Abstract
Background:
Epilepsy is described as a chronic neurological disorder characterized by recurrent seizures of cerebral origin, presenting with episodes of sensory, motor or autonomic phenomenon with or, without loss of consciousness. A recent meta-analysis of published and unpublished studies puts an overall prevalence rate of epilepsy in India at 5.59 per 1,000 populations. There have been studies that report clinical benefits of the use of folic acid as an adjuvant to the anti-epileptic therapy in the prevention of anti-epileptic drug induced gingival enlargement. However, studies conducted in the past have also reported precipitation of epileptic attacks in patients on folic acid adjuvant therapy due to fall in sera levels of phenytoin due to drug interactions. The study was planned to investigate the association of phenytoin induced gingival enlargement and sera levels of folic acid in epileptic patients on phenytoin therapy so as to justify the use of folic acid as a routine adjuvant to the usual anti-epileptic therapy to prevent this inevitable adverse effect without destabilizing the ongoing regimen leading to the precipitation of seizures in an otherwise stable patient (breakthrough seizures).
Materials and Methods:
A total of 100 patients between the ages 18 and 50 years were clinically diagnosed with epilepsy prior to the start of phenytoin therapy were included based on selection criteria and written informed consents were obtained. Assessment of serum folic acid levels and gingival enlargement was performed prior to the start of and after 1 year of phenytoin therapy.
Statistical Analysis Used:
The statistical analysis was carried out using t-test and the baseline serum folate levels and the serum folate levels obtained after 1 year of phenytoin therapy were correlated with the respective grades of gingival enlargement using Pearson's coefficient formula.
Results:
The results of the study confirmed a significant association between low serum folate levels with increasing severity as well as an early onset of phenytoin induced gingival enlargement.
Conclusions:
The results of the study suggest a higher incidence of gingival enlargement with an early onset and increased severity in phenytoin treated epileptic patients with a positive correlation with falling serum folic acid levels as the duration of the therapy increases.
Keywords: Breakthrough seizures, epilepsy, folic acid, gingival enlargement, phenytoin
Introduction
Epilepsy is described as a chronic neurological disorder characterized by recurrent seizures of cerebral origin, presenting with episodes of sensory, motor or, autonomic phenomenon with or, without loss of consciousness.[1] Epilepsy, from the Ancient Greek (epilēpsía)- “to seize”, is a common chronic neurological disorder characterized by recurrent unprovoked seizures. The Ancient Greek epilēpsía, is derived from epilambαnein meaning" to take hold of", which in turn, was combined from epí “upon” and lambαnein “to take”.[2,3] The worldwide prevalence of active epilepsy is 4 to 10 per 100,000 populations[2,3,4,5,6,7,8,9] while the incidence of epilepsy ranges from 40 to 70 per 100,000 in most developed countries and 100 to 190 per 100,000 in developing countries.[4,10,11,12,13,14]
Approximately 60% of all epilepsies are idiopathic or, cryptogenic, that is without a discernible cause. Almost any type of brain pathology can cause seizures/epilepsy. Cerebro-vascular disease is the most commonly identified cause among adults, while peri-natal insults seem to be most common among children. The etiology of seizures is multi-factorial in any given individual and is best thought of as an interaction between genetically determined seizure thresholds, underlying predisposing pathologies or, metabolic derangements and acute precipitating factors. These seizures are transient signs and/or symptoms of abnormal, excessive or, synchronous neuronal activity in the brain.
Epilepsy is usually controlled, but cannot be cured completely with medication. However, over 30% of people with epilepsy do not have seizure control even with the best of the available medications. Despite tremendous advances in the management of epilepsy, phenytoin still remains the drug of choice. Phenytoin (diphenylhydantoin) was first synthesized by German chemist Heinrich Biltz in 1908.[15] In 1938, Merritt H Houston and Putnam Tracy[16,17,18,19] discovered phenytoin's usefulness for controlling seizures without the sedative effects associated with phenobarbital that was commonly being used in those times. The primary site of action of the drug appears to be the motor cortex where spread of seizure activity is inhibited. Possibly by promoting sodium efflux from neurons, phenytoin tends to stabilize the threshold against hyper-excitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient. This includes the reduction of post-tetanic potentiation at synapses. Loss of post-tetanic potentiation prevents cortical seizure foci from detonating adjacent cortical areas. Phenytoin reduces the maximal activity of brain stem centers responsible for the tonic phase of tonic-clonic (grand mal) seizures. Phenytoin's inhibitory effect is dependent on the voltage and frequency of neural cell firing by selectively blocking the neurons that are firing at high frequency.[16,17,18,19]
In 1938, Merritt and Putnam published their noteworthy data using phenytoin to treat major, absence and psychomotor seizures. Since that time, phenytoin has been demonstrated to be a highly effective anti-convulsant.[16,17,18,19] However, the long term administration of phenytoin has been seen to lead to a number of adverse effects. Gingival enlargement is a frequently reported adverse effect of phenytoin.[20,21] Approximately 40-50% of the patients treated with phenytoin develop esthetically disfiguring enlargement of the gingivae. Whenever occurs, this adverse effect of phenytoin, lasts throughout the period of therapy and continues further with a severe reduction in the quality of life of the affected individual. The pseudo-pockets that are formed as a result of gingival enlargement increase plaque retentive areas which further predispose the patient towards an enhanced susceptibility for inflammatory changes in the gingivae, dental caries and periodontal diseases.[22,23]
The etio-pathogenesis of phenytoin induced gingival enlargement is still not clearly understood, however, many studies indicate its multi-factorial etiology[24,25] including oral hygiene status of the affected epileptic patients. It has also been seen that phenytoin is not only responsible for the initiation of the enlargement of the gingival tissue but has also been noted to interfere with folic acid metabolism especially, absorption thereby leading to a significant decrease in the plasma as well as the tissue levels of folates, one of the several proposed etiologies behind drug induced gingival enlargements.[26]
There have been several studies that suggest severe folate deficiencies resulting out of long term phenytoin usage. Folates, on the other hand, have been blamed for a significant decrease in the serum concentration of phenytoin severe enough to precipitate seizures.[27,28,29] Based on the conclusions drawn from the various studies correlating decreased plasma and tissue folate levels with phenytoin induced gingival enlargement, folic acid has been tried, both topically and systemically, to prevent this inevitable adverse effect of long term phenytoin therapy.[30] There has been consistent void, however, in research in assessing serum folic acid levels in epileptic patients and their correlation with the onset and severity of phenytoin induced gingival enlargement starting from the beginning of phenytoin treatment. Hence, the present study was designed to investigate the association of phenytoin induced gingival enlargement with serum folate levels in phenytoin treated epileptic patients. The aim of the study was to find an etiological role, if any, of folic acid behind anti-epileptic drug, phenytoin induced gingival enlargement so as to initiate research on an important question that has been raised in the past regarding the safety of the use of folate adjuvants in the prevention of this inevitable side effect of the drug, although associated with a risk of precipitation of epileptic attacks due to a significant propensity for drug interactions leading to decreased sera phenytoin levels. The objectives of the study were to study the incidence and assess the scores of gingival enlargement in epileptic patients before and after 1 year of phenytoin therapy; to assess serum folic acid levels before and after 1 year of phenytoin therapy and to correlate the scores of gingival enlargement with serum folic acid levels in patients on phenytoin therapy.
Materials and Methods
Source of data
A total of 100 patients visiting the Department of Neurology, Victoria Hospital, Bangalore during the period of January 2009 to December 2009 clinically diagnosed with epilepsy were selected prior to the start of phenytoin therapy based on the defined inclusion and exclusion criteria.
Method of collection of data
Selected epileptic patients in the age group of 18-50 years, who were clinically diagnosed with epilepsy and being started with phenytoin therapy and who were with the full complement of teeth without any carious or periodontal involvement or any other pathological process in the teeth and the jaws, were explained in detail about the planned study and written informed consents were obtained. The patients were exclusively being started on phenytoin therapy and were not supposed to take any other medication apart from phenytoin throughout the course of study. Patients who required polytherapy with other drugs in combination with phenytoin were excluded from the study. These patients were subjected to a detailed history and a thorough clinical examination using a specially prepared proforma.
Epileptic patients with other systemic diseases; with pre-existing gingival enlargements due to any reasons as idiopathic, inflammatory, neoplastic, endocrinal, chronic vitamin C deficiency, mouth breathing or pregnancy; epileptic patients on any type of pharmacologic therapy including multi-vitamins or folate antagonists and who had a history of dental treatment and trauma to teeth were excluded from the study.
Methodology
Based on the selection criteria, 100 patients clinically diagnosed with epilepsy, were enrolled in the study with their written informed consents and then subjected to thorough oral prophylaxis, routine hematological examination and serum folic acid level assessment. Before the start of study, the ethical clearance was obtained by the Ethical Committee of the Institution as well as from Bangalore Medical College and Research Institute and Associated Hospitals, Bangalore.
Assessment of serum folic acid
Assessment of serum folic acid level was carried out by chemiluminescent method using immulite kit [Figure 1] prior to the start of phenytoin therapy. A gap of a minimum of 10 h after the last meal followed by intake of the drug was considered as the standard fasting period in the patients. For this, following an overnight fasting period, 5 ml of venous blood was taken from patients from the antecubital vein using a sterile disposable syringe in the sitting position between 8 am and 10 am Serum was immediately separated by ultracentrifugation. The supernatant was discarded and the rest of the sample was stored at −20°C. The control group was assessed based on their inclusion according to age and sex. They were free of any systemic disease process and were not on any drugs including supplementation with synthetic vitamin supplements or the drugs, which could have interfered with the absorption of such nutrients.
Figure 1.

Immulite 1000 system, Siemens REF LKFO1, lot 0305, SMN 10380902 equipment for assessment of serum folic acid levels by chemiluminiscent immunoassay system with running samples
After a gap of 1 week, these patients were thoroughly examined and their gingival status assessed using the index originally described by Angelopoulos and Goaz and later, modified by Miller and Damm, the Gingival Overgrowth/GO Index [Figure 2].
Figure 2.
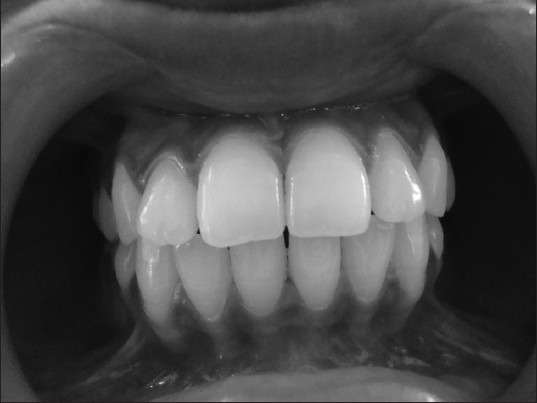
Clinical picture of gingival status in a male epileptic patient before the start of phenytoin therapy, after 1 week of oral prophylaxis
Assessment of gingival enlargement
The gingival status was assessed using the Gingival Over growth/GO Index. There were a total of four investigators who assessed gingival enlargements and were blinded to the purpose of the study as well as blinded to the sera levels of folic acid. The height of gingival tissue was measured from the cemento-enamel junction to the free gingival margin. The grades for gingival enlargement were assessed in relation to the six anterior teeth in both the maxillary and mandibular arches based on the findings of the previous studies of the more common involvement of the anterior segments of the jaws, both on the mesial and the distal inter-proximal aspects and the greater score among them was selected to be included to refer to the peak effect of the drug.
After 3 [Figures 3–5] months, the patients were reviewed and their gingival scores re-assessed using the same criteria. The same procedure was repeated at the end of 1 year [Figure 5] of phenytoin therapy and serum folic acid levels assessed before the morning dose of phenytoin. Results were tabulated and subjected to statistically analysis.
Figure 3.
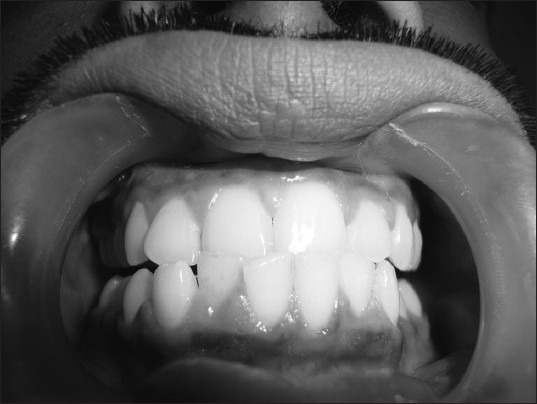
Clinical picture of gingival enlargement in a male epileptic patient (Grade 1) after 3 months of initiation of phenytoin therapy
Figure 5.
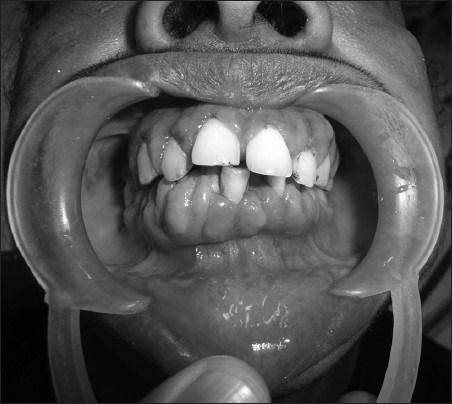
Clinical picture of bulbous gingival enlargement in a female epileptic patient with more prominent involvement of the interdental papillae (Grade 3) after 1 year of phenytoin therapy
Figure 4.
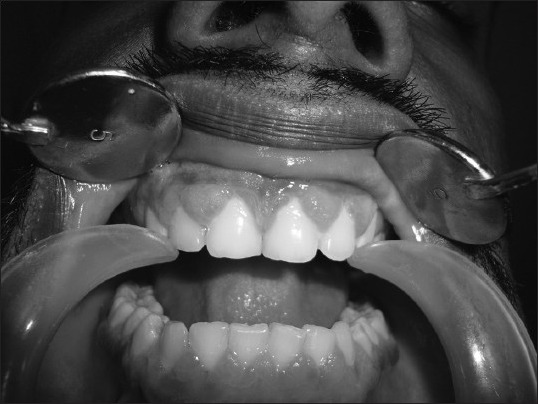
Clinical picture of gingival enlargement in a male epileptic patient (Grade 2) after 6 months of phenytoin therapy
Method of statistical analysis
The statistical analysis was carried out using t-test and the baseline serum folate levels and the serum folate levels obtained after 1 year of phenytoin therapy were correlated with the respective grades of gingival enlargement using Pearson's coefficient formula.
Results
The present study was designed in the Department of Oral Medicine and Radiology, Government Dental College and Research Institute, Bangalore during the period of January 2009 to December 2009 to assess the correlation between phenytoin induced gingival enlargement and serum folate levels. Selected epileptic patients to be enrolled in the study based on defined inclusion and exclusion criteria were explained in detail about the planned study and written informed consents were obtained.
The study consisted of a total of 60 patients with 40 male (66.67%) and 20 female (33.33%) patients. The mean age of the study group was 30.08 years with an age range of 18-50 years. The mean age of the 40 male patients included in the study was 30.26 years with an age range of 18-50 years while for the 20 female patients with an age range of 20-36 years, the mean age was calculated to be 29.5 years.
The study revealed a higher incidence of gingival enlargement in phenytoin treated epileptic patients with the observation of gingival enlargement in all patients in the test group after 1 year of phenytoin administration, though to varying grades. The study also observed serum folic acid levels in selected epileptic patients prior to the start of and after 1 year of phenytoin therapy in addition to the age and sex matched controls. Assessment of serum folic acid level was done by chemiluminiscent method using Immulite kit.
Average serum folate level in the test group in our study was 7.68 ± 2.11ng/mL prior to the start of phenytoin therapy with an average serum folate level of 14.46 ± 2.81 ng/mL for the age and sex matched 40 control samples. The mean difference was calculated as −7.530 with a t-value of −14.94 Table 1 and Graph 1].
Table 1.
Depicting comparison of mean serum folate levels in the test and control groups before initiation of phenytoin treatment

Graph 1.
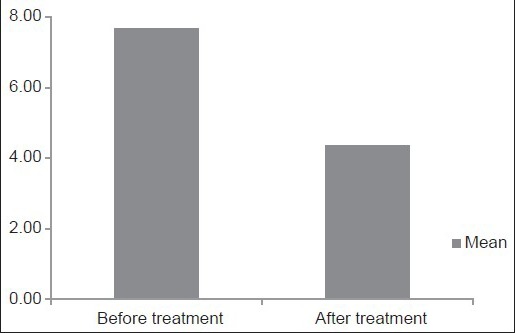
Column diagram depicting comparison of mean serum folate levels in the test and control groups after 1 year of phenytoin treatment
The gingival status was assessed using the index originally described by Angelopoulos and Goaz and later, modified by Miller and Damm, Gingival Overgrowth/GO Index prior to the start of and after 1 year of phenytoin therapy. The average score of gingival enlargement prior to the start of phenytoin therapy in our study was found to be 1.86 ± 0.32 [Table 2] [Graph 2].
Table 2.
Depicting comparison of average grades of gingival enlargement before and after 1 year of phenytoin treatment
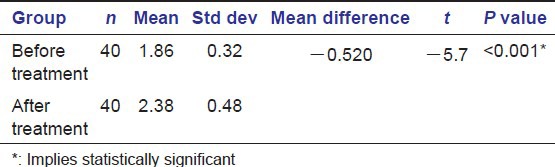
Graph 2.
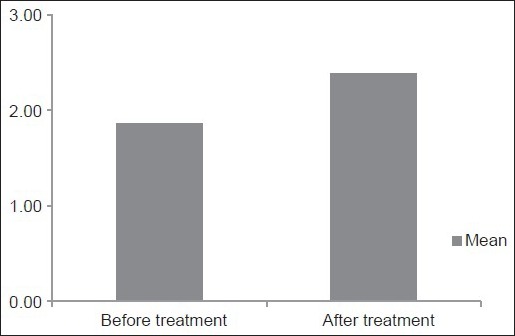
Column diagram depicting comparison of average grades of gingival enlargement before and after 1 year of phenytoin treatment
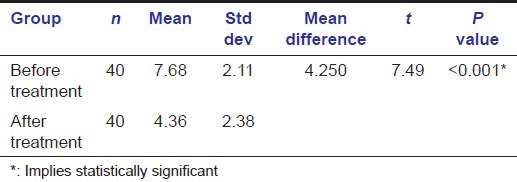
After 1 year of phenytoin therapy, the average serum folate level in the study group was found to be 4.36 ± 2.38 ng/mL with an average serum folate level of 15.82 ± 3.26 ng/mL for the age and sex matched 40 control samples [Table 3]. There was a drop-out of 20 patients during the therapy as the patients didn’t turn-up for the follow-up. Average grade for gingival enlargement after 1 year of phenytoin treatment was found to be 2.38 ± 0.48 [Table 2].
Table 3.
Depicting comparison of mean serum folate levels in the test and control groups after 1 year of phenytoin treatment

The statistical analysis was done using t-test and the baseline serum folate levels and the serum folate levels obtained after 1 year of phenytoin therapy were then correlated with the respective grades of gingival enlargement using Pearson's coefficient formula.
The results arrived found the reduction in mean serum folate levels before and after 1 year of phenytoin treatment to be statistically significant [Tables 1, 3, 4]. The increase in mean gingival enlargement from before to after 1 year of phenytoin therapy was also found to be statistically significant [Table 2]. In either case, the P value came out to be less than 0.001 with the level of significance kept at 0.05 [Tables 2 and 4]. A positive correlation was also noted between the mean serum folate levels and the mean gingival enlargement before and after 1 year of phenytoin treatment.
Table 4.
Depicting comparison of mean serum folate levels in the test group before and after 1 year of phenytoin treatment
Discussion
Despite tremendous advances in the management of epilepsy in the recent decade, the anti-epileptic drug phenytoin still remains the prime drug in the management of epileptic patients in India.[31,32] Chronic administration of phenytoin has been associated with a number of adverse effects.[33,34,35] Phenytoin-induced gingival overgrowth is one such most frequently reported gingival lesion which was first described in 1939.[20,21]
Numerous reports suggest that phenytoin induced gingival enlargement is more commonly seen in younger age groups. This is in concordance with the observations of the several epidemiological studies conducted by Steinberg and Steinberg, 1982, Dahllof and Modeer, 1986, Stinnett et al., 1987 and more recently, Thomason et al., 1992. Also, both genders have been reported to be equally susceptible to phenytoin induced gingival enlargement in the literature.[20] The above mentioned observations were confirmed in our study as well.
The incidence of phenytoin induced gingival enlargement as reported by a study conducted by Kimball was found to be 57% while other studies conducted in relation to incidence of phenytoin induced gingival enlargement have revealed wide incidence ranges of 20-40%[36,37] in some studies to 6-79% in others[33,34,35,36,37,38,39,40,41,42] while 3-93% in few other studies[35,43] and 50% in institutionalized epileptic patients (Seymour, 1993) as reported in the literature. The incidence of gingival overgrowth in the normal population has been reported to be between 4-7.5%.[44] This wide range of variability may be attributed to the small number of the cases reported in some publications to large variations in phenytoin dosages to variations in the length of phenytoin exposure and to differences in the age of the patients included in the various studies as well. The study revealed a high incidence of gingival enlargement in epileptic patients on phenytoin therapy with the observation of varying grades of gingival enlargement in all patients in test group after 1 year of phenytoin administration.
Drug induced gingival enlargement normally begins at the interdental papillae and is more frequently found in the anterior segments of the jaws though it often involves all the surfaces of teeth and is generalized in its distribution.[34,40,41] Gradually, gingival lobulations are formed that may appear inflamed or more fibrotic in nature depending on the degree of local factors’ induced secondary inflammatory changes. All the clinical features of phenytoin induced gingival enlargement were confirmed in our study as well wherein we observed a predominantly firm and fibrotic nature of the gingival enlargement in most of the patients with local factors’ induced secondary inflammatory changes having a minor role to play in the clinical picture of the phenytoin induced lesions of gingival enlargement as the oral hygiene was being meticulously maintained. The observations of our study also revealed that the inter-dental papillae were the most common sites of involvement for the phenytoin induced gingival enlargements. The tissues affected were though not subjected to a detailed histo-pathological analysis as the patients were not subjected to surgical therapeutic options for the treatment that carries a high probability for recurrence.[20,31,34] Also, significant was the observation that the gingival enlargement induced by phenytoin was usually generalized with involvement of all surfaces of the teeth in all the quadrants but was more severe in the anterior segments of the jaws as per the observations of the prior studies possibly because of a relative lack of oral hygiene maintenance in these areas of the jaws.
A review of the gingival enlargement indices proposed in the literature clearly demonstrates their diversity, from the most simple gingival enlargement index proposed to the most elaborate one. Different authors have used different criteria for grading the gingival enlargement in their studies however there is no universal criteria that can be adopted for the same as every criteria has a more or less subjective methodological approach for the assessment of gingival enlargement and depends on the author's discretion for following the same. The majority of the indices used to quantify gingival enlargement are difficult to reproduce because they lack an objective criteria to differentiate between the degree of horizontal and vertical overgrowth.
In our study, the gingival status was assessed using the index originally described by Angelopoulos and Goaz[24,25] and later, modified by Miller and Damm, the Gingival Overgrowth/GO Index.[20] Other criteria for assessing the gingival enlargement were not followed for their being with too extensive methodologies and yet with highly subjective nature of assessment of gingival status. The results obtained however could not be compared with the observations of other studies as the indices followed were either different, modified Harris and Ewalt index in a study conducted by Prasad et al., on the role of folic acid in the prevention of phenytoin induced gingival enlargement on sixty epileptic children in the age range of 8-13 years[30] or even using the same index in a cross sectional study conducted by Brunet et al., on fifty nine patients on anti-epileptic medications using the GO and MB indices[20] owing to the subjectivity of the assessment procedure and a lack of reproducibility with local factors further playing a confounding role in the observations.
In our study, assessment of serum folic acid was done by chemiluminiscent method using Immulite kit prior to the start of and after 1 year of phenytoin therapy. Other methods used to assess serum folate levels that have been described in the literature include the one of immunoassay method and the less reliable and a relatively less sensitive assay of serum folate levels using Lactobacillus casei as the test organism. Average range of serum folate levels in normal controls as standardized by few studies has been found to be 3-17 ng/mL.
Serum folate levels were earlier quantified by means of a radioimmunoassay method using a SimulTRAC Radioassay kit in a study conducted by Majola et al.,[45] involving a total of a hundred and thirty four patients on the factors influencing phenytoin-induced gingival enlargement. The average serum folate levels in the study ranged from1.2 ng/mL to 14.7 ng/mL with a mean value of 4.6 ng/mL.
The mean serum folate level as assayed with the help of Lactobacillus casei method was found to be 4.76 ng/mL in the normal controls and 3.96 ng/mL in the epileptic patients in the study conducted by Reynolds et al.,[46] involving thirty three normal controls, thirty four epileptic outpatients, nineteen of whom also suffered from psychiatric illness, thirty three epileptic inpatients with psychiatric illness and thirty non-epileptic inpatients with psychiatric illness on folate metabolism in epileptic and psychiatric patients.
Serum folate levels were on the other hand found to be 8.8 ± 3.6 ng/mL with a range of 2.9-16.1 ng/mL in controls while 4.1 ± 1.6 ng/mL with a range of 1.2-6.7 ng/mL in sixteen amongst a total of seventy five epileptic patients on phenytoin therapy in the study conducted by Sener Ufuk et al.,[47] on the effects of common anti-epileptic drug mono-therapy on serum levels of homocysteine, vitamin B12, folic acid and vitamin B6. Serum folate levels in this study were measured by the immunoassay method using commercial kit, Immulite, DPC, United States. Normal range of serum folates in healthy adults as estimated by Lactobacillus casei method has been standardized to be 2.5-15 ng/mL.
The wide variations found in the mean and average range of serum folate levels in different age groups and genders from the previous studies likely reflected the differences among the study samples in terms of age and health status as well as differences in the assessment procedures.
While correction of folate deficiency is required in such cases, administration of large doses of folic acid has been seen to decrease blood levels of phenytoin, potentially interfering with seizure control.[48,49,50,51,52,53] The mean decrease in total serum phenytoin level after addition of 1mg of daily folic acid given orally is about 20% while it might reach as high as 40% in case of a daily 5 mg supplementation, a reduction severe enough to lead to breakthrough seizures in a stable epileptic patient. Pharmacokinetic studies of this interaction strongly suggest that folic acid is a co-factor in the metabolism of phenytoin. Higher levels of folic acid appear to increase the affinity of the metabolizing enzymes, thus greatly increasing the efficiency of phenytoin degradation.[53] In addition to interfering with the anti-convulsant drug metabolism, high dose folic acid itself may be epileptogenic, although not confirmed in later studies, the exact mechanism behind this largely remaining obscure. Intra-venous administration of 14.4 mg of folic acid has been seen to induce a tonic-clonic seizure in an epileptic patient although other patients experienced no adverse effects even when administered 75 mg of folic acid in one dose.[28] Precipitation of epileptic seizures has been seen in a female epileptic patient prescribed 0.8 mg of folic acid on a daily basis so as to correct folate deficiency in view of her expected future pregnancy[54] although a dose of 100-1,000 mcg/day has been found by most of the neuro-physicians to be sufficient to prevent folate deficiency without impairing seizure control.[55]
Also, numerous studies in the past suggest the possible role of folic acid in the prevention of phenytoin-induced gingival enlargement as well as its recurrence following a surgical removal.[56,57,58] In a study conducted by Arya et al., a hundred and twenty paediatric patients that developed gingival hyperplasia due to phenytoin use were included and followed for 6 months. During the study, sixty-two patients were treated with folic acid against fifty eight patients who were kept on placebo. Gingival hyperplasia was found to be significantly reduced in patients treated with folic acid. However, a limitation of the study was that folate levels were not measured in either the control or, treatment groups.[59] To add, the results of most of the studies indicate that topical folates lead to a significantly inhibited gingival hyperplasia than either systemic folate or placebo groups.[57] A recent study however has also concluded that systemic folic acid prescribed along with phenytoin reduces the incidence and delays the onset and severity of phenytoin-induced gingival enlargement.[30]
This is however a preliminary study with the results of the study suggesting a higher incidence and severity of gingival enlargement in phenytoin treated epileptic patients with a positive correlation between serum folic acid levels and gingival enlargement before and after 1 year of phenytoin administration. No available published reports with similar methodology have been found in the literature.
Based on this discussion, it can be concluded that modest doses of folic acid can be used to treat folate deficiency in epileptic patients to overcome the associated adverse effects however larger clinical trials with greater sample sizes and an adequate representation of patients from different age groups and sex are required to arrive at a definitive conclusion before folic acid could be declared as a safer and standardized adjuvant to conventional anti-epileptic therapy.
Conclusion
This is a preliminary study which aims at the assessment of serum folate levels in epileptic patients who are on long term phenytoin therapy and their association with phenytoin induced gingival enlargement. The statistical analysis of the results suggests a higher incidence and increased severity of gingival enlargement in epileptic patients on phenytoin therapy; a positive correlation between gingival enlargement and average serum folate levels before and after phenytoin administration; and a significant drop in serum folate levels after 1 year of phenytoin treatment. Thus, the use of folic acid as an adjuvant to phenytoin therapy in the prevention of phenytoin-induced gingival enlargement can be considered but calls for further studies in future keeping in mind the precipitation of epileptic attacks seen in a significant number of patients on folic acid adjuvant therapy secondary to fall in the sera levels of phenytoin due to the propensity for drug interactions between the two. Since this is only a baseline study, the results of the study encourage for further studies with larger sample sizes and estimation of tissue level folates to conclude the results.
Acknowledgments
We thank all the people who directly and indirectly contributed for the study as the study required intense efforts from the people outside our Department including Department of Neurology and Department of Clinical Biochemistry, Bangalore Medical College and Research Institute and Associated Hospitals.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Sridharan R. Epidemiology of epilepsy. Current Science. 2002;82:664–70. [Google Scholar]
- 2.Sridharan R, Murthy BN. Prevalence and pattern of epilepsy in India. Epilepsia. 1999;40:631–6. doi: 10.1111/j.1528-1157.1999.tb05566.x. [DOI] [PubMed] [Google Scholar]
- 3.Blume W, Lüders H, Mizrahi E, et al. Glossary of descriptive terminology for ictal semiology: Report of the ILAE task force on classification and terminology. Epilepsia. 2001;42:1212–8. doi: 10.1046/j.1528-1157.2001.22001.x. [DOI] [PubMed] [Google Scholar]
- 4.Granieri E, Rosati G, Tola R, et al. IBID. 1983;24:502–14. doi: 10.1111/j.1528-1157.1983.tb04921.x. [DOI] [PubMed] [Google Scholar]
- 5.Li SC, Schoenberg BS, Chun-Cheng W, et al. IBID. 1985;26:391–94. [Google Scholar]
- 6.Haerer AF, Anderson DW, Schoenberg BS. IBID. 1986;27:66–75. doi: 10.1111/j.1528-1157.1986.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 7.Osuntokun BO. IBID. 1987;28:272–9. doi: 10.1111/j.1528-1157.1987.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 8.Hauser WA, Annegers JF, Kurland L. IBID. 1991;32:429–45. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 9.Aziz H, Guvener A, Akhtar SW, et al. IBID. 1997;38:716–22. doi: 10.1111/j.1528-1157.1997.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 10.Mani KS, Geeta R, Srinivas HV, et al. The Yelandur study: A community based approach to epilepsy in rural south India: Epidemiological aspects. Seizure. 1998;7:281–88. doi: 10.1016/s1059-1311(98)80019-8. [DOI] [PubMed] [Google Scholar]
- 11.Mani KS. Epidemiology of epilepsy in Karnataka, India. Neurosci Today. 1997;1:167–74. [Google Scholar]
- 12.Cockerell OC, Shorvon SD, editors. London: Current Medical Literature Ltd; 1996. Epilepsy Current Concepts; pp. 1–13. [Google Scholar]
- 13.Placencia M, Shorvon SD, Paredes V, et al. Epileptic seizures in an Andean region of Ecuador: Incidence and prevalence and regional variation. Brain. 1992;115:771–82. doi: 10.1093/brain/115.3.771. [DOI] [PubMed] [Google Scholar]
- 14.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia. 1993;34:453–68. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 15.Biltz H. Constitution of the products of the interaction of substituted carbamides of Benzil. Berl Dtsch Chem Gesamte. 1908;41:1379–93. [Google Scholar]
- 16.Merrit HH, Putman TH. Sodium diphenylhydantoinate in the treatment of convulsive disorders. J Am Med Assoc. 1938;111:1068–73. [Google Scholar]
- 17.Merritt HH, Putnam TJ. Sodium diphenylhydantoinate in the treatment of convulsive seizures: Toxic symptoms and their prevention. Arch Neurol Psychiatry. 1939;42:1053–8. [Google Scholar]
- 18.Merritt HH, Putnam TJ. Sodium diphenylhydantoinate in the treatment of convulsive seizures: Toxic symptoms and their prevention. Trans Am Neurol Assoc. 1939;65:158–62. [Google Scholar]
- 19.Merritt HH, Putnam TJ. Further experiences with the use of sodium diphenylhydantoinate in the treatment of convulsive disorders. Am J Psychiatry. 1940;96:1023–7. [Google Scholar]
- 20.Brunet L, Miranda J, Farré M, et al. Gingival enlargement induced by drugs. Drug Saf. 1996;15:219–31. doi: 10.2165/00002018-199615030-00007. [DOI] [PubMed] [Google Scholar]
- 21.Hassell TM. Epilepsy and the Oral Manifestations of Phenytoin Therapy. In: Myers H, editor. Monographs in Oral Science. Basel: S, Karger; 1981. [PubMed] [Google Scholar]
- 22.Brown RS, Sein P, Corio R, et al. Nitrendipine- induced gingival hyperplasia: First case report. Oral Surg Oral Med Oral Pathol. 1990;10:533–6. doi: 10.1016/0030-4220(90)90406-i. [DOI] [PubMed] [Google Scholar]
- 23.Whitehead N, Reyner F, Lindernbaum J. Megaloblastic changes in the cervical epithelium. J Am Med Assoc. 1973;226:1421–4. [PubMed] [Google Scholar]
- 24.Angelpoulous AP, Goaz PW. Incidence of diphenylhydantoin gingival hyperplasia. Oral Surg Oral Med Oral Pathol. 1972;34:898–906. doi: 10.1016/0030-4220(72)90228-9. [DOI] [PubMed] [Google Scholar]
- 25.Angelopoulos AP. Diphenhydantoin gingival hyperplasia: A clinico-pathological review of incidence, clinical features and histopathology. J Can Dent Assoc. 1975;41:103–6. [PubMed] [Google Scholar]
- 26.Vogel RI. Gingival hyperplasia and folic acid deficiency from anticonvulsive drug therapy: A theoretical relationship. J Theor Biol. 1977;67:269–78. doi: 10.1016/0022-5193(77)90199-0. [DOI] [PubMed] [Google Scholar]
- 27.Berg MJ, Rivey MP, Vern BA, et al. Phenytoin and folic acid: Individualized drug-drug interaction. Ther Drug Monit. 1983;5:395–9. doi: 10.1097/00007691-198312000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Ch’ien LT, Krumdieck CL, Scott C-WJ, et al. Harmful effect of mega-doses of vitamins: Electroencephalogram abnormalities and seizures induced by intra-venous folate in drug-treated epileptics. Am J ClinNutr. 1975;28:51–8. doi: 10.1093/ajcn/28.1.51. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds EH. Effects of folic acid on the mental state and fit-frequency of drug-treated epileptic patients. Lancet. 1967;1:1086–8. doi: 10.1016/s0140-6736(67)92654-2. [DOI] [PubMed] [Google Scholar]
- 30.Prasad VN, Chawla HS, Goyal A, et al. Folic acid and phenytoin induced gingival overgrowth-Is there a preventive effect. J Indian Soc Pedo Prev Dent. 2004;22:82–91. [PubMed] [Google Scholar]
- 31.Hassessian A, Marcucci G, Guimarães J., Júnior Freqüência da hyperplasia gengival medicamentosa em 48 pacientes tratados com nifedipina. Revista Abo Nacional, Rio de Janeiro. 2003;11:28–32. [Google Scholar]
- 32.Scheinfeld N. Phenytoin in cutaneous medicine: Its uses, mechanisms and side effects. Dermatol Online J. 2003;9:6. [PubMed] [Google Scholar]
- 33.Ciancio SG. Gingival hyperplasia and diphenylhydantoin. J Perio. 1972;43:411. doi: 10.1902/jop.1972.43.7.411. [DOI] [PubMed] [Google Scholar]
- 34.Hassell TM, Burtner A Paul, McNeal Donald, et al. Hypertrophic Oral problems and genetic aspects of individuals with epilepsy. Periodontol. 2000;6:68. doi: 10.1111/j.1600-0757.1994.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 35.Kapur RN, Girgis S, Little TM, et al. Diphenylhydantoin-induced gingival hypertrophy and its relationship to dose and serum level. Dev Med Child Neurol. 1973;15:483–7. doi: 10.1111/j.1469-8749.1973.tb05070.x. [DOI] [PubMed] [Google Scholar]
- 36.Cals MJ, Bories PN, Devanlay M, et al. Extensive laboratory assessment of nutritional status in fit, health-conscious, elderly people living in the Paris area. J Am Coll Nutr. 1994;6:646–57. doi: 10.1080/07315724.1994.10718461. [DOI] [PubMed] [Google Scholar]
- 37.Weggemans RM, de Groot LCPGM, Haller J. Factors related to plasma folate and vitamin B12. The Senega study. Int J Food Sci Nutr. 1997;48:141–50. doi: 10.3109/09637489709006974. [DOI] [PubMed] [Google Scholar]
- 38.Benton D, Haller J, Fordy J. The vitamin status of young British adults. Int J Vit Nutr Res. 1997;67:34–40. [PubMed] [Google Scholar]
- 39.Ferro-Luzzi A, Mobarhan S, Maiani G, et al. Habitual alcohol consumption and nutritional status of the elderly. Eur J Clin Nutr. 1988;42:5–13. [PubMed] [Google Scholar]
- 40.Hallmon WW, Rossmann JA. The role of drugs in the pathogenesis of gingival overgrowth: A collective review of current concepts. Periodontol. 1999;21:176–96. doi: 10.1111/j.1600-0757.1999.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 41.Marshall RI, Bartold PM. A clinical review of drug induced gingival overgrowth. Aust Dent J. 1999;44:219–32. doi: 10.1111/j.1834-7819.1999.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg IH, Bowman BB, Cooper BA, et al. Folate nutrition in the elderly. Am J Clin Nutr. 1982;36:1060–6. doi: 10.1093/ajcn/36.5.1060. [DOI] [PubMed] [Google Scholar]
- 43.Lennox WG. The drug therapy of epilepsy. J Am Med Assoc. 1940;114:1347–54. [Google Scholar]
- 44.Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 45.Majola MP, Mc Fadyen ML, Connolly C, et al. Factors influencing phenytoin-induced gingival enlargement. J Clin Periodontol. 2000;27:506–12. doi: 10.1034/j.1600-051x.2000.027007506.x. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds EH, Preece J, Johnson AL. Folate metabolism in epileptic and psychiatric patients. J Neurol Neurosurg Psychiat. 1971;34:726–32. doi: 10.1136/jnnp.34.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sener Ufuk, Yasar Zorlu, Karaguzel Oguz, et al. Effects of common anti-epileptic drug mono-therapy on serum levels of homocysteine, Vitamin B 12 , folic acid and Vitamin B 6. Seizure. 2006;15:79–85. doi: 10.1016/j.seizure.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Furlanut M, Benetello P, Avogaro A, et al. Effects of folic acid on phenytoin kinetics in healthy subjects. Clin Pharmacol Ther. 1978;24:294–7. doi: 10.1002/cpt1978243294. [DOI] [PubMed] [Google Scholar]
- 49.Gaby AR. Natural approaches to epilepsy. Altern Med Rev. 2007;12:9–24. [PubMed] [Google Scholar]
- 50.Mattson RH, Gallagher BB, Reynolds EH, et al. Folate therapy in epilepsy: A controlled study. Arch Neurol. 1973;29:78–81. doi: 10.1001/archneur.1973.00490260022002. [DOI] [PubMed] [Google Scholar]
- 51.O’Hare J, O’Driscoll D, Duggan B, et al. Increase in seizure frequency following folic acid. J Ir Med Assoc. 1979;72:241–242. [Google Scholar]
- 52.Seligmann H, Potasman I, Weller B, et al. Phenytoin-folic acid interaction: A lesson to be learnt. Clin Neuropharmacol. 1999;22:226–31. [PubMed] [Google Scholar]
- 53.Steinweg DL, Bentley ML. Seizures following reduction in phenytoin level after orally administered folic acid. Neurol. 2005;64:82. doi: 10.1212/01.WNL.0000163997.67472.C5. [DOI] [PubMed] [Google Scholar]
- 54.Guidolin L, Vignoli A, Canger R. Worsening in seizure frequency and severity in relation to folic acid administration. Eur J Neurol. 1998;5:301–3. doi: 10.1046/j.1468-1331.1998.530301.x. [DOI] [PubMed] [Google Scholar]
- 55.Hiilesmaa VK, Teramo K, Granstrom ML, et al. Serum folate concentrations during pregnancy in women with epilepsy: Relation to anti-epileptic drug concentration, number of seizures, and fetal outcome. Br Med J. 1983;287:577–99. doi: 10.1136/bmj.287.6392.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown RS, Di Stanislao PT, Beaver WT, et al. The administration of folic acid to institutionalized epileptic adults with phenytoin-induced gingival hyperplasia: A double-blind, randomized, placebo-controlled, parallel study. Oral Surg Oral Med Oral Pathol. 1991;71:565–8. doi: 10.1016/0030-4220(91)90363-h. [DOI] [PubMed] [Google Scholar]
- 57.Drew HJ, Vogel RI, Molofsky W, et al. Effect of folate on phenytoin hyperplasia. J Clin Periodontol. 1987;14:350–6. doi: 10.1111/j.1600-051x.1987.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 58.Poppell TD, Keeling SD, Collins JF, et al. Effect of folic acid on recurrence of phenytoin-induced gingival overgrowth following gingivectomy. J Clin Periodontol. 1991;18:134–9. doi: 10.1111/j.1600-051x.1991.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 59.Arya R, Gulati S, Kabra M, et al. Folic acid supplementation prevents phenytoin-induced gingival overgrowth in children. Neurol. 2011;76:1338–43. doi: 10.1212/WNL.0b013e3182152844. [DOI] [PMC free article] [PubMed] [Google Scholar]


