Abstract
The purpose of this study was to (a) Determine the cellular transport and uptake of amine-terminated generation 3 (G3) poly(amido amine) (PAMAM) dendrimers across an in vitro model of the pulmonary epithelium, and the ability to modulate their transport by forming nanoblends of the dendrimers with biodegradable solid polymeric nanoparticles (NPs) and (b) to formulate dendrimer nanocarriers in portable oral inhalation devices and evaluate their aerosol characteristics. To that end, fluorescein isothiocyanate (FITC)-labeled G3 PAMAM dendrimer nanocarriers (DNCs) were synthesized, and also encapsulated within poly lactide-co-glycolide nanoparticles (NPs). Transport and uptake of both DNCs encapsulated within NPs (nanoblends) and unencapsulated DNCs were tracked across polarized monolayers of airway epithelial cells, Calu-3. DNCs were also formulated as core-shell microparticles in pressurized metered-dose inhalers (pMDIs) and their aerodynamic properties evaluated by Andersen cascade impaction. The apparent permeability of DNCs across the airway epithelial model was similar to that of a paracellular marker of comparable molar mass—order of 10−7 cm s−1. The transport and cellular internalization of the DNCs can be modulated by formulating them as nanoblends. The transport of the DNCs across the lung epithelium was completely suppressed within the time of the experiment (5 h) when formulated as blends. The encapsulation also prevents saturation of the cellular internalization profile. Nanoblending may be a potential strategy to modulate the rate of transport and cellular uptake of DNCs, and thus be used as a design strategy to achieve enhanced local or systemic drug delivery.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-014-9588-5) contains supplementary material, which is available to authorized users.
KEY WORDS: Calu-3, dendrimers, nanoparticles, nanoblends, oral inhalation, pressurized metered-dose inhalers, transport modulation
INTRODUCTION
Oral inhalation (OI) is not only the most sensible route for the regional administration of drugs to the lungs, but it has been also recognized as a potential alternative platform for the systemic delivery of therapeutic molecules through the lungs [1]. Some of the potential advantages of using the lungs as a portal to the systemic circulation include the lower enzymatic activity in the lung tissue [2, 3], which may improve drug bioavailability [2, 4], and the large absorptive area and high vascularization of the alveolar region, which is expected to facilitate the transport of therapeutics into the blood stream [1]. Literature reports indicate that the peak in the systemic circulation of many, if not most, small-molecule therapeutics delivered to the lungs via OI is in the order of minutes, or even shorter [1, 5]. Such fast transport rates may actually be a challenge at times, as most commercial OI formulations in the market today target the lungs, and short residence times translate to more frequent and higher dosage requirements, which may lead to more pronounced side effects [6, 7]. The reverse problem is also observed in cases when the lung epithelium works as an effective (and dominant) barrier to the transport of therapeutics that is intended for systemic delivery [5, 8].
Novel OI formulations that take full advantage of controlled delivery systems may help modulate the transport of therapeutics across the lung epithelium, and thus improve the effectiveness of existing OI therapies [9]. Innovative formulations such as those that allow for the controlled delivery of therapeutics may also help augment the inhaled drug market share. Polymeric nanocarriers (PNCs) are especially suited within this context [10–13]. PNCs may help overcome many of the drug delivery challenges facing OI therapies; as cellular uptake and transport across the epithelium, the main barriers to the systemic circulation, may be potentially modulated by carefully designing the carriers, as has been demonstrated for other epithelia [13–15]. Important design characteristics of PNCs that may be used to improve the control over the transport of therapeutics across epithelial barriers include their architecture (e.g., dendrimer nanocarriers (DNCs) vs. polymeric nanoparticles (NPs)), size, surface charge, and surface chemistry (e.g., presence of targeting or non-fouling moieties) [14, 16, 17]. While significant attention has been given to the transport of PNCs across traditional epithelial barriers [9, 14, 18], little is known on how PNCs (and DNCs) interact with the lung epithelium. Similarly, the ability to deliver PNCs to the lungs using portable inhalers has been only recently demonstrated, while a substantial body of work is available in the literature regarding the formulation of PNCs for other delivery routes [14, 16, 19].
Based on the challenges and opportunities described above, the goal of this work was to investigate the uptake and transport of DNCs across polarized Calu-3 monolayers, an in vitro model of the lung airway epithelium, and the ability to further modulate the cellular uptake and transport of the DNCs by forming polymeric blends between DNCs and polymeric NPs. Generation 3 (G3), amine-terminated, poly(amido amine) (PAMAM) dendrimers (G3-NH2), and poly (d,l-lactide-co-glycolide) (PLGA) NPs were selected as models in this study due to the extensive literature available for these systems as drug delivery carriers. Transport studies were performed across polarized monolayers grown onto porous inserts at the air interface culture condition (AIC). Cellular internalization of the DNCs, polymeric NPs, and their blends were determined by flow cytometry and cell lysis, also on polarized cell monolayers. Another goal of this study was to demonstrate the ability to formulate DNCs in portable OI formulations that show aerosol characteristics conducive to enhanced deep lung deposition. Core-shell structures containing the DNCs were prepared, and the aerosol characteristics of corresponding formulations in pressurized metered-dose inhalers (pMDIs) quantitatively determined.
MATERIALS
Carboxyl-terminated PLGA (COOH-PLGA, 50:50, molecular weight (MW) 31.3–45.0 KDa) was purchased from Birmingham Polymers (Birmingham, AL). G3 PAMAM dendrimer (G3-NH2), containing 32 surface groups (MW 6,909 Da), fluorescein isothiocyanate (FITC), glutaraldehyde (25%), Triton-X-100 (reagent grade), bovine serum albumin (BSA), osmium tetroxide (OsO4, 4% in water), penicillin-streptomycin (100 U/mL, AB), poly(vinyl alcohol) (PVA, 87%–90% hydrolyzed), Alcian Blue, Trypan Blue, DPX® mounting media, N-Hydroxysuccinimide (NHS), N,N′-dicyclohexylcarbodiimide (DCC), and dialysis membranes (Spectra/Por, MWCO 1 kDa) were all procured from Sigma. Ethylenediamine was purchased from Fluka. Deionized (DI) water with a resistivity of 18.0 MΩ cm (NanoPure Diamond, Barnstead) was used in all experiments. BCA® protein assay was purchased from Pierce. Phosphate buffered saline (PBS, 10X) was purchased from Fisher Scientific and was diluted to 1X using DI water. Hank’s balanced salt solution (HBSS 1X, pH 7.3) supplemented with 0.01-M HEPES was prepared. Rabbit Anti ZO-1 (Mid) primary antibody (product #A11010), Alexa Fluor® 546-labeled goat anti-rabbit IgG (product #402200), 4′,6-diamino-2-phenylidole diacetate (DAPI) and the cell viability assay, MTT (Vybrant® cell proliferation assay kit), were purchased from Molecular Probes. Transwell® inserts (Corning, 0.4-μm pore size, polyester, 0.33 cm2), cell culture flasks (75 cm2), 24-well culture plates, cover glass slides (18 mm2), and 96-well flat-bottomed culture plates (Cellstar), and trypsin supplemented with 0.25% ethylene diamine tetraacetic acid (EDTA) were purchased from VWR International. Dulbecco’s modified Eagle’s medium (DMEM, product #10569) was procured from Invitrogen. Fetal bovine serum (FBS, non-heat inactivated) was purchased from Atlanta Biologicals. Metering valves were kindly provided by Valois (DF30) and 3M (EPDM Spraymiser). Pressure-proof vials (5 mL, 6800318) were purchased from West Pharmaceutical Services. Pharma Grade (>99.99% purity) hydrofluoroalkane, 1,1,1,2,3,3,3-heptafluoropropane (HFA227) was a gift from Solvay Fluor und Derivate GmbH & Co. 2H,3H-perfluoropentane (HPFP, Vertrel XF) was purchased from TMC Industries. Glutaraldehyde was diluted from 25% to 2.5%, and osmium tetroxide diluted to 1% using DI water prior to their utilization. G3-NH2 dendrimers were dried in vacuum overnight to remove methanol prior to usage. Anhydrous dimethylsulfoxide (anh. DMSO) and anhydrous dichloromethane (anh. DCM) were obtained from EMD Chemicals. All other chemicals used in this work were procured from EMD, were of analytical grade, and were used as received, unless otherwise noted.
METHODS
Conjugation of FITC to G3-NH2
Conjugation of FITC to G3-NH2 was performed according to a procedure described earlier [20]. Briefly, a known mass of dendrimer was dissolved in PBS (5 mL). A known mass of FITC (concentration <5 mg mL−1) was solubilized in acetone and reacted with the aforementioned solution of G3-NH2 under darkness for 24 h, resulting in the FITC-conjugated G3-NH2 (G3-NH2-FITC). The organic phase was evaporated by gently sparging nitrogen, and the remaining contents from the flask were transferred to a dialysis bag and dialyzed extensively against DI water for 48 h, with periodic changing in DI water every 6 h, to remove any unreacted FITC. The contents of the dialysis bag were then transferred to a centrifuge tube, flash frozen using liquid N2 and lyophilized (Labconco FreeZone) for 48 h to obtain the final product. The presence and the extent of FITC conjugation onto the dendrimer was ascertained by 1H NMR (DMSO-d6 Varian 400 MHz).
Preparation of G3-NH2-FITC-Loaded PLGA NPs
Loading of the G3-NH2-FITC into the NPs to form the nanoblends was accomplished using a modified version of the double emulsion solvent evaporation technique [21]. Briefly, 0.9 mg of the FITC-labeled dendrimer was dissolved in 50 μL of DI water. The aqueous solution was emulsified over an ice bath with 80 mg of PLGA dissolved in 6 mL of an organic mixture containing ethyl acetate and acetone (4:1 ratio) using a sonicating probe (Omni Ruptor 250, Omni Inc.) set at 50 W power for 2 min. The primary emulsion formed was added dropwise to 5 mL of an ice-cold, 5% PVA solution under heavy vortexing (3,000 rpm). The secondary emulsion formed was emulsified over an ice bath using the probe set to the same power, for another 1 min. The emulsified mixture was diluted in about 150 mL of chilled 1% PVA solution and stirred overnight to evaporate the organic solvent. The NPs were recovered by centrifugation (26,000×g for 60 min at 4°C, Sorvall Legend X1R) and washed thrice with DI water to remove residual PVA. The washed NPs were redispersed in DI water, flash frozen in liquid N2, and lyophilized for 36 h to obtain the final product. The recovered G3-NH2-FITC-loaded NPs (the nanoblend) were characterized for size, surface charge and morphology using the Zetasizer Nano (Malvern), and scanning electron microscopy (SEM). For the light scattering (LS) measurements, 2 mg of NPs were gently dispersed in 1.5 mL of 1X HBSS using a probe sonicator over an ice bath. For SEM, a few drops of the aforementioned dispersion were deposited on the surface of a cover glass and subsequently evaporated overnight. The cover glass was then sputter coated with gold for 25 s (Ernst Fullam) and imaged using SEM (Hitachi S-2400) at a voltage of 25 kV.
Loading Efficiency of the G3-NH2-FITC into PLGA NPs
Loading efficiency of the FITC-conjugated PAMAM dendrimers from the NPs was determined using fluorescence spectroscopy. Five milligrams of the G3-NH2-FITC-loaded PLGA NPs was dissolved in 2-mL methylene chloride. The organic phase was then contacted with an equal volume of 1X HBSS and the mixture was subject to vortexing for 1 min at 3,000 rpm followed by extraction (LabQuake, Thermo Fisher) overnight under gentle mixing. The aqueous phase was isolated after extraction and subjected to fluorescence spectroscopy (Synergy Mx, BioTek Instruments, λex = 490 nm and λem = 520 nm). The readings obtained were compared against a previously prepared calibration curve of the G3-NH2-FITC to quantify the extent of the conjugate in the NPs. This procedure was done in triplicate, and the value reported here is an average of the three independent runs.
Controlled Release Studies of the G3-NH2-FITC from the NPs
The sustained release of the G3-NH2-FITC from the PLGA NPs was performed in 1X HBSS maintained at 37°C. Five milligrams of the G3-NH2-FITC-loaded PLGA NPs (ca. 50 μg G3-NH2-FITC) were incubated in 5 mL of buffer maintained at constant temperature (37°C) in a water bath. At predetermined times, the suspension was centrifuged (26,000×g, for 30 min at 4°C) and 200 μl of the HBSS was sampled from the supernatant. The amount of the G3-NH2-FITC released was estimated by fluorometry, as described in the previous section. An equal volume of HBSS was added to the release media in order to compensate for the buffer removed. The system was then redispersed and replaced back into the water bath for further sampling. The release profile is presented as a cumulative estimate of the G3-NH2-FITC conjugate released over a period of 24 h. The release of G3-NH2-FITC from the NPs in presence of mucus-laden HBSS was also evaluated. Synthetic regular mucus was prepared according to a methodology available in the literature and was diluted in the ratio 1:1000 in HBSS. The ratio 1:1000 (mucus/HBSS) was estimated by determining the volume of mucus on confluent Calu-3 monolayers seeded in the insert, which was calculated based on the thickness of the mucosal layer in the airways—estimated to be 8 μm, and the surface area of the insert, which is 0.33 mm2 [1]. The release studies were conducted as described for the release in HBSS. The measurements were conducted in triplicate, and the values reported here are averages of three independent runs.
Preparation of FITC-Conjugated PLGA NPs
FITC was conjugated to PLGA according to a procedure detailed in the literature [22]. Briefly, COOH-PLGA (200 mg, 5 μM) was solubilized in anh. dichloromethane (DCM, 5 mL) and allowed to react with DCC (1.5 mg, 7 μM) and NHS (1 mg, 7 μM) overnight in order to activate the terminal carboxyl group on PLGA. The dicyclohexylisourea (DCU) formed as a precipitate was filtered, and the resulting product was allowed to react with ethylenediamine (1 mg, 16 μM) in DCM in order to convert the succinimidyl ester group on PLGA to primary amine. The product obtained was purified by precipitating in diethyl ether, and the resulting solid was dried under vacuum. This amine-terminated PLGA was allowed to react with FITC (2.2 mg, 6 μM) overnight in anh. DMSO (5 mL). The resulting product was purified by dialysis against water, and lyophilized to recover the final product. The polymer thus prepared was characterized using 1H NMR to ascertain the conjugation of FITC to the polymer backbone–carboxyl group. NPs of the resulting polymer (FITC-conjugated PLGA) were prepared using emulsification solvent evaporation technique as detailed earlier. The NPs recovered were characterized for size and surface charge as described in previous sections.
Cell culture
The human airway epithelial cell line Calu-3, was purchased from the American Type Culture Collection (ATCC). Cells from passages 35–45 were used in the transport experiments. Calu-3 cells were plated in 75-cm2 culture flasks, in DMEM supplemented with 10% FBS and 1% AB, and allowed to grow to confluence. Once the confluence of the monolayer in the culture flasks was ascertained visually, they were subcultured and reseeded in Transwell® inserts at a density of 0.5 × 106 cells per well, and allowed to proliferate in a humidified atmosphere maintained at 37°C and 5% CO2. The cells were grown initially under liquid culture conditions (LCC) for 48 h, after which the medium on the apical side was removed and the cells were allowed to grow under an air interface culture (AIC). The culture medium on the basolateral side was replaced every 2 days, and the integrity of the Calu-3 monolayer was ascertained by determining its transepithelial electrical resistance (TEER). The measurements were conducted using an electrovoltohmmeter (EVOM, WPI Inc.,) equipped with chopstick electrodes. TEER of the monolayer was monitored every day until the values peaked to a high value and leveled, indicating that the monolayer had reached confluence. Additional techniques, including electron microscopy and immunocytochemical analysis were employed to confirm monolayer integrity and to ascertain the presence of tight junctions.
Electron Microscopy and Immunocytochemistry
Confluent Calu-3 monolayers were subjected to SEM to visually ascertain the morphology of the cell monolayers. Fixing and staining of the cells was done according to a procedure enlisted in the literature [23]. Once the confluence of cell monolayers was confirmed by TEER, select inserts were isolated and incubated in a 1:1 mixture of 2.5% glutaraldehyde (in DI water) and cell culture medium for 5 min at room temperature. The inserts were gently rotated in this medium during incubation. The mixture was replaced with the fixative, 2.5% glutaraldehyde, and was incubated for 30 min at room temperature. The cells were then stained with a 1% solution of OsO4 for a period of 90 min after which they were washed in increasing gradients of ethanol (20%, 40%, 60%, 80% and 100%, all v/v), each for a period of 10 min. The dehydrated monolayers were lyophilized for a period of 48 h, sputter coated with gold (Ernst Fullam) and imaged using SEM (JEOL, JSM-6510LV-LGS) at 25 kV.
Alcian blue stain was used in confirming the secretion of glycoproteins on the surface of Calu-3 cells. Confluent Calu-3 monolayers were isolated and were washed with HBSS after which they were incubated for a period of 20–30 min in 1% Alcian Blue (in 3% acetic acid) at room temperature. After incubation, the staining reagent was removed from the apical side, and the monolayers were washed with excess HBSS until the rinsate ran clear. The membrane from the inserts were carefully removed and mounted on microscope slides using DPX mountant, and imaged using an optical microscope (Diaphot 300, Nikon).
Immunocytochemical staining was conducted on confluent Calu-3 monolayers to detect and confirm the presence of the zona occludens (ZO-1) protein that populates the tight junctions of confluent Calu-3 monolayers [23, 24]. The procedure was performed according to the protocol prescribed by the manufacturer. Briefly, select monolayers, whose TEER values had peaked and leveled, were washed with 1X PBS twice and were fixed in a 4% solution of paraformaldehyde for 20 min at room temperature. The fixed monolayers were washed with PBS and permeabilized with 0.5% Triton-X-100 solution containing 1% BSA (blocking agent), in HBSS for a duration of 30 min. The permeabilizer was removed and cells were washed with PBS, and subsequently incubated for 60 min in a 6% BSA solution in HBSS to block non-specific interactions. The cells were then incubated in rabbit anti ZO-1 (mid) primary antibody (5 μg mL−1) dissolved in the blocking agent for 45 min followed by another 45-min incubation in an Alexa Fluor® 546 labeled goat anti-rabbit IgG in blocking solution. The cells were washed with PBS and counterstained with DAPI (0.1 μg mL−1in DI water), mounted on glass slides using DPX mounting media and stored at 4°C overnight. The stained monolayers were observed using a fluorescent microscope (Zeiss AxioCam mRM, Carl Zeiss) to visually ascertain the presence of ZO-1. The images were captured using the software, AxioVision (Zeiss).
Cytotoxicity Studies
The viability of Calu-3 cells in the presence of G3-NH2-FITC and G3-NH2-FITC-loaded NPs was estimated using MTT assay, according to a protocol outlined by the manufacturer. Briefly, Calu-3 cells were seeded at a density of 10,000 cells per well in 96-well culture plates, and allowed to grow for a period of 24 h. Cells were then washed twice with HBSS and the medium was replaced with 100 μL of HBSS containing varying concentrations of either G3-NH2-FITC or the NPs containing the conjugates. The cells were allowed to incubate in the test solutions for a period of 7 h. This duration was selected as it was 2 h longer than the time utilized for the transport experiment. After the duration of the test period, cells were washed thrice with 1X HBSS and incubated at 37°C in 100 μL of HBSS containing 20 μL of MTT solution for a period of 4 h. The incubation was followed by replacing 75 μL of the solutions in the wells with 50 μL of DMSO and allowing the cells to stand for 10 min in order to dissolve the formazan crystals. The well plates were read at an absorbance of 540 nm using a plate reader (Synergy Mx, BioTek Instruments). Cell monolayers exposed to only 1X HBSS were used as controls. Cell viability was determined by comparing the absorbance values of cells exposed to G3-NH2-FITC and G3-NH2-FITC-loaded into NPs to that of the control.
IN-VITRO TRANSPORT, CELLULAR UPTAKE, AND MASS BALANCE STUDIES
Transport
Transport of bare G3-NH2-FITC and G3-NH2-FITC from blends with the polymeric NPs was determined across confluent Calu-3 monolayers using a strategy similar to that reported in the literature for therapeutics and other probe molecules [23, 25]. Once an acceptable in vitro epithelial barrier was established as described above, the transport experiments commenced. Prior to starting the transport studies, the medium on the apical and basolateral sides were replaced with warm 1X HBSS and the monolayers were allowed to equilibrate at 37°C for a period of 45 min. The TEER of the monolayer was recorded, after which the PNCs were pulsed onto the cell monolayers in 0.2-mL HBSS solution. For the case of bare G3-NH2-FITC, the 0.2-mL HBSS solution contained 25 μg of dendrimers. For the case of the dendrimer-NP blends, the 0.2-mL HBSS solution contained 3 mg of NPs. The NP concentration was selected based on the loading of the G3-NH2-FITC—so as to correspond to a total mass of dendrimer of 25 μg. These experiments guarantee the same mass/M concentration of dendrimer in both systems. The basolateral side (receptor compartment) was fed with 0.6-mL fresh 1X HBSS. The transport across the cell monolayer to the basolateral side was monitored over a 5-h period. At predetermined times, inserts were transferred to wells containing fresh 1X HBSS in the receptor compartment and the transport study was continued. This was done in order to maintain sink conditions during the duration of the experiment. The amount of G3-NH2-FITC that traversed onto the basolateral side was quantified by fluorescence spectroscopy (λex = 485 nm; λem = 520 nm). The apparent permeability (Papp) was determined according to Eq. (1).
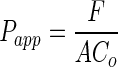 |
1 |
Where F is the flux (rate of cumulative mass of G3-NH2-FITC transported), A is the area of the insert (0.33 cm2), and Co is the initial concentration of G3-NH2-FITC on the apical side of the insert. F was calculated by plotting the cumulative mass of G3-NH2-FITC transported as a function of time, and corresponds to the slope from the resultant curve [23, 25].
Cellular Uptake and Mass Balance
Cellular uptake was determined in two ways. Here, we report the procedure for the uptake determined from the cell lysate. This is an absolute determination (total concentration of the species of interest), and was used to perform an overall mass balance with respect to the amount of the dendrimer. In the next section, we discuss cellular uptake determined by flow cytometry. The concentration of G3-NH2-FITC and G3-NH2-FITC encapsulated in the NPs into Calu-3 cells was quantified at the end of the transport experiments—at the 5-h time point. After completion of the transport studies, the apical media was removed and stored under darkness at 4°C. The cell monolayers were washed with ice-cold HBSS to arrest uptake and remove non-bound NPs and conjugates. The particles washed during this rinse were also collected and accounted for as non-internalized. The amount of G3-NH2-FITC in the fluids collected was measured by fluorescence spectrometry as described in earlier sections. For bare G3-NH2-FITC, the apical fluid collected was centrifuged (1,500 rpm, 6 min) and directly read using a fluorometer, whereas for the dendrimer-NP blends, the apical fluid laden with NPs was contacted with organic solvent (methylene chloride) to break down the polymeric matrix and release the dendrimer. The mixture was allowed to stand until the organic and the aqueous phases separated. The aqueous phase was subsequently analyzed for the conjugate released as detailed above. The monolayers were subsequently lysed by allowing them to stand in 2% triton-X-100 in HBSS for 24 h under darkness. In case of bare G3-NH2-FITC, lysed cell monolayers were centrifuged (800 rpm, 5 min) and the supernatant was analyzed for the G3-NH2-FITC using fluorometry (λex = 485 nm and λem = 520 nm, Synergy Mx, Biotek Instruments). In case of the G3-NH2-FITC loaded into NPs, the lysate was centrifuged (800 rpm, 5 min), the supernatant separated and contacted with methylene chloride to dissolve the internalized NPs and extract the G3-NH2-FITC into the aqueous phase. After a 24-h extraction, the aqueous phase was analyzed for G3-NH2-FITC using fluorometry. The results were normalized with respect to the total protein content using BCA protein assay, according to the protocol given by the manufacturer. By determining the concentration of the G3-NH2-FITC in the apical side of the monolayer, the concentration that was internalized within the cell, and the amount transported onto the basoleteral side, a mass balance of the G3-NH2-FITC could thus be performed.
Flow Cytometry
Flow cytometry analysis was conducted on polarized Calu-3 cells according to methodology detailed in the literature, with some modifications [26]. Calu-3 cells (passages 35–45) were seeded at a density of 1 × 106 cells per well in 24-well plates, and allowed to grow to confluence. The monolayer confluence was confirmed by staining select wells for ZO-1 and subjecting them to fluorescence spectrometry as detailed earlier, a day before commencing the uptake studies. The presence of ZO-1 protein suggested that the cells were confluent. Prior to pulsing G3-NH2-FITC or NPs loaded with G3-NH2-FITC, the cell monolayers were incubated with 1X HBSS (at 37°C) for a period of 30 min. The cells were then incubated with (i) G3-NH2-FITC or (ii) NPs loaded with G3-NH2-FITC or (iii) FITC-PLGA NPs in HBSS for varying time periods (5, 4, 3, 2, and 1 h, and 30 and 15 min). Care was taken to ensure that the concentration of G3-NH2-FITC was the same for both systems. The concentration FITC-PLGA was the same as that of NPs loaded with G3-NH2-FITC. After incubation for a specified duration, the test solutions were removed and the cell monolayers were washed thrice with ice-cold HBSS. The extracellular fluorescence was quenched with trypan blue (0.25% w/v in HBSS), and the monolayers were washed again, before being subjected to trypsinization (trypsin supplemented with 0.25% EDTA). The detached cells were centrifuged (1,200 rpm, 6 min) to obtain the cell pellet which was subsequently resuspended in 1 mL of fresh HBSS and analyzed using a flow cytometer (BD LSR II (BD Biosciences, San Jose, CA)), with the samples excited with a Coherent Sapphire laser (488 nm) and detected through a 530/30 bandpass filter (FITC). Only viable cells were gated for fluorescence analysis. As controls for G3-NH2-FITC, 1X HBSS was utilized, whereas for NP system, blank PLGA NPs were used. At least 6,000 events were counted for each condition. All experiments were repeated/performed independently by two different investigators in our laboratories (impendent runs), with three wells each time, 4 weeks apart from each other. The results shown here are thus averages of six wells. The cellular entry was expressed in percentages as a function of time by plotting the mean fluorescence intensity (MFI) values of cells gated for FITC from histograms sourced from the fluorescence-activated cell sorting (FACS) data. In addition, the rate of cellular entry was also plotted as a function of time by normalizing the MFI data with respect to the 5-h MFI values, which were set at 100%.
Preparation and Characterization of G3-NH2-FITC-Loaded Core-Shell Particles
Synthesis and characterization of a biodegradable co-oligomer, oligo(lactide) grafted chitosan (OLA-g-CS) that forms the shell encapsulating the G3-NH2-FITC has been documented in detail elsewhere [27]. The CS was depolymerized from 310 kDa to a MW of about 1 kDa according to procedure described in the literature, with a degree of deacetylation of 77% as estimated by 1H NMR [28]. Ring opening polymerization was used in grafting lactide chains onto the degraded CS backbone. The resulting co-oligomer had a MW of 1.7 kDa, with at least three OLA chains (8 LA units, 21% graft percentage) onto each CS backbone, as determined, using 1H NMR. Preparation of G3-NH2-FITC loaded core-shell particles was accomplished via emulsification diffusion. Briefly, 30 mg of the co-oligomer was dissolved in 0.9-mL DI water and to this solution, 1.2 mg of the dendrimer was added. This aqueous solution was emulsified with 19 mL of ethyl acetate in a bath sonicator (VWR P250D, 180 W) for 8 min at 17°C. The resulting water in oil emulsion was quickly added to a large volume of ethyl acetate (200 mL). The high solubility of water in ethyl acetate causes it to migrate from the dispersed droplet phase into the bulk phase. Being interfacially active [27], the co-oligomer resides at the interface, templating the G3-NH2-FITC as spherical particles with core-shell morphology, where the co-oligomer becomes the shell and dendrimers the core. Core-shell particles thus formed were collected by centrifugation (Sorvall Legend X1R, 5,000 rpm, 20 mins), air dried, and stored in a desiccator prior to usage. The particles were characterized for shape and size using dynamic light scattering (DLS) and SEM. A known amount (2 mg) of particles was dispersed in 1.5 mL of HPFP at 18°C in a bath sonicator and subject to DLS analysis. A small quantity of the dispersion was deposited onto cover glass slides, dried in a light stream of air, and sputter coated with gold for 40 s prior to imaging them under SEM (Hitachi S-2400) at 25 kV. The loading of the dendrimer conjugates in the core of the particles was determined using fluorescent spectroscopy. A known mass of the particles (3 mg) containing the G3-NH2-FITC and the water-soluble shell was dissolved in a known volume (3 mL) of HBSS. The resulting solution was analyzed using fluorescence plate reader at excitation and emission wavelengths of 495 and 520 nm, respectively, (those corresponding to FITC) and compared against a previously prepared calibration curve of the G3-NH2-FITC to quantify their loading.
Physical Stability of G3-NH2-FITC Loaded Core-Shell Particles in HFA Propellant
A known mass of the core-shell particles containing the G3-NH2-FITC was loaded into pressure-proof glass vials (West Pharma, 6800318), and crimp sealed using metering valves (DF30, Valois). The propellant was fed into the pressure-proof vials using a manual high-pressure pump (HiP 50-6-15) and a homemade aerosol filler. The volume of HFA added was such that the final concentration in the vial was 2 mg mL−1. The formulation was gently agitated in a low-intensity sonicating bath (VWR P250D set to 180 W) between 16 and 18°C for a few minutes to break up larger aggregates. Digital images of the dispersion were captured immediately after ceasing the mechanical energy input, and later at predetermined times. The physical stability of the dispersion was qualitatively estimated as a function of the dispersion age. As a comparison, bare G3-NH2-FITC was also formulated in pMDIs (0.15 mg mL−1) as described above for core-shell particles, and its dispersion stability also evaluated as a function of time.
Aerodynamic Properties of G3-NH2-FITC Core-Shell Formulations
An eight-stage Andersen Cascade Impactor (ACI, Copley Scientific) operating at a flow rate of 28.3 L min−1 was used to evaluate the aerosol characteristics of the G3-NH2-FITC-loaded core-shell formulations. Formulations of G3-NH2-FITC alone were also tested for comparison. The studies were carried out at room temperature and 52% relative humidity. Freshly prepared canisters of the formulations were dispersed with the help of a sonicating bath maintained at 18°C for 30 min. Several shots of the formulation were fired to waste prior to subjecting them to the impaction experiments. Twenty shots were then fired into the ACI at an interval of 10 s between each actuation. After the study, the amount of dendrimer deposited on the actuator (Ac), the induction port (IP), and each of the stages of the ACI was estimated by rinsing the stage plates in 20 mL of 1X HBSS overnight, and subjecting the resulting solution to fluorometry. Three independent runs (different canisters) were conducted, and the results reported here are averages of those runs. In addition to the fine-particle fraction (FPF), other relevant aerosol properties including the mass median aerodynamic diameter (MMAD), and geometric standard deviation (GSD) were also determined from the ACI results.
RESULTS AND DISCUSSIONS
Synthesis and Characterization of G3-NH2-FITC, and FITC-Conjugated PLGA
FITC was tagged onto dendrimers in order to allow their detection during transport and uptake studies. FITC conjugation to G3-NH2 was accomplished by linking the isothiocyanate group of FITC to the amine group of G3-NH2 [29]. Characterization of the prepared G3-NH2-FITC was done using 1H NMR. The NMR spectrum of the prepared conjugates, along with the reaction scheme is shown in the Supplementary Material Figure S1.
A singlet observed at 2.19 ppm can be attributed to the methylene (−CH2–) protons of G3-NH2. This peak was used as an internal standard to estimate the extent of FITC conjugation, and was set at 120(H) protons [30]. Multiplets between 7.82–8.22 ppm can be attributed to the carbamate (−NH-) peaks of the G3-NH2. The presence of FITC can be confirmed by the appearance the multiplet at 6.42–6.61 ppm, attributed to the aromatic protons (4H) on FITC [20]. The ratio of the integral area of the signals between 6.42–6.61 to the integral area of the methylene protons of the G3-NH2, weighted by the respective number of protons gives the number of FITC molecules per G3-NH2, in this case, determined to be 1.7. Conjugation of FITC to G3-NH2 surface reduced its zeta potential from 21.2 to 15.5 mV. The number of FITC molecules that can be tethered to G3-NH2 surface can be tailored by altering molar ratio of the reactants [26]. FITC-conjugated PLGA was synthesized so as to evaluate the rate of cellular uptake of PLGA NPs and to compare with the uptake results of the dendrimer-NP blends as discussed below. The conjugation was also characterized by 1H NMR. The presence of multiplet peaks between 6.5 and 6.7 ppm can be attributed to the presence of FITC (spectrum not shown), which was conjugated to the carboxyl group (converted to a primary amine) on the PLGA terminus.
Loading of G3-NH2-FITC onto PLGA NPs, Characterization of the NPs, and Release of the Dendrimers from the Polymeric Nanoblends
Loading moieties into biodegradable polymeric matrices can lead to sustained release of encapsulate over extended periods of time [31]. Sustained and controlled release of therapeutics from the NPs is an effective methodology to ensure retention and prolonged therapeutic effect at the target site, and has further relevance in the delivery of high potency, and/or more costly drugs such as chemotherapeutics [31]. Such strategies have also been demonstrated for large therapeutics such as biomacromolecules, with molecular weight and hydrophilicity similar to that of DNCs investigated in this work [32]. Incorporating dendrimer conjugates within polymeric NPs (nanoblends) can potentially augment the capabilities of the dendrimers and PNC as potent drug delivery vehicles, by providing an avenue for further control of the release and transport of the DNCs across biological barriers, and consequently, that of the therapeutic cargo conjugated to the DNCs. Additionally, these NPs can act as protective depots for fragile molecules that may be conjugated to the dendrimers, thereby ensuring greater retention of their activity [31, 32]. The encapsulation of dendrimers in polymeric NPs can also be used to mediate the interaction of the dendrimers with both extra and intracellular barriers present in the lung epithelia. As drugs and other moieties are grafted onto DNCs, the surface chemistry of the carriers is altered, which may also impact their interaction with both intra and extracellular barriers [33]. The encapsulation of the dendrimers into NPs may thus serve as a strategy/general platform for the transport of different dendrimer nanocarriers.
The double emulsion solvent evaporation technique was employed to load the G3-NH2-FITC onto the NPs. The particles formed were lyophilized and characterized using SEM and LS. A representative SEM micrograph (×10,000) of the NPs and the size distribution as determined by LS (as inset) is shown in Fig. 1a. LS experiments were conducted in 1X HBSS as the medium, which is the same solvent medium used in the uptake and transport experiments. Particle size as determined by LS was 175 ± 28 nm, with a polydispersity of 0.08. SEM images indicated that the particles formed were smooth spheres with an average size of 221 ± 35 nm, as determined with the ImageJ software (v1.4, NIH). The zeta potential of the particles was found to be −10.1 ± 2.4 mV. The loading of the G3-NH2-FITC into the NPs was estimated using fluorometry as discussed in Methods. The amount of G3-NH2-FITC loaded into the NPs was determined to be 7.8 ± 1.2 μg mg−1 NPs. This average is an estimate of three independent runs (different batches of NPs). The overall encapsulation efficiency of the G3-NH2-FITC into the NPs was found to be 10% ± 1.8%. The relatively low loading of the G3-NH2-FITC into the NPs can be attributed to the hydrophilicity of the dendrimer, which enhances the rapid partition of the G3-NH2-FITC to the external aqueous phase during the dilution procedure, as observed for other drugs and biomacromolecules such as proteins and peptides [34]. It must be noted that the procedure detailed for loading G3-NH2 here has not been optimized. However, further improvements to the process can be made to enhance G3-NH2 loading by incorporation of appropriate stabilizers, altering polymer/dendrimer concentrations, and adjusting pH of the external phase during the emulsification process [31, 35]. The NPs of FITC-conjugated PLGA had a size of 245 ± 40 nm and a zeta potential of −12 ± 3.1 mV as determined by LS.
Fig. 1.
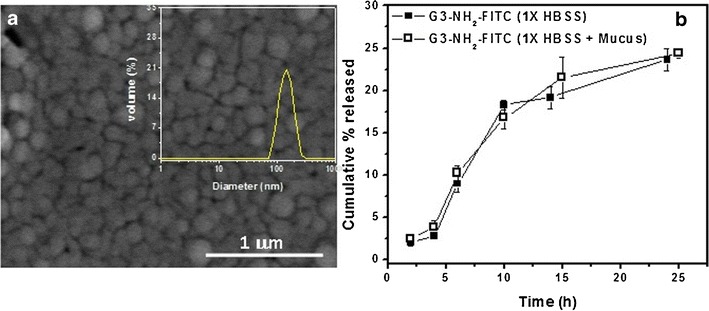
a SEM micrograph of the G3-NH2-FITC-loaded PLGA NPs prepared using double emulsion solvent evaporation technique. inset Size distribution of the G3-NH2-FITC-loaded PLGA NPs as determined by dynamic light scattering (DLS). b Sustained release profile of G3-NH2-FITC from PLGA NPs in 1X HBSS (pH 7.3) and mucus containing 1X HBSS (pH 7.4) measured at 37°C. (Inset) Illustration indicating release of the G3-NH2-FITC from PLGA NPs. Error bars that do not show are smaller than symbol sizes. Results reported here are averages of three independent runs
There have been a few studies in the literature that have reported the formulation of dendrimers into particulate carriers [36]. Most of those studies have focused on the encapsulation of G3-NH2-plasmid DNA complex (dendriplexes) into PLGA microparticles [36]. One of the important findings from those studies was the ability to sustain the release of these dendriplexes from the polymeric core over extended periods of time, suggesting that formulating dendrimer conjugates into polymeric matrices can be a potential strategy to further enhance their efficacy as drug delivery agents [36]. To the best of our knowledge, there have been no previous articles reporting the encapsulation of dendrimers into polymeric NPs.
Release studies of the G3-NH2-FITC from the polymeric matrix were conducted in 1X HBSS, the medium in which the transport experiments were to be conducted, as described in the earlier sections. Additionally, release studies were conducted in synthetic mucus-laden HBSS, in order to simulate an environment that the NPs will encounter in the vicinity of the cell milieu and understand the impact of the surroundings on the G3-NH2-FITC release. These experiments were conducted in triplicate. The release studies were conducted for a period of 24 h, with supernatant removed and tested at periodic time points for the released conjugates. A plot of cumulative release of G3-NH2-FITC from PLGA NPs as a function of time is shown in Fig. 1b. It is observed that a sustained release of G3-NH2-FITC can be achieved from the NPs within the time span investigated, with about 24% of the conjugate being released into the buffer by 24 h. A similar release profile was also exhibited by the NPs when the studies were conducted in the mucus-dissolved buffer. In order to put these release studies in perspective, it would be instructive to compare the results to compounds of similar size as the DNCs that have been encapsulated into PLGA NPs, with PLGA of similar molecular weight. Interestingly, insulin (MW 5,808 Da) loaded into PLGA (MW 50 kDa) NPs exhibited a similar release profile as the G3-NH2-FITC reported in this work [37]. However, in our work, we observed a more subtle, sustained release of the conjugates during the initial times as opposed to a sudden burst release, which has been reported for the aforementioned and several other systems [38]. This can be attributed to the relatively low loading efficiency of the conjugates within the NPs, and the MW of the polymer. The effect of lower loading of encapsulates into NPs on the release characteristics has been extensively reported in literature. For instance, PLA NPs containing varying payloads of savoxepine, a potent antipsychotic compound, displayed dual release profiles [39]. An initial burst and faster release characteristics were observed for NPs with higher encapsulated payloads, while those with lower drug contents displayed little or no burst release, and considerably, slower release profiles [40]. This behavior was also observed for a protein, bovine serum albumin (BSA) loaded into PLGA microspheres. Another factor that could possibly influence the release characteristics of G3-NH2-FITC from the polymeric matrix is the presence of surface-associated PVA, which is usually present on the smaller NP surface, and which has been shown to hinder the diffusion of encapsulated compounds from within the NPs [41]. This is an important observation, as sustained-release profiles of moieties from polymeric matrices is expected to be primarily governed by diffusion processes during the early stages of release [41].
Cytotoxicity of G3-NH2-FITC and G3-NH2-FITC-Loaded NPs on Calu-3 Cells
The cytotoxicity of the conjugates and the dendrimer-NP blends were tested on Calu-3 cells. Cell viability was determined using the MTT assay as described in Methods. The cell viability results are plotted as a function of the concentration of G3-NH2-FITC and of the dendrimer-NP blend. The results are illustrated in the supplemental information as Figure S2. The results reported here are those for a period of 7 h, as transport studies conducted in this work lasted 5 h.
The results show that neither the bare conjugate dendrimer nor the conjugate formulated as NPs induced any appreciable cytotoxicity on Calu-3 cells within the concentration ranges investigated. It has been well established that cytotoxicity of dendrimers are concentration, charge and generation dependent, with cationic dendrimers of higher generations exhibiting greater cell kill than their anionic and lower generation counterparts [42]. As there have been no previous cytotoxicity studies of G3-NH2-FITC onto Calu-3 cells, it would be appropriate to compare our results to studies performed on Caco-2 cells, which is a colon cancer cell line that exhibits certain characteristics somewhat similar to Calu-3 (possess microvilli and form tight epithelial junctions) [14]. Experiments conducted on Caco-2 cells show that G3-NH2-FITC are non-toxic up to a concentration of 10 μmol (~69 mg) per milliliter, which is much higher than the concentration range investigated in this work [43].
The combination of G3-NH2-FITC and PLGA NPs did not exert any toxic effect on the cell monolayers within the concentration tested either, as shown in Figure S2b. This is not surprising as previous cell viability studies on Calu-3 cells have reported that PLGA NPs did not induce any pronounced cytotoxic effects even at 48 h after exposure for concentrations of up to 5 mg of NPs per milliliter [44]. The non-toxic nature of the G3-NH2-FITC-loaded NPs suggests that the encapsulation of dendrimer conjugates within NPs may possibly also be seen as a viable strategy to alleviate the potential toxicity associated with higher concentrations and/or high dendrimer generation, and their corresponding conjugates. The encapsulation of dendrimers in NPs may also alleviate issues regarding to the solubility of dendrimer nanocarriers in physiological environment in cases when their surface is modified with hydrophobic therapeutics.
Characterization of the Calu-3 Monolayers for Transport Studies
The human airway epithelial cell line Calu-3 was used to assess the permeability and uptake of the G3-NH2-FITC and G3-NH2-FITC encapsulated into PLGA NPs. Calu-3 is a well differentiated and characterized cell line derived from human bronchial sub mucosal glands [23]. Besides producing tight junctions, Calu-3 is one of the few respiratory cell lines capable of expressing many important characteristics of the bronchial epithelium in vitro, such as the production of airway surface liquid, mucin excretion, cilia, and other immunologically active substances that make Calu-3 an appropriate candidate for elucidating tracheobronchial permeability in vitro [23, 45]. Calu-3 cells were grown under a liquid-covered culture for 48 h, after which the medium in the apical compartment was removed and the cells were allowed to grow under AIC. It has been shown that when grown at AIC, Calu-3 cells exhibit a greater resemblance to the native epithelium (microvilli/cilia, mucins and other relevant glycoproteins), and hence that particular method of culture was employed in this study [23].
The TEER of the Calu-3 monolayers was measured to ascertain their confluence. As described earlier, before measuring the TEER, 200 μl of the culture medium was added to the apical chamber for the measurements as the cells were cultured at AIC. The well plate was allowed to equilibrate in a humidified atmosphere for 30 min, and the TEER then recorded. A plot of the TEER afforded by the cell monolayer as a function of time (days in culture) is shown in Fig. 2d.
Fig. 2.
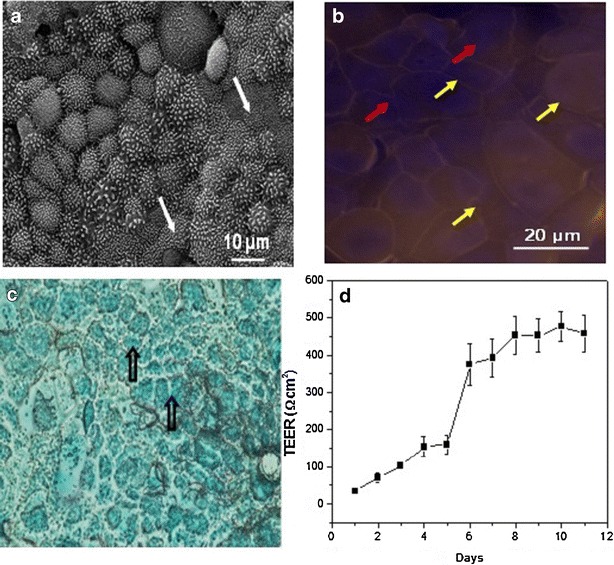
a Representative SEM micrograph of Calu-3 monolayers grown under an air interface culture (AIC). Monolayers were isolated once the TEER values peaked and leveled. Cell monolayers were fixed and stained with osmium tetroxide, and dehydrated in increasing gradients of ethanol after which they were lyophilized. White arrows indicate the presence of microvilli on the monolayer surface. b Fluorescent microscope image of Calu-3 monolayers showing the presence of tight junctions (yellow arrows) populating the cell periphery. Red arrows indicate the location of the nuclei (stained with DAPI - blue). Cells were seeded at a density of 0.5 × 106 per well and were grown under AIC. The cells were stained for the tight junctional protein, zona occludens-1 (ZO-1) labeled with Alexa Fluor® 546 dye (orange) and counterstained with DAPI for nuclei. c Optical microscope image of a Calu-3 monolayer stained with Alcian blue, indicating the presence of glycoproteins (mucins, unfilled black arrows). d Variation in transepithelial electrical resistance (TEER) across Calu-3 monolayers (n = 12) grown in Transwell® inserts (0.4-μm pore size and 0.33-cm2 area) under an air interface culture (AIC) as a function of time. The TEER values were obtained after equilibrating the apical chamber with the culture medium for 30 min. The TEER reported here was corrected for the resistance of the blank Transwell® insert
The TEER of the Calu-3 monolayers is seen to increase steadily above baseline by day 5, and to reach confluence by day 8. The values leveled by day 11, indicating that the monolayer had reached the level of confluence required to conduct the transport studies [23]. These results compare favorably with the results reported in the literature for Calu-3 cells cultured under AIC, which are deemed confluent for TEER values peaking beyond ca. 300 Ω cm2, values which are dependent to some extent to the culture conditions and cell passage [23, 46].
In addition to determining the TEER of the monolayers, immunocytochemical (IC) analysis and electron microscopy studies were conducted to visually ascertain the presence of tight junctions and morphology of the confluent Calu-3 monolayers. In this work, IC analysis and electron microscopy experiments were performed a day prior to commencement of the transport studies. The cell monolayers were prepared for both studies as detailed in the Methods section. Figure 2a is a representative electron micrograph of a Calu-3 monolayer fixed and stained with osmium tetroxide, and reveals that the confluent Calu-3 monolayers displayed some of the requisite morphology possessed by confluent Calu-3, such as microvilli (filled white arrows).
IC analysis was performed on the cell monolayers to detect the presence of zona occludens protein-1 (ZO-1), which is a major protein expressed in the tight junctions of confluent Calu-3 cells. The presence of the protein was detected by staining the fixed cells with an anti ZO-1 antibody labeled with Alexa Fluor® 546 [23]. The nuclear stain, DAPI, was utilized as a counterstain. A representative fluorescent microscope image of the fixed and stained Calu-3 monolayer is shown in Fig. 2b. The presence of orange boundaries (denoted by the yellow arrows) around the cells can be attributed to the expression of ZO-1 protein. Calu-3 cells when cultured under AIC have been shown to secrete a greater amount of glycoproteins (mucins) on their surface, which can be detected by staining the cell surface with Alcian blue. Figure 2c is an optical microscope image captured after the cells were stained with Alcian blue and fixed. The heavy blue staining on the monolayer surface (open black arrows) indicates the presence of mucosal glycoproteins on Calu-3 cultures grown under AIC. The observations reported here are in agreement with those reported in earlier studies characterizing confluent Calu-3 monolayers [23, 46]. Once the monolayers were characterized and their morphology ascertained, they were subject to transport experiments, which are discussed next.
In Vitro Transport and Uptake
In vitro transport of bare G3-NH2-FITC and those loaded into NPs were conducted on confluent Calu-3 cell monolayers for a period of 5 h. A summary of the Papp (y1 axis) results as a function of time for bare G3-NH2-FITC, and those formulated as blends within PLGA NPs across Calu-3 is shown in Fig. 3.
Fig. 3.
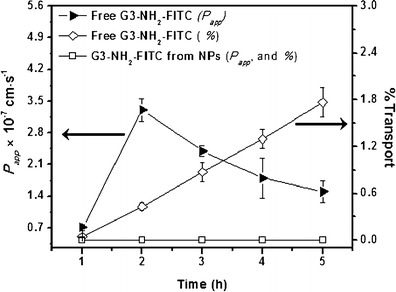
Apparent permeability (y 1; P app) and percentage transported (y 2) of G3-NH2-FITC and G3-NH2-FITC-loaded into NPs, plotted as a function of time (n = 4). Error bars that do not show are smaller than symbol sizes
The y2 axis in the plot denotes the cumulative mass of G3-NH2-FITC transported (in percentage) across the cell monolayer as a function of time after pulsing. At the end of the 5-h time point, the Papp of the G3-NH2-FITC across Calu-3 was found to be 1.4 × 10−7 cm s−1. About 1.7% of the conjugates had traversed across the monolayer within 5 h. An increase in the Papp value from 0.7 to (a peak of) 2.8 × 10−7 cm s−1 was observed at 2 h, followed by a subsequent decrease to 1.4 × 10−7 cm s−1 by the end of the experiment, indicating the presence of an “activation” time for transport. This coincides with a minimum in the TEER (Fig. 4), which may indicate a preference with regards to transport route.
Fig. 4.
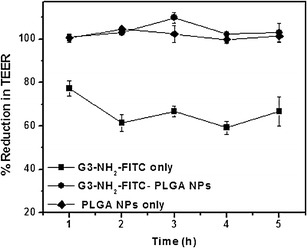
Variation in TEER of Calu-3 monolayers as a function of time, upon incubation with bare G3-NH2-FITC, bare PLGA NPs, and NPs loaded with G3-NH2-FITC. Results are reported as percentage reduction in TEER compared to control (cells incubated in blank HBSS) (n = 4)
On the other hand, for the system comprised of G3-NH2-FITC formulated as blends with the PLGA-NPs, no transport was observed within the duration of the experiment. Slow release of the conjugates (low concentration of the G3-NH2-FITC in the donor chamber) from the NPs is expected to help mediate the transport of the DNCs across the monolayer. From the sustained release studies discussed above, it is estimated that only ca. 5% of the encapsulated conjugate is released from the polymeric matrix within 5 h. Because the concentration of the DNCs is expected to impact their permeability [14], the encapsulation of the dendrimer within the NPs should help decrease the rate of transport. Moreover, the NPs themselves are not expected to be transported across the Calu-3 monolayer, further reducing the potential for transport of the DNCs [47].
Cell monolayers incubated with bare G3-NH2-FITC and G3-NH2-FITC loaded PLGA NPs were lysed after the transport experiments so as to quantify cellular uptake. The results indicated that 16% ± 1.7% of the bare G3-NH2-FITC (0.08 nmol) and 19% ± 2.1% (0.1 nmol) of the G3-NH2-FITC from the NPs were internalized into the cells during the study. It is quite interesting to observe that the dendrimer internalization seems to be greater with NPs compared to free dendrimers. A mass balance of the dendrimers was accomplished by quantifying the contents of the apical chamber for G3-NH2-FITC after the completion of the transport studies, and combining those values with those obtained from analyzing the contents internalized into cells and the contents transported across to the basolateral side. The total amount recovered from these analyses (for both systems) was 85%—out of the total pulsed onto the cells. In comparison, earlier translocation studies conducted on Calu-3 cells utilizing polymeric NPs of similar size as those discussed here reported an internalization of 11%, with a recovery of up to 93% [47] The fact that recovery in both studies falls short of 100% can be attributed to losses associated with the fraction of carriers that maybe bound to the cell surface despite washing—that fraction will settle down along with the cell debris during the centrifugation. Losses may also arise due to adsorption of particles to other surfaces, and also quenching of the fluorophore [47].
While no previous results for internalization or transport of dendrimers across Calu-3 cells have been reported yet, it is interesting to put the results of this work in perspective by comparing with other cell types. The transport of G3-NH2-FITC across Calu-3 cells is observed to be qualitatively different than that reported for Caco-2 cells, where no apparent maximum in permeability has been reported [14]. However, the magnitude of the Papp of the G3-NH2-FITC across the Calu-3 monolayer was observed to be similar to that reported across Caco-2 monolayers, which was ca. 0.8 × 10−7 cm s−1 at 150 min [48]. While these numbers seem to be in agreement, caution should be exercised when drawing such comparisons, as factors including cell type, donor chamber concentration, extent of fluorescent labeling and duration of the study can greatly influence the overall rate of transport [14, 43, 49]. For instance, in the aforementioned studies, typical donor concentrations employed were at least 0.1 mM, which is considerably larger compared to this study (3.2 nmol). The similarities in these numbers at such dramatically different concentrations of the nanocarriers highlight the impact of the cell type on transport. For instance, at concentrations of 0.1 mg mL−1 G3-NH2-FITC dendrimers had Papp values of 5.5 × 10−7 cm s−1 across Madin-Darby canine kidney cells (MDCK) [50]. These values were markedly different when compared to what was observed for both Caco-2 and Calu-3 cells. Another factor that can also influence the permeability of G3-NH2-FITC is the donor chamber concentration and incubation time. For Caco-2 cells, at two different donor concentrations of 10 and 1 mM, the Papp values were determined to be 2.7 × 10−7 and 0.6 × 10−7 cm s−1, respectively [48]. In another study, transport of G2-NH2 (generation 2 dendrimers) studied on MDCK cells at increasing donor chamber concentrations from 50 to 300 μg resulted in ca. fourfold increase of the Papp values [50]. These studies highlight the dependence of Papp on the initial donor chamber concentration.
To put these results in perspective, a comparison of the Papp values can be also made against some of the most common paracellular and transcellular markers. FITC-dextran, a common paracellular marker, of similar MW to the dendrimers studied here (10 KDa) was reported to have a Papp value of ca. 2 × 10−7 cm s−1 across Calu-3 monolayers cultured under AIC—similar to what was observed in this case, while small lipophilic compounds that primarily traverse across Calu-3 monolayers via transcellular route, have much higher permeabilities, with Papp in the range of 10−5 to 10−6 cm s−1 [23].
TEER measurements were used to understand the impact of the nanocarriers onto the monolayer integrity. The results for G3-NH2-FITC, NP containing G3-NH2-FITC, and NPs alone are shown in Fig. 4.
From the plot, a marked reduction (~60% of control) in the TEER of the Calu-3 cell monolayers incubated with G3-NH2-FITC was observed. Similar results have been reported when studying the transport of dendrimer conjugates across Caco-2 cell lines, where a strong association between paracellular transport (through the cellular tight junctions) and TEER reduction was proposed [49]. The integrity of the tight junctions populating the cell monolayers has been shown to be altered in the presence of positive charges such as the amine surface groups of G3-PAMAM dendrimers [49]. Our experiments revealed that replacing the G3-NH2-FITC laden media with culture medium after 5 h resulted in the revival of TEER to 80% of the control after 3 days. This is an important find as these results, coupled with the lack of cytotoxicity on Calu-3 cells within the concentration ranges tested, reaffirm the potential of G3-NH2 dendrimers as drug carriers for lung delivery.
The TEER reduction was also evaluated for the cell monolayers incubated with G3-NH2-FITC loaded NPs. The studies revealed that there was no significant reduction in the TEER values of the cells incubated with the G3-NH2-FITC loaded NPs compared to that of the control. The same observation was recorded for blank PLGA NPs pulsed onto Calu-3 cells. These results agree with earlier studies where it has been shown that PLGA NPs incubated on Calu-3 and on other epithelial cells did not alter the TEER, suggesting that the NPs do not interfere with the tight junctional integrity [51]. An impact on the TEER seems to correlate well with the ability of the nanocarrier to transport across the monolayer. This is also clearly a concentration-dependent effect, as when the dendrimers are released in small concentrations from the NPs, they do not significantly affect the TEER, and are also not transported across the monolayer either.
Uptake Studies with FACS
Flow cytometry was employed in order to quantitatively determine the rate and extent of internalization of bare G3-NH2-FITC, G3-NH2-FITC-loaded NPs, and PLGA-FITC NPs into polarized Calu-3 cells. The additional system of PLGA-FITC NPs was added in order to decouple the effect of NP internalization to that of the internalization of the FITC-dendrimer released from the NPs into the medium during the experiment, as discussed in more detail below. For each system, at least 6,000 events were counted.
The results of the mean fluorescent intensity (MFI) values obtained from FACS for each system (G3-NH2-FITC, G3-NH2-FITC-loaded NPs, and PLGA-FITC NPs) are summarized in Fig. 5 as a function of time. The results from Fig. 5a are recast in Fig. 5b as a percentage relative to the total internalization at 5 h (which was set as 100%) in order to better understand the rate of cellular entry. Note that this is done in spite of the fact that not all curves seem to have leveled off at that time, but nonetheless, it will prove useful in this discussion. The results in Fig. 5c serves to highlight the effect of monolayer polarization on cellular uptake—the uptake results for a selected system (G3-NH2-FITC) are summarized in Fig. 5c for polarized vs. non-polarized Calu-3 monolayers.
Fig. 5.
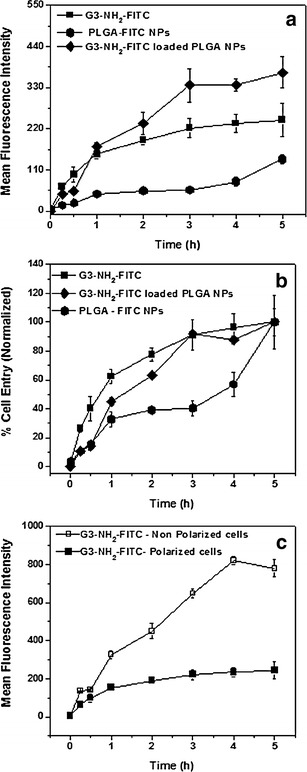
a Cellular entry of G3-NH2-FITC, G3-NH2-FITC-loaded PLGA NPs, and PLGA-FITC NPs into Calu-3 as a function of time, as determined by FACS—mean fluorescence intensity values (MFI). The results shown here are averages of six replicates for each system, with at least 6,000 events counted per system; b Rate of cell entry vs. time obtained by recasting MFI values relative to their cellular uptake at 5 h; c Effect of cell polarization on the cellular entry of G3-NH2-FITC. The error bars represent standard deviation (n = 6 wells per time point; 6,000 events for each well)
A sustained increase in cellular uptake, followed by a subsequent leveling off was observed in the case of G3-NH2-FITC nanocarriers—Fig. 5a. The leveling off in the cellular uptake was consistent with previous studies, where a similar trend with respect to the uptake of FITC-conjugated cationic dendrimers was observed on A549 cells [26]. This phenomenon was attributed to the fact that cationic dendrimers are typically internalized via adsorptive endocytosis, mediated by the presence of negatively charged proteoglycans on the cell membrane [26]. The uptake kinetics for this mode of internalization follows a curvilinear pattern likely due to saturation of membrane binding sites. A similar observation was also reported in B16f10 melanoma cells [52]. Interestingly, the internalization for cationic dendrimers in A549 cells leveled off within an hour after pulsing [26]. However, in this work, we observed a more gradual leveling off in the rate of internalization, with ca. only 60% being internalized after 1 h—Fig. 5b. This could possibly be attributed to differences in dendrimer generation and cell type, as both can influence cellular uptake [14, 53]. Another factor that could have potentially affected the rate of internalization is that, different from A549 cells, Calu-3 cells express mucins, which are negatively charged proteins that may interact with the dendrimers via electrostatic interactions, effectively hindering their transport to the cell surface.
When compared to the G3-NH2-FITC nanocarriers, the NPs (FITC-PLGA NPs) are seen to be internalized at a much lower rate—Fig. 5b. It has been previously reported that particles in the size range of 30-nm diffuse unhindered across mucosal layers, while larger particles diffused at a much slower pace [54]. It seems, therefore, that despite their positive charge, G3-NH2-FITC, being much smaller than NPs, traverse the mucus layer at a much faster rate compared to what has been observed for NPs. It is also interesting to observe that there seems to be an induction period in terms of the internalization of the NPs, which was not observed for the free dendrimers. After an initial internalization, very little, in terms of NPs, seems to be taken up between 1 and 3 h, when the rate of internalization starts increasing again. Even after 5 h, the trend in the uptake curve appears to suggest that NPs continue to be internalized. This could be potentially related to the difficulty in diffusing through the mucosal layer at early times, as discussed above, but the ability of those particles to eventually traverse the mucus layer and to start being subsequently internalized.
The G3-NH2-FITC-NP nanoblends internalized at a rate similar to that of the FITC-PLGA NPs in the first 30 min of experiments, after which, the rate of internalization more closely resembled that of the free G3-NH2-FITC—Fig. 5b. These results suggest that initial dendrimer internalization happened mostly as a blend (within the NPs). Different from the G3-NH2-FITC, the uptake of PLGA NPs into the cell milieu is mostly expected to occur via internalization mechanisms distinct to those of positively charged dendrimers (at least how it is mediated at the cell surface), such as pinocytosis or clathrin-mediated endocytosis, owing to the negative charge and size of NPs [55]. This may help explain the variation in the rate of uptake when compared to that of bare G3-NH2-FITC. However, after 1 h, even when the release of the dendrimer from the NPs is relatively small at ca. 1.5%, the internalization of (free) dendrimers released from the NPs, and not as blends, seem to take over the internalization process. It is also interesting to see that even after 5 h, the internalization process does not seem to have leveled off, different from the case of free dendrimers. This may be due to the fact that the internalization of the NPs increases at later times—after an induction period as discussed for the FITC-PLGA NPs. The increasing presence of free dendrimer released from the NPs is also likely to contribute to the fact that the curve seems to not level off at 5 h.
Given that the same mass/M concentration of dendrimer is added to the system for both free dendrimer and dendrimer encapsulated within the NPs (dendrimer in the blends), the MFI for the respective curves shown in Fig. 5a can be directly compared. The dual mode of uptake of the dendrimer when formulated as blends (within the NPs as a blend, and as free dendrimer) seems to facilitate the internalization of a greater amount of dendrimer into the cell, thus effectively preventing the saturation seen in the case of free dendrimer. This is especially attractive as a drug delivery strategy as one can control not only the rate of delivery, but also the total cellular uptake. The ability to enhance uptake may be achieved by eliciting different endocytic pathways as can be done by varying the surface characteristics of nanocarriers, and this is currently under investigation [42, 55].
Another important aspect of the FACS study was to elucidate the effect of Calu-3 polarization on cellular uptake. Polarized Calu-3 cells are expected to form tight junctions and exhibit all requisite morphological features inherent to them, which also includes a different composition of the apical and basolateral membrane [56]. Because cellular uptake is surface-mediated, the composition of the apical side of the membrane is expected to impact uptake. Calu-3 was cultured to form polarized monolayers as described in the earlier sections. In case of the non-polarized cells, the cells were seeded at the same density in 24-well plates and allowed to proliferate for a period of (only) 4 days prior to pulsing them with the G3-NH2-FITC, when the cells were determined to be non-confluent. A plot of the MFI as a function of time for both non-polarized and polarized cells is shown in Fig. 5c. From the figure, a drastic increase in cellular uptake was observed for the non-polarized Calu-3 cells, with no signs of saturation in internalization by 5 h, in sharp contrast to the results observed for the polarized layer. The results thus emphasize the relevance in performing the internalization studies on polarized monolayers.
In summary, the transport and internalization results described above suggest that the G3-NH2-FITC can not only be effectively transported across Calu-3 monolayers, but also efficiently internalized in the cell milieu. The results also show that further mediation in terms of both cellular uptake into Calu-3 cells, and transport across the respective monolayers may be achieved by nanoblending the DNCs within polymeric NPs. In the sections that follow, we present a strategy to formulate dendrimer nanocarriers into propellant-based OI formulations (pressurized metered-dose inhalers, pMDIs), with aerosol characteristics conducive to deep lung deposition. Together with a recent publication from our group that demonstrates the ability to formulate solid polymeric NPs in pMDIs, the results demonstrate the potential in using the nanocarriers discussed here as platforms for the delivery of therapeutics to the lungs for local or systemic delivery [28, 57].
Preparation and Characterization of G3-NH2-FITC Loaded Core-Shell Particles
Given the fact that the dendrimer nanocarriers are highly positively charged, we expected them not to be easily dispersible in the low dielectric HFAs [58]. Another potential issue in formulating the nanocarriers in propellant-based inhalers that needed to be considered is the fact that the optimum size of the aerosol particles for deep lung deposition (a few microns in diameter) falls far outside the size of the dendrimer nanocarriers (a few nanometers in diameter) [1, 9]. These will be considered in more detail in the discussion that follows. In this section, we present a particle engineering technology that was pursued to formulate the dendrimer nanocarriers as micron-sized particles with enhanced physical stability in the propellant HFA, as an attempt to address the concerns mentioned above.
Loading of G3-NH2-FITC into core-shell particles was accomplished via a modified emulsification-diffusion methodology [27]. OLA-g-CS is a water soluble co-oligomer that has been shown to aid in the formation of stable particle dispersions in propellant HFAs due to the presence of ester groups, which are highly HFA-philic [27, 57]. A representative SEM micrograph of the G3-NH2-FITC loaded core-shell particles prepared as discussed in Methods is shown in Fig. 6. The size distribution of these particles, as determined by DLS in a model HFA (HPFP, which is liquid at ambient conditions), is shown as an inset in the same figure.
Fig. 6.
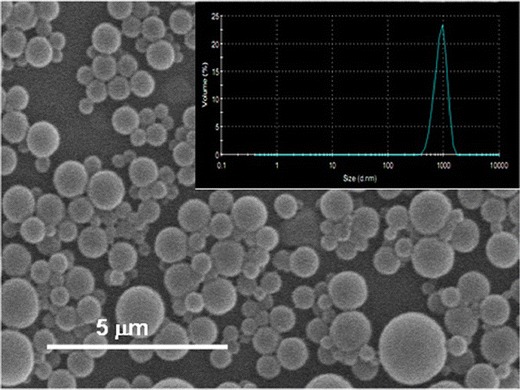
SEM micrograph of the G3-NH2-FITC-loaded core-shell particles prepared via emulsification-diffusion. (Inset) Size distribution of G3-NH2-FITC-loaded core-shell particles prepared using emulsification diffusion, as estimated by DLS, in HPFP, a model (liquid at ambient conditions) propellant HFA
The SEM micrograph indicates the formation of smooth, fairly polydisperse (but with a single peak) particles. The average size of the particles was determined to be 1.0 ± 0.2 μm, with a polydispersity of 0.3. The geometric size range of the particles formed here is well within the range prescribed for efficient deep-lung deposition of inhaled aerosols [1, 27, 57]. The encapsulation efficiency of the G3-NH2-FITC was determined using fluorometry. The loading of G3-NH2-FITC in the core-shell particles was determined to be 0.06 ± 0.01 mg of dendrimer per milligram co-oligomer, which corresponds to an encapsulation efficiency of 37% ± 5%. While we have not attempted to encapsulate the G3-NH2-FITC-loaded NPs in such core-shell particles, we have recently demonstrated the applicability of similar methodology in the encapsulation of polymeric NPs containing a fluorescent probe, which is of similar size and surface characteristics, and thus expect that strategy to be also applicable to the to the G3-NH2-FITC-loaded PLGA NPs. [57].
Physical Stability of G3-NH2-FITC-Loaded Core-shell Particles in HFA227
The stability of freshly prepared G3-NH2-FITC-loaded core-shell particles and bare G3-NH2-FITC dispersions in HFA227 was assessed by visually ascertaining the rate of sedimentation of the formulations as a function of time. Digital photographs were captured at predetermined times after the mechanical energy for dispersing the particles ceased, in order to assess the quality of the dispersions. Images captured at t = 0 h and t = 2 h are shown as inset in Fig. 7a. The physical stability of formulations prepared using bare G3-NH2-FITC is also shown as insets in Fig. 7b. The concentration of the core-shell particles was 2 mg mL−1 (corresponding to 0.14 mg of the conjugate). A similar concentration of the G3-NH2-FITC (0.15 mg mL−1) was utilized for the bare formulation.
Fig. 7.
Anderson cascade impaction results of the G3-NH2-FITC formulated a as core-shell microparticles, and b as bare G3-NH2-FITC in HFA 227, at 298 K and saturation pressure of the propellant. Particle concentration was 2 mg mL−1 for the core-shell preparation while the concentration for the bare G3-NH2-FITC formulation was 0.15 mg mL−1. AC, IP, and F refer to actuator, induction port, and filter, respectively. (Insets) a Dispersion stability of G3-NH2-FITC formulated as core-shell particles in HFA 227 at 2 mg mL−1, 298 K, and saturation pressure of the propellant mixture. The digital images were obtained as soon as the mechanical energy input for dispersing the system was halted, and as a function of time after that. b Formulations prepared using bare G3-NH2-FITC at 0.15 mg mL−1 concentration
From the images, we observed that the dispersions formulated using the core-shell particles are seen to possess superior physical stability when compared to the bare G3-NH2-FITC formulation. The bare G3-NH2-FITC formulated in the propellant creamed within a few seconds, and remained as aggregate, even after further mechanical energy input. For the core-shell formulation, creaming of the particles due to gravitational forces was observed—these are micron particles compared to the nanometer-sized dendrimers. These particles form loose aggregates that can be easily broken up and redispersed by simple manual agitation, indicating that the particles did not irreversibly flocculate.
Aerosol Characteristics of G3-NH2-FITC-Loaded Core-shell Particles
Cascade impaction studies were conducted using an ACI in order to quantitatively assess the aerosol quality of the pMDI formulations, and the effect of the proposed particle engineering technology. ACI is an in vitro testing device comprised of eight stages, with each stage representing a region of the lung, as benchmarked by in vivo and in vitro studies [57]. Actuating the formulation through the device under vacuum at a constant flow rate deposits the particles (free dendrimers or core-shell particles containing the G3-NH2-FITC) on different stages depending on the aerosol size—larger aerosol particles are retained on the earlier stages of the impactor, while the smaller ones are retained in the later stages [59]. The amount of the G3-NH2-FITC deposited on these stages was quantified by fluorometry, and relevant aerosol properties, including the FPF, MMAD, and GSD, were determined. FPF (fraction on stages 3-F of the ACI) is a measure of the efficiency of deposition in the deep lungs. MMAD is defined as the median of the airborne particle mass distribution with respect to the aerodynamic diameter. MMAD is always accompanied by GSD, which characterizes the variability of the particle size distribution [59].
The ACI results for both the core-shell and the bare G3-NH2-FITC formulation are summarized in Table S1 included in the supplementary information, and recast as a percentage in Fig. 7a, b. The figure in percentage is easier for the visualization and comparison of the different formulations, but does not contain the raw information required for the various calculations. Therefore, we include both figure and table (in the Supplementary Material). The values reported here are averages of three independent experiments—three different canisters, with 20 actuations per canister.
The FPF for the formulation with the G3-NH2-FITC-loaded core-shell particles was 55% ± 5%. We also achieved high recovery efficiency for this formulation - mass collected relative to the estimated amount delivered based on the formulation concentration and reservoir volume—80% ± 8%. In comparison, the bare G3-NH2-FITC formulation had a very poor recovery efficiency (ca. 5%), making a reliable estimation of the FPF and other aerosol properties impractical. The low recovery for the formulation with free dendrimer can be attributed to the fact that the system is not well dispersed within the canister, and other losses due to perhaps the small sizes of the dendrimers. From what was collected, a large fraction was detected in the induction port and filter regions of the actuator, indicating the aggregation and erratic dispersion of these carriers within the propellant. These results further corroborate our earlier hypothesis, where, through a combination of sedimentation rate experiments and colloidal probe microscopy, we have shown that there is a direct correlation between the physical stability of the formulations in the propellant, and the corresponding aerosol characteristics [27]. The aerosol properties of the G3-NH2-FITC core-shell formulation compare favorably with those of commercial pMDI formulations containing dispersed crystals of small molecular weight therapeutics [27]. For instance, Ventolin HFA®, a commercial formulation for asthma and chronic obstructive pulmonary disease (COPD) reported an FPF of 46% under the same conditions—note we have not attempted to optimize the FPF through changes in hardware nor particle size/distribution, when we would expect to achieve even larger FPFs. The MMAD and GSD of the core-shell formulation, was calculated to be 3.0 and 2.5 μm, respectively, which is in good agreement with values required for enhanced deposition to the deep lungs [8].
These results suggest that G3-NH2-FITC formulations with good aerosol characteristics can be prepared using the proposed particle engineering strategy. Such platforms have thus the potential to be utilized to treat local ailments of the lungs, or in the treatment of systemic disorders through careful carrier design—transport modulation, as discussed above.
CONCLUSIONS
In this work, we demonstrate that the internalization and transport of G3-NH2-FITC across a model of the pulmonary epithelium can be modulated by forming nanoblends of the dendrimer within polymeric nanoparticles. The internalization of the blends is dramatically different compared to that of the free dendrimers. While saturation in cellular uptake is observed in the case of free G3-NH2-FITC, the same is not seen for the solid NPs blends containing the dendrimers, within the time frame of the experiments—5 h. Another distinguishing feature in the uptake of the blends and dendrimers is that while there seems to be an induction period over which the uptake of the NPs is slow, as expected, due to the larger size of the NPs, the presence of a mucus layer covering the Calu-3 cells, and the negative surface charge of the NPs, the total uptake of the dendrimers blended into the NPs increases at later times, and ends up being larger than the loading of free dendrimers at 5 h—and it does not seem to have reached saturation at that time. These results thus indicate that the formation of nanoblends may help overcome saturation, and thus enhance cellular uptake, by exploring alternative internalization mechanisms. The formulation of dendrimers as nanoblends is also seen to be very efficient in preventing the transport across the monolayer, as seen by a decrease in the apparent permeability from 10−7 cm s−1 for the free dendrimers, to zero for dendrimers formulated as nanoblends. It is also relevant to mention that within the concentration range investigated in this work, both the dendrimer and nanoblends were seen to be fairly non cytotoxic to Calu-3 cells. These results are particularly exciting as dendrimers have been suggested as effective drug delivery carriers for several chemotherapeutics, biomolecules, imaging agents, and other relevant high potency drugs. These results, combined with the ability to efficiently formulate free dendrimers or solid NPs (our previous work) in pMDIs, highlight the potential of these nanocarriers for the OI delivery of a range of therapeutics for the treatment of regional and systemic diseases.
Electronic supplementary material
(DOC 336 kb)
ACKNOWLEDGMENTS
We would like to acknowledge the National Science Foundation (NSF-CBET grant no. 0933144) and the NanoIncubator at WSU for financial support. We would also like to thank Solvay Fluor und Derivate GmbH & Co. for providing the propellant HFAs, and 3M and Valois for the metering valves. We thank the groups of Prof. Verani, Prof. Matthew, and Prof. Huttemann from Wayne State University, and Prof. Kannan’s group, now at Johns Hopkins, for giving us access to their laboratory equipment, in particular, the FTIR, plate reader, fluorescence microscope, and DLS. The microscopy, imaging, and cytometry resources core is supported, in part by NIH center grant P30CA22453 to the Karmanos Cancer Institute, Wayne State University, and the Perinatology Research Branch of the National Institutes of Child Health and Development, Wayne State University.
REFERENCES
- 1.Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6(1):67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 2.Andrade F, Rafael D, Videira M, Ferreira D, Sosnik A, Sarmento B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv Drug Deliv Rev. 2013 doi: 10.1016/j.addr.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cipolla D, Gonda I, Chan HK. Liposomal formulations for inhalation. Thera Deliv. 2013;4(8):1047–1072. doi: 10.4155/tde.13.71. [DOI] [PubMed] [Google Scholar]
- 4.Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev. 2006;58(9–10):1030–1060. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Siekmeier R, Scheuch G. Systemic treatment by inhalation of macromolecules—principles, problems, and examples. Journal of physiology and pharmacology. Official J Polish Phys Soc. 2008;59(Suppl 6):53–79. [PubMed] [Google Scholar]
- 6.Laube BL, Janssens HM, de Jongh FH, Devadason SG, Dhand R, Diot P, et al. What the pulmonary specialist should know about the new inhalation therapies. European Resp J. 2011;37(6):1308–1331. doi: 10.1183/09031936.00166410. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigo GJ, Castro-Rodriguez JA. Safety of long-acting beta agonists for the treatment of asthma: clearing the air. Thorax. 2012;67(4):342–349. doi: 10.1136/thx.2010.155648. [DOI] [PubMed] [Google Scholar]
- 8.Rogueda PG, Traini D. The nanoscale in pulmonary delivery. Part 1: deposition, fate, toxicology and effects. Exp Opinion Drug Deliv. 2007;4(6):595–606. doi: 10.1517/17425247.4.6.595. [DOI] [PubMed] [Google Scholar]
- 9.Rytting E, Nguyen J, Wang X, Kissel T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Exp Opinion Drug Deliv. 2008;5(6):629–639. doi: 10.1517/17425247.5.6.629. [DOI] [PubMed] [Google Scholar]
- 10.Tsapis N, Bennett D, Jackson B, Weitz DA, Edwards DA. Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc Natl Acad Sci U S A. 2002;99(19):12001–12005. doi: 10.1073/pnas.182233999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sung JC, Pulliam BL, Edwards DA. Nanoparticles for drug delivery to the lungs. Trends Biotech. 2007;25(12):563–570. doi: 10.1016/j.tibtech.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Bai S, Thomas C, Rawat A, Ahsan F. Recent progress in dendrimer-based nanocarriers. Critical Rev Thera Drug Carrier Sys. 2006;23(6):437–495. doi: 10.1615/CritRevTherDrugCarrierSyst.v23.i6.10. [DOI] [PubMed] [Google Scholar]
- 13.Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42(3):1147–1235. doi: 10.1039/c2cs35265f. [DOI] [PubMed] [Google Scholar]
- 14.Kitchens KM, El-Sayed ME, Ghandehari H. Transepithelial and endothelial transport of poly (amidoamine) dendrimers. Adv Drug Deliv Rev. 2005;57(15):2163–2176. doi: 10.1016/j.addr.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Contreras L, Sung JC, Muttil P, Padilla D, Telko M, Verberkmoes JL, et al. Dry powder PA-824 aerosols for treatment of tuberculosis in guinea pigs. Antimicro Agents Chemother. 2010;54(4):1436–1442. doi: 10.1128/AAC.01471-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 17.Saad M, Garbuzenko OB, Ber E, Chandna P, Khandare JJ, Pozharov VP, et al. Receptor targeted polymers, dendrimers, liposomes: which nanocarrier is the most efficient for tumor-specific treatment and imaging? Official J Controlled Release Soc. 2008;130(2):107–114. doi: 10.1016/j.jconrel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jevprasesphant R, Penny J, Attwood D, D'Emanuele A. Transport of dendrimer nanocarriers through epithelial cells via the transcellular route. Official J Controlled Release Soc. 2004;97(2):259–267. doi: 10.1016/j.jconrel.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Mignani S, El Kazzouli S, Bousmina M, Majoral JP. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: a concise overview. Adv Drug Deliv Rev. 2013 doi: 10.1016/j.addr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Kolhe P, Misra E, Kannan RM, Kannan S, Lieh-Lai M. Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. International J Pharm. 2003;259(1–2):143–160. doi: 10.1016/S0378-5173(03)00225-4. [DOI] [PubMed] [Google Scholar]
- 21.Cartiera MS, Johnson KM, Rajendran V, Caplan MJ, Saltzman WM. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials. 2009;30(14):2790–2798. doi: 10.1016/j.biomaterials.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Jeong JH, Chun KW, Park TG. Target-specific cellular uptake of PLGA nanoparticles coated with poly(L-lysine)-poly(ethylene glycol)-folate conjugate. ACS J Surf Colloids. 2005;21(19):8852–8857. doi: 10.1021/la0502084. [DOI] [PubMed] [Google Scholar]
- 23.Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res. 2006;23(7):1482–1490. doi: 10.1007/s11095-006-0255-0. [DOI] [PubMed] [Google Scholar]
- 24.Fiegel J, Ehrhardt C, Schaefer UF, Lehr CM, Hanes J. Large porous particle impingement on lung epithelial cell monolayers—toward improved particle characterization in the lung. Pharm Res. 2003;20(5):788–796. doi: 10.1023/A:1023441804464. [DOI] [PubMed] [Google Scholar]
- 25.Grainger CI, Greenwell LL, Martin GP, Forbes B. The permeability of large molecular weight solutes following particle delivery to air-interfaced cells that model the respiratory mucosa. Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2009;71(2):318–324. doi: 10.1016/j.ejpb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Perumal OP, Inapagolla R, Kannan S, Kannan RM. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials. 2008;29(24–25):3469–3476. doi: 10.1016/j.biomaterials.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Bharatwaj B, Panyam J, da Rocha SR. Core-shell particles for the dispersion of small polar drugs and biomolecules in hydrofluoroalkane propellants. Pharm Res. 2008;25(2):289–301. doi: 10.1007/s11095-007-9466-2. [DOI] [PubMed] [Google Scholar]
- 28.Conti DS, Bharatwaj B, Brewer D, da Rocha SR. Propellant-based inhalers for the non-invasive delivery of genes via oral inhalation. Official J Controlled Release Soc. 2012;157(3):406–417. doi: 10.1016/j.jconrel.2011.09.089. [DOI] [PubMed] [Google Scholar]
- 29.Majoros IJ, Thomas TP, Mehta CB, Baker JR., Jr Poly(amidoamine) dendrimer-based multifunctional engineered nanodevice for cancer therapy. J Med Chem. 2005;48(19):5892–5899. doi: 10.1021/jm0401863. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y, Klutz AM, Jacobson KA. Systematic investigation of polyamidoamine dendrimers surface-modified with poly(ethylene glycol) for drug delivery applications: synthesis, characterization, and evaluation of cytotoxicity. Bioconjug Chem. 2008;19(8):1660–1672. doi: 10.1021/bc700483s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–347. doi: 10.1016/S0169-409X(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 32.Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliv Rev. 2011;63(10–11):943–955. doi: 10.1016/j.addr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 33.de la Fuente M, Csaba N, Garcia-Fuentes M, Alonso MJ. Nanoparticles as protein and gene carriers to mucosal surfaces. Nanomedicine. 2008;3(6):845–857. doi: 10.2217/17435889.3.6.845. [DOI] [PubMed] [Google Scholar]
- 34.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly (D, L-lactide-co-glycolide) and its derivatives. Official J Controlled Release Soc. 2008;125(3):193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. Official J Controlled Release Soc. 2001;70(1–2):1–20. doi: 10.1016/S0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XQ, Intra J, Salem AK. Conjugation of polyamidoamine dendrimers on biodegradable microparticles for nonviral gene delivery. Bioconjug Chem. 2007;18(6):2068–2076. doi: 10.1021/bc070116l. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Zhang SM, Chen PP, Cheng L, Zhou W, Tang WX, et al. Controlled release of insulin from PLGA nanoparticles embedded within PVA hydrogels. J Mater Sci Mater Med. 2007;18(11):2205–2210. doi: 10.1007/s10856-007-3010-0. [DOI] [PubMed] [Google Scholar]
- 38.Mao S, Xu J, Cai C, Germershaus O, Schaper A, Kissel T. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. International J Pharm. 2007;334(1–2):137–148. doi: 10.1016/j.ijpharm.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Cohen-Sela E, Chorny M, Koroukhov N, Danenberg HD, Golomb G. A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticles. Official J Controlled Release Soc. 2009;133(2):90–95. doi: 10.1016/j.jconrel.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 40.Allemann E, Leroux JC, Gurny R, Doelker E. In vitro extended-release properties of drug-loaded poly(DL-lactic acid) nanoparticles produced by a salting-out procedure. Pharm Res. 1993;10(12):1732–1737. doi: 10.1023/A:1018970030327. [DOI] [PubMed] [Google Scholar]
- 41.Panyam J, Dali MM, Sahoo SK, Ma W, Chakravarthi SS, Amidon GL, et al. Polymer degradation and in vitro release of a model protein from poly(D, L-lactide-co-glycolide) nano- and microparticles. Official J Controlled Release Soc. 2003;92(1–2):173–187. doi: 10.1016/S0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 42.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15(5–6):171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Jevprasesphant R, Penny J, Attwood D, McKeown NB, D'Emanuele A. Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharm Res. 2003;20(10):1543–1550. doi: 10.1023/A:1026166729873. [DOI] [PubMed] [Google Scholar]
- 44.Csaba N, Caamano P, Sanchez A, Dominguez F, Alonso MJ. PLGA:poloxamer and PLGA:poloxamine blend nanoparticles: new carriers for gene delivery. Biomacromolecules. 2005;6(1):271–278. doi: 10.1021/bm049577p. [DOI] [PubMed] [Google Scholar]
- 45.Ehrhardt C, Fiegel J, Fuchs S, Abu-Dahab R, Schaefer UF, Hanes J, et al. Drug absorption by the respiratory mucosa: cell culture models and particulate drug carriers. Official J International Soc Aero Med. 2002;15(2):131–139. doi: 10.1089/089426802320282257. [DOI] [PubMed] [Google Scholar]
- 46.Haghi M, Young PM, Traini D, Jaiswal R, Gong J, Bebawy M. Time- and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Development Indus Pharm. 2010;36(10):1207–1214. doi: 10.3109/03639041003695113. [DOI] [PubMed] [Google Scholar]
- 47.Madlova M, Jones SA, Zwerschke I, Ma Y, Hider RC, Forbes B. Poly(vinyl alcohol) nanoparticle stability in biological media and uptake in respiratory epithelial cell layers in vitro. Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2009;72(2):437–443. doi: 10.1016/j.ejpb.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 48.El-Sayed M, Ginski M, Rhodes C, Ghandehari H. Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. Official J Controlled Release Soc. 2002;81(3):355–365. doi: 10.1016/S0168-3659(02)00087-1. [DOI] [PubMed] [Google Scholar]
- 49.Kitchens KM, Kolhatkar RB, Swaan PW, Eddington ND, Ghandehari H. Transport of poly (amidoamine) dendrimers across Caco-2 cell monolayers: influence of size, charge and fluorescent labeling. Pharm Res. 2006;23(12):2818–2826. doi: 10.1007/s11095-006-9122-2. [DOI] [PubMed] [Google Scholar]
- 50.Tajarobi F, El-Sayed M, Rege BD, Polli JE, Ghandehari H. Transport of poly amidoamine dendrimers across Madin-Darby canine kidney cells. International J Pharm. 2001;215(1–2):263–267. doi: 10.1016/S0378-5173(00)00679-7. [DOI] [PubMed] [Google Scholar]
- 51.Mura S, Hillaireau H, Nicolas J, Kerdine-Romer S, Le Droumaguet B, Delomenie C, et al. Biodegradable nanoparticles meet the bronchial airway barrier: how surface properties affect their interaction with mucus and epithelial cells. Biomacromolecules. 2011;12(11):4136–4143. doi: 10.1021/bm201226x. [DOI] [PubMed] [Google Scholar]
- 52.Seib FP, Jones AT, Duncan R. Comparison of the endocytic properties of linear and branched PEIs, and cationic PAMAM dendrimers in B16f10 melanoma cells. Official J Controlled Release Soc. 2007;117(3):291–300. doi: 10.1016/j.jconrel.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 53.D'Emanuele A, Attwood D. Dendrimer–drug interactions. Adv Drug Deliv Rev. 2005;57(15):2147–2162. doi: 10.1016/j.addr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, et al. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci U S A. 2009;106(46):19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hillaireau H, Couvreur P. Nanocarriers' entry into the cell: relevance to drug delivery. Cell Molec Life Sci. 2009;66(17):2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bucior I, Mostov K, Engel JN. Pseudomonas aeruginosa-mediated damage requires distinct receptors at the apical and basolateral surfaces of the polarized epithelium. Infec Immu. 2010;78(3):939–953. doi: 10.1128/IAI.01215-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bharatwaj B, Wu L, Whittum-Hudson JA, da Rocha SR. The potential for the noninvasive delivery of polymeric nanocarriers using propellant-based inhalers in the treatment of Chlamydial respiratory infections. Biomaterials. 2010;31(28):7376–7385. doi: 10.1016/j.biomaterials.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Wu L, Peguin RP, da Rocha SR. Understanding solvation in hydrofluoroalkanes: ab initio calculations and chemical force microscopy. J Phys Chem B. 2007;111(28):8096–8104. doi: 10.1021/jp071205y. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell JP, Nagel MW. Cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. The official Journal International Society Aerosols Medicine. 2003;16(4):341–377. doi: 10.1089/089426803772455622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 336 kb)


