Abstract
The concept of increased blood vessel (BV) density proximal to glucose sensors implanted in the interstitial tissue increases the accuracy and lifespan of sensors is accepted, despite limited existing experimental data. Interestingly, there is no previous data or even conjecture in the literature on the role of lymphatic vessels (LV) alone, or in combination with BV, in enhancing continuous glucose monitoring (CGM) in vivo. To investigate the impact of inducing vascular networks (BV and LV) at sites of glucose sensor implantation, we utilized adenovirus based local gene therapy of vascular endothelial cell growth factor-A (VEGF-A) to induce vessels at sensor implantation sites. The results of these studies demonstrated that 1) VEGF-A based local gene therapy increases vascular networks (blood vessels and lymphatic vessels) at sites of glucose sensor implantation; and 2) this local increase of vascular networks enhances glucose sensor function in vivo from 7 days to greater than 28 days post sensor implantation. This data provides “proof of concept” for the effective usage of local angiogenic factor (AF) gene therapy in mammalian models in an effort to extend CGM in vivo. It also supports the practice of a variety of viral and non-viral vectors as well as gene products (e.g. anti-inflammatory and anti-fibrosis genes) to engineer “implant friendly tissues” for the usage with implantable glucose sensors as well as other implantable devices.
Keywords: Diabetes, Gene Therapy, Blood and Lymphatic Vessels, Wound Healing, Glucose Sensors
INTRODUCTION
The development of implantable glucose sensors for continuously monitoring blood glucose levels (i.e. continuous glucose monitoring (CGM)) and insulin infusion systems (i.e. subcutaneous insulin infusion, SCII) have significantly enhanced the management of blood glucose levels in patients with diabetes. Unfortunately current commercial sensors have a limited functional lifespan in vivo of only 3–7 days. It is generally believed that much of the loss of sensor performance in vivo is thought to be the result of sensor induced tissue reactions, i.e. inflammation, fibrosis, and fibrosis-induced vessel regression, at the site of sensor implantation(1–4). In fact, it has often been argued that the loss of blood vessels proximal to the sensor (i. e. fibrosis induced vessel regression) at the sensor implantation site is one of the major causes of the loss of effective CGM in vivo. Interestingly despite the critical role of lymphatic vessels in maintaining tissue function in normal and inflamed tissue, the role of lymphatic vessel and fibrosis induced lymphatic vessel regression in sensor function and CGM has received little to no attention. Since having both blood vessels and lymphatic vessels is critical to establish a functional vascular network in tissues, understanding the contributions of both BV and LV to CGM in vivo is critical to developing rationale approaches to enhance and extend CGM.
Interestingly, although there have been significant discussions related to the importance of angiogenesis and neovascularization in sensor function, in actuality there have only been limited sensor studies to investigate this effect. For example, studies by Ward et al. (4) demonstrated that local delivery of the recombinant angiogenic factor VEGF at sites of varying distance from an implanted glucose sensor was not only associated with neovascularization but it also promoted better sensor performance. Studies by Norton et al. (5) supported the potential of recombinant VEGF induced vessel formation at sites of sensor implantation to enhance its performance in vivo, although actual sensor functional measurements were not performed. However in all these cases, the vessel regression occurred with the termination of recombinant VEGF delivery. Alternatively, two gene therapy studies by Klueh et al. have demonstrated that local VEGF gene therapy induced neovascularization and extended sensor function in a short-term ex ova chicken embryo chorioallantoic membrane (CAM) model (6, 7). These ex vivo studies only addressed the impact of neovascularization on sensor function in a chicken CAM model over a 6–8 day study, and did not address the existence or role of lymphatic vessels on short-term sensor function.
These data and concepts have led us to hypothesize that local VEGF-A gene therapy at sites of glucose sensor implantation can extend glucose sensor performance in mammalian models of CGM by inducing vascular networks composed of both BV and LV at sites of glucose sensor implantation. To test this hypothesis in mammalian systems, we utilized our murine model of CGM (8) and adenovirus based local VEGF-A gene therapy. For these studies we evaluated the impact of direct injection of adenovirus vectors containing the VEGF-A gene (Adv-VEGF-A) as well as control genes and viral vectors at sensor implantation sites on CGM over a 28 day time period. Histologic analysis of BV and LV density at the various sensor implantation sites demonstrated that injections of Adv-VEGF-A 1) enhanced BV and LV density surrounding the implanted sensor when compared to control injections, and 2) this local increase of vascular networks enhanced glucose sensor performance in vivo. This exciting data provides “proof of concept” that increasing vascular networks at sites of glucose sensor implantation using gene therapy enhances long-term performance of glucose sensors in mammalian models of CGM.
MATERIALS AND METHODS
Glucose Sensors, Implantation and Murine Continuous Glucose Sensor System
Modified Abbott Navigator glucose sensors polarized at 200 mV versus a silver-silver chloride reference electrode were obtained from Abbott Diabetes Care. These newly developed glucose sensors (i.e. modified Abbott Navigator glucose sensors) have an extended in vitro lifespan of greater than 2 months, and greater than 28 days in vivo (9). Glucose sensors were implanted into adult female C57BL/6 mice (Jackson Laboratories, Bar Harbor Maine) and continuous glucose monitoring (CGM) was undertaken for a period up to 28 days as described recently (8–10). The Institutional Animal Care and Use Committee of the University of Connecticut Health Center (Farmington, CT) approved all mice studies.
VEGF-A Viral Vector and Injection Procedure
Dr. J.A. Nagy (Beth Israel Deaconess Medical Center, Boston, Mass) kindly provided the adenovirus containing mouse VEGF-A(164) used for these studies (11). For each treatment (injection), sensors were implanted in the mice. On 3 consecutive days (days 6, 7 & 8) post sensor implantation 30 μl of Adv-LacZ or Adv-VEGF-A, for a total of approximately 1.3x1010 viral particles in saline, was injected at the implanted sensor tip, i.e. the sensing element of the sensor. The injection procedure was similar as described in a previous publication (9). After the initial 3 injections were completed there were no further treatments. Additional controls included mice that received no injections. Both pre and post injection, the mice were allowed to roam freely as previously described by our laboratories (12). It should be noted that CGM was done for 28 days with 21 days post-adenovirus treatment/injection i.e. (days 7–28) to minimize any impact of acquired immunity against adenovirus infected tissue cells (13).
CGM and CGM Analysis
Blood glucose reference measurements were obtained periodically over the 28-day implantation period, using blood obtained from the tail vein and a FreeStyle Blood Glucose monitor (14). These reference blood measurements were used to calculate the mean absolute relative difference (MARD) over a four-week experiment for the three treatment groups of mice. See Figure 1 for the MARD calculation method (15). Based on the following criteria sensors were incorporated into the final analyses: total number of sensors implanted (T); low initial sensor output (LO), sensor performance issues (SP) (i.e. broken wire or excessive electrical noise); implantation site infection (ISI); mouse death (MD); sensors included in study (F). Sensor outcomes for the no-treatment mice was T=44; LO=6; SP=10; ISI=1; MD=1 and F=26. Sensors outcomes for the LacZ treated mice was T=34; LO=5; SP=3; ISI=0; MD=3 and F=23. Sensors outcomes for the VEGF treated mice was T=29; LO=5; SP=6; ISI=1; MD=2 and F=15. It should be noted that the larger number of control sensors was a result of additional control sensors run for each of the study groups.
Figure 1. Mean Absolute Relative Difference (MARD) calculations.
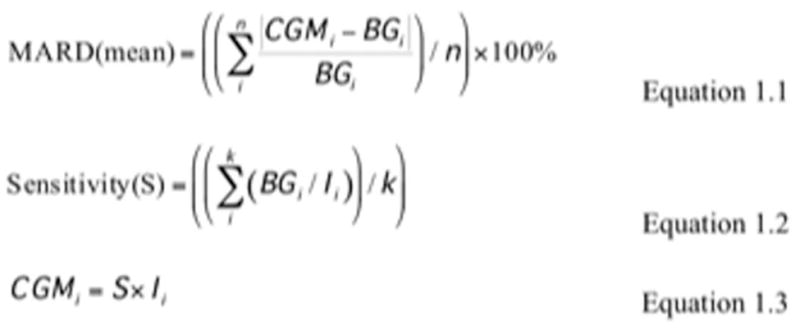
In Figure 1, equations 1.1 to 1.3 illustrate how mean absolute relative differences (MARDs) are calculated by taking the mean of a series of absolute relative differences between the continuous glucose measurement (CGM in mg/dL), calculated from the measurement of electrical current (I in nAmp) within the implanted sensor, and the blood glucose reference measurement (BG in mg/dL) from a standard clinical glucometer (FreeStyle Blood Glucose monitor). Sensitivity (S in mg/dL•nAmp) is calculated for each mouse experiment, based on the BG and I measurements in an initial reference stage of the experiment, i.e. k in Equation 1.2 is approximately 5, for the first initial 5 measurements across 2 days.
Quantitation of Blood and Lymphatic Vessel Density at Sites of Sensor Implantation
To evaluate the impact of Adv-VEGF-A and control treatments at the sensor implantation sites, tissue samples were obtained from the implantation sites of the various treatment/injection groups and fixed in zinc (Zn) buffer. The resulting samples were processed for immunohistochemistry (IHC) for vessel detection and quantitation. Mouse blood vessels were detected using anti-mouse CD31, and lymph vessels were detected using anti-mouse podoplanin immunoglobulin (16)(16). Non-immune IgG was used as a specificity control for both antibodies. High-resolution digital images with representative histological sections around the sensor implantation site were selected using the Aperio ImageScope (v10.2.2.2352) software. A standard unit of area around the identifiable sensor implantation site was selected within the 20x magnified digital images, and blood and lymph vessels were then visually identified within the selected area, from positive CD31 and podoplanin immuno-histology staining, with Trichrome staining as a reference. The percent area of the histology around the sensor represented by blood or lymph vessels was calculated by encircling the positively identified blood or lymph vessels as a region of interest (ROI) within a separate image analysis software package, ImageJ (version 1.43u). The areas of the ROIs, i.e. the blood or lymph vessels, were calculated by the image analysis software, which then were summed and presented as a percentage of total area. The final calculations were conducted within Microsoft Excel for Mac 2011 (version 14.1.4). Boxplots were produced in IBM SPSS Statistics 19 (release 19.0.0) to illustrate the quartiles of percent blood or lymph vessel area of the three treatment groups.
Regression Analysis of Lymph Vessel Density and Glucose Sensor Function
To calculate the statistical relationship between blood and/or lymph vessel density and glucose sensor function, a simple linear regression between percent blood and/or lymph vessel area and mean MARD values was conducted, to obtain ratios between change in MARD value per 1% increase in blood and/or lymph vessel area, their standard errors, statistical significance, and coefficient of determination values (R2). The error distributions of all three linear regression models, as described by the models’ residuals, were normally distributed. Microsoft Excel for Mac 2011 (version 14.1.4) and IBM SPSS Statistics 19 (release 19.0.0) were the software packages used for these calculations and statistical analyses respectively.
RESULTS
Impact of Local VEGF-A Gene Therapy on CGM
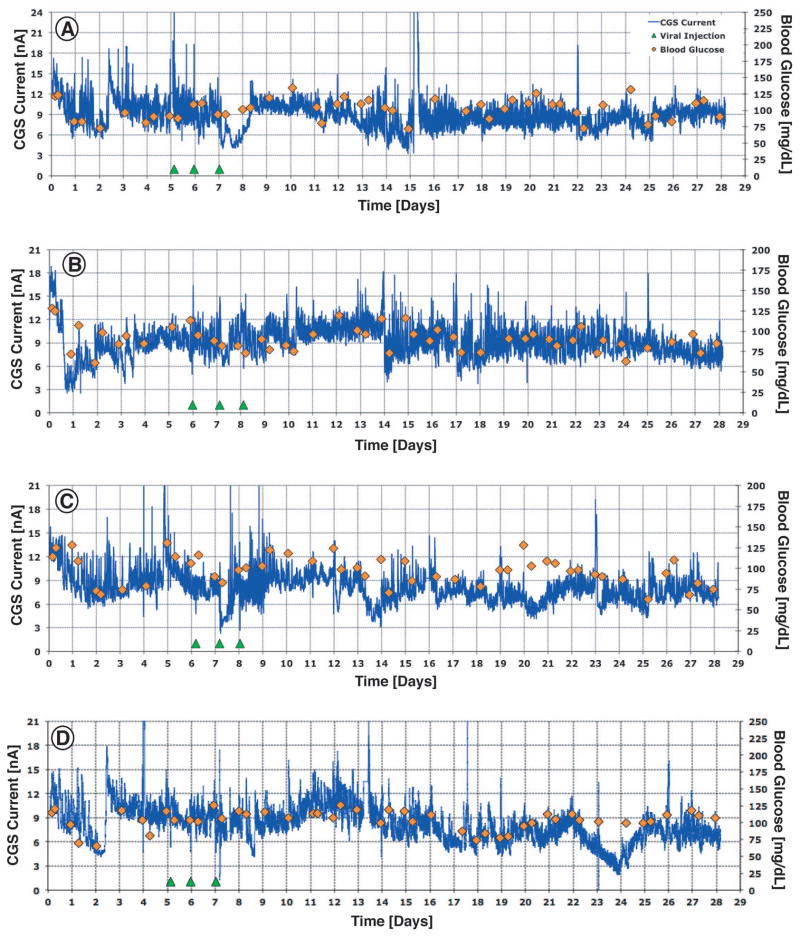
To determine the impact of local VEGF-A gene therapy on sensor function and CGM in our murine model (12)(12), we administered mice with either no injections (Figure 2), direct injections of Adv-LacZ (control virus with unrelated gene, Figure 3), or adenoviral vectors containing mouse VEGF-A gene (Adv-VEGF-A, figure 4) at sites of glucose sensor implantation in vivo. For each injection treatment (Adv-LacZ or Adv-VEGF-A) sensors were implanted in mice, and on 3 consecutive days (days 6, 7 & 8 post sensor implantation, green triangles in Figures 3,4) 30 μl of Adv-LacZ or Adv-VEGF-A was injected at the implanted sensor tip, i.e. the sensing element of the sensor (see Figure 3,4). CGM output in nA (blue lines, Figure 2–4) was recorded for all sensors and actual blood glucose levels were determined using the Abbott Freestyle external monitor (orange diamonds in Figure 2–4). As can be seen in Figures 2–3 sensors implanted in mice with treatments of no injection (Figure 2) and Adv-LacZ (Figure 3) injection showed periods where the mouse blood glucose (orange diamonds) did not correspond to sensor output (continuous blue line). On the other hand, sensors implanted in mice with Adv-VEGF-A treatment did not experience these sensor performance losses (Figure 4). Although these CGM results are exciting, they only provide a representative snap shot of the entire data set of a generalized population of treated and untreated mice. Therefore statistical analysis of the CGM data, i.e. mean MARD analysis, can more meaningfully condense the data and represent these treated and untreated populations.
Figure 2. 28 day Glucose Sensor Function in untreated normal C57BL/6 mice.
Figures 2a, 2b, 2c, and 2d are representative of 28 days post sensor implantation CGM in C57BL/6 mice with no injections. Sensor output is expressed as CGS output (nA) and is represented by the blue lines. Orange diamonds represents Blood glucose levels. A good sensor performance is seen when the measured blood glucose levels (orange diamonds) line up with the sensor response (blue line). Examples for poor sensor performance were seen in Figure 2A days 13–18 or Figure 2B days 3–7. For this study we evaluated a total of 26 C57BL/6 mice.
Figure 3. 28 day Glucose Sensor Function in Adv-LacZ adenovirus injected C57BL/6 mice.
Figures 3a, 3b, 3c, and 3d are representative of CGM in Adv-LacZ injected C57BL/6 mice (viral vector control) for up to 28 days post sensor implantation. Sensor output is expressed as CGS output (nA) and is represented by the blue lines. Orange diamonds represents Blood glucose levels. For this study we evaluated a total of 23 C57BL/6 mice.
Figure 4. 28 day Glucose Sensor Function in Adv-VEGF-A adenovirus injected C57BL/6 mice.
Figures 4a, 4b, 4c, and 4d are representative of CGM in Adv-VEGF-A injected C57BL/6 mice for up to 28 days post sensor implantation. Sensor output is expressed as CGS output (nA) and is represented by the blue lines. Orange diamonds represents Blood glucose levels. For this study we evaluated a total of 15 C57BL/6 mice.
Mean Absolute Relative Difference (MARD) Analysis
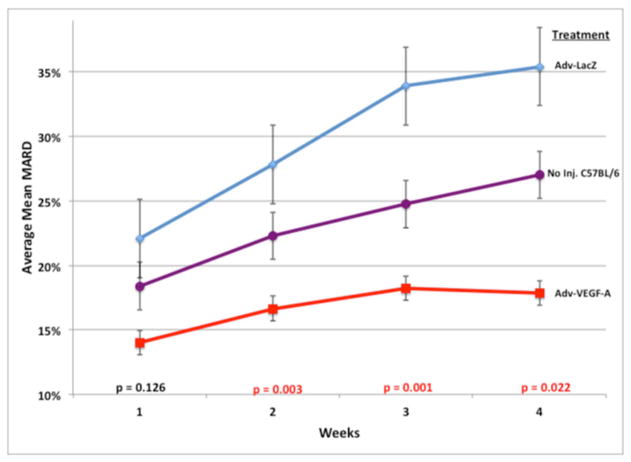
To assess the impact of local VEGF-A gene therapy, we analyzed the resulting CGM data using standard MARD analysis (17). We evaluated the effect of adenoviral vector delivered VEGF-A (Adv-VEGF-A) on glucose sensor function by measuring and calculating the MARD between blood glucose measurements from implanted glucose sensors and standard clinical glucometers over the entire course of a four week experiment for all the mice in the three treatment groups. We observed a statistically significant difference among the three treatment groups, as per the Kruskal-Wallis test (p= 0.001). Figure 5 demonstrates the difference and trends in the MARD values between the treatment groups over the course of the 4 week or 28 day experiment. Adv-VEGF-A treated mice had lower MARD values than all the other control groups, demonstrating its effectiveness in improving glucose sensor function. In addition the difference between the Adv-VEGF-A MARD values and the other treatment groups grew over the four weeks. In concert with the timing of the injections around the end of the first week, we observe that the statistical difference between the groups, as evaluated by Kruskal-Wallis tests, is not significant during the first week (p= 0.126), but become statistically significant in weeks 2, 3, and 4 (p= 0.003, 0.001, and 0.022 respectively). As shown in Table 1, we observed that the Adv-VEGF-A treated mice had a total (all weeks) mean MARD of 17.44 +/− 5.72%, whereas the No Injection C57BL/6 control mice had intermediate MARD of 23.50 +/− 9.83%, and Adv-LacZ had the worst mean MARD of 31.49 +/− 14.50%. The Adv-VEGF-A treated mice had significantly (p<0.05) lower total mean MARD than every other treatment group, as measured by Mann-Whitney U tests. In addition, the Adv-VEGF-A mean MARD data was normally distributed, and had a smaller standard deviation than every other group. The sample sizes are relatively large for such investigations with approximately 15 or more mice in each group.
Figure 5. MARD Trend Analysis at Sensor Implantation Sites from Untreated (no injection), Adv-LacZ Injected, and Adv-VEGF-A Injected.
Figure 5 represents the trends in average mean MARDs from weeks 1 to 4, by treatment group. p-values at the bottom represent the significance of the difference among the three treatment groups in average mean MARD value for each individual week, by the Kruskal-Wallis test (as non-parametric equivalent to ANOVA). The error bars around each data point represent the standard error of the mean MARD for the particular time point and treatment group. Three injections of approximately 1.3x1010 adenoviral vector particles bearing VEGF-A or LacZ were conducted at the end of the first week, and no injections at all were conducted on the untreated controls.
Table 1. Average Mean Absolute Relative Difference (MARD) values for all four weeks of CGM in mice.
Average Mean Absolute Relative Difference (MARD) values for all four weeks of mice treated with adenoviral vectors bearing VEGF-A or LacZ (Adv-VEGFa or Adv-LacZ), and their untreated (no injection) controls. Error values following the +/− are standard deviations from the average of the individual treatment group’s MARD values. p-values within the boxes represent the statistical significance of the comparisons of the two treatment groups indicated in the axes, calculated by Mann-Whitney U tests, as non-parametric equivalents to student t-tests. Mann-Whitney U tests were conducted because only the Adv-VEGF-A treated group had normally distributed mean MARD values. To test for statistical differences among all three treatment groups at once, a Kruskal-Wallis test was conducted on the MARD values, as a non-parametric equivalent to analysis of variance (ANOVA).
| Total mean MARD data | No Injection: C57BL/6 Average Mean MARD = 23.50 +/− 9.83% (n=26) | Ad-LacZ: C57BL/6 Average Mean MARD = 31.49 +/− 14.50% (n=23) | Ad-VEGFa: C57BL/6 Average Mean MARD =17.44 +/− 5.72% (n=15) |
|---|---|---|---|
| No Injection: C57BL/6 Average Mean MARD = 23.50 +/− 9.83% (n=26) | ---------- | p = 0.021 | p = 0.045 |
| Ad-LacZ: C57BL/6 Average Mean MARD = 31.49 +/− 14.50% (n=23) | ---------- | p = 0.0005 | |
| Ad-VEGFa: C57BL/6 Average Mean MARD = 17.44 +/− 5.72% (n=15) | ---------- |
Blood Vessel Density and Regression Analysis
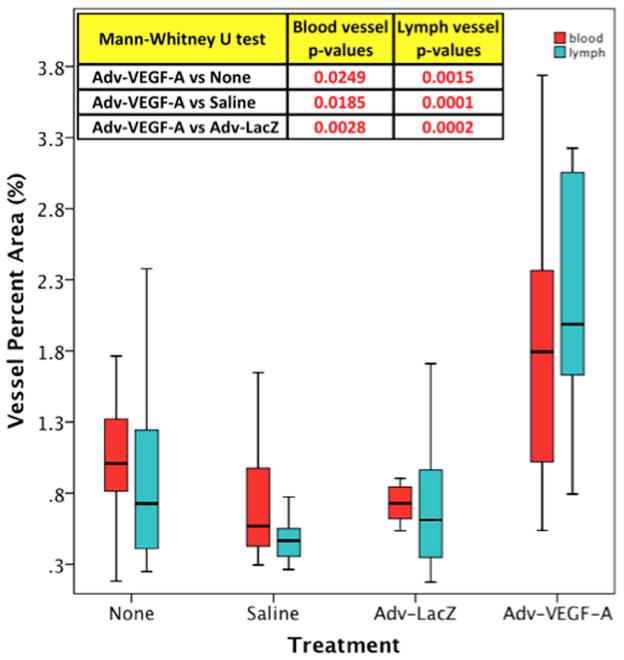
The measured and calculated density of blood vessels among three treatment groups showed a statistically significant increase in the percent area of tissue surrounding the implanted sensor which were identifiable blood vessels, as per immunohistology. The mean percent area, which was blood vessel, for the Adv-VEGF-A treated group of mice was 2.15 +/− 1.55%, whereas the Adv-LacZ and no treatment groups had mean percent blood vessel areas of 0.77 +/−0.23% and 1.03 +/− 0.41%, respectively. Consequently Adv-VEGF-A induced a 1.9 and 2.4 fold increase in mean blood vessel percent area at sensor implantation sites when compared to non-injected or Adv-LacZ injected mice, respectively. The statistical significance of the difference among the three treatment groups, measured by the Kruskal-Wallis statistical test, was a p-value of 0.005. In Mann-Whitney U tests directly comparing the Adv-VEGF-A group to the other two treatment groups specifically, also showed that the Adv-VEGF-A mean percent area was statistically greater than the Adv-LacZ and no treatment groups, with p-values of 0.003, and 0.025 respectively. Figure 6 demonstrates the relative distributions of blood vessel density, through a boxplot of the percent blood vessel area of the respective treatment groups in the boxes colored red. To determine if there was a significant statistical relationship between the observed increase in blood vessel density and improved sensor function, we conducted a linear regression analysis between blood vessel density and MARD values. For those mice among all treatment groups that survived the entire four-week time course of the experiment and whose blood vessel density around the sensor was measured, a functional improvement of 9.12 +/− 2.77% decrease in MARD for every 1% increase in blood vessel area was observed. This ratio was statistically significant with a p-value of 0.005 and a coefficient of determination R2 value of 0.40.
Figure 6. Blood and Lymphatic Vessel Density Boxplot at Sensor Implantation Sites from Untreated (no injection), Adv-LacZ Injected, and Adv-VEGF Injected C57B/6 mice.
Figure 6 is a boxplot of blood and lymphatic vessel density, as measured by percent area, by treatment group. p-values in the table at the top represent Mann-Whitney U test p-values (as non-parametric equivalents to student t-tests) for the significance of the difference between the paired comparisons with Adv-VEGF-A treated mice blood and lymph vessel densityMann-Whitney U tests were conducted because separately only the no injection treatment group had normally distributed blood vessel percent area values, and only the Adv-VEGF-A treatment group had normally distributed lymph vessel percent area values. To determine if there is a statistical difference among all three treatment groups, a Kruskal-Wallis test was conducted for the blood vessel percent area values (since those values as a complete group were non-parametric), and the traditional ANOVA analysis was conducted for lymph vessel percent area values (since those values as a complete group were normally distributed).
Analysis of the Impact of Adv-VEGF-A on Lymphangiogenesis at Implantation Sites
Immunohistochemistry (IHC) for lymph vessel detection and quantitation was conducted using anti-mouse podoplanin (16). The mean percent area as lymph vessel, for the Adv-VEGF-A treated group of mice was 2.12 +/− 0.81%, whereas the Adv-LacZ and no treatment groups had mean percent lymph vessel areas of 0.67 +/−0.46% and 0.91 +/− 0.66%, respectively. The statistical significance of the difference among the three treatment groups, measured by the ANOVA statistical test (since the entire group of lymph percent area values were normally distributed), was a p-value of essentially zero or 3.1 x 10−5. Figure 6 demonstrates the relative distributions of lymph vessel density, through a boxplot of the percent lymph vessel area of the respective treatment groups in the boxes colored turquoise. Adv-VEGF-A induced a 2.3 and 3.2 fold increase in mean lymph vessel percent area L-Angio at sensor implantation sites when compared to non-injected (p=0.0015) or Adv-LacZ (p=0.0002).
Lymph Vessel Density and Regression Analysis
To determine if there was a significant statistical relationship between the observed increase in lymph vessel density only and improved sensor function, we conducted a linear regression analysis between lymph vessel density and MARD values. For those mice among all treatment groups that survived the entire four week time course of the experiment and whose lymph vessel density around the sensor was measured, a functional improvement of −4.29 +/− 1.70% decrease in MARD for every 1% increase in lymph vessel area was observed. This ratio was statistically significant with a p-value of 0.024 and had a coefficient of determination R2 value of 0.30.
Blood and Lymph Vessel Correlation
Blood and lymphatic vessels from histological samples of all treatment groups were also quantified in counts per square millimeter of mouse back tissue, proximal to the glucose sensor implantation site, as per immunohistology following staining of selected samples against CD31 and podoplanin antigens, to identify blood and lymph vessels respectively. Simple linear regression analysis was conducted on these counts per square millimeter of blood and lymphatic vessels in mouse back tissue. The ratio of lymphatic to blood vessels (1.06 +/− 0.25) suggests that lymphatic and blood vessel growth, formation, and maintenance track in a 1 to 1 correspondence, which is consistent with other observations, particular in cornea experimental models (18)(18). Particularly in response to VEGF-A, the vessel growth of both blood and lymph vessels are biologically coordinated. As seen in Table 2, the linear regression model of this blood and lymph coordination from our histological observations was very significant (p=0.0001), particularly since the sample size for this model was relatively large (n=39). R2 represents the statistical coefficient of determination between the blood and lymphatic vessel variables, as counts per square millimeter, and was 0.33.
Table 2. Statistical comparison of lymphatic and blood vessel counts per square millimeter.
Simple linear regression analysis was conducted on matching histological samples from all treatment groups, whose lymphatic and blood vessels were quantified in counts per square millimeter of mouse back tissue, proximal to the glucose sensor implantation site. The calculated regression coefficient suggests a ratio of lymphatic to blood vessels in a near 1 to 1 correspondence. The significance is expressed as a p-value, with statistical significance achieved at p <0.05. R2 represent the coefficient of determination between the blood and lymphatic vessel variables, as counts per square millimeter. The error distribution of this linear regression model, as described by the model’s residuals, is normally distributed.
| Statistical analysis of BV and LV counts | |
|---|---|
| Ratio #/mm2 LV/BV | 1.063 |
| Standard Error | 0.250 |
| Significance | 0.0001 |
| R2 | 0.328 |
| Sample Size | 39 |
Combined Linear Regression Model with Blood and Lymph Vessels
Quantified increases in both blood and lymph vessels, as measured in percent area as blood or lymph vessel, were individually determined to decrease MARD values by the ratios described above, and therefore improve glucose sensor function, but these previous analyses did not account for both vessel types at the same time in the same regression model. To test the combined blood and lymph vessel model, a simple linear regression analysis was then conducted to determine the contribution of both blood and lymph vessel density together, by inputting both the blood and lymph vessel density variables into the same linear regression model. The results for those mice among all treatment groups that survived the entire four week time course of the experiment and whose blood and lymph vessel density around the sensor was measured, a functional improvement of 7.13 +/− 4.74% decrease in MARD for every 1% increase in blood vessel area, and a 1.28 +/− 2.59% decrease in MARD for every 1% increase in lymph vessel area was observed. This ratio was statistically significant with a p-value of 0.0297. R2 represents the coefficient of determination between MARD and both the percent area as blood vessel and percent area as lymphatic vessel variables, as per this linear regression model was 0.4, approximately the same as for the linear regression model for MARD with the blood vessel variable alone. These studies demonstrate that Adv-VEGF-A increases both H-Angio and L-Angio at sensor implantation sites when compared to non-injected and various control treated sensor implantation sites. Equally important is the fact that sensor performance was also enhanced by Adv-VEGF-A when compared to all controls (see total mean MARD, Table 1). These studies establish “proof of principle” that increasing H-Angio and L-Angio with Adv-VEGF-A significantly increases sensor function and CGM.
DISCUSSION
Sensor implantation induced tissue reactions include inflammation and fibrosis, both of these tissue reactions are known to ultimately cause the loss of blood vessels at the site of sensor implantation. In fact, it is likely that both fibrosis itself and fibrosis-induced vessel regression work synergistically to limit effective CGM in vivo. Specifically fibrosis not only induces vessel regression at sites of sensor implantation, but fibrosis is also known to slow glucose diffusion between blood vessels and the implanted glucose sensor. This fibrosis based inhibition of glucose diffusion results in the loss of “real time” blood glucose measurements because of the element of time delay (19). As such, both fibrosis and the loss of blood vessels near the sensor, make it impossible for the sensor to measure “real time” blood/interstitial glucose levels. Although it is known that inflammation and fibrosis can cause lymphatic vessel regression(20, 21), the impact of inflammation-fibrosis induced lymphatic vessel regression at sites of sensor implantation, and the impact of that regression on long-term sensor function has not been addressed previously.
Overcoming Vascular Network Regression in vivo
Among the possible solutions to overcoming the negative impact of inflammation and fibrosis on sensor function is to suppress inflammation and fibrosis or induce new vascular networks at the site of sensor implantation in the skin. Inflammation and fibrosis are extremely complex multi-factorial systems that are extremely difficult to control long-term. Alternatively, efforts to overcome fibrosis induced vessel regression in injured tissues such as ischemic hearts and limbs have focused on the usage of recombinant angiogenic factors (AF) to induce new vascular networks (22). For example, it is known that VEGF is a potent mitogen for endothelial cells and is responsible for sprouting and proliferation of endothelial cells to form new, but immature vessels in vivo. VEGF alone can induce angiogenesis, but VEGF alone is unable to induce true neovascularization, i.e. vessel maturation allowing long-term vessel formation in vivo. Based on the literature, maturation of immature blood vessels require additional growth factors and/or cytokines, as well as cells such as pericytes, in order to mature and sustain long-term blood vessels in vivo. Promising candidates of maturation factors are angiopoietins (e.g. Ang1 or Ang2) (23), PDGF and/or FGF (24–26). Thus, truly long-term or sustained blood vessels for implantable devices such as sensors will require the use of both angiogenic and maturation factors and cells.
One of the additional challenges is that recombinant VEGF induced angiogenesis is short-lived after the local delivery of VEGF is ended or discontinued. Previous sensor studies using recombinant VEGF alone demonstrated that when VEGF delivery ceased, vessel regression occurred at sites of glucose sensor implantation (4, 27). Additionally, blockade of VEGF can induce vessel regression in tumors (28–30). Using this concept, anti-VEGF cancer therapy strategies to induce blood vessel regression were developed to “starve” tumors in vivo. Also this approach can prolong the life of patients by several month, the success of VEGF-targeted therapies is still insufficient. Recently the focus has been on the concept that “normalization” of the tumor vasculature (also known as vessel remodeling) will lead to a better clinical outcome by decreasing tumor metastasis, but further clinical data is need to understand the importance of vessel regression (tumor starvation) and vessel normalization in tumor growth, metastasis and treatment (31). Moreover, a recent study reported that VEGF’s effects are sustained up to one month after discontinuing its delivery using local microsphere delivery of VEGF (32). Unfortunately an increase of only one month is not useful in most issues of tissue engineering or implantable devices. Additionally, this study did not address vessel maturation. Due to foreign body reactions (FBR) induced by the microsphere used in this study, the impact of FBR-wound healing induced neovascularization is unclear.
Ultimately the limitations of recombinant VEGF have spurred efforts to utilize local AF gene therapy to create and sustain vascular networks at sites of disease, tissue injury, and ischemia. Additional efforts to utilize angiogenic factors in organ and stem cell transplantation is under significant investigation by numerous laboratories (33), and there is great hope for local AF gene therapy in these situations, as well as in vascular network dependent implantable devices such as glucose sensors. Our current study is focused on the initiating of vascular networks (i.e. angiogenesis) at site of sensor utilizing local VEGF gene therapy as a “proof of concept” that increasing blood vessel density at sites of glucose sensor implantation using local gene therapy enhances and extends glucose sensor performance in vivo. The impact of long-term or sustained vessel formation thru the use multiple AF and maturation factors on in vivo sensor function is a goal for future studies.
Vascular Networks and Glucose Sensor Function
Although the role of angiogenesis and neovascularization in implantable sensor function and lifespan in vivo has seen significant discussion, currently there are only a few publications, which have focused on the impact of angiogenesis on in vivo sensor function (3, 4). Additionally most of these initial studies do not evaluate glucose sensor function. To our knowledge presently there are no VEGF-CGM studies in the literature, only single point evaluation of glucose sensor function in vivo. Generally these studies have focused on the effect of recombinant VEGF induced vessel density and sensor performance and were only evaluated at pre-determined time points (e.g. every 2 weeks) rather than continuously (12). For example, Ward et al. delivered recombinant VEGF at various distances to the sensor element and found that sensor performance was best for units closes to the VEGF infusion port. Alternatively, Klueh et al. demonstrated that using local VEGF gene delivery in an ex ova chicken model (i.e. chicken chorioallantoic membrane (CAM) model) increased vessel density at the sites of implantation and enhanced sensor performance for short periods of time ex ova (7). Obviously translating these gene therapy results from the chicken embryo to long-term mammalian models of CGM is crucial. Additionally it should be noted that to our knowledge, none of these studies or other studies in the literature have addressed lymphatic vessel regression/formation and glucose sensor function or CGM in vivo. Our results statistically demonstrate an approximately one to one correspondence in the number of blood to lymph vessels, similar to other corneal experimental models, in which blood and lymph vessel track each other (18). This evidence contributes to the possibility that the angiogenesis and lymphangiogenesis should be viewed typically as one united physiological process with two branches, which in experimental contexts can be separated or induced in isolation. One can clearly consider more potent lymphangiogenic factors, (e.g VEGF-C and VEGF-D) and inhibitors in mammalian CGM systems such as our mouse model. Adding these potent lymphangiogenic factors or inhibitors to in vivo CGM model may reveal the unique functions of lymphatic vessels in tissue reactions at sensor implantation sites and sensor function, e.g. anti-inflammatory effects. These in vivo results also describe a numerical, statistical signature to the relative contributions of blood and lymph vessels to a defined process. Our analysis demonstrates the statistical dominance of blood vessels over lymphatic vessels with respect to blood glucose concentration, specifically a numerical signature of 7.1% and 1.3% respectively, or in a ratio of those two numbers, the BV are 5.6 times more dominant than LV.
Our data demonstrated that Adv-LacZ group has a significantly higher MARD as compared to the no injection control. We believe that this difference is a result of lower blood vessel and lymphatic vessel density at the Adv-LacZ adenovirus treated implantation site when compared to the non-injected site. Although adenoviruses are defective and cannot propagate, they are able to infect cells and can cause some tissue inflammation. We hypothesize that in the case of the Adv-VEGF-A the increased lymphangiogenesis / lymphatic vessel density at the sensor implantation site increased drainage of inflammatory cell and tissue debris and thereby decreasing inflammation and tissue injury at the tissue location of the device. In the case of the Adv-LacZ adenovirus infection there was no increase in lymphatic vessel density at the sensor implantation site but in fact a slight loss in lymphatic vessel density when compared to no injection sites. Additional analysis of blood vessel density also indicated that the Adv-LacZ adenovirus injection sites had lower blood vessel density likely a result of the tissue reactions. It is possible that the lower blood vessel density resulted in less efficient blood flow and glucose diffusion at the Adv-LacZ injected site therefore resulting in poorer MARD values when compared to the non-injected sites.
Translation of “proof of concept” to the bedside
The goal of our present studies is to demonstrate “proof of concept” of local gene therapy to enhance and extend CGM in mammalian systems. We choose VEGF-adenovirus based gene therapy since adenoviral vectors do not integrate into the genome, and therefore the expression is ultimately transient. This finite lifetime of adenoviral vectors can actually be a beneficial safety feature, since there are reported safety issues or concerns with gene therapy, e.g. VEGF (34). The limitation to the adenoviral vector’s life would prevent any adverse reactions from progressing to any appreciable degree. Future studies using various AF and maturation genes can be used to refine vascular network formation and lifespans. Additionally, the usage of alternative gene delivery, such as adenovirus associated viral vectors (AAV) or non-viral vector methods (e.g. electroporation, bubbles, liposomes) will help to define and optimize “sensor friendly” tissue engineering at sites of sensor implantation. Using other gene therapy vehicles such as AAV would not only extend local gene expression in vivo, but also limit the adverse immune reaction one could expect from such local gene therapy vectors, especially in comparison to that observed with adenoviral vectors. Our present studies also underscores the potential uses of local gene therapy to control inflammation and fibrosis, which would also likely aid in enhancing and extending sensor performance and CGM performance in vivo. Ultimately the combination of local delivery of factors, drugs, and genes will likely be the key to development of accurate long term CGM, which will be the foundation for closed loop technology and provide effective blood glucose management in the future.
CONCLUSION
The aim of this research was to determine the impact of increased BV and/or LV at sites of sensor implantation as it relates to glucose sensor performance. Once it is determined what factors and pathways play an important role in the control of vascular network formation a more appropriate gene delivery strategy needs to be explored. That said, it should be noted that “gene guns” have been used experimentally for local gene delivery in preclinical studies. The most likely gene delivery strategy for this local gene will be to incorporate the genetic material (DNA, siRNAs, viral vectors, etc.) into a biocompatible sensor coating (natural or synthetic), which is applied on the outside of the glucose sensor prior to implantation.
Acknowledgments
We would like to acknowledge Jackman Frailey for his indispensible care and maintenance of the CGM murine models. The National Institute of Diabetes and Digestive Kidney Diseases, and the American Diabetes Association provided support for these studies.
References
- 1.Novak MT, Yuan F, Reichert WM. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal Bioanal Chem. 2010;398(4):1695–705. doi: 10.1007/s00216-010-4097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dungel P, Long N, Yu B, Moussy Y, Moussy F. Study of the effects of tissue reactions on the function of implanted glucose sensors. J Biomed Mater Res A. 2008;85(3):699–706. doi: 10.1002/jbm.a.31593. [DOI] [PubMed] [Google Scholar]
- 3.Ward WK, Quinn MJ, Wood MD, Tiekotter KL, Pidikiti S, Gallagher JA. Vascularizing the tissue surrounding a model biosensor: how localized is the effect of a subcutaneous infusion of vascular endothelial growth factor (VEGF)? Biosens Bioelectron. 2003;19(3):155–63. doi: 10.1016/s0956-5663(03)00180-5. [DOI] [PubMed] [Google Scholar]
- 4.Ward WK, Wood MD, Casey HM, Quinn MJ, Federiuk IF. The effect of local subcutaneous delivery of vascular endothelial growth factor on the function of a chronically implanted amperometric glucose sensor. Diabetes Technol Ther. 2004;6(2):137–45. doi: 10.1089/152091504773731320. [DOI] [PubMed] [Google Scholar]
- 5.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J Biomed Mater Res A. 2007;81(4):858–69. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klueh U, Dorsky DI, Kreutzer DL. Uses of Vascular Endothelial Cell Growth Factor Gene Transfer to Enhance Biosensor Function In Vivo. J Biomed Mater Res; Student Research Award in the Ph. D. Science Degree Candidate category, 29th annual meeting of the Society for Biomaterials; Reno, Nevada. April 29–May 3, 2003; 2003. pp. 1072–86. [DOI] [PubMed] [Google Scholar]
- 7.Klueh U, Dorsky DI, Kreutzer DL. Enhancement of implantable glucose sensor function in vivo using gene transfer-induced neovascularization. Biomaterials. 2005;26(10):1155–63. doi: 10.1016/j.biomaterials.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Klueh U, Liu Z, Cho B, Ouyang T, Feldman B, Henning TP, et al. Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technol Ther. 2006;8(3):402–12. doi: 10.1089/dia.2006.8.402. [DOI] [PubMed] [Google Scholar]
- 9.Klueh U, Kaur M, Qiao Y, Kreutzer DL. Critical role of tissue mast cells in controlling long-term glucose sensor function in vivo. Biomaterials. 2010;31(16):4540–51. doi: 10.1016/j.biomaterials.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klueh U, Liu Z, Feldman B, Kreutzer DL. Importance of Interleukin 1 / Interleukin 1 Receptor Antagonist in Short Term Glucose Sensor Function in Vivo. Diabetes Science and Technology. 2010;4(5):1073–86. doi: 10.1177/193229681000400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Manseau EJ, et al. VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb Symp Quant Biol. 2002;67:227–37. doi: 10.1101/sqb.2002.67.227. [DOI] [PubMed] [Google Scholar]
- 12.Klueh U, Liu Z, Feldman B, Henning TP, Cho B, Ouyang T, et al. Metabolic biofouling of glucose sensors in vivo: role of tissue microhemorrhages. J Diabetes Sci Technol. 2011;5(3):583–95. doi: 10.1177/193229681100500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seregin SS, Amalfitano A. Improving adenovirus based gene transfer: strategies to accomplish immune evasion. Viruses. 2010;2(9):2013–36. doi: 10.3390/v2092013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham DC, Stenken JA. In Vivo Glucose Sensing. Wiley; 2009. p. 454. [Google Scholar]
- 15.Obermaier K, Schmelzeisen-Redeker G, Schoemaker M, Klotzer HM, Kirchsteiger H, Eikmeier H, et al. Performance evaluations of continuous glucose monitoring systems: precision absolute relative deviation is part of the assessment. J Diabetes Sci Technol. 2013;7(4):824–32. doi: 10.1177/193229681300700404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ismail JA, Poppa V, Kemper LE, Scatena M, Giachelli CM, Coffin JD, et al. Immunohistologic labeling of murine endothelium. Cardiovasc Pathol. 2003;12(2):82–90. doi: 10.1016/s1054-8807(02)00166-7. [DOI] [PubMed] [Google Scholar]
- 17.Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31(6):1160–4. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regenfuss B, Bock F, Cursiefen C. Corneal angiogenesis and lymphangiogenesis. Curr Opin Allergy Clin Immunol. 2012;12(5):548–54. doi: 10.1097/ACI.0b013e328357b4a2. [DOI] [PubMed] [Google Scholar]
- 19.Ward WK, Troupe JE. Assessment of chronically implanted subcutaneous glucose sensors in dogs: the effect of surrounding fluid masses. Asaio J. 1999;45(6):555–61. doi: 10.1097/00002480-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25(4):443–7. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 21.Huggenberger R, Detmar M. The cutaneous vascular system in chronic skin inflammation. J Investig Dermatol Symp Proc. 2011;15(1):24–32. doi: 10.1038/jidsymp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobek V, Taltynov O, Pinterova D, Kolostova K. Gene therapy of the ischemic lower limb--Therapeutic angiogenesis. Vascul Pharmacol. 2006;44(6):395–405. doi: 10.1016/j.vph.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials. 2013;34(36):9201–9. doi: 10.1016/j.biomaterials.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies NH, Schmidt C, Bezuidenhout D, Zilla P. Sustaining neovascularization of a scaffold through staged release of vascular endothelial growth factor-A and platelet-derived growth factor-BB. Tissue engineering Part A. 2012;18(1–2):26–34. doi: 10.1089/ten.tea.2011.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiological reviews. 2011;91(3):1071–121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutton C, Young YW, Williams R, Meedeniya AC, Mackay-Sim A, Goss B. Combined VEGF and PDGF treatment reduces secondary degeneration after spinal cord injury. Journal of neurotrauma. 2012;29(5):957–70. doi: 10.1089/neu.2010.1423. [DOI] [PubMed] [Google Scholar]
- 27.Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the development of a vehicle for localized and controlled drug delivery for implantable biosensors. J Diabetes Sci Technol. 2008;2(6):1016–29. doi: 10.1177/193229680800200611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamin LE. The controls of microvascular survival. Cancer metastasis reviews. 2000;19(1–2):75–81. doi: 10.1023/a:1026552415576. [DOI] [PubMed] [Google Scholar]
- 29.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. The American journal of pathology. 2004;165(1):35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. The Journal of clinical investigation. 1999;103(2):159–65. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nature reviews Drug discovery. 2011;10(6):417–27. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 32.Daugherty AL, Rangell LK, Eckert R, Zavala-Solorio J, Peale F, Mrsny RJ. Sustained release formulations of rhVEGF(1)(6)(5) produce a durable response in a murine model of peripheral angiogenesis. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2011;78(2):289–97. doi: 10.1016/j.ejpb.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Naderi H, Matin MM, Bahrami AR. Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl. 2011;26(4):383–417. doi: 10.1177/0885328211408946. [DOI] [PubMed] [Google Scholar]
- 34.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102(8):898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]