, 22 still show an association between decline in BMI and mortality. Other factors, such as poorer psychological well-being, including loss of appetite, or the body’s inability to take up and benefit from nutrients might also be on the causal pathway.
Genetic risk score for obesity
Studies based on children and young adults have shown that the fat mass and obesity associated gene (FTO) and constructed OGRSs predict accelerated increases in BMI.23–25 We find that this predictive relationship holds true through the midlife period, but not in late life. However, our findings are not in accordance with a study including participants from five European countries in which FTO was not found to be associated with a change in weight over seven years in midlife.26 It is possible that the influence of obesity genes can only be detected with a longer follow-up time, or by evaluating several genetic variants together, as we did. The OGRS was not associated with a change in BMI in late life (Table 2 and Table 3). Hence, it may be that other genetic variants, including those associated with late-life diseases, are more important for BMI changes in late life. Genome-wide association studies of elderly populations that focus on change, and not just cross-sectional analyses, could add valuable knowledge about the less well-understood origins of weight changes in late life.
Cohort differences
We found generational differences in BMI where the later born cohort had higher BMI. These birth cohort differences are often suggested to be due to changes in lifestyle, specifically higher caloric intake and less physical activity. However, neither physical exercise nor high intake of fruit and vegetables (a rough proxy for a healthy diet) were associated with average BMI or with change in BMI when all factors were considered (Table 2 and Table 3). The observed influence of birth cohort may instead reflect trends that are difficult to assess, such as decreased overall lower energy expenditure or increased use of caloric sweeteners.27
Cohorts born after1925 gained weight at a steeper rate in midlife. Interestingly, persons born before 1926 were heavier in early midlife (Figure 3b), especially women. One possible explanation for this result is that persons born before 1926 had more physically demanding lives, for example by being a farmer or a farmer’s wife, which were very common occupations during the first half of the 20th century in Sweden. Within the normal BMI range, greater muscle mass results in a higher BMI. For women, cohort differences may also be associated with childbirth patterns; the cohorts born before 1926 had more children and also gave birth earlier in life. In fact, women who had given birth to more children experienced a steeper increase in BMI until the age of 65 years, and a higher BMI at the age of 65 years, than women who had given birth to fewer children. Reasons for the steeper weight gain among women who have given birth is probably multifactorial. For example, women who have given birth (and more births) may not be able to return to pre-birth weights as easily and hence accumulate weight for each successive birth. Further, the reproductive years might be associated with lower physical activity28, 29 and dietary changes.29
While the cohort difference in BMI was preserved in late life for men, for women, both the level of BMI and the BMI trajectories were strikingly similar for both cohorts after the age of 65 years. Rapid weight gain during midlife has been suggested to have health consequences as negative as having a consistently higher BMI, or even worse.30 Hence, the negative effects of weight gain may have occurred earlier and ultimately caused weight loss, bringing the subjects to the same level of BMI as the earlier born cohort. The women in TwinGene who were born before 1926 exhibited a different growth pattern from the other female trajectories. Their pattern most likely reflects a selection effect, as the youngest persons in this cohort were 79 years old when data collection for the TwinGene study started.
Disorders
In the presence of genetic and lifestyle factors, CVD and T2DM were still strongly associated with a higher mean BMI and a steeper increase in BMI until the age of 65 years (Table 2, Table 3, and Figure 3c). Accordingly, both high average BMI and increase in BMI across midlife should be seen as important risk factors for lifetime cardiometabolic disease. Both in clinical practice and in research, weight changes, and not only current BMI, should be considered when health risks are evaluated.
Obesity has been suggested as a potential risk factor for cancer of different types.31, 32 The association between cancer and BMI is complex, and is dependent on the age of exposure and cancer type,33 as indicated in the current study. While cancer was associated with a less steep increase in BMI until the age of 65 years among men in TwinGene, no such association was found among women or the men in SATSA. It is possible that reversed causality influences the association between BMI and cancer in TwinGene, as weight loss BMI might be an indication of underlying cancer, as has been reported for prostate cancer, for example.33
Although not consistently observed as significant across the studies, respiratory diseases were associated with higher BMI at age 65 years and steeper increase in BMI until the age of 65 years. Higher BMI might be a risk factor for respiratory diseases, as adiposity might affect the function of the respiratory system.34 However, higher BMI and weight gain might also be a consequence of the medical treatment of respiratory diseases, for example with steroids.35
Lifestyle factors
The lifestyle factors that were most strongly associated with BMI and the BMI trajectories were not consistent across age or sex. As previously shown,36 among men, alcohol use was especially associated with a steeper increase in BMI during midlife (Table 2), which likely is related to increased energy intake. Among women, smoking was associated with lower BMI and a lower increase in BMI until the age of 65 years (Table 3), confirming previous findings.37 In the presence of genetic factors and disease, few other lifestyle factors were associated with either the average BMI or the BMI curve; this observation was particularly true for changes in BMI in old age (Table 2 and Table 3). Studies focusing on weight changes in late life are therefore warranted.
Strengths and limitations
The strengths of this study include a population-based design with rich assessments of multiple factors over a long follow-up. However, the societal changes occurring over this timeframe with respect to lifestyle factors limits the generalizability of these results to more recent birth cohorts. The stability of the change points needs to be discussed, as there is a gap in measurements. This is more so a problem in TwinGene than in SATSA. Therefore, we characterized the adult BMI curve on SATSA where almost 50% of the sample had data before and after the first change point at 65 years and similarly about 20% of the sample had data around the second change point at 80 years. Thorough analyses confirmed these findings, among both men and women.
Another limitation is that different assessment methods of height and weight were used. BMI values bases on self-reported height and weight usually result in an underestimation of the actual BMI.38 The influence of different assessment methods would particularly influence the expected BMI levels at the change points between the assessment methods, as we have previously shown that the change in self-report bias is small to negligible over time.15 Still, the use of self-reported BMI in early midlife likely underestimates actual BMI, hence, a flatter BMI trajectory from early midlife to late midlife would be expected if height and weight were measured. However, given the careful control of the influence of assessment methods in the analysis, we do believe that the impact of the assessment methods is small and does not substantially influence the results.
All evaluated lifestyle factors were self-reported, and we lacked sufficient coverage to evaluate these behaviors as time-varying covariates. Similarly, the National Patient Register did not cover all parts of Sweden until 1987, and we were therefore not able to differentiate between early and late onset T2DM and CVD.
Conclusions
There are two turning points in BMI in adulthood, one at age 65 years and one at age 80 years. While genetic factors, birth cohort, and cardiometabolic diseases generally predicted higher average BMI and a steeper increase in BMI in midlife, after age of 65 years T2DM and dementia predicted decline in BMI. This study shows that the factors influencing change in BMI differ across the adult life course. Current health recommendations need to be adjusted accordingly.
Acknowledgments
Funding
TwinGene is supported by the Swedish Research Council (M-2005 1112), GenomEUtwin (EU/QLRT-2001-01254; QLG2-CT-2002-01254), NIH DK U01-066134, The Swedish Foundation for Strategic Research (SSF), and the Heart and Lung foundation (no. 20070481). SATSA is supported by the National Institute of Aging (AG04563, AG10175), the MacArthur Foundation Research Network on Successful Aging, the Swedish Council for Working Life and Social Research (FAS) (97:0147:1B and 2009-0795), and the Swedish Research Council (825-2007-7460, 825-2009-6141). Data analyses are supported by FAS 2010-0704, Future Leaders of Aging Research in Europe (FLARE) postdoctoral grant 2010-1852 (A.K.D.), and the European Community's Seventh Framework Programme (FP7/2007–2013; ENGAGE Consortium, grant agreement HEALTH-F4-2007- 201413). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author statement
A. K. D. was responsible for drafting the manuscript. A.K.D. and C.A.R. analyzed the data. A.K.D., C.A.R., and N.L.P. designed the current study. P.K.E.M., and N.P.L. designed, initiated, and directed TwinGene. N.L.P. designed, initiated, and directed SATSA, and C.A.R. and A.K.D. have directed later waves of data collection of SATSA. T.F. created the obesity genetic risk scores. All of the authors are responsible for the intellectual content of the manuscript.
Anna K. Dahl, Chandra A. Reynolds, Patrik K.E. Magnusson, and Nancy L. Pedersen report no conflicts of interest to disclose. Tove Fall has received speaker fees from MSD (Merck).
Data sharing statement: There are no additional data available.
References
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Silventoinen K, Kaprio J. Genetics of tracking of body mass index from birth to late middle age: evidence from twin and family studies. Obes Facts. 2009;2(3):196–202. doi: 10.1159/000219675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison AC, Bare LA, Chambless LE, Ellis SG, Malloy M, Kane JP, et al. Prediction of coronary heart disease risk using a genetic risk score: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2007;166(1):28–35. doi: 10.1093/aje/kwm060. [DOI] [PubMed] [Google Scholar]
- 4.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69(8):739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 5.Somes GW, Kritchevsky SB, Shorr RI, Pahor M, Applegate WB. Body Mass Index, Weight Change, and Death in Older Adults. Am J Epidemiol. 2002;156(2):132–138. doi: 10.1093/aje/kwf019. [DOI] [PubMed] [Google Scholar]
- 6.Magnusson PK, Almqvist C, Rahman I, Ganna A, Viktorin A, Walum H, et al. The Swedish Twin Registry – establishment of a biobank and other recent developments. Twin Res. Hum. Gen. 2013;16(01):317–329. doi: 10.1017/thg.2012.104. [DOI] [PubMed] [Google Scholar]
- 7.Finkel D, Pedersen N. Processing speed and longitudinal trajectories of change for cognitive abilities: the Swedish adoption/twin study of aging. Aging Neurpsych Cogn. 2004;11(2–3):325–345. [Google Scholar]
- 8.Institute B. NP Annotation and Proxy Search [Google Scholar]
- 9.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Gene. 2007;81 doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl A, Hassing LB, Fransson E, Berg S, Gatz M, Reynolds CA, et al. Being overweight in midlife is associated with lower cognitive ability and steeper cognitive decline in late life. J Gerontol A Biol Sci Med Sci. 2010;65(1):57–62. doi: 10.1093/gerona/glp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muthén LK, Muthén BO. Mplus User’s Guide. 6 edn. Los Angeles: Muthén & Muthén; 1998–2010. [Google Scholar]
- 13.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2 edn. Newbury Park, CA.: Sage; 2002. [Google Scholar]
- 14.Dahl AK, Reynolds CA. Accuracy of Recalled Body Weight – A Study with 20-years of Follow-up. Obesity. 2013;21:1293–1298. doi: 10.1002/oby.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahl AK, Hassing LB, Fransson EI, Pedersen NL. Agreement between self-reported and measured height, weight and body mass index in old age-a longitudinal study with 20 years of follow-up. Age Ageing. 2010;39(4):445–451. doi: 10.1093/ageing/afq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SAS Institute Inc. SAS system for Microsoft Windows. 9.1 ed. SAS Institute Inc.; 2002–2003. [Google Scholar]
- 17.McArdle JJ, Nesselroade JR. Growth curve analysis in contemporary psychological research. In: Schinka J, Velicer W, editors. Comprehensive handbook of psychology. Vol. 2. New York: Wiley; 2003. pp. 447–480. [Google Scholar]
- 18.Dey DK, Rothenberg E, Sundh V, Bosaeus I, Steen B. Height and body weight in the elderly: a 25-year longitudinal study of a population aged 70 to 95 years. Eur J Nutr. 1999;53:905–914. doi: 10.1038/sj.ejcn.1600852. [DOI] [PubMed] [Google Scholar]
- 19.Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163(10):938–949. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutrition research reviews. 2009;22(1):93–108. doi: 10.1017/S0954422409990035. [DOI] [PubMed] [Google Scholar]
- 21.Marengoni A, Winblad B, Karp A, Fratiglioni L. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am J Public Health. 2008;98(7):1198–1200. doi: 10.2105/AJPH.2007.121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahl AK, Fauth EB, Ernsth-Bravell M, Hassing LB, Ram N, Gerstorf D. Body Mass Index, Change in Body Mass Index, and Survival among Old and Very Old Persons. J Am Geriatr Soc. 2013;61:512–518. doi: 10.1111/jgs.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haworth CM, Carnell S, Meaburn EL, Davis OS, Plomin R, Wardle J. Increasing heritability of BMI and stronger associations with the FTO gene over childhood. Obesity (Silver Spring) 2008;16(12):2663–2668. doi: 10.1038/oby.2008.434. [DOI] [PubMed] [Google Scholar]
- 24.Belsky DW, Moffitt TE, Houts R, Bennett GG, Biddle AK, Blumenthal JA, et al. Polygenic risk, rapid childhood growth, and the development of obesity: evidence from a 4-decade longitudinal study. Arch Pediatr Adolesc Med. 2012;166(6):515–521. doi: 10.1001/archpediatrics.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjelmborg JB, Fagnani C, Silventoinen K, McGue M, Korkeila M, Christensen K, et al. Genetic influences on growth traits of BMI: a longitudinal study of adult twins. Obesity (Silver Spring) 2008;16(4):847–852. doi: 10.1038/oby.2007.135. [DOI] [PubMed] [Google Scholar]
- 26.Vimaleswaran KS, Angquist L, Hansen RD, van der AD, Bouatia-Naji N, Holst C, et al. Association Between FTO Variant and Change in Body Weight and Its Interaction With Dietary Factors: The DiOGenes Study. Obesity. 2012;20(8):1669–1674. doi: 10.1038/oby.2012.49. [DOI] [PubMed] [Google Scholar]
- 27.Popkin BM, Nielsen SJ. The Sweetening of the World's Diet. Obes Res. 2003;11(11) doi: 10.1038/oby.2003.179. [DOI] [PubMed] [Google Scholar]
- 28.Kern ML, Reynolds CA, Friedman HS. Predictors of physical activity patterns across adulthood: a growth curve analysis. Personality & social psychology bulletin. 2010;36(8):1058–1072. doi: 10.1177/0146167210374834. [DOI] [PubMed] [Google Scholar]
- 29.Laroche HH, Wallace RB, Snetselaar L, Hillis SL, Cai X, Steffen LM. Weight Gain among Men and Women Who Have a Child Enter Their Home. Journal of the Academy of Nutrition and Dietetics. 2013 doi: 10.1016/j.jand.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zajacova A, Ailshire J. Body Weight Trajectories and Mortality Among Older Adults: A Joint Growth Mixture – Discrete Time Survival Analysis. The Gerontologist. doi: 10.1093/geront/gns164. EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 33.Moller E, Adami HO, Mucci LA, Lundholm C, Bellocco R, Johansson JE, et al. Lifetime body size and prostate cancer risk in a population-based case-control study in Sweden. Cancer causes & control : CCC. 2013 doi: 10.1007/s10552-013-0291-0. [DOI] [PubMed] [Google Scholar]
- 34.Murugan AT, Sharma G. Obesity and respiratory diseases. Chronic respiratory disease. 2008;5(4):233–242. doi: 10.1177/1479972308096978. [DOI] [PubMed] [Google Scholar]
- 35.Schols AM, Soeters PB, Mostert R, Pluymers RJ, Wouters EF. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1268–1274. doi: 10.1164/ajrccm.152.4.7551381. [DOI] [PubMed] [Google Scholar]
- 36.Wannamethee SG, Shaper AG. Alcohol, body weight, and weight gain in middle-aged men. Am J Clin Nutr. 2003;77(5):1312–1317. doi: 10.1093/ajcn/77.5.1312. [DOI] [PubMed] [Google Scholar]
- 37.Botoseneanu A, Liang J. The effect of stability and change in health behaviors on trajectories of body mass index in older Americans: a 14-year longitudinal study. J of Gerontol A Biol Sci Med Sci. 2012;67(10):1075–1084. doi: 10.1093/gerona/gls073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8(4):307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]