Abstract
Objectives:
Melanoma is the deadliest skin cancer, and its incidence has been increasing faster than any other cancer. Although immunogenic, melanoma is not effectively cleared by host immunity. In this study, we investigate the therapeutic, anti-melanoma potential of the histone deacetylase inhibitor (HDACi) Panobinostat (LBH589) by assessing both its cytotoxic effects on melanoma cells as well as enhancement of immune recognition of melanoma.
Methods:
Utilizing murine and human melanoma cell lines, we analyze the effects of LBH589 on proliferation and survival. Additionally, we analyze expression of several immunologically relevant surface markers and melanoma differentiation antigens, and the ability of LBH589 treated melanoma to activate antigen specific T-cells. Finally, we assess the in vivo effects LBH589 in a mouse melanoma model.
Results:
Low nanomolar concentrations of LBH589 inhibit the growth of all melanoma cell lines tested, but not normal melanocytes. This inhibition is characterized by increased apoptosis as well as a G1 cell cycle arrest. In addition, LBH589 augments the expression of MHC and co-stimulatory molecules on melanoma cells leading to an increased ability to activate antigen specific T-cells. Treatment also increases expression of melanoma differentiation antigens. In vivo, LBH589 treatment of melanoma-bearing mice results in a significant increase in survival. However, in immunodeficient mice, the therapeutic effect of LBH589 is lost.
Conclusions:
Taken together, LBH589 exerts a dual effect upon melanoma cells by affecting not only growth/survival but also by increasing melanoma immunogenicity. These effects provide the framework for future evaluation of this HDAC inhibitor in melanoma treatment.
Keywords: Melanoma, LBH589, Panobinostat, histone deacetylase inhibitors, HDAC
Introduction
Skin cancers are the most common malignancies in the US, and melanoma accounts for the majority of skin cancer-related mortality. Moreover, melanoma is one of the few cancers that has continued to increase in incidence over time and, therefore, so too have deaths from this neoplasm [1]. Currently, metastatic melanoma has a 5-year survival of 15%, and few therapies exist which provide significant survival benefit [2].
Overcoming tolerance mechanisms and directing the immune response towards a pro-inflammatory response represent major hurdles in developing effective immunotherapy strategies against cancer. An important factor determining T-cell activation, and the type of immune response, is the type and quality of effector cell interactions with antigen presenting cells (APCs). Three signals between APCs and T-cells direct the ensuing immune response. These are 1) presentation of antigen though MHC, 2) co-stimulatory molecules, and 3) cytokine signaling. While classically associated with professional APCs, it has become increasingly evident that tumor cells harbor APC-like functions [3-5]. However, it is likely that this potential function is negatively affected by the immunosuppressive microenvironment that characterizes the tumor site, through the expression of inhibitory ligands such as PD-L1 and/or production of anti-inflammatory cytokines such as IL-10, VEGF or TGF-β [6, 7]. Strategies that are able to shift this micro-environment towards a pro-inflammatory phenotype may provide for more effective anti-tumor immunity.
HDAC inhibitors (HDACi) have demonstrated selective anti-proliferative properties and cytotoxicity to tumor cells compared with minimal effects on normal cells [8]. Indeed, two HDACi have already been approved for the treatment of patients with refractory cutaneous T-cell lymphoma. However, their therapeutic efficacy in solid malignancies including melanoma remains to be fully explored. In addition to their direct cytotoxic effects, there is growing evidence supporting previously unknown immunoregulatory properties of HDACi. In the context of melanoma, studies have already demonstrated clear immunoregulatory effects of these compounds [9] [10].
We sought to characterize the effects of the pan-HDACi LBH589 to assess its therapeutic potential for the treatment of melanoma, Herein we evaluated its direct anti-tumor effects in addition to its ability to modulate tumor immunogenicity. To this end, we found that LBH589 exerted direct cytotoxic effects on melanoma cells by the induction of apoptosis and G1 cell cycle arrest, and that it also enhanced the APC function of melanoma cells through upregulation of various immune-relevant receptors/ligands. This resulted in enhanced activation of antigen specific T-cells. Additionally, LBH589 resulted in enhanced expression of melanoma antigens that have been previously described to be recognized by tumor infiltrating lymphocytes [11]. Importantly, these in vitro effects translated into therapeutic benefit in vivo, with LBH589 treatment resulting in a significant increase in survival. Survival advantages were abrogated in immune deficient mice, highlighting the importance of the immunoregulatory effects of LBH589 in the anti-tumor response.
Materials and Methods
Mice
C57BL/6 mice were purchased from NCI laboratories, and B6.129S7-Rag1tm1Mom/J immunodeficient mice were purchased from Jackson laboratories. For in vivo tumor studies, mice were injected subcutaneously into the shaved flank with 100,000 B16 melanoma cells suspended in Hank’s Balanced Salt Solution (Invitrogen). All animal studies were performed in compliance with protocols approved by the IACUC at the University of South Florida.
Cells
Melanoma cell lines were obtained from the ATCC and cultured in RPMI 1640 supplemented with 10% FBS, 100I.U./mL penicillin, and 100μg/mL streptomycin. The human melanocyte cell line, HEMn-LP, was obtained from Invitrogen (Carlsbad, CA) and grown in manufacturer suggested media, Medium 254 supplemented with HMGS. All cell lines were grown under humidified conditions at 37° and 5% CO2.
HDACi
MGCD0103 was purchased from Selleck Chemicals (Houston, TX), and Trichostatin A (TSA) from Sigma Aldrich (St. Louis, MO). LBH589 was kindly provided by Novartis (Basel, Switzerland). For in vitro use, LBH589 was reconstituted in DMSO at greater than 1000x the final effective dose. For in vivo studies, LBH589 was dissolved in a 5% dextrose solution and sonicated to aide dissolution.
Determination of IC50 by MTS
Cells were plated at 5×103/well in 96-well flat bottom plates. The following day, media was changed to that containing LBH589 or DMSO vehicle diluted in complete medium. Cells were incubated for 72 hours. Density of viable, metabolically active cells was quantified using a standard MTS assay purchased from Promega (Fitchburg, WI) as per manufacturer’s instructions. Absorbance at 490nM was measured spectrophotometrically with background subtraction at 670nM. All values were then normalized and expressed as a percentage of DMSO control growth.
Flow Cytometry
For surface marker analysis, melanoma cells were treated with LBH589 or DMSO for 48 hours. Cells were stained with phycoerythryn (PE), fluorescein isothiocyanate (FITC) or allophycocyanin (APC) conjugated antibodies against MHC I, MHC II, CD40, CD80, or CD86. Conjugated antibodies were purchased from eBioscience (San Diego, CA). Cells were suspended in buffer containing DAPI (50ng/mL) for viability.
For apoptosis analysis, melanoma cells treated with LBH589 or DMSO for 48 hours. Cells were then stained with Annexin V according to the manufacturer’s (BD Biosciences) protocol. Cells were stained with FITC conjugated Annexin V concomitantly with propidium iodide (PI) viability staining.
For cell cycle analysis, melanoma cells were treated with indicated doses of LBH589 or DMSO control for 48 hours. Cells were washed and resuspended in 75% ethanol overnight. Samples were then washed and resuspended in PBS containing 0.1% Triton X-100. Finally, samples were treated with RNAse A and stained with PI.
A minimum of 10,000 events were collected for all experiments using an LSR II (BD Biosciences, Franklin Lakes, NJ) or FACScan flow cytometer and subsequently analyzed using FlowJo software.
Quantitative Reverse Transcriptase PCR
Cells were plated overnight then treated for 24 hours with LBH589 or DMSO. Cells were then lysed using TRIzol® from Invitrogen. RNA was isolated using a standard phenol-chloroform separation protocol, and cDNA generated using iScript™ from Bio-Rad (Hercules, CA). Expression was assessed by qRT-PCR using a SYBR Green system. Primers for human GAPDH (forward: GAAGGTCGGAGTCAACGGATT, reverse: ATGGGTGGAATCATATTGGAAC) and mouse 18s ribosomal RNA from Qiagen (Shanghai, China) were used for reference genes. Primers for human gp100 (forward: TGGAGAGGTGGTCAAGTGTC , reverse: TGGCAATACCTTTTGGCTTC), mart1 (forward: AAGGAAGGTGTCCTGTGCC, reverse: TCAGCCGTGGTGTAAGAGTG), tyrp1 (forward: GACATGCAGGAAATGTTGC, reverse: CATCAAGTCATCCGTGCAGA), and tyrp2 (forward: GCAAGTGCACAGGAAACTTTG, reverse: CCGAATCACTGGTGGTTTCT) were utilized for human cells. Primers for mouse gp100 (forward: CATCAATGGGAGCCAGGTG, reverse: TTCGGAGGTTTAGGACCAGA), mart1 (forward: GGAAGGTGTCCTGTGCTGA, reverse: TGACATAGGAGCGTCTGTGC), tyrp1 (forward: GCAGCTCTGTGCTGTATTTTCA, reverse: GGGGGAGGACGTTGTAAGAT), and tyrp2 (forward: GTGCGACAGCTTGGATGACTA, reverse: CAGGCAATCTTGCACATTTTT) were utilized for murine cells.
T-cell Activation Studies
B16 melanoma cells were treated with indicated doses of LBH589 or DMSO for 48 hours. Cells were then washed with media and subsequently ovalbumin (OVA) specific antigen specific CD4+ T-cells (OTII) plus OVA peptide (Ile-Ser-Gln-Ala-Val-His-Ala-Ala-His-Ala-Glu-Ile-Asn-Glu-Ala-Gly-Arg) were added. Twenty-four hours later, supernatant was collected and assessed for cytokine production by a standard ELISA protocol.
Statistical Analysis
Significance of melanoma growth inhibition and T-cell cytokine production was determined by one-way ANOVA using GraphPad Prism 5.0 software. In addition, GraphPad Prism 5.0 software was used to determine significance of survival curves. Values of p<0.05 were considered significant.
Results
HDAC inhibitors have direct anti-melanoma activity in vitro
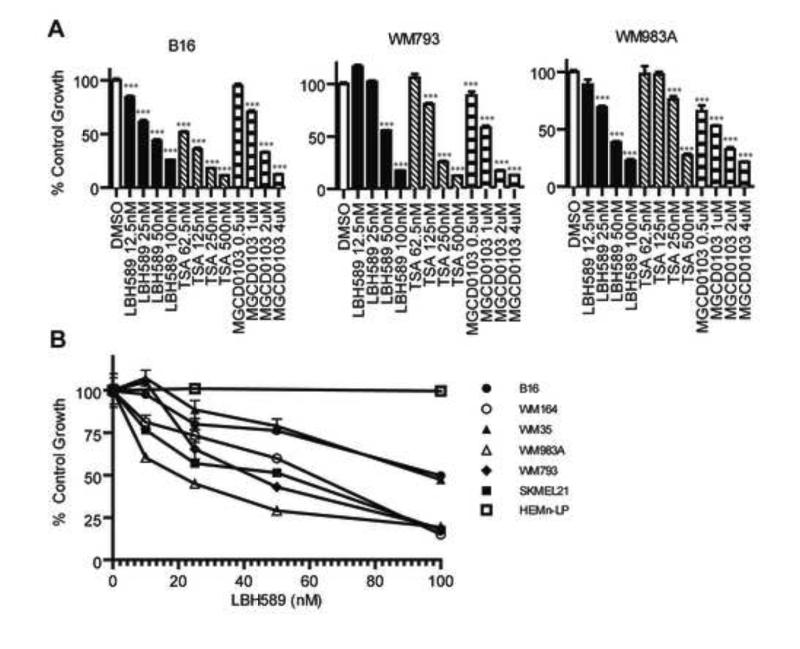
We initially determined the direct anti-melanoma efficacy of various HDACi. As shown in Figure 1A, the pan-HDAC inhibitors LBH589 and TSA in addition to the class 1 specific HDACi MGCD0103 inhibited the growth of murine melanoma B16 and two human melanoma lines, WM793 and WM983A. LBH589 had a potent effect on melanoma growth with IC50 values in the low nanomolar range.
Figure 1.
LBH589 is a potent inhibitor of melanoma cell growth in vitro.(A) Two human (WM793 and WM983A) and one murine (B16) melanoma cell lines were plated in triplicate and treated for 72 hours with indicated with indicated doses of LBH589 (black), TSA (diagonal striped), MGCD0103 (horizontal striped) or DMSO control (white). An MTS assay to determine relative amounts of metabolically active cells. Values were converted to a percentage of DMSO treatment growth for each cell line. Means +/− SEM were graphed and doses compared to DMSO control (***p<0.001). (B) Indicated melanoma cell lines, along with the human melanocyte cell line HEMn-LP, were plated in triplicate and treated for 72 hours with LBH589. Using an MTS assay, dose response curves were generated.
To further assess the anti-melanoma activity of LBH589, its effects on additional human melanoma cell lines were assessed in vitro. All treated cell lines displayed significant, dose-dependent impairment in growth (Figure 1B). IC50 values ranged from 25nM to 100nM. Importantly, LBH589 inhibited the growth of human melanoma cells with different genotypic and phenotypic characteristics such as cell lines with BRAF mutations (WM793, WM983A, WM35 and WM164), melanoma cells with constitutively activated STAT3 (WM793) and the CDKN2A mutant cell line SKMEL21 [12].
To determine the tumor selectivity of LBH589 upon cell growth inhibition we next assessed whether this compound impaired the growth of a non-transformed, human melanocyte cell line. As shown in Figure 1B (open squares) at the concentrations used, no impairment in the growth of normal melanocytes was observed.
To mechanistically dissect the inhibitory effect of LBH589, we investigated two known effects of HDACi, cell death and cell cycle arrest [13, 14]. Measurement of viability and surface expression of phosphatidylserine evidenced a marked increase in cellular death in the three cell lines analyzed (Fig 2A). To investigate any potential cell cycle inhibitory effects of LBH589, propidium iodide staining of DNA in fixed cells 48-hours post LBH589 treatment was used to determine cell cycle distribution. Consistent with previous reports of HDACi anti-tumor effects in other malignancies [15], LBH589 induced a G1 cell cycle arrest in melanoma lines WM793 and WM983A (Figure 2B). Additionally, a G2 arrest is apparent in WM983A, but not WM793 cells.
Figure 2.
LBH589 induces both apoptosis and a G1 cell cycle arrest in melanoma cells. Indicated melanoma cell lines were plated overnight. The following day, cells were treated with indicated concentrations of LBH589 or equivalent volume of DMSO for 48 hours. (A) Cells were assessed for viability and phosphatidylserine translocation by means of propidium iodide and annexin V staining respectively and analyzed by flow cytometry. (B) Cell cycle distribution of cells was determined by propidium iodide staining of fixed cells. Analysis and percent distributions were determined using FlowJo software. DMSO treatment is shown in black, 25nM LBH589 in blue, and 50nM in red.
LBH589 augmented expression of immunologically relevant molecules in melanoma cells, enhancing T-cell activation
Previous reports have suggested an immunologic effect of HDACi on tumor cells. To address if such effects result from LBH589 treatment, we stained melanomas for various immunologically relevant molecules. In B16 cells LBH589 treatment resulted in upregulation of MHC class I, MHC class II as well as the costimulatory molecules CD40, CD80 and modest upregulation of CD86 (Figure 3A, row 1). In WM793 LBH589 treatment resulted in similar upregulation of MHC class I and class II. However, while modest upregulation of CD40 and CD86 were noted, no differences in CD80 were seen (Figure 3A, row 2). As well, in WM983A upregulation of MHC class I was seen, but no changes in class II were observed. Increases in CD40 and CD86 expression were also seen, but no alterations were seen in CD80 levels (Figure 3A, row 3).
Figure 3.
LBH589 increases the immunogenicity of melanoma. (A) Indicated melanoma cell lines were plated overnight. The following day, cells were treated with 50nM LBH589 or equivalent volume of DMSO for 48 hours. Cells were assessed by flow cytometery for expression of indicated surface markers. Expression levels of DMSO treated cells are shown in grey outline, treatment with 50nM LBH589 is shown in black outline and unstained auto-florescence is shown in solid grey. Data shown are representative of results from three independent experiments. (B) B16 cells were treated with indicated doses of LBH589 for 48 hours. Cells were washed, then irradiated, loaded with ovalbumin peptide and plated with naöve OTII T-cells for 24 hours. Supernatant was collected and analyzed by ELISA for IFN-γ and IL-2 production. Cytokine levels are compared against DMSO treatment group (**p<0.01, ***p<0.001). (C) B16 and WM793 cells were plated overnight, then treated for 24 hours with indicated doses of LBH589 or DMSO. Expression of mRNA for indicated melanoma antigens was assessed by qRT-PCR. Data shown are representative of results from three independent experiments.
Next, we determined whether this enhanced expression of immunologically relevant molecules rendered melanoma cells better able to activate antigen-specific T-cells. To answer this question B16 murine melanoma cells (H-2b) were treated with LBH589 and then cultured with naöve OVA-specific CD4+ T-cells (OT-II) with the presence of cognate OVA-peptide. CD4+ T-cells encountering cognate peptide onLBH589-treated B16 cells produced significantly higher levels of both IL-2 and IFN-γ as compared to T-cells encountering antigen in untreated or DMSO treated melanoma cells (Figure 3B).
While enhanced expression of costimulatory molecules and MHC molecules can aid in directing the type and magnitude of the immune response, recognition of tumor by T-cells is essential to generating an anti-tumor immune response. Consequently, we analyzed expression of several melanoma differentiation antigens in LBH589 treated melanoma. In both B16 and WM793 cells expression of gp100, mart1, tyrp1 and tyrp2 was enhanced (Figure 3C).
The in vivo anti-melanoma efficacy of LBH589 requires an intact adaptive immune system
Given these effects of LBH589 in vitro on melanoma cells, we assessed whether this would translate to a relevant anti-melanoma effect in vivo. C57BL/6 mice were challenged with B16 melanoma cells injected subcutaneously. When tumors became palpable (> 3 mm), melanoma bearing mice were randomly assigned to receive either 5% dextrose in PBS vehicle control, or LBH589 (25mg/kg), each administered three times weekly by intraperitoneal (ip) injection. As shown in Figure 4A, a significant increase in survival was observed in those mice treated with LBH589.
Figure 4.
In vivo, LBH589 administration prolongs survival in immunocomptent, but not immunodeficient, melanoma bearing mice. C57BL6 mice or immunodeficient B6.129S7-Rag1tm1Mom/J were injected subcutaneously with B16 melanoma. Ten days following inoculation, tumors were visible and treatment with 25mg/kg LBH589 or dextrose vehicle control commenced. Treatment was administered three times weekly by intraperitoneal injection.
To address the importance of enhancement of immunogenicity in generating a survival advantage, C57BL/6 SCID mice, lacking T and B-cells, were also challenged with B16 melanoma cells. Unlike immunocompetent melanoma bearing, no survival advantage was observed in immunodeficient animals treated with LBH589. These results indicate that the antitumor effect of LBH589 required an intact adaptive immune system and points to the immunological anti-tumor effects triggered by LBH589 as playing a dominant role in its in vivo anti-melanoma activity.
Discussion
In the past several years, a number of therapeutic approaches have sought to improve the weak antigen-presenting capabilities of solid tumors by either genetically modifying these cells to enforce the expression of adhesion/costimulatory molecules [16] or by using cytokines such as IFNs to upregulate the expression of MHC molecules in tumor cells in vivo [17]. Although these approaches generated productive immune responses, the duration and magnitude of these effects were transient and not strong enough to eradicate solid malignancies. Novel therapies endowed with the dual ability to influence tumor growth as well as its immunogenicity, such as shown here, might be more successful in triggering durable antitumor immune response against solid malignancies, including metastatic melanoma.
Previously our group demonstrated that the pan-HDACi LAQ824, which belongs to the same family of hydroxamic acid derivatives as LBH589, augmented the expression of MHC and costimulatory molecules in professional APCs by inhibiting the production of the immunosuppressive cytokine IL-10 and by increasing the production of several pro-inflammatory mediators. Such an effect resulted in the generation of inflammatory APCs that effectively activate antigen-specific CD4+ T-cells and restore the responsiveness of anergic T-cells [18]. As shown here, the positive immunological effects triggered by this family of HDAC inhibitors is not limited to professional APCs, since treatment of melanoma cells with LBH589 also resulted in increased immunogenicity and effective T-cell activation. However, it remains to be determined whether the changes observed in LBH589-treated melanoma cells involved the same mechanism(s) identified in professional APCs. Some evidence presented here point to some mechanistic differences. For instance, in APCs treated with LBH589 we have observed a consistent upregulation of MHC and B7.2 costimulatory molecules. This effect is however not always seen in human melanoma cells lines. It is possible that alternate epigenetic mechanisms are responsible for silencing CD86 in human melanoma cells, which might not be solely reverted by HDAC inhibition.
In this study, we have described a dual anti-melanoma effect of the HDAC inhibitor LBH589: direct cytotoxicity and augmentation of melanoma immunogenicity. The direct cytotoxic effects of LBH589 are mediated by a G1 cell cycle arrest and induction of apoptosis in melanoma cells. Intriguingly, as seen in WM983A, LBH589 also is capable of inducing a G2 arrest in some melanomas. While G2 arrests are less common in HDAC inhibitor treated cells than G1 arrests, such arrests are known to occur [19]. Melanoma cell lines are highly heterogeneous[20], and it is likely that mutational differences in WM983A cells result in this G2 arrest. Future work is needed to test this hypothesis.
The immunological effects of LBH589 are largely due to augmentation of the APC function of melanoma cells through up-regulation of immune-relevant receptors/ligands leading to increased activation of antigen specific T-cells. Additionally, robust increases in expression of melanoma differentiation antigens result from LBH589 treatment. While enhancement of APC function by way of HDAC inhibitors has previously been reported[21], few reports of this enhanced antigen expression exist [22]. The implications of this HDAC inhibitor mediated antigen upregulation are potentially far reaching. For example, upregulation of melanoma antigens could be utilized to improve T-cell adoptive therapy by way of enhancing ex vivo T-cell expansion or in vivo tumor recognition. Further experiments will need to address such utilization, if antigen upregulation is also seen in other malignancies, and if treatment with other HDAC inhibitors produces similar results.
Importantly, the demonstrated in vitro properties of LBH589 treatment translate into positive in vivo effects. LBH589 induces a significant increase in survival in melanoma bearing mice. However, in mice lacking adaptive immunity this survival advantage is lost, highlighting the importance of the immunomodulatory effectors of LBH589.
Our demonstration that LBH589 is an effective therapeutic strategy in a murine model of melanoma provides the basis for evaluating the efficacy of this compound in human melanoma. The additional demonstration of aberrant expression of several HDACs in human melanoma cells [23, 24] provides further support for the evaluation of the therapeutic efficacy of LBH589 in this disease. Compared to LAQ824, for which clinical development was stopped, LBH589 has a better safety profile. An additional advantage of LBH589 is that it is an oral agent. These properties have led to the clinical development of LBH589, which is currently undergoing evaluation in a phase III clinical trial for patients with multiple myeloma.
The previously unknown dual positive effects of LBH589 upon melanoma cells, together with our recent findings confirming that LBH589 is more potent than LAQ824 in inhibiting melanoma cell proliferation (data not shown), provide the framework for the future evaluation of LBH589 either as a single agent or in combination with other immunomodulatory agents such as ipilimumab in human metastatic melanoma.
Acknowledgements
We extend our appreciation to The Flow Cytometry Core and Animal Facilities at H. Lee Moffitt Cancer Center and Research Institute for providing valuable technical assistance.
Financial Support: This work was supported by PHS grants CA153246 (AV), CA100850 (EMS) and by a generous grant from the Donald A. Adam Comprehensive Melanoma Research Center (CMRC).
Footnotes
Conflicts of Interest: Peter Atadja is currently employed by Novartis Pharmaceuticals
References
- 1.Rebecca Sigel DN, Ahmedin Jemal. Cancer Statisics. Vol. 63. A Cancer Journal for Clinicians; CA: 2013. 2013. pp. 11–30. [Google Scholar]
- 2.Korn EL, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–34. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 3.Hersey P, et al. Expression of the co-stimulatory molecule B7 on melanoma cells. International journal of cancer. Journal international du cancer. 1994;58(4):527–32. doi: 10.1002/ijc.2910580413. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, et al. Production of IL-10 by melanoma cells: examination of its role in immunosuppression mediated by melanoma. International journal of cancer. Journal international du cancer. 1994;56(5):755–60. doi: 10.1002/ijc.2910560524. [DOI] [PubMed] [Google Scholar]
- 5.Propper DJ, et al. Low-dose IFN-gamma induces tumor MHC expression in metastatic malignant melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9(1):84–92. [PubMed] [Google Scholar]
- 6.Liu CZ, et al. Overexpression and immunosuppressive functions of transforming growth factor 1, vascular endothelial growth factor and interleukin-10 in epithelial ovarian cancer. Chin J Cancer Res. 2012;24(2):130–7. doi: 10.1007/s11670-012-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azuma T, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111(7):3635–43. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks PA. The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Expert Opin Investig Drugs. 2010;19:1049–1066. doi: 10.1517/13543784.2010.510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan AN, Gregorie CJ, Tomasi TB. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer immunology, immunotherapy : CII. 2008;57(5):647–54. doi: 10.1007/s00262-007-0402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vo DD, et al. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009;69(22):8693–9. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadrup SR. The antigen specific composition of melanoma tumor infiltrating lymphocytes? OncoImmunology. 2012;1(6):935–936. doi: 10.4161/onci.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198(1):63–9. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu L, et al. Histone deacetylase inhibitors trigger a G2 checkpoint in normal cells that is defective in tumor cells. Mol Biol Cell. 2000;11(6):2069–83. doi: 10.1091/mbc.11.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insinga A, et al. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med. 2005;11(1):71–6. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- 15.Woan KV, et al. Modulation of antigen-presenting cells by HDAC inhibitors: implications in autoimmunity and cancer. Immunol Cell Biol. 2012;90(1):55–65. doi: 10.1038/icb.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonia SJ, et al. Phase I trial of a B7-1 (CD80) gene modified autologous tumor cell vaccine in combination with systemic interleukin-2 in patients with metastatic renal cell carcinoma. J Urol. 2002;167(5):1995–2000. [PubMed] [Google Scholar]
- 17.Brouwer RE, et al. Loss or downregulation of HLA class I expression at the allelic level in acute leukemia is infrequent but functionally relevant, and can be restored by interferon. Hum Immunol. 2002;63(3):200–10. doi: 10.1016/s0198-8859(01)00381-0. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, et al. Histone deacetylase inhibitor LAQ824 augments inflammatory responses in macrophages through transcriptional regulation of IL-10. Journal of immunology. 2011;186(7):3986–96. doi: 10.4049/jimmunol.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strait KA, et al. Histone deacetylase inhibitors induce G2-checkpoint arrest and apoptosis in cisplatinum-resistant ovarian cancer cells associated with overexpression of the Bcl-2-related protein Bad. Mol Cancer Ther. 2005;4(4):603–11. doi: 10.1158/1535-7163.MCT-04-0107. [DOI] [PubMed] [Google Scholar]
- 20.Paraiso KH, et al. The HSP90 inhibitor XL888 overcomes BRAF inhibitor resistance mediated through diverse mechanisms. Clin Cancer Res. 2012;18(9):2502–14. doi: 10.1158/1078-0432.CCR-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan AN, Gregorie CJ, Tomasi TB. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol Immunother. 2008;57(5):647–54. doi: 10.1007/s00262-007-0402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodyear O, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116(11):1908–18. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- 23.Pan L, et al. HDAC4 inhibits the transcriptional activation of mda-7/IL-24 induced by Sp1. Cell Mol Immunol. 2010;7(3):221–6. doi: 10.1038/cmi.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark M, Hayward N. Genome-wide loss of heterozygosity and copy number analysis in melanoma using high-density single-nucleotide polymorphism arrays. Cancer Res. 2007;67(6):2632–42. doi: 10.1158/0008-5472.CAN-06-4152. [DOI] [PubMed] [Google Scholar]