Abstract
Pain-related self-efficacy and pain-related fear have been proposed as opposing predictors of pain-related functional outcomes in youth with chronic pain. Self-efficacy is a potential resiliency factor that can mitigate the influence pain-related fear has on outcomes in youth with chronic pain. Drawing from theoretical assertions tested among adults with chronic pain, this study aimed to determine whether pain-related self-efficacy mediates the adverse influence of pain-related fear on functional outcomes in a sample of youth with chronic headache. In a cross-sectional design of 199 youth with headache, self-efficacy was strongly associated with fear, disability, school impairment, and depressive symptoms. Pain intensity and self-efficacy were only modestly related, indicating level of pain has less influence on one’s confidence functioning with pain. Self-efficacy partially mediated relationships between pain-related fear and both functional disability and school functioning but did not mediate the relationship between pain-related fear and depressive symptoms. These results suggest that one’s confidence in the ability to function despite pain and fear avoidance both uniquely contribute to pain-related outcomes in youth with chronic headache. These results further suggest that treatment for chronic headache in youth must focus on not only decreasing pain-related fear but also on enhancing a patient’s pain-related self-efficacy.
Perspective
Pain-related self-efficacy is an important resiliency factor impacting the influence of pain-related fear on functional disability and school functioning in youth with headache. Enhancing self-efficacy may be a key mechanism for improving behavioral outcomes. Clinicians can reduce pain-related fear and enhance pain-related self-efficacy through interventions that encourage accomplishment and self-confidence.
Keywords: pain-related self-efficacy, pain-related fear, mediation analysis, youth, headache
Headache is the most common complaint in pediatrics36, with results of a recent systematic review indicating that 60% of children and adolescents worldwide experience headaches of varying frequency and duration1. The majority of pediatric headache patients continue to exhibit headaches up to 40 years after their initial presentation6. Thus, headaches are a persistent problem in youth22. Within the literature examining biopsychosocial stressors during pediatric chronic pain experiences, pain-related fear and pain-related self-efficacy have been identified as two important cognitive factors8; 41.
According to the Fear-Avoidance Model of Pain49, fear emerges when stimuli related to pain are perceived as threatening, with heightened fear leading to behavioral avoidance and increased disability. Disuse and depression may follow, fueling a cycle of more pain, fear, and disability. In this context, fear-avoidance has been characterized as a psychological risk factor for increased pain and diminished activity7; 29. By contrast, self-efficacy refers to the confidence an individual has regarding their ability to perform a particular behavior4. Pain-related self-efficacy consists of one’s beliefs, while in pain, about abilities to function effectively13; 8; 14. Self-efficacy has been characterized as a protective psychological resource or resiliency factor associated with lower pain and better physical functioning in adults with chronic pain54; 32; 45; 40.
Heightened pain-related fear has been associated with poor pain-related outcomes such as decreased functional disability and increased depression42. Pain -related self-efficacy has been associated with positive pain-related outcomes including better school attendance20; 21. Thus, understanding the effects of diminished pain-related fear and enhanced pain-related self-efficacy among youth suffering from chronic headache could improve their clinical care and promote strong, lasting health-related behavioral outcomes.
Research among adults experiencing chronic pain, particularly those with low back pain, has examined the mediating role of pain self-efficacy in the relationship between pain-related fear and chronic pain outcomes13; 14; 53; 15; 11. Several studies53; 11 have shown that self-efficacy may have a more direct link than fear or avoidance of movement to pain and disability, prompting Woby and colleagues to propose a revised fear-avoidance model that directly incorporates the mediating role of self-efficacy53. In this modified model, it is suggested that avoidance behavior is likely to occur when pain-related fear leads to a reduction in functional self-efficacy whereas avoidance behavior is less likely to occur when pain-related fear does not lead to this kind of reduction. Their presumption is that self-efficacy can change, or weaken, the relationship between fear and pain-related outcomes through its function, specifically, as a mediator in the relations between pain-related fear and pain intensity and pain-related fear and disability.
The influence of pain-related self-efficacy within the context of the Fear-Avoidance Model of pain has yet to be examined in youth with chronic pain, including headache. If pain-related self-efficacy can be shown to be an important beneficial influence mitigating the negative effects of fear in youth with long-term pain, then self-efficacy could be a promising target in the development of interventions to improve functional outcomes for these youth. This study proposes that pain-related self-efficacy among youth with chronic headache will play a mediating role in relations between pain-related fear and pain-related disability. We hypothesize that pain-related self-efficacy, a demonstrated cognitive protective factor, will partially mediate the relationships observable between (1) pain-related fear and functional disability, (2) pain-related fear and school functioning, and (3) pain-related fear and depressive symptoms, thereby influencing both physical and psychological well-being in youth with chronic headache.
Method
Participants
All English-speaking patients 8 – 17 years of age who underwent a multidisciplinary pain evaluation for headache at a tertiary pediatric headache clinic in a large, urban northeast pediatric hospital between September, 2011 and May, 2013 were eligible for the study. One parent of each child also participated. Of the 234 children and adolescents and parents approached to participate in this study, 205 consented and 199 completed the pain-related self-efficacy measure for inclusion in these analyses, resulting in an 88% consent rate and 85% completion rate. All participants were evaluated at their first, or initial, contact with the clinic.
Measures
Pain-related self-efficacy
The Pain Self-Efficacy Scale (PSES-C)8 is a 7-item measure assessing children’s self-efficacy for functioning normally when in pain. Example items include “How sure are you that you can take care of yourself when you have pain?” and “How sure are you that you can do well in school when you have pain?” Items are scored on a five-point Likert scale with higher scores indicating poor self-efficacy. Initial validation provided support for the measure’s validity and reliability8. In the current study, the coefficient alpha level of the total score was 0.90.
Pain-related fear
The 24-item Fear of Pain Questionnaire (FOPQ-C)41 assesses children’s self-reported perceptions of pain-related fears and avoidance behaviors. The FOPQ-C consists of 24 items and two subscales: Fear of Pain (“I worry when I am in pain”; 13 items) and Avoidance of Activities (“I avoid making plans because of my pain”; 11 items). Items are rated on a five-point scale from 0=strongly disagree to 4=strongly agree. Higher total scores indicate higher levels of pain-related fear and avoidance. The FOPQ-C has demonstrated validity and reliability.41 In the current study, the coefficients alpha level of the total score was 0.94.
Pain intensity
During the pain evaluation, children were asked to provide their average pain rating on an 11-point verbal numeric rating scale from 0 (no pain) to 10 (most pain possible). This is a reliable and valid method for obtaining children’s self-report of pain in this age group50.
Functional disability
The Functional Disability Inventory (FDI)51 is a self-report scale that assesses children’s difficulty in physical and psychosocial functioning due to physical health. The instrument consists of 15 items concerning perceptions of activity limitations during the past two weeks; total scores are computed by summing the items. Higher scores indicate greater disability. The FDI has demonstrated reliability and validity10. Alpha reliability for the current sample was 0.91.
School functioning
The Pediatric Quality of Life Inventory (PedsQL)44 school functioning subscale is a five-item parent-reported measure of adolescent school functioning. Items all begin with the stem, “In the past one month, how much of a problem has your child had with …” and response options range from never (0) to always (4). Example items are “Paying attention in class” and “Keeping up with school work”. Raw scores are then transformed into standard scores on a scale of 0–100, with higher scores indicating better functioning (less impairment). Alpha reliability in this sample was 0.86.
Depressive symptoms
The Children’s Depression Inventory (CDI)24; 25 is a well-validated 27-item self-report measure of children’s depressive symptoms that has been widely used in pediatric pain studies. It is a recommended outcome measure for clinical trials in pediatric chronic pain31. Items are rated on a three-point scale from 0 to 2 and were summed to obtain a total score that was converted to a T-score. Higher scores indicated higher levels of depressive symptoms. Alpha reliability in this sample was 0.90.
Procedure
Participants were recruited during their multidisciplinary headache clinic appointment within a large tertiary children’s hospital. The hospital’s Institutional Review Board approved the study. Patients and their parents were approached by a research assistant during their evaluation and were asked to consent/assent both for this particular study and also if their responses to clinic measures could be used for research purposes.
Statistical Analyses
Data were analyzed with parametric tests using PASW 18.0 for Windows (SPSS Inc, Chicago, IL). Descriptive statistics including the means, standard deviations, and ranges were conducted to examine underlying assumptions of normality for all variables of interest. Internal consistency ratings were calculated as well. Pearson product moment correlations were used to examine relationships among the proposed outcomes and the mediator.
A regression analysis with Preacher and Hayes’ bootstrap script37 (with n=5000 bootstrap samples) (http://afhayes.com/spss-sas-and-mplus-macros-and-code.html) was employed to assess the indirect effects, specifying a 95% Bias Corrected and Accelerated Confidence Interval (BCACI)55. In assessing mediation with this method, the total effect (weight c; a regression coefficient) of an independent variable (IV) on a dependent variable (DV) is composed of a direct effect (weight c′; a regression coefficient) of the IV on the DV and an indirect effect (weight a × b) of the IV on the DV through a proposed mediator (M). Weight a signifies the effects of the IV on the M while weight b reflects the effect of the M on the DV, partialling out the effect of the IV. Mediation is demonstrated if the BCACIs do not contain zero. It is important to note that total mediation is not the goal, thus we tested for partial mediation. Average pain was entered as a control variable in the mediation analyses for all three proposed outcomes using Preacher and Hayes’ bootstrap script.37 In addition, age was included as a control variable in the same manner together with average pain in the mediation assessment for school functioning. Average pain, age, and gender were included likewise as control variables in the mediation assessment for depressive symptoms. Neither age nor gender was included as a control variable in the mediation analysis for functional disability because neither variable was significantly correlated with functional disability.55
Results
Participant characteristics
Basic demographic (e.g., age, gender) information was collected from patient charts (Table 1). Parents completing the study questionnaires were predominantly mothers (91%). Headache diagnoses assigned individually by one of three clinic-affiliated physicians who conducted the medical portion of the clinical evaluations for these participants were used, based on the International Classification of Headache Diagnoses-II (ICHD-II): migraine (25%); tension-type headache (24%); migraine and tension (23%); other primary headache disorders (largely new daily persistent headache), (12%); attributed to trauma or injury to the head/neck (8%); and other (e.g., occipital neuralgia; 8%). Duration of pain varied greatly from 1 month to 194 months, with median duration of pain 19 months. Included here are 11 patients (5.5% of the total sample) reporting a duration of pain less than 3 months; because of their referral to a tertiary care clinic, we consider, regardless of duration of pain, their headache diagnosis to be complex enough to be worthy of inclusion in these analyses. A large percentage of parents were well educated (i.e., college graduate or higher: 73% for mothers, 66% for fathers).
Table 1.
Participant demographic characteristics.
| VARIABLE | RANGE | MEAN (SD) | FREQUENCY |
|---|---|---|---|
| Gender | |||
| Male | 27% | ||
| Female | 73% | ||
| Ethnicity | |||
| Caucasian | 90% | ||
| African American | 3% | ||
| Asian | 2% | ||
| Hispanic | 3.5% | ||
| Parent marital status | |||
| Married | 78% | ||
| Single | 6% | ||
| Divorced/separated | 14% | ||
| Spouse deceased | 2% | ||
| Duration of pain (months) | 1 – 194 | 34 (37) |
Preliminary analyses
Average pain was related to higher levels of pain-related fear, functional disability, and depressive symptoms. Age was related to higher levels of pain-related fear and depression and worse school functioning. Gender was related to higher levels of fear and depression. Pain intensity was modestly correlated with pain-related self-efficacy (Table 2). Pain-related fear was correlated with lower levels of pain-related self-efficacy, functional disability and depression. With regards to self-efficacy and our outcomes of interest, poorer self-efficacy was associated with higher levels of functional disability, depression, and worse school functioning.
Table 2.
Intercorrelations, means, standard deviations, and ranges for pain-related fear, pain-related self-efficacy, and disuse/disability.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | N | M | SD | Range |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age (years) | -- | 0.12 | .16* | 0.06 | 0.14 | −0.3** | .29** | 199 | 13.8 | 2.53 | 8 – 18 |
| 2. Pain Intensity | -- | .22** | .15* | .23** | −0.12 | .15* | 195 | 5.9 | 1.65 | 2 – 10 | |
| 3. Pain-Related Fear | -- | .68** | .54** | −.42** | .54** | 199 | 37.3 | 18.82 | 0 – 93 | ||
| 4. Pain-Related Self-Efficacy | -- | .59** | −.39** | .40** | 199 | 19.5 | 6.52 | 0 – 35 | |||
| 5. Functional Disability | -- | −.40** | .39** | 194 | 17.5 | 10.98 | 0 – 46 | ||||
| 6. School Functioning | -- | .41** | 189 | 52.7 | 22.43 | 0 – 100 | |||||
| 7. Depressive Symptoms | -- | 195 | 9.32 | 7.63 | 0 – 39 |
Mediation Analyses
Does self-efficacy mediate the relation between pain-related fear and outcomes?
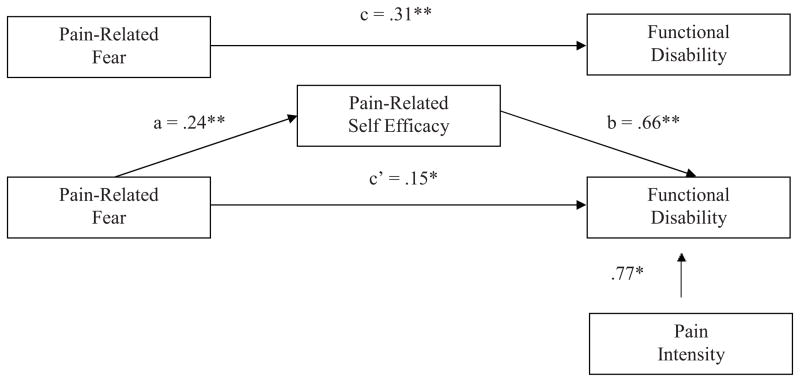
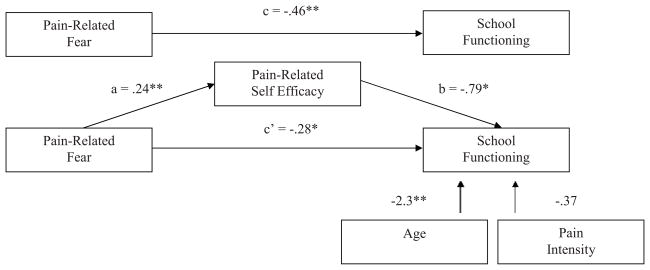
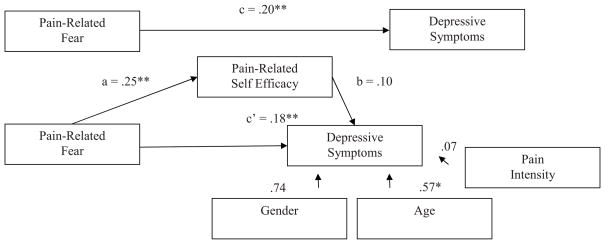
Figure 1 presents the bootstrap results of the mediation effect of pain-related self-efficacy on the pain-related fear-functional disability relationship. In the direct model, the pain-related fear-functional disability relationship (c path) was statistically significant. In the mediation model, the pain-related fear-pain-related self-efficacy path (a path) was significant and the pain-related self-efficacy-functional disability (b path) was significant. With self-efficacy in the model, pain-related fear continued to be a significant predictor of functional disability (c’ path). Bootstrapping results confirmed a partial mediation effect (β = .16; BCACI = .09 – .23). Pain-related self-efficacy partially mediated the pain-related fear-functional disability link in youth with chronic headache while controlling for average pain intensity. The same pattern of results emerged for school functioning with a partial mediation effect (β = −.19, BCACI = −.34 – −.05) (Figure 2). Pain-related self-efficacy partially mediated the relationship between pain-related fear and school functioning in youth with chronic headache while controlling for pain intensity and age. Results for depressive symptoms were somewhat different (Figure 3). When taking into account pain-related fear, the pain-related self-efficacy-depressive symptoms path (b path) was not significant. There was no evidence of mediation (β = .03, p < .001; BCACI = −.01 – .07) while controlling for pain intensity, age, and gender, thus not supporting our hypothesis.
Figure 1.
Tests of the theorized mediation model for functional disability. Upper figure: the total effect (pain-related fear predicting disability). Lower figure: the indirect effect, with pain-related self-efficacy as mediator, and pain-intensity as a control variable. Indirect effect (β) = .16. Unstandardized regression coefficients are displayed. Bias-corrected and accelerated confidence intervals .09 – .23, CI95, bootstrap re-samples = 5,000. * p < .05, **p < .001.
Figure 2.
Tests of the theorized mediation model for school functioning. Upper figure: the total effect (pain-related fear predicting school functioning). Lower figure: the indirect effect, with pain-related self-efficacy as mediator, and pain-intensity and age as control variables. Indirect effect (β) = −.34. Unstandardized regression coefficients are displayed. Bias-corrected and accelerated confidence intervals −.34 – −.05, CI95, bootstrap re-samples = 5,000. * p < .05, **p < .001.
Figure 3.
Tests of the theorized mediation model for depressive symptoms. Upper figure: the total effect (pain-related fear predicting depressive symptoms). Lower figure: the indirect effect, with pain-related self-efficacy as mediator, and pain-intensity, age, and gender as control variables. Indirect effect (β) = .03. Unstandardized regression coefficients are displayed. Bias-corrected and accelerated confidence intervals −.01 – .07, CI95, bootstrap re-samples = 5,000. * p < .05, **p < .001.
Discussion
Psychological factors are well known to influence the development and maintenance of chronic pain18; 27, including among youth experiencing chronic headache19; 23; 52. In the current study we examined whether self-efficacy, a demonstrated influential cognitive appraisal, may be a protective factor that can mitigate the influence of fear on negative functional outcomes (e.g., disability, depression) as has been observed in adult pain patients53; 11. If so, these results would suggest that one of the mechanisms by which fear exerts an influence on outcomes may be through one’s confidence in performing activities, or lack thereof and could direct implications on how cognitive behavioral treatment is delivered among highly fearful pediatric headache patients. This is the first report testing the co-existing effects of fear avoidance and pain-related self-efficacy in youth with chronic pain.
Our hypotheses were partially supported, making our results consistent with previous findings, including those of Woby and colleagues, of a mediating role for self-efficacy in the relation between fear-avoidance and functional outcomes.53 Self-efficacy partially mediated the pain-related fear disability and school functioning links and did not mediate the relation between pain-related fear and depressive symptoms. For global functional disability and for the more specific domain of school functioning, these findings demonstrate that both fear-avoidance and self-efficacy uniquely contribute to these outcomes providing two tangible targets for psychological intervention. Our results support a potential modification to the fear-avoidance model to include self-efficacy beliefs as a positive, protective factor expanding upon the current unelaborated ‘confrontation’ side of the model that can partially mitigate the inhibitory, or risk mechanisms more strongly associated with the negative development of disability and school impairment in children with chronic headache over time. The protective function of self-efficacy found in this study expands upon previous literature for individuals with chronic pain54; 32; 40.
Our finding that pain-related self-efficacy failed to mediate relations between fear-avoidance and depressive symptoms suggests that pain-related self-efficacy may be more closely related to pain specific functional outcomes than to emotional distress. In other words, a strong belief in one’s ability to cope with pain may promote resilience in daily functioning but may not be sufficiently protective against psychological problems such as depressed mood, which may onset secondary to pain or may exist alongside but somewhat separate from the pain experience33; 3. It may be true as well in our research that, because self-efficacy was significantly associated with depressive symptoms at the bivariate level, whatever association that was there could be accounted for by the same underlying construct that pain-related fear is assessing (i.e. emotional distress). We do suspect from this research that pain self-efficacy is a more salient predictor of behavior, rather than mood, in youth experiencing chronic pain. We acknowledge that this conclusion is contrary to the findings of Sanchez (2011)39, who reported depression as a significant predictor of self-efficacy in a sample of adults with fibromyalgia. This is likely due to the fact that they did not include pain-related fear in their evaluation of self-efficacy’s impact on depressive symptoms that appears to usurp the influence of self-efficacy.
Among other findings, it was quite encouraging that pain intensity only modestly correlated with pain-related self-efficacy. This is contrary to previous findings that strongly associate pain intensity negatively with self-efficacy in adult chronic pain43; 38; 13; 16. This is more consistent with research that has found a nominal relationship between pain acceptance and levels of pain26; 9; 17, suggesting that resiliency factors such as self-efficacy and pain acceptance can promote positive pain-related outcomes regardless of pain level.
Findings from this study clearly identify potential behavioral targets for clinician intervention. It is imperative to assess and treat both fear and self-efficacy in youth exhibiting chronic pain. Others have found that pain-related self-efficacy ratings are likely to improve following cognitive behavioral management of low back pain and that these changes are associated with better outcomes such as reduced disability35; 2. In terms of a specific type of treatment, it has been suggested that graded in vivo exposure therapies may be particularly effective types of treatment for youth with chronic pain. Gradually exposing fearful patients to activities that they perceive as threatening and/or harmful can lead to reductions in disability, fear of movement, and catastrophizing46–48. Successfully accomplishing a given task has been described as a method for increasing self-efficacy because it relates directly to the enhancement of personal mastery experiences4; 5. Directly exposing youth to activities during graded exposure to fear-eliciting activities may indirectly promote self-efficacy, in that it requires patients to engage in, and successfully accomplish, those same feared activities. Similarly, cognitive behavioral strategies demonstrated to be effective at treating chronic headache, enhancing levels of self-efficacy, and both diminishing catastrophizing and stress (e.g., mindfulness therapy12, biofeedback34, and stress management/relaxation therapy28) hold promise as effective therapies for youth with chronic headache. Clinicians should consider both reducing pain-related fear and enhancing pain-related self-efficacy through use of these cognitive-behavioral interventions that effectively promote adaptive functioning in daily life among youth.
There are limitations to the current study. We collected our data from self-report measures, which have limitations including social desirability bias30 and shared method variance. Additionally, we recognize that our design was cross-sectional; thus, all uses of the term ‘predictor’ are as a statistical construct and cannot be interpreted as indicative of causality. Because our sample included only youth reporting headache as their primary pain problem, it also is unclear the extent to which the results can be generalized to a wider sample of youth reporting chronic pain more broadly. It is our view that longitudinal studies overcoming these limitations and investigating the extent to which self-efficacy mediates relationships between pain-related fear and outcomes like disability and mood over time in a wider group of chronic pain patients in more diverse clinical settings are warranted. Both observational and self-report measurement methods should be utilized in these subsequent studies.
Given that chronic headache is known to be a widespread and persistent complaint among youth world-wide, it is clear that resources should be allocated to further understand processes like self-efficacy that may promote lasting resilience in youth with chronic headache. Our work suggests that cognitive behavioral interventions that reduce chronic pain effectively should include an understanding of the effects of pain-related self-efficacy and fear-avoidance on the maintenance and exacerbation of functional disability and school impairment. Such intervention could improve long-term health and insure more lasting wellbeing among youth suffering from the debilitating effects of chronic or lasting headache.
Acknowledgments
The authors wish to thank the children and parents who participated in the study.
Footnotes
Disclosures: The authors received funding from the Sara Page Mayo Endowment for Pediatric Pain Research and Treatment (C.B. Berde, MD, PhD), and the Department of Anesthesiology, Perioperative and Pain Medicine at Boston Children’s Hospital in support of this work. Additional support came from a NIH K23 career development award (HD067202) to LS. There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abu-Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: a systematic review of population-based studies. Dev Med Child Neurol. 2010;52:1088–1097. doi: 10.1111/j.1469-8749.2010.03793.x. [DOI] [PubMed] [Google Scholar]
- 2.Altmaier E, Russel D, Kao C, Lehmann T, Weinstein J. Role of self-efficacy in rehabilitation outcome among chronic low back pain patients. J Counseling Psychol. 1993;40:5. [Google Scholar]
- 3.Asghari A, Julaeiha S, Godarsi M. Disability and depression in patients with chronic pain: pain or pain-related beliefs? Arch Iran Med. 2008;11:263–269. [PubMed] [Google Scholar]
- 4.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 5.Bandura A. The exercise of control. New York, NY: WH Freeman; 1997. Self-efficacy. [Google Scholar]
- 6.Bille B. A 40-year follow-up of school children with migraine. Cephalalgia. 1997;17:488–491. doi: 10.1046/j.1468-2982.1997.1704488.x. discussion 487. [DOI] [PubMed] [Google Scholar]
- 7.Buer N, Linton SJ. Fear-avoidance beliefs and catastrophizing: occurrence and risk factor in back pain and ADL in the general population. Pain. 2002;99:485–491. doi: 10.1016/S0304-3959(02)00265-8. [DOI] [PubMed] [Google Scholar]
- 8.Bursch B, Tsao JC, Meldrum M, Zeltzer LK. Preliminary validation of a self-efficacy scale for child functioning despite chronic pain (child and parent versions) Pain. 2006;125:35–42. doi: 10.1016/j.pain.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho S, McCracken LM, Heiby EM, Moon DE, Lee JH. Pain acceptance-based coping in complex regional pain syndrome Type I: daily relations with pain intensity, activity, and mood. J Behav Med. 2012 doi: 10.1007/s10865-012-9448-7. [DOI] [PubMed] [Google Scholar]
- 10.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the Functional Disability Inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Costa LC, Maher CG, McAuley JH, Hancock MJ, Smeets RJ. Self-efficacy is more important than fear of movement in mediating the relationship between pain and disability in chronic low back pain. Eur J Pain. 2011;15:213–219. doi: 10.1016/j.ejpain.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Day MA, Thorn BE, Ward LC, Rubin N, Hickman SD, Scogin F, Kilgo GR. Mindfulness-based cognitive therapy for the treatment of headache pain: a pilot study. Clin J Pain. 2013 doi: 10.1097/AJP.0b013e318287a1dc. [DOI] [PubMed] [Google Scholar]
- 13.Denison E, Asenlof P, Lindberg P. Self-efficacy, fear avoidance, and pain intensity as predictors of disability in subacute and chronic musculoskeletal pain patients in primary health care. Pain. 2004;111:245–252. doi: 10.1016/j.pain.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Denison E, Asenlof P, Sandborgh M, Lindberg P. Musculoskeletal pain in primary health care: subgroups based on pain intensity, disability, self-efficacy, and fear-avoidance variables. J Pain. 2007;8:67–74. doi: 10.1016/j.jpain.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Desrochers G, Bergeron S, Khalife S, Dupuis MJ, Jodoin M. Fear avoidance and self-efficacy in relation to pain and sexual impairment in women with provoked vestibulodynia. Clin J Pain. 2009;25:520–527. doi: 10.1097/AJP.0b013e31819976e3. [DOI] [PubMed] [Google Scholar]
- 16.Dohnke B, Knauper B, Muller-Fahrnow W. Perceived self-efficacy gained from, and health effects of, a rehabilitation program after hip joint replacement. Arthritis Rheum. 2005;53:585–592. doi: 10.1002/art.21324. [DOI] [PubMed] [Google Scholar]
- 17.Esteve R, Ramirez-Maestre C. Pain fear avoidance and pain acceptance: a cross-sectional study comparing their influence on adjustment to chronic pain across three samples of patients. Ann Behav Med. 2013 doi: 10.1007/s12160-013-9499-1. [DOI] [PubMed] [Google Scholar]
- 18.Gatchel RJ, Turk DC. Psychosocial factors in pain: Critical perspectives. Guilford Press; 1999. [Google Scholar]
- 19.Heinen F. Headaches in childhood and adolescence. Neuropediatrics. 2013;44:1–2. doi: 10.1055/s-0032-1333436. [DOI] [PubMed] [Google Scholar]
- 20.Heyne D, King N, Tonge B, Rollings S, Pritchard M, young D, Myerson N. The self-efficiency questionnaire for school situations: Development and psychometric evaluation. Behaviour Change. 1998;15:31–40. [Google Scholar]
- 21.Heyne D. School refusal. In: Fisher J, O’Donohue W, editors. Practitioners’ guide to evidence-based psychotherapy. New York, NY: Kluwer Academic/Plenum Publishers; 2006. [Google Scholar]
- 22.Kaczynski KJ, Claar RL, Lebel AA. Relations between pain characteristics, child and parent variables, and school functioning in adolescents with chronic headache: a comparison of tension-type headache and migraine. J Pediatr Psychol. 2013;38:351–364. doi: 10.1093/jpepsy/jss120. [DOI] [PubMed] [Google Scholar]
- 23.Kashikar-Zuck S, Zafar M, Barnett KA, Aylward BS, Strotman D, Slater SK, Allen JR, Lecates SL, Kabbouche MA, Ting TV, Hershey AD, Powers SW. Quality of life and emotional functioning in youth with chronic migraine and juvenile fibromyalgia. Clin J Pain. 2013 doi: 10.1097/AJP.0b013e3182850544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- 25.Kovacs M. Children’s Depression Inventory. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- 26.Kranz D, Bollinger A, Nilges P. Chronic pain acceptance and affective well-being: a coping perspective. Eur J Pain. 2010;14:1021–1025. doi: 10.1016/j.ejpain.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Leeuw M, Houben RM, Severeijns R, Picavet HS, Schouten EG, Vlaeyen JW. Pain-related fear in low back pain: a prospective study in the general population. Eur J Pain. 2007;11:256–266. doi: 10.1016/j.ejpain.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Lemstra M, Stewart B, Olszynski WP. Effectiveness of multidisciplinary intervention in the treatment of migraine: a randomized clinical trial. Headache. 2002;42:845–854. doi: 10.1046/j.1526-4610.2002.02202.x. [DOI] [PubMed] [Google Scholar]
- 29.Leonhardt C, Lehr D, Chenot JF, Keller S, Luckmann J, Basler HD, Baum E, Donner-Banzhoff N, Pfingsten M, Hildebrandt J, Kochen MM, Becker A. Are fear-avoidance beliefs in low back pain patients a risk factor for low physical activity or vice versa? A cross-lagged panel analysis. Psychosoc Med. 2009;6:Doc01. doi: 10.3205/psm000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan DE, Claar RL, Scharff L. Social desirability response bias and self-report of psychological distress in pediatric chronic pain patients. Pain. 2008;136:366–372. doi: 10.1016/j.pain.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Logan DE, Claar RL, Guite JW, Kashikar-Zuck S, Lynch-Jordan A, Palermo TM, Wilson AC, Zhou C. Factor structure of the Children’s Depression Inventory in a multisite sample of children and adolescents with chronic pain. J Pain. 2013 doi: 10.1016/j.jpain.2013.01.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miles CL, Pincus T, Carnes D, Taylor SJ, Underwood M. Measuring pain self-efficacy. Clin J Pain. 2011;27:461–470. doi: 10.1097/AJP.0b013e318208c8a2. [DOI] [PubMed] [Google Scholar]
- 33.Nash JM, Williams DM, Nicholson R, Trask PC. The contribution of pain-related anxiety to disability from headache. J Behav Med. 2006;29:61–67. doi: 10.1007/s10865-005-9033-4. [DOI] [PubMed] [Google Scholar]
- 34.Nestoriuc Y, Martin A, Rief W, Andrasik F. Biofeedback treatment for headache disorders: a comprehensive efficacy review. Appl Psychophysiol Biofeedback. 2008;33:125–140. doi: 10.1007/s10484-008-9060-3. [DOI] [PubMed] [Google Scholar]
- 35.Nicholas MK, Wilson PH, Goyen J. Comparison of cognitive-behavioral group treatment and an alternative non-psychological treatment for chronic low back pain. Pain. 1992;48:339–347. doi: 10.1016/0304-3959(92)90082-M. [DOI] [PubMed] [Google Scholar]
- 36.Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, Bohnen AM, Suijlekom-Smit LW, Passchier J, der Wouden JC. Pain in children and adolescents: A common experience. Pain. 2000;87:51–58. doi: 10.1016/S0304-3959(00)00269-4. [DOI] [PubMed] [Google Scholar]
- 37.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 38.Reid MC, Williams CS, Gill TM. The relationship between psychological factors and disabling musculoskeletal pain in community-dwelling older persons. Journal of the American Geriatrics Society. 2003;51:1092–1098. doi: 10.1046/j.1532-5415.2003.51357.x. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez AI, Martinez MP, Miro E, Medina A. Predictors of the pain perception and self-efficacy for pain control in patients with fibromyalgia. The Spanish Journal of Psychology. 2011;14:366–373. doi: 10.5209/rev_sjop.2011.v14.n1.33. [DOI] [PubMed] [Google Scholar]
- 40.Shipton E, Ponnamperuma D, Wells E, Trewin B. Demographic characteristics, psychosocial measures, and pain in a sample of patients with persistent pain referred to a new zealand tertiary pain medicine center. Pain Med. 2013;14:1101–1107. doi: 10.1111/pme.12113. [DOI] [PubMed] [Google Scholar]
- 41.Simons L, Sieberg C, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): Assessment of pain-related fear among children and adolescents with chronic pain. Journal of Pain. 2011 doi: 10.1016/j.jpain.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Simons LE, Kaczynski KJ. The Fear Avoidance model of chronic pain: examination for pediatric application. J Pain. 2012;13:827–835. doi: 10.1016/j.jpain.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Hout J, Vlayen J, Heuts P, Sillen W, Willen A. Functional disability in nonspecific low back pain: the role of pain-related fear and problem-solving skills. International Journal of Behavioral Medicine. 2001;8:134– 148. [Google Scholar]
- 44.Varni JW, Seid M, Knight TS, Uzark K, Szer IS. The PedsQL 4.0 Generic Core Scales: sensitivity, responsiveness, and impact on clinical decision-making. J Behav Med. 2002;25:175–193. doi: 10.1023/a:1014836921812. [DOI] [PubMed] [Google Scholar]
- 45.Viniol A, Jegan N, Leonhardt C, Strauch K, Brugger M, Barth J, Baum E, Becker A. Study protocol: Transition from localized low back pain to chronic widespread pain in general practice: identification of risk factors, preventive factors and key elements for treatment--a cohort study. BMC Musculoskelet Disord. 2012;13:77. doi: 10.1186/1471-2474-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. Graded exposure in vivo in the treatment of pain-related fear: a replicated single-case experimental design in four patients with chronic low back pain. Behaviour Research and Therapy. 2001;39:151–166. doi: 10.1016/s0005-7967(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 47.Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. The treatment of fear of movement/(re)injury in chronic low back pain: further evidence on the effectiveness of exposure in vivo. Clinical Journal of Pain. 2002;18:251–261. doi: 10.1097/00002508-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Vlaeyen JW, De Jong JR, Onghena P, Kerckhoffs-Hanssen M, Kole-Snijders AM. Can pain-related fear be reduced? The application of cognitive-behavioural exposure in vivo. Pain Res Manag. 2002;7:144–153. doi: 10.1155/2002/493463. [DOI] [PubMed] [Google Scholar]
- 49.Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 50.von Baeyer CL. Numerical rating scale for self-report of pain intensity in children and adolescents: recent progress and further questions. Eur J Pain. 2009;13:1005–1007. doi: 10.1016/j.ejpain.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 52.Weeks RE. Application of behavioral therapies in adult and adolescent patients with chronic migraine. Neurol Sci. 2013;34 (Suppl 1):S11–17. doi: 10.1007/s10072-013-1360-6. [DOI] [PubMed] [Google Scholar]
- 53.Woby SR, Urmston M, Watson PJ. Self-efficacy mediates the relation between pain-related fear and outcome in chronic low back pain patients. Eur J Pain. 2007;11:711–718. doi: 10.1016/j.ejpain.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Wright LJ, Zautra AJ, Going S. Adaptation to early knee osteoarthritis: the role of risk, resilience, and disease severity on pain and physical functioning. Ann Behav Med. 2008;36:70–80. doi: 10.1007/s12160-008-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao X, John G, Lynch J, Chen Q. Reconsidering Baron and Kenny: Myths and truths about mediation analysis. Journal of Consumer Research. 2010;37:197–206. [Google Scholar]