Introduction
The same populations of women at risk for sexual acquisition of HIV are also at risk for pregnancy; many of these women use contraception. Combating the spread of HIV is a major global goal[1] ideally achievable with development of highly effective dual protection methods that prevent both sexual acquisition of HIV and unwanted pregnancy. Currently, emerging evidence[2] suggests that some commonly used contraceptives may increase risk of sexual HIV acquisition and transmission. There are several biologically plausible mechanisms by which hormonal contraceptives (HC) could increase HIV risk including disrupting epithelial barriers (thinning of the epithelium or altering epithelial integrity), causing changes in inflammatory responses that could in turn enhance HIV replication locally,[3] or altering the vaginal microbiota which itself effects local immunity and genital inflammation. The objective of this manuscript is to review the evidence linking contraceptives to genital tract changes and to identify research gaps to be addressed with future studies.
The anatomic, immune, and microbiological milieu of the female genital tract may be important determinants of both HIV acquisition and sexual transmission.[4] The likelihood of HIV acquisition when a susceptible individual is sexually exposed is driven by several factors, including viral load of the transmitting sexual partner,[5] however, host factors are also important in acquisition risk. One potentially important host factor in HIV acquisition risk is the number of accessible cellular targets available for viral entry, a necessary step prior to systemic dissemination.[3] Genital tract immune cells are common portals of entry for HIV[3, 6] yet little is known about the effects of HC on genital architecture, immune cell populations, and microbiota in the genital mucosa, all of which may relate to susceptibility. Given mounting data[7] suggesting women using systemic HC may have increased susceptibility to HIV, this is a significant knowledge gap.
There are strong data supporting significant changes in epithelial thickness in animals exposed to depot medroxyprogesterone acetate (DMPA) and, in fact, DMPA dosing is required in several animal models to induce susceptibility to HIV/SIV infection. Interestingly, multiple investigators have examined genital tract tissues in women who use DMPA and have not confirmed a similar epithelial thinning in women.[8-11] Other architectural features, such as epithelial tight junctions or adherens proteins, may be important barriers to HIV transmission and if altered with use of HC, may contribute to HIV risk. One study has reported that these epithelial architectural features are not altered with HC use.[11]
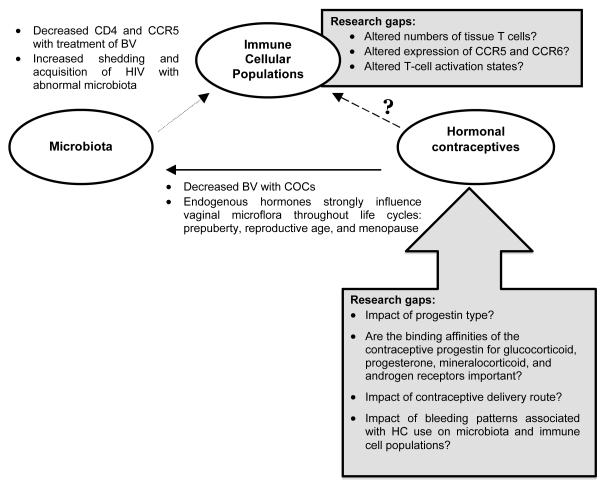
Other possible mechanisms by which HC could increase HIV acquisition risk include: altering HIV target immune cells resident within the local tissues; interfering with innate or adaptive immunity within the genital tract tipping transmission probability towards establishing infection; or creating inflammation that promotes transmission by disrupting tight junctions between epithelial cells and increasing the number and activation state of target immune cells. Additionally, HC could alter co-receptor expression on target immune cells required for HIV cell entry thus making exposed cells more permissive to infection. Finally, genital tract infection and endogenous microbiota composition may confound the relationship between sex hormones and immune cellular populations within the genital tract since HC use alters vaginal microflora,[12-17] but it is not known if these microbiota alterations result in changes to HIV susceptibility (Figure 1).
Figure 1.
Model Relationships: Contraceptives, Microbiota and Genital Tract Immune C ells
To date, little research has been specifically designed to elucidate if HC use causes cellular and/or microbiological changes in the genital tract that could increase the efficiency of HIV transmission. Although regulatory approval of these contraceptives required rigorous data supporting contraceptive efficacy and safety, there are limited data on the non-contraceptive effects that these drugs have on the genital tract.
Do Hormonal Contraceptives Differ in the Types and Concentrations of Progestins Present?
Progestogens are compounds that induce a secretory endometrium in order to support pregnancy. The term progestogen is inclusive of the only naturally occurring progestogen, progesterone, as well as the wide array of synthetic progestogens, collectively called progestins. Some progestins are highly related to progesterone, such as medroxyprogesterone acetate (MPA), and other progestins are more closely related to testosterone, such as norethindrone, levonorgestrel, desogestrel and gestodene (Fig. 2). Progesterone-related progestins in particular induce profound suppression of estradiol levels via negative feedback on hypothalamic-pituitary-ovarian axis and bind more tightly to the glucocorticoid receptors compared with other progestins.[18, 19] With MPA use, estradiol levels may become as low as those measured in menopausal women. A major determinant of progestogen action is the binding affinity of each progestogen for the progesterone receptor and for other steroid receptors, including the glucocorticoid receptor.[19, 20]
Figure 2.
Systemic Progestin Concentration Varies with Contraceptive Method
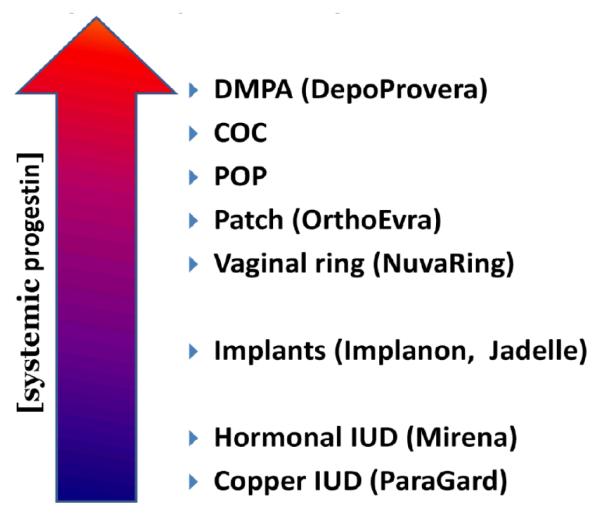
There are a wide variety of progestins used in modern hormonal contraceptives and they belong to three main chemical families: progesterone derivatives (pregnanes), 19-nortestosterone derivatives (estranges and gonanes), and spironolactone derivatives (Table 1). Progestins impact many aspects of human physiology and so biological potency varies based on the end-point measured. Clinically, progestins are largely used for contraception with ovulation inhibition and endometrial transformation being the most typical endpoint evaluated. Gonane derivatives are the most active with respect to these contraceptive endpoints generally with potencies >100x that of progesterone. Little to nothing is known regarding whether the type of progestin, or the delivery route, or both influence the HC effect on genital tract tissues, cells, and microbiota.
Table 1.
Contraceptive Progestins
| Progestin-type | Steroid class | Progestin generation |
Class members | Common Contraceptives |
Delivery route(s) |
Notes |
|---|---|---|---|---|---|---|
| Progesterone derivatives |
Pregnanes | n/a | MPA | DMPA | Injectable* | |
|
| ||||||
| Testosterone derivatives |
Estranes | 1st | Norethindrone | Multiple progestin only and combined oral formulations |
Oral | Of the testosterone derivative progestins: Lowest progestational effect; lowest potency; shortest half-life (T1/2). |
|
|
||||||
| Norethindrone acetate |
Multiple combined oral formulations |
Oral | ||||
|
|
||||||
| Norethindrone enanthate§ |
NET-En | Injectable | ||||
|
|
||||||
| Ethynodiol diacetate |
Several combined oral formulations |
Oral | ||||
|
|
||||||
| Gonanes | 2nd | Norgestrel | Multiple combined oral formulations |
Oral | 2nd generation progestins have higher andreogenic effects compared with other progestins |
|
|
|
||||||
| Levonorgestrel | Multiple combined oral formulations |
Oral | ||||
|
|
||||||
| Mirena®/Skyla® | Intrauterine | |||||
|
|
||||||
| Norplant®/Jadelle®§ | Subdermal implants |
|||||
|
|
||||||
| 3rd | Desogestrel | Multiple combined oral formulations |
Oral | 3rd generation progestins have higher affinity for the progesterone receptor and lower affinity for the androgen receptor compared with 1st and 2nd gen. progestins |
||
|
|
||||||
| NuvaRing® | Intravaginal | |||||
|
|
||||||
| Implanon®/Nexplanon® | Subdermal Implants |
|||||
|
|
||||||
| Norgestimate | Multiple oral formulations | Oral | ||||
|
|
||||||
| Gestodene§ | Several oral formulations | Oral | ||||
|
| ||||||
| Spironolactone derivatives |
n/a | 4th | Drospirenone | Multiple oral formulations | Oral | Increased anti- mineralocorticoid and anti- androgenic properties compared with other progestins |
Intramuscular and subcutaneous injectable formulations available
Not available in the United States
The glucocorticoid receptor is a protein present in the cytosol of almost all cells that regulates genes controlling development, metabolism, and immune response. The glucocorticoid receptor is activated when bound by cortisol or synthetic glucocorticoids, including progestogens. Activation of glucocorticoid receptors generally results in immune suppression as is seen with cortisol use, and MPA can have similar immunosuppressive effects.[21] Interestingly, progestins that are classified together often have significantly different non-contraceptive biological effects. For instance, apart from progesterone and the related pregnanes (MPA), the progestin that has the highest relative binding affinity for glucocorticoid receptors is gestodene, a gonane, but the other gonanes, such as levonorgestrel, have very low relative binding affinities for glucocorticoid receptors.[19] Understanding the relative binding affinities of the various progestins for glucocorticoid receptors, mineralocorticoid receptors and androgen receptors and the subsequent immune and genital tract biological effects of these bound receptors is critical in evaluating the biological plausibility of HIV risk and HC. Given the paucity of data into understanding these non-contraceptive effects of HC use, this is a significant knowledge gap.
Are HIV Target Cells in the Genital Tract Impacted by HC Use?
Genital tract lymphocytes and APCs expressing surface receptors compatible with HIV entry are targets for the establishment of HIV infection and fluctuate with hormonal change, including pregnancy.[22-26] These critical immune cellular populations in the female genital tract have not yet been carefully assessed in the setting of contraceptive use, many of which contain hormones. CD4 T lymphocytes are thought to be the primary targets for initial HIV infection in the female genital tract.[27-33] HIV infects discrete subsets of CD4 T cells all of which express phenotypic receptors and co-receptors necessary for HIV to gain intracellular access.[9, 34-36] CCR5 is the predominant target co-receptor for initial infection and CXCR4 appears to play a more prominent role in continuation of an already established infection.[9]
CCR5 expression on CD4 T cells in the human female genital tract[22-24, 37] may be increased in women who use combined oral contraceptive pills (COCs)[25] offering a potential biological link between HIV susceptibility and hormonal status. CCR5 expression on immune cells is influenced by sex hormones and may account for the observed increased risk of HIV acquisition during pregnancy.[26, 38-40] Progesterone both activates and suppresses mucosal immune cells[41, 42] since it increases recruitment of inflammatory cells[29, 30, 43] and decreases the cellular functions of natural killer cells and cytotoxic T-cells.[31, 44, 45] Ex-vivo studies have demonstrated that incubation of cervical tissue in media containing progesterone stimulates CCR5 expression.[23] However, data describing genital tract immune cell populations, including CCR5 receptor expression, in women who use various hormonal contraceptives are lacking.
Several investigators have sought to characterize and quantify immune cells within the female reproductive tract. However, a comparison of the various methods and populations studied to date[25, 46-51] highlights the heterogeneity in participants, tissues, and collection methods, making it difficult to directly compare data. Many of the published studies collected tissues from gynecologic surgery specimens that are more likely to come from older women with a variety of pathologic conditions, including malignancies that may not be relevant to the genital tract immune cell populations in women at the highest risk of HIV. Consensus data from healthy women remains elusive, and data from African women of reproductive age are particularly lacking.
Endogenous and exogenous sex hormones may modulate the expression of HIV coreceptors on the surface of mucosal immune cells, and the expression of surface co-receptors may be critical to the cellular permissiveness to HIV entry and replication. Modulation by sex hormones may or may not be tissue dependent, and since the tissues permissive to HIV infection are not well delineated, it remains important to obtain data on cells from all of the relevant mucosal surfaces that may be sexually exposed: the lower genital tract, the upper genital tract, and the distal GI tract. Women are at risk of HIV acquisition from anal receptive intercourse.[52] The impact of hormonal contraceptives on immune cells in the distal GI tract is an important research gap that should be addressed but which will not be discussed further in this review.
What is the Evidence that Vaginal Microflora Can Alter Immune Cell Populations?
Hormonal contraceptive usage can alter genital tract microflora[8, 13, 17, 53-55] (Fig. 1) and abnormal vaginal flora can impact susceptibility to HIV and other sexually transmitted infections including Herpes Simplex Virus (HSV) and Human Papilloma Virus (HPV).[56-58] HSV and HPV are also associated with increased risk of HIV acquisition.[59, 60] [61] HSV is an example of a genital infection that significantly alters local immune cell composition.[49, 62] HSV modifies the relationship between risk of HIV acquisition and hormonal contraceptive use, with HSV negative women having a greater increase in risk of HIV acquisition.[63]
Bacterial vaginosis (BV) is a common condition in which the predominant lactobacilli, which comprise the microflora of healthy women, are replaced by a heterogeneous group of microbiota. Women with BV have bacterial communities with species richness and diversity. While no single bacterial species is present in all women with BV, G. vaginalis is present in most women (97%).[64] Other bacteria present in significant numbers in women with BV include A. vaginae (92%), L. iners (86%) and Eggerthella species (85%), in addition to other anaerobic gram positive bacteria.[64] BV has been linked to increased risk of HIV acquisition,[65, 66] increased shedding of HIV[67] and increased transmission of HIV from HIV infected women to their uninfected partners.[68, 69]
Treatment of BV has been shown to decrease HIV target immune cells within the cervix.[70] Rebbapragada reported that when 15 HIV infected women with BV were successfully treated with oral metronidazole, there was a significant decrease in activated CD4 T cells in cervical cytobrush samples obtained before and after treatment.[70] They also reported an increase in DC-SIGN+ immature dendritic cells among HIV+ women following treatment of BV. These data are some of the only available that directly link changes in vaginal microflora with changes in immune cell populations in the genital tract.[8, 13, 17, 53-55] Additional research exploring how changes in the vaginal microbiota impact the numbers, distribution, activation status and co-receptor expression of genital immune cell populations are needed in order to establish the biological basis of how flora changes alter risk of HIV.
What is the Evidence that Contraceptive Hormones Cause Changes in Vaginal Microbiota, Including Bacterial Vaginosis and Yeast Vaginitis?
An important and understudied mechanism by which contraceptives could alter HIV risk is by impacting the vaginal microflora and vaginal infections, which can in turn cause changes in the vaginal ecosystem and enhance HIV risk. Both BV and yeast vaginitis cause changes that could enhance susceptibility to HIV, although the data linking BV to enhanced HIV risk is more consistent than that of yeast vaginitis. In addition, women with BV are more susceptible to HSV-2,[71, 72] and women with HSV-2 are significantly more likely to acquire HIV.[73] Similarly, women with BV are more susceptible to HPV.[74] Most studies that have reported associations between BV and HIV risk or changes in immune cells, have not controlled for these viral infections that may also have synergy with BV.
In addition to changes in genital tract immune cells associated with BV described above, women with BV are known to have changes in the levels of pro-inflammatory and anti-inflammatory cytokines and secretory leukocyte protease inhibitor (SLPI) in cervical and vaginal fluid.[75-80]. While interleukin-1β (IL-1β) is consistently increased in BV, other cytokines have been less consistently linked with alterations in vaginal flora. Among 81 women, increases in IL-1β, tumor necrosis factor, interferon-γ, IL-2, IL-4, IL-10, and GM-CSF were present among women with BV and declined with treatment and restoration of a Lactobacillus-dominant flora.[77] The absence of IL-8 elevation in BV is consistent with the lack of increased neutrophils in this condition,[78] but why it is low when IL-1β and TNF are elevated is unclear.[78] Most of the data linking contraceptive use with changes in the microbiota and vaginal infections are derived from cross-sectional studies of women presenting to clinics with symptoms or are secondary analyses derived from clinical trials of other interventions or longitudinal cohort data. Thus, the studies to date reporting changes in the vaginal flora and/or incident infections are usually very small observational cohort studies following women for 2-6 months after initiation of a new contraceptive method, or they are large secondary analyses of larger studies. The smaller cohort studies are generally of insufficient size or length of follow up to detect large or clinically significant changes in flora. A major deficiency of the larger secondary analyses is that the researchers group contraceptives together out of necessity since some contraceptive types are not used by many of the study participants. For example, IUD users are often grouped together even though the IUD types many be significantly different. As noted above, the exposure to progestin differs substantially among hormonal contraceptive methods, so grouping of methods may obscure real biological differences between contraceptive groups.
Women who enroll in cross-sectional studies may be using no contraception, may be using condoms alone for contraception or may be using hormonal methods or IUDs along with condoms. Therefore, in these studies it is methodologically unclear which group of women should be used as the comparator group when assessing the effects of contraceptives on vaginal infections. Women of reproductive age who do not use contraceptives and women who rely on condoms alone are likely behaviorally and demographically different from those women who are using a highly effective contraceptive method. In some studies, the authors have compared those women using oral contraceptives to everyone else,[13] creating a binary variable, while in other studies women using condoms or those using non-hormonal methods have served as the comparator group.[14]
The association between contraceptive use and prevalent BV has been evaluated in a large number of cross-sectional studies,[13, 81-84] and selected studies published over the past 15 years are summarized in Table 3. As shown, women in these studies using oral contraceptives and those reporting consistent condom use have generally had a decreased risk of BV, while those reporting IUD use have had inconsistent associations with prevalent BV.[81, 82] One of the largest and most recent studies evaluating the impact of contraceptive methods on BV was performed by Riggs.[13] These authors recruited 3,077 women of reproductive age from gynecologic and family planning clinics in Birmingham, AL for a 1-year prospective longitudinal study. Gram stains were used to quantify vaginal flora. In this study, there was decreased BV prevalence among oral contraceptive users (odds ratio, OR 0.76; confidence interval, CI 0.63-0.90) and among those using hormonal injections/implants (OR 0.64; CI 0.53-0.76). An increased risk for BV (OR 1.38; CI 1.11-1.71) was observed among those women who had tubal ligation, and condom users had no change in their BV risk (Table 3). As noted in Tables 3 and 4, Riggs et al grouped those women using DMPA and those having implantable contraceptives together, even though the type, concentration and delivery route of progestins delivered in these HCs differs significantly.[13]
Table 3.
Cross-sectional Studies Linking Contraceptive Use with Prevalent BV
| Author, Year | Group | Odds Ratio or RR (95% Confidence interval) |
|---|---|---|
| Riggs, 2007[ 13 ] | Oral contraceptives | 0.76 (0.63 – 0.90) |
| DMPA/Implant | 0.64 (0.53 – 0.76) | |
| Tubal ligation | 1.38 (1.11 – 1.71) | |
| Condom | 1.0 (0.86 – 1.16) | |
|
Shoubnikova,
1997[ 81 ] |
Combined oral contraceptives | 0.4 (0.2 – 0.8) |
| IUD | 0.7 (0.3 – 1.6) | |
| Condom | 0.3 (0.1 – 0.9) | |
| Calzolari, 2000[ 82 ] | Combined oral contraceptives | 0.43 (0.22 – 0.76) |
| IUD | 2.98 (1.66 – 5.34) | |
| Condom | 0.56 (0.33 – 0.96) | |
| Holzman, 2001[ 83 ] | Hormonal Contraceptives | 0.5 (0.2 – 0.8) |
|
Hutchinson,
2007[ 84 ] |
Condoms | 0.55 (0.34 – 0.88) |
Table 4.
Longitudinal Studies of Contraceptives and Acquisition of Bacterial Vaginosis
| Author, Year | Group | RR (95% CI) |
|---|---|---|
| Barbone, 1990[ 12 ] | COC Users vs.IUD/tubal ligation | 0.84 (0.63 – 1.10) |
| Riggs, 2007[ 13 ] | COC User | 1.04 (0.78 – 1.39) |
| DMPA/Implant Yes | 1.07 (0.79 – 1.45) | |
| Tubal ligation Yes | 1.43 (1.02 – 2.07) | |
| Condom Yes | 1.02 (0.79 – 1.31) | |
| Rifkin, 2009[ 14 ] | COC User vs No hormones | 0.66 (0.39 – 1.10) |
| Progestin only OC vs No Hormones |
0.42 (0.20 – 0.88) | |
| Madden, 2012[ 15 ] | IUD Users vs COC, ring or patch | 1.28 (0.53 – 3.06) |
|
Bradshaw,
2012[ 16 ] |
COC User | 0.62 (0.70 – .95) |
| Inconsistent condom use | 1.9 (1.0-3.3) | |
| Baeten, 2001[ 17 ] | COC vs. no contraception | 0.8 (0.7-1.0) |
| DMPA vs. no contraception | 0.7 (0.5-0.8) |
Six longitudinal studies that assessed BV incidence or recurrence are summarized in Table 4. These authors reported that women using oral contraceptives had a reduced incidence of trichomoniasis when compared to the comparator group of women using either an IUD or having had a tubal ligation (RR 0.56, 95% CI 0.39-0.81).[12] In this same study, there was a trend toward decreased incident BV among women using oral contraceptives (Table 4). Rifkin et al enrolled 330 women recruited from a sexually transmitted clinic and evaluated incident BV among women using progestin only oral contraceptives or combined oral contraceptives compared to women who reported no exogenous hormones.[14] As summarized in Table 4, women using either type of oral contraceptives had significantly reduced rates of BV. In a study of 948 Kenyan sex workers, Baeten et al reported a decreased incidence of BV among women using oral contraceptives.[17] Bradshaw et al performed a secondary analysis of a randomized trial evaluating a probiotic treatment for BV and found that both consistent condom use and use of an estrogen-containing contraceptive reduced recurrent BV.[16]
The least-well studied contraceptive method with respect to incident BV is the IUD. As summarized in Tables 3 and 4, IUD usage was not reported to be associated with prevalent BV by Shoubnikova,[81] but was linked with a nearly threefold increased prevalence of BV by Calzolari.[82] The first study of both copper and levonorgestrol IUD users and incident BV diagnosed by Gram stain Nugent criteria was reported by Madden et al in 2012.[15] In this study, women using IUDs were less likely to report condom usage than were women who reported using oral contraceptives, vaginal rings or patches. They were also more likely to report irregular bleeding, which was also associated with an increased incidence of BV. By univariate analysis, IUD users were significantly more likely to acquire BV. However, after adjustment for irregular bleeding, IUD usage was no longer associated with an increased risk of BV. Even though this study had too few cases of incident BV to clearly assess the impact of the hormonal vs. the non-hormonal IUD, the analysis demonstrated that factors such as bleeding and condom usage could confound the associations between contraceptive method and BV.
A number of prospective-cohort studies have evaluated vaginal microflora over 2-6 months of contraceptive use. For example, Gupta et al and Eschenbach et al reported that the vaginal microflora was unchanged 1-2 months after initiating COC use.[85, 86] Miller et al, reported that among 38 women initiating DMPA use, there was a statistically significant decline in colonization by lactobacilli producing hydrogen peroxide, although BV did not increase in these women over 6 months of follow-up.[8] There is little published data available on the impact of vaginal rings on the vaginal ecosystem. In one crossover study of 64 women randomized to receive either the hormonal contraceptive ring or oral contraceptives, and who were then crossed over to the other method, participants were more than twice as likely to be colonized by more than 100,000 colony forming units of hydrogen peroxide producing lactobacilli while using the ring.[87] However, in this study and a subsequent randomized trial of 500 women comparing vaginal microflora among women using hormonal contraceptive rings or hormonal contraceptive patches, there was no statistically significant change in vaginal microflora over time as assessed by Nugent score.[88] Thus, there is no evidence suggesting the vaginal ring has any clinically significant impact on vaginal microflora and/or acquisition of vaginal infections.
The data gathered to date are clearly insufficient to draw any conclusions about the impact of many contraceptives on vaginal infections. Although, the strongest data suggest a consistent association between oral contraceptives and reduced BV, the mechanism for this protective benefit is unclear. The well-controlled studies where women are followed after initiating their contraceptive method are very small, and the measures of the microbiota rely on clinical signs of BV, the Nugent criteria or very selected evaluation of individual constituents of the microbiota. There is almost no data that assess the impact of the hormonal vs. the non-hormonal IUD on the microbiota, and most studies have grouped different hormonal methods together for convenience. There are extremely limited data on the impact of the copper IUD on the vaginal microbiota even though it is the only non-hormonal long acting reversible contraceptive available at this time. It is therefore difficult to assess the independent role of progestins, or to ascertain from the existing studies whether there is any dose relationship between changes in the bacterial flora and contraceptive hormones.
Is There an Association Between Contraceptive Use and Yeast Colonization or Yeast Vaginitis?
Yeast vaginitis has been linked with an increased risk of HIV among Zimbabwean and Ugandan women who had vaginal yeast at the visit prior to and at the visit during which HIV was detected (hazard ratio 2.97, 95% CI 1.67-5.28).[89] As summarized in Table 4, there has been an inconsistent association between oral contraceptive use and yeast vaginitis. Women using COCs have been reported to have an increase in yeast vaginitis,[12, 17] a decrease in yeast colonization[85] or no effect on yeast colonization[16] depending on the study size and design. There is a similar inconsistent relationship between DMPA usage and yeast with some studies reporting decreased colonization[8] and decreased yeast vaginitis,[17] and other authors reporting increased yeast colonization in women using DMPA.[90] The association between yeast colonization and yeast vaginitis and contraceptive deserves further study given the lack of adequate data on hormonal and non-hormonal IUDs, implants, and vaginal rings.
Summary and identification of key knowledge gaps
Although there is a large body of published literature on the impact of contraceptive methods on genital immunity and vaginal microflora, these studies have several important limitations. The study of non-contraceptive effects of hormonal contraceptive use has been complicated by the sheer diversity of available contraceptive hormones, the unaccounted for differences in local tissue and systemic hormone exposure associated with different methods, and the differing routes of delivery, all of which may impact HIV risk. Few studies have accounted for how changes in bleeding patterns associated with some types of contraceptives and decreased condom usage, both of which can impact the genital microflora, altered the relationship between contraceptives and flora changes. Importantly, most studies evaluating the impact of contraception on genital immune cells or changes in the vaginal flora have been secondary analyses of randomized trials or small, well-controlled prospective studies that have been too small to detect clinically significant changes.
With concern that effective contraceptives such as DMPA could increase the risk of HIV for women living in high HIV prevalence areas, some experts have advised that reliance on DMPA should be decreased. However, there are a number of research gaps that should be addressed to inform decisions about DMPA and about alternative contraceptives, which have been less studied, may be less effective, and may not have a better safety profile with respect to HIV risk. While diversifying the contraceptive mix for women is a laudable goal, women and contraceptive providers need access to better research to guide these recommendations. Some of the most critical research gaps identified in this review include the following:
The relative binding affinities of the various progestins used in HC for glucocorticoid receptors, mineralocorticoid receptors and androgen receptors and the subsequent immune and genital tract biological effects of these bound receptors.
The changes in genital tract immune cell populations, including CCR5 receptor expression, in women initiating use of a new contraceptive. The full range of available contraceptives should be evaluated.
How changes in vaginal microflora impact the number, distribution, activation status, and co-receptor expression of immune cells in the reproductive tract.
How bleeding patterns associated with contraceptive methods such as the IUD alter vaginal flora, and whether these changes lead to vaginal flora patterns that enhance HIV risk.
The impact of hormonal vs. non-hormonal IUDs, and implantable contraceptives, on the microflora and immune cell populations, especially since these are highly effective contraceptive methods that are most likely to be recommended instead of DMPA.
Table 2.
Hormonal Content, Delivery Route and Progestin Concentrations with Various Contraceptives
| Method | Progestin | Estrogen | Delivery route | [Serum P] | [LocalTissue P] |
|---|---|---|---|---|---|
| DMPA | MPA 150mg | None | Injection | 1-7 ng/mL | Unknown |
| COCs | Various | EE | Oral | 1-6 ng/mL | 0.2-3 ng/g* |
| LNG-IUD | LNG | None | Intrauterine | 0.1-0.4 ng/mL | 1-5 ng/g |
| Cu-IUD | None | None | Intrauterine | n/a | n/a |
| Net-En | Norethindrone enanthate 200mg |
None | Injection | 1-15ng/mL | Unknown |
| CycloFem | MPA 25mg | E2C 5mg | Injection | 0.5-2 ng/mL | Unknown |
| Jadelle | Levonorgestrel | None | Subdermal | 0.7-0.9 ng/mL | Unknown |
| Nexplanon | Etonogestrel | None | Subdermal | 0.8-0.9ng/mL | Unknown |
| NuvaRing | Etonogestrel 11.7mg | EE 2.7mg | Intravaginal | 1-2 ng/mL | 0.2-1ng/g |
| OrthoEvra | Norelgestromin 6mg | EE 0.75mg | Transdermal | 0.3-1.5 ng/mL | Unknown |
MPA= medroxyprogesterone acetate; LNG= levonorgestrel; EE=ethinyl estrodiol; E2C=estradiol cypionate; Net-En-norethindrone enanthate
only available data for COCs: 150mcg/20mcg desogestrel/EE pill formulation
Note: the primary active metabolite of desogestrel is etonogestrel
NuvaRing: releases 150ug ENG and 15ug EE/day
Table 5.
Association Between Contraceptive Use and Vaginal Colonization by Yeast or Yeast Vaginitis in Longitudinal Cohort Studies
| Author, Year | No. of Women |
Findings |
|---|---|---|
| Barbone, 1990[ 12 ] | 818 | COC users: Non-significant increase in yeast vaginitis compared to IUD/tubal ligation group (RR 1.28, 95%, CI 0.98-1.90) |
| Gupta, 2000[ 85 ] | 103 | COC users: Decrease in yeast colonization (16% to 5%) done month after COC initiation, p = 0.01 |
|
Eschenbach,
2000[ 86 ] |
30 | COC users: No change in vaginal yeast colonization between COC initiation and 2 months (10% vs. 10%, p = 0.90) |
| Miller, 2000[ 8 ] | 38 | DMPA Users: Decrease in yeast colonization (21% to 8%; p =0.02) between baseline and 6 months after initiation |
| Baeten, 2001[ 17 ] | 948 | COC users increased risk of yeast vaginitis (hazard ration 1.5, 95% CI 1.2-1.9); No increase in yeast vaginitis among DMPA users |
| Beigi, 2004[ 90 ] | 1248 | DMPA users: Increased risk of vaginal colonization, (adjusted OR 1.4, 95%, CI 1.1 – 1.7, p = 0.02) |
Acknowledgments
Sources of support: US National Institutes of Health/National Institute of Allergy and Infectious Diseases grant support (NIH R01-AI102835)
Footnotes
Conflicts of interest: Dr. Hillier is a consultant for Merck. Dr. Achilles has no conflicts of interest.
References
- 1.United Nations Millennium Development Goals Report. New York: 2011. [Google Scholar]
- 2.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. The Lancet Infectious Diseases. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 4.Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, MacDonald K, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. Journal of Reproductive Immunology. 2008;77:32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 6.Hladik F, Doncel GF. Preventing mucosal HIV transmission with topical microbicides: Challenges and opportunities. Antiviral Research. 88:S3–S9. doi: 10.1016/j.antiviral.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heffron R, Donnell D, Rees H, Celum C, Were E, Mugo N, et al. Hormonal contraceptive use and risk of HIV-1 transmission: a prospective cohort analysis. 6th International AIDS Society Conference; Rome, Italy. 2011. [Google Scholar]
- 8.Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;96:431–439. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 9.Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60:15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 10.Bahamondes L, Trevisan M, Andrade L, Marchi NM, Castro S, Diaz J, et al. The effect upon the human vaginal histology of the long-term use of the injectable contraceptive Depo-Provera. Contraception. 2000;62:23–27. doi: 10.1016/s0010-7824(00)00132-3. [DOI] [PubMed] [Google Scholar]
- 11.Chandra N, Thurman AR, Anderson S, Cunningham TD, Yousefieh N, Mauck C, et al. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retroviruses. 2013;29:592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbone F, Austin H, Louv WC, Alexander WJ. A follow-up study of methods of contraception, sexual activity, and rates of trichomoniasis, candidiasis, and bacterial vaginosis. Am J Obstet Gynecol. 1990;163:510–514. doi: 10.1016/0002-9378(90)91186-g. [DOI] [PubMed] [Google Scholar]
- 13.Riggs M, Klebanoff M, Nansel T, Zhang J, Schwebke J, Andrews W. Longitudinal association between hormonal contraceptives and bacterial vaginosis in women of reproductive age. Sex Transm Dis. 2007;34:954–959. [PubMed] [Google Scholar]
- 14.Rifkin SB, Smith MR, Brotman RM, Gindi RM, Erbelding EJ. Hormonal contraception and risk of bacterial vaginosis diagnosis in an observational study of women attending STD clinics in Baltimore, MD. Contraception. 2009;80:63–67. doi: 10.1016/j.contraception.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Madden T, Grentzer JM, Secura GM, Allsworth JE, Peipert JF. Risk of bacterial vaginosis in users of the intrauterine device: a longitudinal study. Sex Transm Dis. 2012;39:217–222. doi: 10.1097/OLQ.0b013e31823e68fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56:777–786. doi: 10.1093/cid/cis1030. [DOI] [PubMed] [Google Scholar]
- 17.Baeten JM, Nyange PM, Richardson BA, Lavreys L, Chohan B, Martin HL, Jr., et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185:380–385. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 18.Kontula K, Paavonen T, Luukkainen T, Andersson LC. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem Pharmacol. 1983;32:1511–1518. doi: 10.1016/0006-2952(83)90474-4. [DOI] [PubMed] [Google Scholar]
- 19.Chomont N, Gresenguet G, Levy M, Hocini H, Becquart P, Matta M, et al. Detection of Y chromosome DNA as evidence of semen in cervicovaginal secretions of sexually active women. Clin Diagn Lab Immunol. 2001;8:955–958. doi: 10.1128/CDLI.8.5.955-958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76:636–652. doi: 10.1016/j.steroids.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Huijbregts RP, Helton ES, Michel KG, Sabbaj S, Richter HE, Goepfert PA, et al. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013;154:1282–1295. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rottman JB, Ganley KP, Williams K, Wu L, Mackay CR, Ringler DJ. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am J Pathol. 1997;151:1341–1351. [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson BK, Landay A, Andersson J, Brown C, Behbahani H, Jiyamapa D, et al. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. Am J Pathol. 1998;153:481–490. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeaman GR, Howell AL, Weldon S, Demian DJ, Collins JE, O’Connell DM, et al. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prakash M, Kapembwa MS, Gotch F, Patterson S. Oral contraceptive use induces upregulation of the CCR5 chemokine receptor on CD4(+) T cells in the cervical epithelium of healthy women. J Reprod Immunol. 2002;54:117–131. doi: 10.1016/s0165-0378(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 26.Sheffield JS, Wendel GD, Jr., McIntire DD, Norgard MV. The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci. 2009;16:20–31. doi: 10.1177/1933719108325510. [DOI] [PubMed] [Google Scholar]
- 27.Morrison CS, Chen PL, Kwok C, Richardson BA, Chipato T, Mugerwa R, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24:1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta P, Collins KB, Ratner D, Watkins S, Naus GJ, Landers DV, et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76:9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue T, Kanzaki H, Imai K, Narukawa S, Katsuragawa H, Watanabe H, et al. Progesterone stimulates the induction of human endometrial CD56+ lymphocytes in an in vitro culture system. J Clin Endocrinol Metab. 1996;81:1502–1507. doi: 10.1210/jcem.81.4.8636358. [DOI] [PubMed] [Google Scholar]
- 30.Wieser F, Hosmann J, Tschugguel W, Czerwenka K, Sedivy R, Huber JC. Progesterone increases the number of Langerhans cells in human vaginal epithelium. Fertil Steril. 2001;75:1234–1235. doi: 10.1016/s0015-0282(01)01796-4. [DOI] [PubMed] [Google Scholar]
- 31.Souza SS, Castro FA, Mendonca HC, Palma PV, Morais FR, Ferriani RA, et al. Influence of menstrual cycle on NK activity. J Reprod Immunol. 2001;50:151–159. doi: 10.1016/s0165-0378(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 32.Hild-Petito S, Veazey RS, Larner JM, Reel JR, Blye RP. Effects of two progestin-only contraceptives, Depo-Provera and Norplant-II, on the vaginal epithelium of rhesus monkeys. Aids Research and Human Retroviruses. 1998;14:S125–S130. [PubMed] [Google Scholar]
- 33.Smith SM, Baskin GB, Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis. 2000;182:708–715. doi: 10.1086/315776. [DOI] [PubMed] [Google Scholar]
- 34.Li QS, Duan LJ, Estes JD, Ma ZM, Rourke T, Wang YC, et al. Peak SIV replication in resting memory CD4(+) T cells depletes gut lamina propria CD4(+) T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 35.Saba E, Grivel JC, Vanpouille C, Brichacek B, Fitzgerald W, Margolis L, et al. HIV-1 sexual transmission: early events of HIV-1 infection of human cervicovaginal tissue in an optimized ex vivo model. Mucosal Immunol. 2010;3:280–290. doi: 10.1038/mi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hladik F, Lentz G, Delpit E, McElroy A, McElrath MJ. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J Immunol. 1999;163:2306–2313. [PubMed] [Google Scholar]
- 38.Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 39.Morrison CS, Wang J, Van Der Pol B, Padian N, Salata RA, Richardson BA. Pregnancy and the risk of HIV-1 acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21:1027–1034. doi: 10.1097/QAD.0b013e3280f00fc4. [DOI] [PubMed] [Google Scholar]
- 40.Reid SE, Dai JY, Wang J, Sichalwe BN, Akpomiemie G, Cowan FM, et al. Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: relevance for HIV prevention trials among African women. J Acquir Immune Defic Syndr. 2010;53:606–613. doi: 10.1097/QAI.0b013e3181bc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 43.Ghanem KG, Shah N, Klein RS, Mayer KH, Sobel JD, Warren DL, et al. Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. J Infect Dis. 2005;191:358–366. doi: 10.1086/427190. [DOI] [PubMed] [Google Scholar]
- 44.Clemens LE, Siiteri PK, Stites DP. Mechanism of immunosuppression of progesterone on maternal lymphocyte activation during pregnancy. J Immunol. 1979;122:1978–1985. [PubMed] [Google Scholar]
- 45.White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA, et al. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- 46.Stern JE, Givan AL, Gonzalez JL, Harper DM, White HD, Wira CR. Leukocytes in the cervix: a quantitative evaluation of cervicitis. Obstet Gynecol. 1998;91:987–992. doi: 10.1016/s0029-7844(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 47.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 48.White HD, Prabhala RH, Humphrey SL, Crassi KM, Richardson JM, Wira CR. A method for the dispersal and characterization of leukocytes from the human female reproductive tract. Am J Reprod Immunol. 2000;44:96–103. doi: 10.1111/j.8755-8920.2000.440205.x. [DOI] [PubMed] [Google Scholar]
- 49.Cohen CR, Moscicki AB, Scott ME, Ma Y, Shiboski S, Bukusi E, et al. Increased levels of immune activation in the genital tract of healthy young women from sub-Saharan Africa. AIDS. 2010;24:2069–2074. doi: 10.1097/QAD.0b013e32833c323b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 51.Kaldensjo T, Petersson P, Tolf A, Morgan G, Broliden K, Hirbod T. Detection of Intraepithelial and Stromal Langerin and CCR5 Positive Cells in the Human Endometrium: Potential Targets for HIV Infection. PLoS ONE. 2011;6:e21344. doi: 10.1371/journal.pone.0021344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novak RM, Metch B, Buchbinder S, Cabello R, Donastorg Y, Figoroa JP, et al. Risk behavior among women enrolled in a randomized controlled efficacy trial of an adenoviral vector vaccine to prevent HIV acquisition. AIDS. 2013;27:1763–1770. doi: 10.1097/QAD.0b013e328360c83e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tibaldi C, Cappello N, Latino MA, Masuelli G, Marini S, Benedetto C. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: risk factors and rates of occurrence. Clin Microbiol Infect. 2009;15:670–679. doi: 10.1111/j.1469-0691.2009.02842.x. [DOI] [PubMed] [Google Scholar]
- 54.Pettifor A, Delany S, Kleinschmidt I, Miller WC, Atashili J, Rees H. Use of injectable progestin contraception and risk of STI among South African women. Contraception. 2009;80:555–560. doi: 10.1016/j.contraception.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McClelland RS, Richardson BA, Graham SM, Masese LN, Gitau R, Lavreys L, et al. A prospective study of risk factors for bacterial vaginosis in HIV-1-seronegative African women. Sex Transm Dis. 2008;35:617–623. doi: 10.1097/OLQ.0b013e31816907fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 57.Cohen CR, Duerr A, Pruithithada N, Rugpao S, Hillier S, Garcia P, et al. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS. 1995;9:1093–1097. doi: 10.1097/00002030-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 58.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serwadda D, Gray RH, Sewankambo NK, Wabwire-Mangen F, Chen MZ, Quinn TC, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003;188:1492–1497. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 60.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 61.Auvert B, Marais D, Lissouba P, Zarca K, Ramjee G, Williamson A-L. High-Risk Human Papillomavirus Is Associated with HIV Acquisition among South African Female Sex Workers. Infectious Diseases in Obstetrics and Gynecology. 2011;2011 doi: 10.1155/2011/692012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MasCasullo V, Fam E, Keller MJ, Herold BC. Role of mucosal immunity in preventing genital herpes infection. Viral Immunol. 2005;18:595–606. doi: 10.1089/vim.2005.18.595. [DOI] [PubMed] [Google Scholar]
- 63.Morrison CS, Turner AN, Jones LB. Highly effective contraception and acquisition of HIV and other sexually transmitted infections. Best Pract Res Clin Obstet Gynaecol. 2009;23:263–284. doi: 10.1016/j.bpobgyn.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, et al. Bacterial Communities in Women with Bacterial Vaginosis: High Resolution Phylogenetic Analyses Reveal Relationships of Microbiota to Clinical Criteria. PLoS ONE. 2012;7:e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Low N, Chersich MF, Schmidlin K, Egger M, Francis SC, van de Wijgert JH, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med. 2011;8:e1000416. doi: 10.1371/journal.pmed.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van de Wijgert JH, Morrison CS, Brown J, Kwok C, Van Der Pol B, Chipato T, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African Women. Sex Transm Dis. 2009;36:357–364. doi: 10.1097/OLQ.0b013e3181a4f695. [DOI] [PubMed] [Google Scholar]
- 67.Roccio M, Gardella B, Maserati R, Zara F, Iacobone D, Spinillo A. Low-dose combined oral contraceptive and cervicovaginal shedding of human immunodeficiency virus. Contraception. 2011;83:564–570. doi: 10.1016/j.contraception.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Coleman JS, Hitti J, Bukusi EA, Mwachari C, Muliro A, Nguti R, et al. Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS. 2007;21:755–759. doi: 10.1097/QAD.0b013e328012b838. [DOI] [PubMed] [Google Scholar]
- 69.Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, C.A. S, T. H, et al. Association of bacterial vaginosis with female-to-male HIV-1 transmission among HIV-1 discordant couples in Sub-Saharan Africa. 6th International AIDS Society Conference; Rome, Italy. 2011. [Google Scholar]
- 70.Rebbapragada A, Howe K, Wachihi C, Pettengell C, Sunderji S, Huibner S, et al. Bacterial vaginosis in HIV-infected women induces reversible alterations in the cervical immune environment. J Acquir Immune Defic Syndr. 2008;49:520–522. doi: 10.1097/QAI.0b013e318189a7ca. [DOI] [PubMed] [Google Scholar]
- 71.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2008;35:78–83. doi: 10.1097/OLQ.0b013e318156a5d0. [DOI] [PubMed] [Google Scholar]
- 72.Nagot N, Ouedraogo A, Defer MC, Vallo R, Mayaud P, Van de Perre P. Association between bacterial vaginosis and Herpes simplex virus type-2 infection: implications for HIV acquisition studies. Sex Transm Infect. 2007;83:365–368. doi: 10.1136/sti.2007.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 74.Watts DH, Fazzari M, Minkoff H, Hillier SL, Sha B, Glesby M, et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis. 2005;191:1129–1139. doi: 10.1086/427777. [DOI] [PubMed] [Google Scholar]
- 75.Mitchell CM, Balkus J, Agnew KJ, Cohn S, Luque A, Lawler R, et al. Bacterial vaginosis, not HIV, is primarily responsible for increased vaginal concentrations of proinflammatory cytokines. AIDS Res Hum Retroviruses. 2008;24:667–671. doi: 10.1089/aid.2007.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yudin MH, Landers DV, Meyn L, Hillier SL. Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: a randomized trial. Obstet Gynecol. 2003;102:527–534. doi: 10.1016/s0029-7844(03)00566-0. [DOI] [PubMed] [Google Scholar]
- 77.Cherpes TL, Marrazzo JM, Cosentino LA, Meyn LA, Murray PJ, Hillier SL. Hormonal contraceptive use modulates the local inflammatory response to bacterial vaginosis. Sex Transm Infect. 2008;84:57–61. doi: 10.1136/sti.2007.026625. [DOI] [PubMed] [Google Scholar]
- 78.Cauci S, Guaschino S, De Aloysio D, Driussi S, De Santo D, Penacchioni P, et al. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod. 2003;9:53–58. doi: 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 79.Hedges SR, Barrientes F, Desmond RA, Schwebke JR. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis. 2006;193:556–562. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- 80.Losikoff P, Fichorova R, Snyder B, Rodriguez I, Cu-Uvin S, Harwell J, et al. Genital tract interleukin-8 but not interleukin-1beta or interleukin-6 concentration is associated with bacterial vaginosis and its clearance in HIV-infected and HIV-uninfected women. Infect Dis Obstet Gynecol. 2007;2007:92307. doi: 10.1155/2007/92307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shoubnikova M, Hellberg D, Nilsson S, Mardh PA. Contraceptive use in women with bacterial vaginosis. Contraception. 1997;55:355–358. doi: 10.1016/s0010-7824(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 82.Calzolari E, Masciangelo R, Milite V, Verteramo R. Bacterial vaginosis and contraceptive methods. Int J Gynaecol Obstet. 2000;70:341–346. doi: 10.1016/s0020-7292(00)00217-4. [DOI] [PubMed] [Google Scholar]
- 83.Holzman C, Leventhal JM, Qiu H, Jones NM, Wang J. Factors linked to bacterial vaginosis in nonpregnant women. Am J Public Health. 2001;91:1664–1670. doi: 10.2105/ajph.91.10.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hutchinson KB, Kip KE, Ness RB. Condom use and its association with bacterial vaginosis and bacterial vaginosis-associated vaginal microflora. Epidemiology. 2007;18:702–708. doi: 10.1097/EDE.0b013e3181567eaa. [DOI] [PubMed] [Google Scholar]
- 85.Gupta K, Hillier SL, Hooton TM, Roberts PL, Stamm WE. Effects of contraceptive method on the vaginal microbial flora: a prospective evaluation. J Infect Dis. 2000;181:595–601. doi: 10.1086/315267. [DOI] [PubMed] [Google Scholar]
- 86.Eschenbach DA, Patton DL, Meier A, Thwin SS, Aura J, Stapleton A, et al. Effects of oral contraceptive pill use on vaginal flora and vaginal epithelium. Contraception. 2000;62:107–112. doi: 10.1016/s0010-7824(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 87.Veres S, Miller L, Burington B. A comparison between the vaginal ring and oral contraceptives. Obstet Gynecol. 2004;104:555–563. doi: 10.1097/01.AOG.0000136082.59644.13. [DOI] [PubMed] [Google Scholar]
- 88.Creinin MD, Meyn LA, Borgatta L, Barnhart K, Jensen J, Burke AE, et al. Multicenter comparison of the contraceptive ring and patch: a randomized controlled trial. Obstet Gynecol. 2008;111:267–277. doi: 10.1097/01.AOG.0000298338.58511.d1. [DOI] [PubMed] [Google Scholar]
- 89.Garza-Flores J, Moraks del Olmo A, Fuziwara JL, Figueroa JG, Alonso A, Monroy J, et al. Introduction of cyclofem once-a-month injectable contraceptive in Mexico. Contraception. 1998;58:7–12. doi: 10.1016/s0010-7824(98)00062-6. [DOI] [PubMed] [Google Scholar]
- 90.Beigi RH, Meyn LA, Moore DM, Krohn MA, Hillier SL. Vaginal yeast colonization in nonpregnant women: a longitudinal study. Obstet Gynecol. 2004;104:926–930. doi: 10.1097/01.AOG.0000140687.51048.73. [DOI] [PubMed] [Google Scholar]