Abstract
This randomized clinical trial tested the effectiveness of a single-session of motivational interviewing (MI) to decrease alcohol use during pregnancy, while examining theory-based mechanisms of the intervention. Eligible pregnant women who drank any amount of alcohol in the previous year (n=122) were randomized to an intervention or comparison group. Drinking behaviors, basic psychological need satisfaction, and autonomous motivation to decrease prenatal alcohol use were measured at baseline, 30 day postbaseline, and 30 day postpartum follow-ups. Poisson and linear regression with generalized estimating equations were used to evaluate treatment effects over time. Although MI was not found effective in decreasing alcohol use, low levels of reported alcohol use by the women at baseline left little room for improvement due to the intervention. To prevent fetal alcohol spectrum disorders, future studies will use self-report and biomarkers to more accurately identify women in need of interventions to reduce their risk of alcohol-exposed pregnancies.
1.Introduction
Intrauterine alcohol exposure may result in fetal alcohol spectrum disorders (FASD) (Floyd, O’Conner, Sokol, Bertrand, & Cordero, 2005; Hoyme et al., 2005; Manning & Hoyme, 2007; Stratton, Howe, & Battaglia, 1996), a leading cause of mental retardation, birth defects and neurodevelopmental disabilities in prenatally exposed children. Although 2-5% of all US live births are affected by FASD (May et al., 2009), pregnant women continue to consume alcohol in amounts risky to the developing fetus with recent prevalence of 7.6 % of pregnant women reporting any alcohol use and 1.4% reporting binge drinking (consuming four or more standard drinks on an occasion) in the previous 30 days (Marchetta, Denny, Floyd, Cheal, & Sniezek, 2012). When considering the entire gestational period of 40 weeks, up to 35% of pregnant women consume alcohol at some time during pregnancy (Bobo, Klepinger, & Dong, 2006). With harmful effects of prenatal alcohol exposure found with as little as one drink per week (Sood et al., 2001), the US Surgeon General has advised women to abstain from all alcohol use while pregnant (US DHHS, 2005). Interventions to decrease alcohol consumption before, during, and after pregnancy have been successful in reducing alcohol-exposed pregnancies (AEP), with varying doses and ingredients used (Chang et al., 2005; Fleming, Lund, Wilton, Landry, & Scheets, 2008; Floyd et al., 2007; Handmaker, Miller, & Manicke, 1999; Manwell, Fleming, Mundt, Stauffacher, & Barry, 2000; O’Conner & Whaley, 2007; The Project Choices Intervention Research Group, 2003). Yet the specific mechanisms of these interventions most helpful in assisting pregnant women to decrease or abstain from alcohol use have eluded researchers. The National Institutes of Health strongly recommended testing specific theory-based mechanisms of behavioral change and their role as mediators for future development of individually tailored interventions to assist with behavior change (NIH, 2009). Theory-based interventions that assist pregnant women to decrease alcohol consumption have great potential to prevent FASD.
1.1 Consequences of alcohol use during pregnancy
A diagnosis of FASD encompasses a variety of physical, neurological, behavioral, and psychosocial abnormalities which can be categorized according to severity of outcomes for the alcohol-exposed fetus (Floyd, O’Conner, Sokol, Bertrand, & Cordero, 2005; Hoyme et al., 2005; Manning & Hoyme, 2007; Stratton, Howe, & Battaglia, 1996). Fetal alcohol syndrome (FAS), the most severe form of FASD, occurs in 2 to 7 of 1,000 live births (May et al., 2009). Diagnostic characteristics of FAS include all of the following: facial dysmorphology, growth retardation, and central nervous system abnormalities. Less severe but more frequently occurring are partial FAS, alcohol-related birth defects (ARBD), and alcohol-related neurodevelopmental disorders (ARND) which involve combinations of birth defects, central nervous system malformations, growth retardation, and behavioral/cognitive dysfunctions that may be difficult to diagnose before children are school-age and beyond. The rise in prevalence rates of FASD from 1% (May & Gossage, 2001) to 2-5% (May et al., 2009) is representative of neurobehavioral complications of intrauterine alcohol exposure identified more recently with case ascertainment methods of assessing school-age children. The implications of FASD continue throughout the lifespan and are demonstrated in lower IQ scores (Streissguth, 2007), delinquent/aggressive and anxious/depressed behaviors (Sood et al., 2001), psychiatric and substance use diagnoses, inappropriate sexual behaviors, problems in school, and trouble with the legal system (Streissguth, 2007; Streissguth et al., 2004). The economic societal burden of FAS alone is $2 million per affected child (Lupton, Burd, & Harwood, 2004), but is underestimated due to underdiagnosed cases of FAS and FASD related to practitioner inexperience and lack of knowledge (Stratton et al., 1996).
1.2. Interventions to decrease prenatal alcohol use
FASD can be prevented by women not drinking alcohol while pregnant (US DHHS, 2005). But with rates of identified FASD at 2-5 percent in the U.S. (May et al., 2009), effective interventions that assist pregnant women to decrease prenatal alcohol use are essential to prevent the serious sequelae of intrauterine alcohol exposure. Interventions of varied types, dosages, and ingredients have shown promising results with higher level drinking women during the preconception and postpartum periods. The Project TrEAT (Trial for Early Alcohol Treatment) (Manwell et al., 2000) found two 15-minute physician-provided brief interventions with follow-up phone calls to significantly decrease alcohol use in childbearing age problem drinkers, as well as in the women who became pregnant during the 48 month follow-up when compared to a control group. When the Project TrEAT protocols were modified for use with postpartum women, decreased alcohol use behaviors in postpartum high-risk drinkers were found over a 6 month period (Fleming et al., 2008). The Project Choices (Floyd et al., 2007) provided a series of four motivational interviewing (MI) intervention sessions and one contraceptive counseling session to women at risk for an AEP due to problem drinking, ineffective use of contraception, or both. Women receiving the intervention had two times the odds of reduced risk for AEP at 9 month follow-up by reducing drinking behaviors and through more effective use of contraception over a control group.
Outcomes of interventions provided during pregnancy to decrease prenatal alcohol use have varied depending on the dosage of the intervention. A series of brief interventions provided to women who reported alcohol use at monthly prenatal visits found those receiving the interventions were 5 times more likely to abstain from alcohol use, had infants with higher birth weights and lengths, and had infants with 3 times lower fetal mortality rates (O’Conner & Whaley, 2007). Yet Chang and colleagues (2005) study of a single-session brief intervention to decrease prenatal alcohol use found no differences in drinking behaviors between pregnant women who received the intervention and those who did not, although women who drank at higher levels at baseline demonstrated significantly reduced alcohol use at postpartum follow-up. Provision of a single-session MI intervention by Handemaker et al. (1999) found results similar to Chang and colleagues (2005), with only higher level drinkers having greater decreases in blood alcohol concentrations at 2 month follow-up than a control group.
Yet the mechanisms that evoked changes in drinking behaviors in these previous studies remain unknown. In response to the NIH’s (2009) charge to test theory-based mechanisms of behavior change in development of interventions, a preliminary study by Osterman and Dyehouse (2012) determined the effectiveness of a single-session MI intervention to decrease prenatal alcohol use, while examining theory-based mechanisms of the intervention which may have evoked behavior change. Self-determination theory (SDT; Deci & Ryan, 1985, 2000; Ryan & Deci, 2000, 2002) concepts of basic psychological need satisfaction and autonomous motivations to change behavior were investigated to determine mechanisms of MI helpful in reducing prenatal alcohol use. Although MI was not found effective in decreasing prenatal alcohol use in this study (Osterman & Dyehouse, 2012), this preliminary work provided information essential in development of the current study to test the effectiveness of an SDT-based MI intervention to decrease prenatal alcohol use. A description of the theoretical framework that undergirded the MI intervention in both studies follows.
1.3. An SDT-based MI intervention to decrease prenatal alcohol use
The method used to assist pregnant women in decreasing prenatal alcohol use in Osterman and Dyehouse’s (2012) preliminary study and the current study was motivational interviewing (Miller & Rollnick, 2002, 2013). Motivational interviewing (MI) is a person-centered, directive counseling style which increases a person’s intrinsic motivation for change through the MI spirit of partnership, acceptance, compassion, and evocation. Four principles guide MI - establishing empathy, developing discrepancy, rolling with resistance, and supporting self-efficacy. Acceptance and compassion for a person as competent and capable of making decisions in his/her best interest establishes the foundation of an empathic relationship. Reflective statements, open-ended questions, and linking summaries assist with exploration of discrepancies between a person’s beliefs, values, and current behaviors. Resistance is seen as a sign of evolving self-awareness of concerns and ambivalence for behavior change, at which time potential options and opportunities for change can be evoked. Self-efficacy develops as the client is supported in autonomous, competent decisions through collaborative goal setting and commitment for change. MI has facilitated improvements in health behaviors (Burke, Arkowitz, & Menchola, 2003; Hettema, Steele, & Miller, 2005), such as risky alcohol use (Burke et al., 2003), with non-pregnant women at risk for alcohol-exposed pregnancies (Floyd et al., 2007; The Project CHOICES Intervention Research Group, 2003), and with pregnant women who drink (Handmaker et al., 1999). Yet due to MI’s atheoretical development in clinical practice (Miller, 1999), a theoretical framework is needed to understand the processes and efficacy of MI.
Self-determination theory (SDT; Deci & Ryan, 1985, 2000; Ryan & Deci, 2000, 2002) provides a framework which can increase understanding of the mechanisms of MI (Markland et al, 2005; Vansteenkiste & Sheldon, 2006) that may assist pregnant women in reducing their alcohol use. The theory postulates that motivation to perform a behavior increases when three basic psychological needs are satisfied – the need for autonomy, the need for competence, and the need for relatedness. The more these needs are satisfied, the more intrinsically motivated a person is to engage in a behavior. Although intrinsically motivated behaviors are chosen willingly and for self-satisfaction, other behaviors are performed due to motivators external to a person. Rewards, punishments, and guilt may motivate behavior, yet the outcome may be short-lived due to lack of autonomy in choosing the behavior. External motivators that identify or integrate with a person’s values and beliefs are more sustained due to more autonomous choice and satisfaction of a person’s need for autonomy, competence, and relatedness. External social environments which provide autonomy support, structure, and involvement can satisfy a person’s basic psychological needs leading to autonomous motivations to engage in behaviors. Motivational interviewing can provide such an external social environment through an empathic relationship that promotes self-awareness of discrepancies in beliefs, values, and behaviors and supports autonomous, competent decision-making to engage in healthier behaviors. An MI intervention grounded in SDT which satisfies a pregnant woman’s basic psychological needs has the potential to increase motivation to decrease prenatal alcohol use.
1.4. Preliminary study results
In the preliminary RCT which tested the effectiveness of an MI intervention to decrease prenatal alcohol use, while it examined SDT mechanisms of behavior change, such as basic psychological need satisfaction and autonomous motivations to decrease prenatal alcohol use (n=56) (Osterman & Dyehouse, 2012), MI was not found effective in decreasing drinking behaviors. Nonspecific factors, such as participant motivation for improvement, treatment structures, and provider qualities, may have accounted for this study’s outcomes. First, low levels of reported drinking behaviors and high levels of reported basic psychological need satisfaction at baseline resulted in floor/ceiling effects diminishing room for improvement due to the MI intervention. Lower reporting of alcohol use at baseline may have resulted from exclusion of women screening for dependency symptoms, who were deemed not appropriate for a brief intervention but would require referral per the Screening, Brief Intervention, and Referral to Treatment (SBIRT) guidelines (SAMSHA, 2011). The provision of standard education on risks of prenatal drinking by the researcher to all study eligible women before completion of baseline instruments may also have influenced the women’s self-report and in itself provided an additional intervention. Lastly, the provision of all study procedures by the researcher trained in MI strategies may have resulted in potential crossover of the spirit of MI at other interaction points in the study.
1.5. Current study
The purpose of the current study was to build on preliminary work testing the effectiveness of a single-session MI intervention to decrease alcohol use. Self-determination theory mechanisms which evoked changes in drinking behaviors, such as basic psychological needs satisfaction and autonomous motivation to decrease drinking behaviors, were again examined to determine what evoked changes in prenatal alcohol use. To increase the rigor of this study, modifications in study procedures were made to address the nonspecific factors identified in the preliminary study (Osterman & Dyehouse, 2012). First, in efforts to increase variability in reported levels of drinking, basic psychological needs satisfaction, and autonomous motivation at baseline, women who screened positive for any alcohol use in the previous year, including dependency symptoms, were included in this study. This decision was based on other studies of brief interventions to decrease alcohol exposed pregnancies that included women drinking at all levels of alcohol use, including those with alcohol dependence (Chang et al., 2005; Fleming et al., 2008; Floyd et al., 2007; Handemaker et al., 1999; Manwell et al., 2000; O’Conner & Whaley, 2007). Since the pregnant women were attending prenatal care with no proscriptions regarding standard care received, additional education on risks of prenatal drinking was not provided to eligible women. Lastly, all study procedures, except for the intervention, were provided by trained research assistants. It was hypothesized that pregnant women who received the MI intervention would report significantly greater decreases in drinking behaviors than women in the comparison group. It was anticipated that pregnant women who received the MI intervention would also report greater increases in basic psychological need satisfaction and autonomous motivation to decrease prenatal alcohol use than the comparison group and that these factors would mediate the effectiveness of MI to decrease prenatal alcohol use.
2. Materials and methods
2.1. Study design and procedures
After obtaining institutional review board approval and a Certificate of Confidentiality from the National Institute of Alcohol and Alcohol Abuse (NIAAA)/National Institutes of Health (NIH), this experimental two group, pretest-posttest design study recruited pregnant women at three prenatal clinics located in a midwestern university medical center. Women attending an obstetrical clinic treating low-moderate risk pregnancies, a high-risk perinatal center, or a nurse practitioner/midwifery practice consented to participate in the study (N=184). To determine study eligibility, consenting women completed baseline demographic information and instruments measuring alcohol use for the previous 30 days and previous year. Pregnant women who were 36 weeks or less gestation, between the ages of 18 and 44 years inclusive, able to understand, speak, and read English, who reported any alcohol use in the previous year, and being available for telephone follow-ups at 30 days postbaseline and 30 days postpartum were eligible (n=122). Exclusion criteria included loss or termination of the pregnancy. In an attempt to increase diversity in drinking behaviors, women who screened for alcohol dependency symptoms were not excluded from this study as they were in the preliminary work (Osterman & Dyehouse, 2012). Also, contrary to the preliminary study, no additional education on risks of prenatal drinking was provided by the study to consenting women. Recruitment, consenting, and data collection for the entire study were provided by trained research assistants, with only the MI intervention provided by the researcher.
Women meeting inclusion criteria then entered the intervention phase of the study. A series of envelopes containing instruments measuring basic psychological need satisfaction and autonomous motivation to decrease prenatal alcohol use were sequentially numbered on the outside with a card placed in each envelope using a computer-generated table of random numbers to designate which group the woman would be randomized to – the MI intervention (MI) group or no-intervention comparison (NIC) group. The first envelope was opened for the first woman eligible for the study with the group assignment read after completion of instruments. This was repeated for the remaining women eligible for the study. Women randomized to the MI group received the MI intervention at the same or next scheduled prenatal visit. The MI sessions provided by the researcher lasted approximately 30 minutes and were digitally recorded, transcribed, and reviewed for fidelity and integrity by an MI Network of Trainers (MINT) member. The follow-up phase of the study included a 30 day postbaseline and 30 day postpartum follow-up. At both follow-ups, women in both the MI and NIC groups completed instruments reporting alcohol use, basic psychological need satisfaction, and autonomous motivation to decrease prenatal alcohol use in the previous 30 days by telephone. Women received a US $10 gift card for participation in the eligibility phase of the study. A US $20 gift card was received for participation in the intervention phase of the study and for each follow-up.
2.2. Outcome measurements
2.2.1. Prenatal alcohol use
Eligibility for this study was determined at baseline with the Alcohol Use Disorders Identification Test (AUDIT) (Babor et al., 2001) and the Quick Drinking Screen (QDS). The AUDIT is a 10 item instrument that determines previous year hazardous /harmful drinking behaviors as well as dependency symptoms. Each item is scored 0–4 with a total of 40 points possible. With any alcohol use risky during pregnancy, any positive response on the AUDIT made women eligible for this study. Cronbach’s alphas of .87-.90 support the internal reliability of the AUDIT with pregnant women at risk for prenatal alcohol use (Osterman, 2011; Osterman & Dyehouse, 2012). Cronbach’s alpha for the AUDIT in the current study was .89. Since women were recruited into the study at different weeks of gestation and considering that both the 30 day postbaseline and 30 day postpartum follow-ups occurred in less than a year’s time from baseline recruitment, it was necessary to modify the AUDIT to determine previous 30 day drinking behaviors in assessment of hazardous/harmful drinking behaviors at both follow-ups. This decision was based on a similar modification of the AUDIT by Aseltine and colleagues (2008) in assessment of previous 12 month alcohol use at baseline and previous 3 month alcohol use at follow-up in a community sample of risky drinkers.
The Quick Drinking Screen (QDS) was also administered at baseline and both follow-ups to determine average number of drink days per week and drinks per day in the last 30 days. Good agreement of aggregate reported alcohol use has been found between the QDS and the Timeline Followback (TLFB) with significant intraclass coefficients between the two measures (Dum et al, 2009; Sobell et al., 2003). Non-significant paired samples t-tests were also found, except for number of drink days per week which showed statistically significant differences between the QDS and TLFB, but not clinically significant differences. Although the QDS does not yield as detailed drinking patterns as the TLFB, it is recommended for use with women at risk for alcohol-exposed pregnancies when time does not allow for more lengthy interviews in a medical setting or over the telephone (Dum et al., 2009).
2.2.2. Basic psychological need satisfaction
The Basic Psychological Needs Scale (BPNS) (Gagne, 2003) measured pregnant women’s experiences of basic psychological needs satisfaction. The BPNS is a 21-item 7-point Likert-type scale with specified items totaled and averaged to determine subscales of autonomy, competence, and relatedness. General needs satisfaction, or total needs, is calculated by averaging the results of the three subscales and was used for this study’s purposes. Cronbach’s alphas of .80-.88 support the internal consistency of the BPNS for use with pregnant women at risk for prenatal alcohol use (Osterman, 2011; Osterman & Dyehouse, 2012), with a Cronbach’s alpha in the current study of .88.
2.2.3. Autonomous motivations to decrease prenatal alcohol use
A modified version of the Treatment Self-Regulation Questionnaire (TSRQ; Ryan & Connell, 1989; Ryan, Plant, & O’Malley, 1995) was used to measure the pregnant women’s motivation to decrease risky alcohol use at baseline and both follow-ups. In a preliminary study (Osterman, 2011), the stem of the TSRQ was modified to address a woman’s reasons for decreasing alcohol use during pregnancy (TSRQ-P: alcohol use in pregnancy version). Two items were added to address the woman’s reasons for decreasing prenatal alcohol use in relation to the baby’s health resulting in a 17-item 7-point Likert-type scale. Subscales for autonomous motivation, controlled motivation, and amotivation were determined by averaging total scores on specified items. A Relative Autonomy Index (RAI) represented the pregnant women’s autonomous motivation to decrease alcohol use and was calculated by subtracting the controlled motivation average from the autonomous motivation average. Cronbach’s alphas of .71-.76 support the internal consistency of the total TSRQ-P with pregnant women at risk for prenatal alcohol use (Osterman & Dyehouse, 2012), with a Cronbach’s alpha of .72 for this study.
2.3. Motivational interviewing intervention
Motivational interviewing was the psychotherapeutic strategy used by this study with the goal of assisting pregnant women to decrease alcohol use. Women randomized to the MI group received a one-time session of MI which lasted approximately 30 minutes. The MI session was provided by the researcher, an adult psychiatric-mental health clinical nurse specialist with specialty certification in inpatient obstetrics and trained in MI. A description of the MI intervention to decrease prenatal alcohol use as based in SDT-theory is provided in Table 1.
Table 1.
A Motivational Interviewing (MI) Intervention to Decrease Prenatal Alcohol Use Based in Self-Determination Theory (SDT)
| 3 Basic Psychological Needs (SDT: Deci & Ryan, 1985, 2000) |
4 Principles of MI (Miller & Rollnick, 2002) |
MI Intervention Methods |
|---|---|---|
| Need for Relatedness |
Establishing empathy | In a respectful, caring manner, a pregnant woman's goals for her pregnancy, as well as her beliefs and attitudes about prenatal alcohol use, are discussed. Feedback regarding alcohol use obtained in the initial assessment is provided in a non-judgmental way. |
| Need for Autonomy |
Developing discrepancy |
Simple and complex reflections, open-ended questions, and summarizations are then used to assist the pregnant woman in increased awareness of any incongruence between her goals for the pregnancy and her current drinking behaviors. |
| Rolling with resistance | Resistance may occur due to ambivalence between the pregnant woman's current behaviors and changes needed to meet pregnancy goals. With the woman's permission, neutral information and direction is provided to assist her in development of strategies for behavior change. | |
| Need for Competence |
Supporting self- efficacy |
Respect and acceptance of the pregnant woman as capable of making healthy decisions for herself and her pregnancy is supported. |
Integrity and fidelity of the MI intervention was maintained through use of the Motivational Interviewing Treatment Integrity (MITI) Scale 3.0 (Moyers, Martin, Manuel, Hendrickson, & Miller, 2005; Moyers, Martin, Manuel, Miller, & Ernst, 2007). A random selection of one out of every six digitally recorded MI sessions was coded by a member of the MI Network of Trainers (MINT) for the global dimensions of evocation, collaboration, autonomy/support, direction and empathy demonstrated by the researcher in each MI intervention. Behavioral counts of simple/complex reflections, open/closed questions, and MI adherent/nonadherent statements were also completed. Overall, the clinician demonstrated competency in the areas of global dimensions, MI adherent statements, percentage of complex reflections, and percentage of open-ended questions used during the sessions. Beginning proficiency was demonstrated overall in the ratio of reflections to questions used.
2.4 Analytic approach
Given that many of the pregnant women reported low levels of drinking at baseline and both follow-ups, a preponderance of zeros for each of the count variables (e.g., drink days per week and drinks per day) was observed. Thus, to appropriately model all count outcome variables, Poisson regression was used (Long, 1997). Linear regression was used for models with non-count outcomes (e.g., BPNS and RAI). Given the data’s panel design, generalized estimating equations (GEE) were used for all models examining differences between the MI and NIC groups in prenatal drinking behaviors across the three time points (baseline, 30 day postbaseline follow-up, 30 day postpartum follow-up) (Liang & Zeger, 1986). We did not need to exclude any women from our analyses due to missing data. Using an intention-to-treat analysis, all women who were randomized to either the MI or NIC groups were included in the analysis regardless of how much of the intervention they received or how much data were available for each of them. GEE makes use of the information available from each individual at each time point. One does not need to exclude individuals from the analyses just because they are missing data at one (or more) of the time points. Thus, no women were excluded in our analyses due to missing data.
In addition, before conducting analyses, we explored whether randomization had resulted in statistically equivalent groups. Because of the small number of women reporting alcohol use and the relatively small group sizes, it was possible that randomization would not result in equivalent groups (Kazdin, 2003). If this occurred, any differential change across time (positive or negative) or cross sectional differences between groups might result from non-equivalence at the study’s start rather than from the intervention. Thus, groups were compared on each of the outcome variables at baseline. Significant differences between the groups on drink days per week and the AUDIT were found. A visual examination of the data indicated that two women with particularly high counts on these variables were outliers and were therefore excluded from the analyses. In addition, two women incorrectly completed measures of alcohol use and were withdrawn from the analyses. All other women were included in the analyses. Given the large number of comparisons in the study, an alpha of 0.01 was used to reduce Type I errors.
3. Results
3.1 Sample description
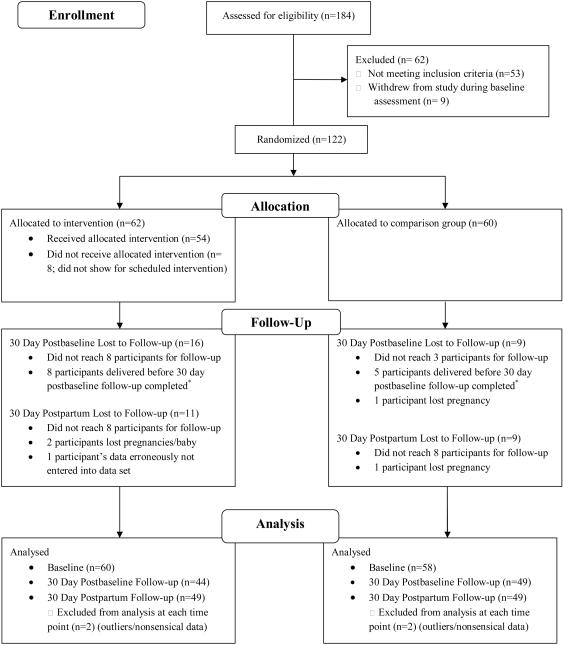
One-hundred and eighty four women consented to participate in the study. These women were primarily African American (63.6%) and single (79.9%) with an average age of 25.4 years. Most had a high school education or higher (73.9%), but an income less than $15,000 per year (73.9%). The average gestation at baseline was 24.3 weeks with women reporting an average of 3.6 pregnancies in their lifetime and an average of 1.9 births (live or stillbirths). The majority of the women reported that the current pregnancy was unplanned (77.7%), yet 71.7% reported that the pregnancy was wanted. Sixty-two women were ineligible for the intervention phase of the study due to not meeting inclusion criteria (n = 53) or withdrawing from the study at baseline (n = 9). Two significant differences were found between eligible women who participated in the intervention phase of the study (n=122) and those who did not (n=62). First, a significant difference in gestational age was found with eligible women being fewer weeks gestation (M = 23.3, SD = 8.77) than those who were ineligible at baseline (M = 26.4, SD = 9.62, t(174) = −2.10, p = .04). Also, a significant difference was found for race with more African American women ineligible for the intervention phase of the study (78.9%) than eligible (59.5%) (X2 (4, N = 178) = 10.97, p = .03). Table 2 provides descriptive statistics for the intervention and comparison groups (n = 122). To examine whether randomization resulted in equivalent groups across demographics and other relevant variables, we used a t-test for continuous variables and a chi-square test for categorical variables. No significant differences between groups were noted in demographic characteristics. Figure 1 provides a flow chart of participants’ involvement in the study.
Table 2.
Characteristics of Pregnant Women in the MI Intervention (MI: n=62) and No-intervention Comparison (NIC: n=60) Groups
| Characteristics | MI group | NIC group | |
|---|---|---|---|
| Age | Mean | 25.27 | 25.55 |
| Standard Deviation | 4.67 | 4.98 | |
| Marital Status | Married | 10(16.1%) | 8(13.3%) |
| Single | 48 (77.4%) | 50 (83.3%) | |
| Divorced | 2 (3.2%) | 0 (0.0%) | |
| Income | < $15,000/year | 44 (71.0%) | 41 (68.3%) |
| $15,000 - | 10 (16.1%) | 12 (20.0%) | |
| $30,000/year | |||
| $30,0001 - | 1 (1.6%) | 2 (3.3%) | |
| $45,000/year | |||
| $45,001 - | 1 (1.6%) | 0 (0.0%) | |
| $60,000/year | |||
| $60,001 - | 1(1.6%) | 1(1.7%) | |
| $75,000/year | |||
| Education | < 12 years | 15(24.2%) | 16 (26.7%) |
| High school graduate | 15(24.2%) | 15 (25.0%) | |
| Some college | 26(42.0%) | 25 (41.7%) | |
| 4 Year college degree | 1 (1.6%) | 2 (3.3%) | |
| Graduate degree | 1 (1.6%) | 0 (0.0%) | |
| Ethnicity | Hispanic | 1 (1.6%) | 3 (5.0%) |
| Not Hispanic | 59 (95.2%) | 52 (86.7%) | |
| Race | Black | 38 (61.3%) | 33 (55.0%) |
| White | 17 (27.4%) | 20 (33.3%) | |
| Multi-racial | 4 (6.5%) | 3 (5.0%) | |
| # of times pregnant | Mean | 3.65 | 3.79 |
| Standard Deviation | 2.01 | 2.78 | |
| # of deliveries (live or stillborn) |
Mean | 2.03 | 1.95 |
| Standard Deviation | 1.62 | 2.03 | |
| # of miscarriages or abortions |
Mean | 0.65 | 0.83 |
| Standard Deviation | 0.90 | 1.13 | |
| Gestation (weeks) | Mean | 23.60 | 23.14 |
| Standard Deviation | 8.72 | 8.72 |
*p < .05
Figure 1.
Flow chart of participant involvement in study. *Women who delivered before the postbaseline follow-up were contacted for the postpartum follow-up only to prevent overlapping of the follow-up assessments.
3.2 Group comparisons of outcome variables across time
Table 3 summarizes the outcome variables of drink days per week, drinks per day, AUDIT, BPNS, and RAI scores for the MI and NIC groups at baseline, 30 day postbaseline, and 30 day postpartum follow-ups after exclusion of outliers and incorrect responses. Low levels of alcohol use by women in both groups were noted over time with overall mean drink days per week ranging from 0 (SD = 0) to 0.16 (SD = 0.57) and mean drinks per day ranging from 0.04 (SD = 0.29) to 0.35 (SD = 0.88).
Table 3.
Mean Outcome Variables for the MI Intervention (MI) and No-intervention Comparison (NIC) Groups at Baseline, 30 Day Postbaseline, and 30 Day Postpartum Follow-ups
| Means (Standard Deviations) |
Baseline | 30 Day Postbaseline | 30 Day Postpartum | |||
|---|---|---|---|---|---|---|
|
|
||||||
| MI | NIC | MI | NIC | MI | NIC | |
| Drink Days per Week | 0.13 (0.57) | 0.12 (0.38) | 0.00 (0.00) | 0.04 (0.29) | 0.16 (0.47) | 0.08 (0.28) |
| Drinks per Day | 0.18 (0.60) | 0.24(0.88) | 0.05 (0.30) | 0.04 (0.29) | 0.35(0.80) | 0.18 (0.49) |
| AUDIT | 4.86 (4.99) | 5.60 (4.87) | 0.45 (0.98) | 0.35 (1.03) | 0.53 (1.37) | 0.41 (0.89) |
| BPNS | 5.69(0.80) | 5.45(0.89) | 6.12 (0.77) | 5.78 (0.96) | 6.19 (0.68) | 6.02 (0.85) |
| RAI | 1.75 (1.30) | 1.76 (1.21) | 1.76 (1.28) | 1.86 (1.10) | 1.93 (1.06) | 2.37(1.19) |
3.3 Poisson regression and GEE
Poisson regression and GEE were used to examine whether the MI intervention significantly decreased women’s drinking behaviors across time, relative to the comparison group. One coefficient in each model examined whether the drinking behavior trajectories generally changed across time. Another examined whether the groups generally differed from each other. The interaction term examined whether the trajectory for one group changed more rapidly than it did for the other (e.g., did women in the intervention group more rapidly reduce their drinking behaviors compared to the comparison group). The analyses suggested that the groups’ drinking behaviors did not change significantly across time for drink days per week or drinks per day, nor did the groups generally differ from each other on these variables. A statistically significant decrease in the AUDIT scores across time (b = −1.86; z = −14.21, p < 0.01) was observed for both groups, as well as statistically significant increases in the RAI and total BPNS scores across time (b = 0.3; z = 3.32, p < 0.01; and b = 0.3; z =5.75, p < 0.01) (see Table 3 for mean AUDIT, BPNS, and RAI scores across time). However, no statistically significant interactions were observed in any of the models.
Before proceeding, caution is suggested when interpreting these findings (null or otherwise) given that apparent non-linear change in drinking behaviors across time was observed. For example, as Table 3 demonstrates, women in both groups reported reduced drinking behaviors at the first follow-up as compared to baseline, followed by increased drinking behaviors at the second follow-up. Due to a limited number of time points and relatively small group sizes, this potential non-linearity could not be modeled. Despite these cautions, this study sheds important light on several issues as discussed below.
4. Discussion
The purpose of this RCT was to determine the effectiveness of MI to decrease alcohol use during pregnancy, while investigating SDT mechanisms which may have evoked a change in the women’s prenatal alcohol use. Modifications were made in this study’s methods to address nonspecific factors that may have potentially influenced preliminary findings (Osterman & Dyehouse, 2012). Women reporting any previous year alcohol use, including women who screened for dependency symptoms with the AUDIT, were recruited in attempts to increase variability in reported drinking behaviors. Standard education on the risks of prenatal alcohol use was not provided to a group of pregnant women receiving standard prenatal care to maintain the rigor of the single-session MI intervention provided and not confound study conclusions. To prevent crossover of the MI spirit into all study methods, recruitment, data collection, and follow-ups were provided by trained RA’s, with the intervention provided by the researcher trained in MI. Yet MI was not found effective in decreasing drinking behaviors or increasing basic psychological need satisfaction and autonomous motivations to decrease alcohol use in pregnant women in this study. These findings did not support study hypotheses that MI would lead to greater decreases in prenatal alcohol use and greater increases in basic psychological need satisfaction and autonomous motivations to reduce prenatal alcohol use. Yet significant, but similar decreases in AUDIT scores over time were found for both the intervention and comparison groups. Significant but similar increases in basic psychological need satisfaction and autonomous motivations to decrease prenatal alcohol use were also noted for both groups. Several factors may have contributed to these findings.
The single-session MI intervention may not have been sufficiently potent to decrease prenatal alcohol use. This outcome was consistent with other studies of single-session interventions which found no overall differences in alcohol use over time in the intervention compared to no-intervention groups (Chang et al., 2005; Handemaker et al., 1999; Osterman & Dyehouse, 2012). Multiple-session interventions have shown more promising results in reducing AEP’s in pregnant and non-pregnant women. O’Conner and Whaley (2007) found significant decreases in pregnant women’s drinking behaviors and increases in positive newborn outcomes after receiving a series of brief interventions. Multiple-session interventions to reduce the occurrence of alcohol-exposed pregnancies before conception and during the postpartum have also had positive results (Floyd et al., 2007; Manwell et al., 2000; The Project CHOICES Intervention Research Group, 2003). It may be that a single-session intervention is not sufficient to decrease prenatal alcohol use. Additional sessions of MI, or booster interventions, could be implemented in future studies to potentially strengthen MI’s effectiveness in decreasing prenatal alcohol use.
Another plausible explanation for the ineffectiveness of a single-session MI intervention to decrease prenatal alcohol use in this study could be the positive effects of alcohol screening to decrease alcohol use in the pregnant women. Aseltine et al. (2008) found significant reductions in typical number of drinks per week in a community sample of at-risk drinkers receiving alcohol screening and feedback only with consumption of almost six fewer drinks per week when compared to controls at 3 month follow-up. These findings emphasized how screening and assessment alone can decrease alcohol consumption in studies when provided for both intervention and control conditions making determination of intervention effects more difficult. The current study used the AUDIT and QDS to screen pregnant women for alcohol use in both the MI and NIC groups at baseline and both follow-ups. It is possible that these screenings may have provided an additional intervention that decreased the pregnant women’s reported alcohol use in both groups.
Evaluation of MI’s effectiveness in decreasing prenatal alcohol use may have been deterred by the low levels of alcohol use reported by women at baseline. Despite inclusion of women screening positive for any alcohol use in the previous year, including dependence symptoms, this study’s findings were consistent with preliminary work (Osterman & Dyehouse, 2012) where MI was not effective in a group of pregnant women reporting lower levels of alcohol use at baseline. Although Chang et al. (2005) and Handemaker et al. (1999) found no overall differences in drinking behaviors at follow-up in pregnant women who received a single-session intervention compared to those who did not, significant reductions in alcohol use were found for women in the intervention group who reported higher levels of alcohol use at baseline in both studies. Ballesteros et al. (2004) found brief alcohol interventions to be more effective with heavier drinkers in primary care settings. In this study, the women’s reports of low levels of alcohol use at baseline left little room for statistical determination of improvement in drinking behaviors due to the intervention (see Table 3). Instead of recruiting women with any alcohol use in the previous year, future studies could recruit women who drink at levels exceeding the NIAAA (2005) recommendations for women before pregnancy recognition, since prepregnancy drinking behaviors are predictive of prenatal drinking behaviors (Bobo et al., 2006; Goransson et al., 2003). Recruitment of women who report higher levels of alcohol use before conception which exceed the NIAAA (2005) recommendations for women of no more than 7 drinks per week or no more than 3 drinks per occasion would increase the number of women with infants at greater risk for FASD.
Considering the low levels of alcohol use reported by women in this study, a potential reason why MI was not found effective in decreasing prenatal alcohol use is that the women were already motivated to change their drinking behaviors. Motivational interviewing is a person-centered, directive counseling style which increases a person’s intrinsic motivation for change (Miller & Rollnick, 2002). It is generally more effective with persons more resistant and less ready for change, and can be counterproductive in those more ready for change (Hettema et al., 2005).For instance, in a group of cocaine dependent patients with co-morbid alcohol abuse/dependence, patients reporting low initial levels of motivation to change who received motivational enhancement therapy (MET; an adaptation of MI which includes client feedback) reported lower alcohol and cocaine relapse, fewer days of alcohol and cocaine use, and less severe alcohol problems than MET patients reporting higher initial motivation to change (Rohsenow et al., 2004). Furthermore, patients with higher initial levels of motivation to change who did not receive MET reported lower cocaine use and less severe alcohol problems than those who did receive MET. Future assessment of pregnant women’s levels of motivation to change using instruments, such as the University of Rhode Island Change Assessment Scale (URICA: DiClemente & Hughes, 1990; McConnaughy, Prochaska, & Velicer, 1983), could identify women in earlier stages of change (pre-contemplative and comtemplative stages) who would benefit more from MI than women reporting higher stages of motivation to change (action and maintenance stages) for whom MI may be contraindicated..
Race may explain the low levels of reported alcohol use by pregnant women in this study. Nonpregnant White women are approximately two times more likely to drink any alcohol and to binge drink than nonpregnant African American women (Marchetta et al., 2012). The likelihood of any alcohol use during pregnancy is similar for both groups, but pregnant White women are more likely to binge drink. The majority of the women in this study were African American, which may explain the low reported levels of alcohol use at baseline, as well as the larger percentage of African American women who were not eligible for the study (78.9%) compared to those eligible (59.5%). But heavy or binge drinking African American women are significantly less likely to reduce drinking or binging after becoming pregnant when compared to White women (Tenkku, Morris, Salas, & Xaverius, 2009). In addition, the CDC Fetal Alcohol Syndrome Surveillance Network (FASSNet) reported FAS rates to be highest for black and American Indian/Alaska Native children (Miller et al., 2002). Yet lower socioeconomic status of the mother has been identified as an important risk factor for FASD (May et al., 2009), with more FAS attributed to minority women living in poverty than to race itself (Abel, 1995). With the majority of women in this study reporting a household income of less than $15,000/year, this supports the need for culturally-sensitive interventions that address the special needs of low income African American women who drink alcohol during pregnancy.
The methods used to assess drinking behaviors in this study may have influenced this study’s results. The AUDIT (Babor et al., 2001), a valid and reliable instrument used with various populations to measure hazardous/harmful/dependent drinking behaviors, was used to measure previous year alcohol use at baseline and previous 30 day alcohol use at both follow-ups. One significant finding of this study was that AUDIT scores decreased similarly over time for both groups, but not due to the MI intervention. This was potentially due to the time frames assessed at baseline, 30 day postbaseline, and 30 day postpartum follow-ups by the AUDIT. Similar to methods used by Aseltine and colleagues (2008), previous year alcohol use was assessed at baseline with the AUDIT, while only previous 30 day alcohol use was assessed at both follow-ups. Further assessment found the baseline AUDIT scores assessing previous year drinking behaviors were not consistent with the low levels of alcohol use reported at baseline for drink days per week and drinks per day in the previous 30 day time period. But higher AUDIT scores at baseline were consistent with national rates of alcohol use in nonpregnant women that are much higher than those reported by pregnant women (51.5% of nonpregnant women reported any alcohol use in the previous 30 days with 15% binge drinking compared to 7.6% of pregnant women drinking any alcohol with 1.4% pregnant women binge drinking) (Marchetta et al., 2012). Since prepregnancy alcohol use is predictive of alcohol use during pregnancy (Bobo et al., 2006; Goransson et al., 2003), screening pregnant women for previous year alcohol use may assist in identification of women high risk for prenatal alcohol use during pregnancy. Yet the decrease in AUDIT scores over time for both groups supported the notion that many women decrease alcohol use after pregnancy recognition (Marchetta et al., 2012).
In addition to the AUDIT (Babor et al., 2001), the QDS asked quantity/frequency questions regarding drink days per week and drinks per day in the last 30 days to determine alcohol use. Women first completed these measures at baseline when they were already pregnant. As mentioned earlier, many women decrease alcohol use after pregnancy recognition (Marchetta et al., 2012), thereby providing one explanation for the low levels of alcohol use reported. Yet pregnant women may have underreported alcohol use due to concerns regarding honest admission while attending prenatal care and potential punitive consequences, such as involvement of children’s protective services, if they reported use (Golden, 2007; Thomas & Rickert, 2004). A certificate of confidentiality as provided in this study would assist in pregnant women’s honest, accurate reporting of alcohol use through protection of information shared in the study against disclosure in a civil, criminal, or other legal proceeding (NIH, 2012). Although good agreement of aggregate reported alcohol use has been found between the QDS and the Timeline Followback (TLFB; Dum et al, 2009; Sobell et al., 2003), use of the TLFB (Sobell & Sobell, 1992) in future studies may decrease the potential for recall bias by increasing women’s recollections and reporting of alcohol consumption through use of a calendar and memory prompts. Another approach to more accurately identifying pregnant women drinking at risky levels in future studies would be the assessment of direct biomarkers of alcohol use. For instance, biological monitoring of ethyl glucoronide (EtG) has found alcohol use exceeding amounts identified by self-report alone in pregnant women (Wurst, Kelso et al., 2008) and other populations (Wurst, Dursteler-MacFarland et al., 2008). Accurate identification of alcohol use in pregnant women is imperative in the provision of interventions to women most at risk for alcohol-exposed pregnancies.
Another finding of this study was the significant increase in BPNS and RAI scores over time, with no intervention effect on the increasing reports of basic psychological need satisfaction or autonomous motivations to decrease prenatal drinking behaviors (BPNS: b = 0.3; z =5.75, p < 0.01 and RAI: b = 0.3; z = 3.32, p < 0.01). According to SDT (Deci & Ryan, 1985, 2000; Ryan & Deci, 2000, 2002), a theoretical explanation for the low levels of alcohol use reported at baseline and both follow-ups is that the women’s higher levels of basic psychological need satisfaction and autonomous motivations to decrease prenatal alcohol use influenced drinking behaviors resulting in the low levels of alcohol use reported (Table 3). To examine this potential relationship, future studies should recruit women reporting higher levels of alcohol use and determine what levels of basic psychological need satisfaction and autonomous motivation to decrease prenatal alcohol use exist. This information could inform intervention development tailored to provide an external social environment of structure, relatedness, and involvement that satisfies a pregnant woman’s basic psychological needs for autonomy, competence, and relatedness leading to increased motivations to decrease alcohol use during pregnancy.
Limitations
A limitation of this study was that all women in the current study were attending prenatal care visits with no proscriptions to the standard care or education provided regarding the risks of prenatal alcohol use. Prenatal care may have provided the external social environment needed to increase the women’s basic psychological need satisfaction leading to autonomous motivations to decrease drinking behavior represented by the high BPNS and RAI scores and low alcohol use reports of women in this study. Future recruitment of a community sample of pregnant women who may not seek prenatal care due to choice, lack of resources, or engagement in high risk behaviors such as prenatal alcohol use, would determine the influence of prenatal care on drinking behaviors.
The inability to interpret the curvilinear relationships in drinking behaviors due to only three time points of data collected was a study limitation. With only one follow-up occurring during pregnancy and one in the postpartum, this limited determination of changes in drinking behaviors that occurred during pregnancy. While drinking behaviors increased at the postpartum follow-up, this could be due to the women resuming drinking behaviors since they are no longer pregnant. In Jagodzinski and Fleming’s (2007) study, 37.8% of women who reported risky drinking prior to pregnancy were engaged in risky drinking at 3 months postpartum. Although most of the preconception risky drinkers abstained from alcohol use after pregnancy recognition (87.9%), these same women reported heavy episodic drinking (18%), frequent drinking only (5%), or both heavy episodic and frequent drinking (15%) in the postpartum period. Risky drinking postpartum women were more likely to have used alcohol after pregnancy recognition (OR = 4.8, 95% CI: 2.2-10.6). Yet a positive finding was that postpartum women who were breastfeeding were less likely to report risky alcohol use in the postpartum (OR = 0.3. 95% CI: 0.2-0.5). These findings demonstrate the resumption of risky drinking behaviors in postpartum women that may have deleterious effects on the mother, infant, and family after the concern for in utero alcohol exposure has ended.
Increasing the number of follow-ups in future studies, particularly near the time of the intervention and during pregnancy, would allow for more frequent assessment of drinking behaviors to sensitively note subtle and timely changes in alcohol consumption potentially due to the intervention, while also assisting in the statistical analysis and interpretation of curvilinear results. Use of daily estimation measures of alcohol use, such as the TLFB (Sobell & Sobell, 1992), would allow for more precise measurements of alcohol use at more frequent assessment points. The recruitment of women up to and inclusive of 36 weeks gestation in the current study limited the ability to increase data collection time points due to recruitment proximity to delivery. Future studies should recruit women earlier in pregnancy to provide interventions that reduce alcohol use as early in pregnancy as possible in the prevention of FASD, as well as to assess progressive effects of the intervention. With risky drinking behaviors resuming after the delivery of the infant, interventions could also address the hazards of postpartum alcohol use to assist women in making healthier decisions about alcohol use after pregnancy and if breastfeeding.
Conclusion
In prevention of the serious, life-long consequences of FASD, provision of effective interventions that assist women to decrease prenatal alcohol use are essential. This study found that a single-session MI intervention was not effective in decreasing alcohol use in pregnant women. Future studies can increase the number of sessions of the intervention to increase potency of the intervention. Theory-based influencers of behavior change should be considered to provide interventions with the greatest potential to decrease prenatal alcohol use in pregnant women less motivated and less ready for change. Yet a major factor affecting determination of the effectiveness of the intervention in this study was the low levels of alcohol use reported by the pregnant women. Whether these reports of prenatal alcohol use were underreported or not is unknown. But the importance of accurately identifying risky alcohol use during pregnancy in women from all cultures and socioeconomic backgrounds cannot be overemphasized. Underreporting of prenatal alcohol use has been substantiated through concurrent use of biomarkers measuring alcohol use (Wurst, Kelso et al., 2008). Therefore, women whose children are at risk of FASD may not be identified as needing interventions through self-report measures. Future use of daily estimation measures of alcohol use, such as the TLFB, in conjunction with biological monitoring of alcohol use can more accurately diagnose risky drinking patterns in women. With FASD affecting 2-5% of all US live births (May et al., 2009), accurate identification of pregnant women who drink is critical in provision of effective interventions to assist them in healthier decisions and behaviors that will impact both them and their children for a life time.
Acknowledgments
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K12HD051953. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to gratefully acknowledge the expectant women at the prenatal centers who participated in this study. Special thanks to research assistants Lauren Herman, Billie Jean Kosak, Diana Nguyen, and Lucinda Romano for their contributions in recruitment and data collection for this study. A limited portion of this manuscript was presented at the Midwest Nursing Research Society 37th Annual Research Conference, Chicago, Illinois.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicology and Teratology. 1995;17(4):437–443. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Aseltine RH, Schilling EA, James A, Murray M, Jacobs DG. An evaluation of National Alcohol Screening Day. Alcohol & Alcoholism. 2008;43(1):97–103. doi: 10.1093/alcalc/agm139. [DOI] [PubMed] [Google Scholar]
- Babor , Higgins-Biddle J, Saunders J, Monteiro M. AUDIT: The alcohol use disorders identification test. 2nd World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- Ballesteros J, Duffy JC, Querejeta I, Arino J, Gonzalez-Pinto A. Efficacy of brief interventions for hazardous drinkers in primary care: Systematic review and meta-analyses. Alcoholism: Clinical and Experimental Research. 2004;28(4):608–618. doi: 10.1097/01.alc.0000122106.84718.67. [DOI] [PubMed] [Google Scholar]
- Bobo JK, Klepinger DH, Dong FB. Changes in the prevalence of alcohol use during pregnancy among recent and at-risk drinkers in the NLSY cohort. Journal of Women’s Health. 2006;15(9):1061–1070. doi: 10.1089/jwh.2006.15.1061. [DOI] [PubMed] [Google Scholar]
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: A meta-analysis of controlled clinical trials. Journal of Consulting and Clinical Psychology. 2003;71(5):843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- Chang G, McNamara T, Orav EJ, Koby D, Lavigne A, Ludman B, Vincitorio N, Wilkins-Haug L. Brief intervention for prenatal alcohol use: A randomized trial. Obstetrics and Gynecology. 2005;105(5):991–998. doi: 10.1097/01.AOG.0000157109.05453.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci E, Ryan R. Intrinsic motivation and self-determination in human behavior. Plenum Press; New York, London: 1985. [Google Scholar]
- Deci E, Ryan R. The “what” and “why” of goal pursuits: Human needs and the self-determination of behavior. Psychological Inquiry. 2000;11(4):227–268. [Google Scholar]
- DiClemente CC, Hughes SO. Stages of change profiles in outpatient alcoholism treatment. Journal of Substance Abuse. 1990;2:217–235. doi: 10.1016/s0899-3289(05)80057-4. [DOI] [PubMed] [Google Scholar]
- Dum M, Sobell LC, Sobell MB, Heinecke N, Voluse A, Johnston K. A quick drinking screen for identifying women at risk for an alcohol-exposed pregnancy. Addictive Behaviors. 2009;(34):714–716. doi: 10.1016/j.addbeh.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Lund MR, Wilton G, Landry M, Scheets D. The Healthy Moms Study: The efficacy of brief alcohol intervention in postpartum women. Alcoholism: Clinical and Experimental Research. 2008;32(9):1600–1606. doi: 10.1111/j.1530-0277.2008.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RL, O'Connor MJ, Sokol RJ, Bertrand J, Cordero JF. Recognition and prevention of fetal alcohol syndrome. Amercian College of Obstetricians and Gynecologists. 2005;106(5):1059–1064. doi: 10.1097/01.AOG.0000181822.91205.6f. [DOI] [PubMed] [Google Scholar]
- Floyd RL, Sobell M, Velasquez MM, Ingersoll K, Nettleman M, Sobell L, Mullen PD, Ceperich S, von Sternberg K, Bolton B, Skarpness B, Nagaraja J. Preventing alcohol-exposed pregnancies: A randomized control trial. American Journal of Preventive Medicine. 2007;32(1):1–10. doi: 10.1016/j.amepre.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne M. The role of autonomy support and autonomy orientation in prosocial behavior engagement. Motivation and Emotion. 2003;27(3):199–223. [Google Scholar]
- Golden J. Message in a bottle: The making of fetal alcohol syndrome. Harvard University Press; Cambridge, MA: 2007. [Google Scholar]
- Goransson M, Magnusson A, Bergman H, Rydberg U, Heilig M. Fetus at risk: Prevalence of alcohol consumption during pregnancy estimated with a simple screening method in Swedish antenatal clinics. Addictions. 2003;98:1513–1520. doi: 10.1046/j.1360-0443.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- Handmaker NS, Miller WR, Manicke M. Findings of a pilot study of motivational interviewing with pregnant drinkers. Journal of Studies on Alcohol. 1999;60:285–287. doi: 10.15288/jsa.1999.60.285. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine Criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodzinski T, Fleming M. Postpartum and alcohol-related factors associated with the relapse of risky drinking. Journal of Studies on Alcohol and Drugs. 2007;68:879–885. doi: 10.15288/jsad.2007.68.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE. Research design in clinical psychology. 4th Allyn and Bacon; Boston, MA: 2003. [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- Long J. Regression models for categorical and limited dependent variables. Sage Publications; Thousand Oaks, CA: 1997. [Google Scholar]
- Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. American Journal of Medical Genetics Part C. 2004;127C:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- Manning MA, Hoyme HE. Fetal alcohol spectrum disorders: A practical clinical approach to diagnosis. Neuroscience and Biobehavioral Reviews. 2007;31:230–238. doi: 10.1016/j.neubiorev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Manwell LB, Fleming M, Mundt MP, Stauffacher EA, Barry KL. Treatment of problem alcohol use in women of childbearing age: Results of a brief intervention trial. Alcoholism: Clinical and Experimental Research. 2000;24(10):1517–1524. [PubMed] [Google Scholar]
- Marchetta CM, Denny CH, Floyd RL, Cheal NE, Sniezek JE, McKnight-Eily LR. Alcohol use and binge drinking among women of childbearing age – United States, 2006 – 2010. Morbidity and Mortality Weekly Report. 2012;61(28):534–538. [PubMed] [Google Scholar]
- Markland D, Ryan R, Tobin V, Rollnick S. Motivational interviewing and self-determination theory. Journal of Social and Clinical Psychology. 2005;24(6):811–831. [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome: A summary. Alcohol Research and Health. 2001;25(3):159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McConnaughy EA, Prochaska JO, Velicer WF. Stages of change in psychotherapy: Measurement and sample profiles. Psychotherapy: Theory, research, and practice. 1983;20(3):368–375. [Google Scholar]
- Miller L, Tolliver R, Druschel C, Fox D, Schoellhorn J, Podvin D, Merrick S, Baio J. Fetal alcohol syndrome – Alaska, Arizona, Colorado, and New York, 1995-1997. Morbidity and Mortality Weekly Report. 2002;51(20):433–435. [PubMed] [Google Scholar]
- Miller WR. Toward a theory of MI. Motivational interviewing newsletter: Updates, education, and training. 1999;6(3):2–4. [Google Scholar]
- Miller W, Rollnick S. Motivational interviewing: Preparing people for change. 2nd The Guilford Press; New York, London: 2002. [Google Scholar]
- Miller W, Rollnick S. Motivational interviewing: Helping people change. 3rd The Guilford Press; New York, London: 2013. [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Hendrickson SML, Miller WR. Assessing competence in the use of motivational interviewing. Journal of Substance Abuse Treatment. 2005;28:19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Miller WR, Ernst D. Revised global scales: Motivational Interviewing Treatment Integrity 3.0 (MITI 3.0) 2007 Retrieved April 5, 2013 from http://casaa.unm.edu/%5Cdownload%5Cmiti3.pdf.
- National Institutes of Health (NIH) Certificates of confidentiality kiosk. 2012 Retrieved May 6, 2013 from http://grants.nih.gov/grants/policy/coc/
- National Institutes of Health (NIH) NIH science of behavior change meeting summary. 2009 Retrieved August 16, 2010 from http://commonfund.nih.gov/documents/SOBC_Meeting_Summary_2009.pdf. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) Helping patients who drink too much: A clinician’s guide. 2005 Retrieved April 5, 2013 from http://pubs.niaaa.nih.gov/publications/practitioner/CliniciansGuide2005/clinicians_guide. htm. [Google Scholar]
- O’Conner MJ, Whaley SE. Brief intervention for alcohol use by pregnant women. American Journal of Public Health. 2007;97(2):252–258. doi: 10.2105/AJPH.2005.077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman R, Dyehouse J. Effects of a motivational interviewing intervention to decrease prenatal alcohol use. Western Journal of Nursing Research. 2012;34(4):434–454. doi: 10.1177/0193945911402523. doi 10.1177/0193945911402523. [DOI] [PubMed] [Google Scholar]
- Osterman R. Feasibility of using motivational interviewing to decrease alcohol consumption during pregnancy. Journal of Addictions Nursing. 2011;22(3):93–102. [Google Scholar]
- Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test: An update of research findings. Alcoholism: Clinical and Experimental Research. 2007;31(2):185–199. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Martin RA, Colby SM, Myers MG, Gulliver SB, Brown RA, Mueller TI, Gordon A, Abrams DB. Motivational enhancement and coping skills training for cocaine users: Effects on substance use outcomes. Addiction. 2004;99:862–874. doi: 10.1111/j.1360-0443.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- Ryan R, Connell J. Perceived locus of causality and internalization: Examining reasons for acting in two domains. Journal of Personality and Social Psychology. 1989;57:749–761. doi: 10.1037//0022-3514.57.5.749. [DOI] [PubMed] [Google Scholar]
- Ryan R, Deci E. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Ryan R, Deci E. Handbook of self-determination research. The University of Rochester Press; Rochester, New York: 2002. [Google Scholar]
- Ryan RM, Plant RW, O'Malley S. Initial motivations for alcohol treatment: Relations with patient characteristics, treatment involvement and dropout. Addictive Behaviors. 1995;20:279–297. doi: 10.1016/0306-4603(94)00072-7. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Agrawal S, Sobell MB, Leo GI, Young LJ, Cunningham JA, Simco ER. Comparison of a quick drinking screen with the Timeline Followback for individuals with alcohol problems. Journal of Studies on Alcohol. 2003;64(6):858–861. doi: 10.15288/jsa.2003.64.858. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J, Templin T, Janisse J, Martier S, Sokol RJ. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. Dose-response effect. Pediatrics. 2001;108(2):e34. doi: 10.1542/peds.108.2.e34. Retrieved April 5, 2013 from http://www.pediatrics.org/cgi/content/full/108/2/e34. [DOI] [PubMed] [Google Scholar]
- Stratton KR, Howe CJ. In: Fetal Alcohol Syndrome: Diagnosis epidemiology, prevention, and treatment. Battaglia FC, editor. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Streissguth A. Offspring effects of prenatal alcohol exposure from birth to 25 years: The Seattle Prospective Longitudinal Study. Journal of Clinical Psychology in Medical Settings. 2007;14:81–101. [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Developmental and Behavioral Pediatrics. 2004;25(4):228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) White paper on screening, brief intervention and referral to treatment in behavioral healthcare. 2011 Apr 1; Retrieved from http://www.samhsa.gov/prevention/sbirt/SBIRTwhitepaper.pdf. [Google Scholar]
- Tenkku LE, Morris DS, Salas J, Xaverius PK. Racial disparities in pregnancy-related drinking reduction. Maternal Child Health Journal. 2009;13:604–613. doi: 10.1007/s10995-008-0409-2. [DOI] [PubMed] [Google Scholar]
- The Project CHOICES Intervention Research Group Reducing the risk of alcohol-exposed pregnancies: A study of a motivational intervention in community settings. Pediatrics. 2003;111(5):1131–1135. [PubMed] [Google Scholar]
- Thomas S, Rickert L. Alcohol and pregnancy policy: Gendered aspects of state legislative policy choices; Paper presented at the annual meeting of the Western Political Science Association; Portland, Oregon. Mar, 2004. [Google Scholar]
- United States Department of Health and Human Services U.S. Surgeon General releases advisory on alcohol use in pregnancy. 2005 Retrieved April 5, 2013 from http://www.surgeongeneral.gov/pressreleases/sg02222005.html. [Google Scholar]
- Vansteenkiste M, Sheldon K. There’s nothing more practical than a good theory: Integrating motivational interviewing and self-determination theory. British Journal of Clinical Psychology. 2006;45:63–82. doi: 10.1348/014466505X34192. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Kelso E, Weinmann W, Pragst F, Yegles M, Poromaa IS. Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT – A pilot study in a population-based sample of Swedish women. American Journal of Obstetrics and Gynecology. 2008;198(4):407.e1–407.e5. doi: 10.1016/j.ajog.2007.10.801. [DOI] [PubMed] [Google Scholar]
- Wurst FM, Dursteler-MacFarland KM, Auwaerter V, Ergovic S, Thon N, Yegles M, Halter C, Weinmann W, Wiesbeck GA. Assessment of alcohol use among methadone maintenance patients by direct ethanol metabolites and self-reports. Alcoholism: Clinical and Experimental Research. 2008;32(9):1552–1557. doi: 10.1111/j.1530-0277.2008.00724.x. [DOI] [PubMed] [Google Scholar]