Abstract
Aim
The aim of this study is to validate high-resolution endovaginal T2- and diffusion-weighted MRI measurements (tumour size, volume and length of uninvolved cervical canal) against histology in patients undergoing trachelectomy.
Patients/interventions
55 consecutive patients 25–44 years with cervical cancer being considered for trachelectomy were prospectively assessed with endovaginal T2-W and diffusion-weighted MRI. Two independent observers blinded to histology recorded maximum tumour dimension, volume and distance from the superior aspect of the tumour to the internal os. Following trachelectomy, pathologist-outlined tumour sections were photographed with a set scale and similar measurements were recorded.
Results
Fifteen of 45 patients subsequently treated with fertility-sparing surgery had residual tumour (median histological volume: 0.28 cm3, IQR = 0.14–1.06 cm3). Sensitivity, specificity, positive and negative predictive values for detecting tumour: Observer1: 86.7%, 80.0%, 68.4%, and 92.3%, respectively; Observer2: 86.7%, 90.0%, 81.0%, and 93.1%, respectively. Size and volume correlated between observers (r = 0.96, 0.84, respectively, p < 0.0001). Size correlated between each observer and histology (observer 1 r = 0.91, p < 0.0001; observer 2 r = 0.93, p < 0.0001), volume did not (observer 1: r = 0.08, p = 0.6; observer 2: r = 0.21, p = 0.16); however, differences between observer measurements and histology were not significant (size p = 0.09, volume p = 0.15). Differences between MRI and histology estimates of endocervical canal length were not significant (p = 0.1 both observers).
Conclusion
In subcentimetre cervical cancers, endovaginal MRI correlates with pathology and is invaluable in assessing patients for fertility-sparing surgery.
Keywords: MRI, Endovaginal, Cervical cancer, Fertility-sparing, Trachelectomy, Volume
Highlights
-
•
High spatial resolution endovaginal MRI of the cervix in patients potentially selected for trachelectomy
-
•
Correlation of MRI with histopathology in subcentimetre cervical tumours
-
•
Interobserver comparison of measured tumour maximum dimension and volume on endovaginal MRI
Introduction
With the introduction of screening programmes in the western world, cancer of the cervix is being diagnosed at an increasingly early stage. In women of a reproductive age, this has increased the demand for fertility-sparing surgical options such as extended cone biopsy and trachelectomy. The former is usually confined to treating cervical intra-epithelial neoplasia (CIN), carcinoma in situ or localised cases of micro-invasive disease (stage 1A) with no lympho-vascular space invasion (LVSI) [1,2]. If disease is more extensive but < 2 cm in maximum dimension, trachelectomy (pioneered by Dargent et al. [3] and modified by Shepherd et al. [4]) is considered which involves cervical amputation and excision of extracervical (lower parametrial) tissue with preservation of the uterine body and ovaries making subsequent pregnancy possible. A cuff of vaginal tissue (1–2 cm) is removed in addition to the cervix and a primary vagino-isthmic/internal os anastomosis created. During the procedure a frozen section of the superior (apical) resection margin is sent to confirm a minimum of 5 mm tumour clearance. When patient selection is vigilant, radical vaginal trachelectomy with pelvic lymph node dissection is similar in efficacy in terms of oncological outcome to radical hysterectomy with no significant differences in 5-year survival, 5-year progression-free survival rate and recurrence rates [5–7].
In patients selected for trachelectomy, it is preferable to leave some healthy cervical stroma in situ so that the risks of cervical incompetence, infection, premature rupture of membranes and premature delivery in subsequent pregnancies are reduced [8]. Most surgeons require 1 cm of tumour free cervix proximal to the tumour but some will accept 5–7 mm. Therefore, pre-operative assessment with MRI to determine the maximum tumour diameter, volume and degree of endocervical extension is of major importance [9–11]. In these small tumours (< 1 cm in maximum dimension) accuracy of detecting tumours is improved using endovaginal T2-W MRI at 1.5 T [12–15] with further improvements evident when diffusion-weighted techniques are added [16]. However, correlation of tumour volume and length of uninvolved endocervical canal in patients undergoing trachelectomy has not been undertaken. Potentially also, extension of endovaginal T2-W and diffusion-weighted techniques to 3.0 T offers further improvements in spatial resolution. The purpose of this study, therefore, was to compare MRI derived measurements of tumour size/volume and length of uninvolved endocervical canal on T2-weighted images viewed in conjunction with diffusion weighted sequences acquired using an endovaginal technique at 3.0 T with measurements made on trachelectomy specimens in order to establish the validity of the preoperative MRI assessment.
Methods
Patients
Over a 25 month period 55 consecutive patients aged 25–44 years (mean ± SD = 30.5 ± 4.9 years) with stage I cervical cancer diagnosed on smear test or who presented with irregular bleeding or abnormal vaginal discharge being considered for fertility-sparing surgery were prospectively assessed with endovaginal MRI. Fifty patients had a diagnostic large loop excision of the transformation zone (LLETZ) or cone biopsy an average of 5.5 weeks (SD ± 2.8 weeks, range 0.5–13 weeks) prior to the MRI study, at which all of the tumour may have been excised.
Image acquisition
Endovaginal images were obtained on a 3.0-T Philips Achieva using a 37 mm ring design solenoidal receiver coil similar to the one previously described for use at 1.5-T [17] but engineered for 3.0 T. The coil was inserted following digital vaginal examination and positioned around the cervix. Air in the vagina introduced during coil insertion was aspirated via a 4-mm diameter tube (Ryles; Pennine Healthcare, London, England) to reduce susceptibility-based artefacts occurring at the air–tissue interface. Each patient was examined supine and coil immobilization achieved using an externally sited clamp with a stand placed between the patients' thighs. Hyoscine butyl bromide (Buscopan) 20 mg IM was administered to reduce artefact from bowel motion.
T2-W images (TR/TE = 4500/80 ms, acquisition matrix = 240, field of view = 100 mm, section thickness = 2 mm with no intersection gap) were obtained in three orthogonal planes to the cervix (sagittal, coronal and transverse) with a 0.42 mm in-plane resolution and 0.36 mm3 voxel size (0.25 mm3 reconstructed). Zonal Oblique Multi-slice (ZOOM) diffusion-weighted (DW) echo-planar images (TR/TE 6500/90, acquisition matrix = 80, field of view = 100 mm) employing b values of 0, 100, 300, 500, and 800 s/mm2 were obtained in three planes to match the T2-W images. In plane resolution = 1.25 mm, voxel size = 3.15 mm3 (0.41 mm3 reconstructed). Twenty four 2 mm thick sections provided coverage of the cervix (acquisition time 4 min 33 s). Isotropic ADC maps were generated with Phillips system software using all b values. Following endovaginal imaging, the internal receiver coil was removed and large field of view images through the abdomen and pelvis was obtained for assessment of the lymph nodes that did not form part of this study.
Image analysis
T2-W and Zoom-DW images were assessed in combination for the presence of tumour (intermediate signal-intensity focal mass on T2-W imaging corresponding to a focal area of restriction on ZOOM-DW imaging) by 2 observers in consensus (1 with 20 years' experience of endovaginal MRI and 1 with 2 years' experience). In patients in whom visible tumour was present on endovaginal MRI, and who subsequently proceeded to fertility-sparing surgery (further knife-cone biopsy or trachelectomy), both observers on separate occasions independently recorded maximum tumour dimensions using an in built 3.0-T workstation measuring tool and drawn on either the sagittal, coronal or axial T2-weighted images with reference to the b = 800 s/mm2 diffusion weighted images and corresponding ADC map (Fig. 1). Tumour volumes were also recorded by drawing regions of interest (ROIs) around the tumour on T2-weighted images in either the sagittal or coronal planes (depending on which plane the tumour was most easily visible) with reference to the b = 800 s/mm2 diffusion weighted images and corresponding ADC map and multiplying the summed area of the tumour ROI on each slice by the slice thickness. In addition, in these patients, the distance from the superior aspect of the tumour to the internal os was recorded on the sagittal T2-weighted images with reference to the corresponding ADC map using the workstation measuring tool (Fig. 2). All measurements were made blinded to the histological findings.
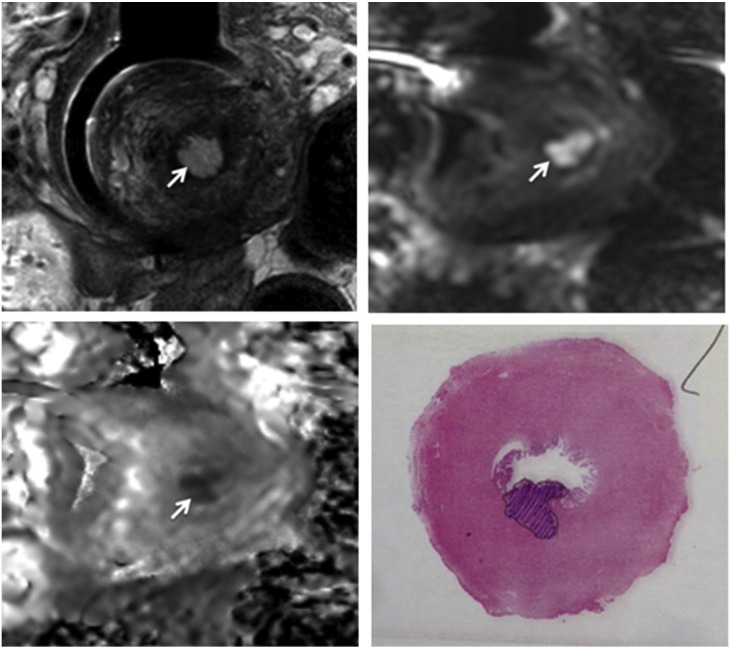
Fig. 1.
Coronal T2-W (left), and corresponding b-800 s/mm2 (middle) and ADC map (right) slices showing a small tumour on the left cervical lip (arrows).
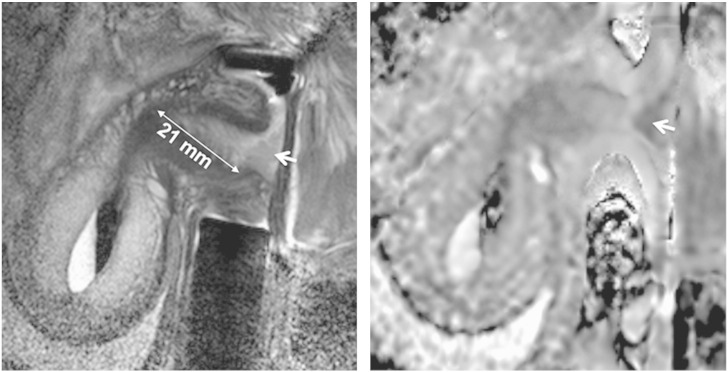
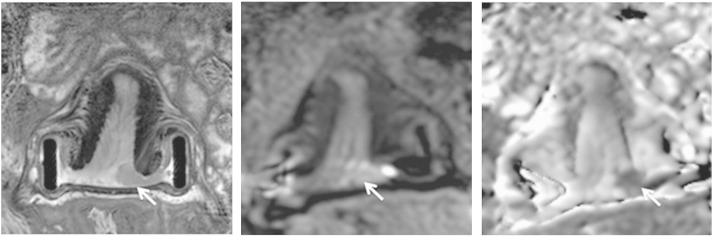
Fig. 2.
Measuring length of normal endocervical canal above tumour on sagittal T2-W images (left) with sagittal ADC maps (right) for reference. Tumour is seen an intermediate signal-intensity mass in A and an area of restricted diffusion on the ADC map (arrows).
Histopathological analysis
All specimens were fixed in formalin and the entire specimen submitted for histology in the following manner: a shave of the proximal endocervical resection margin (at or near the internal os) was obtained and sliced longitudinally (usually in the sagittal plane centrally and the coronal plane laterally). The cervix together with the parametrium was then sliced into transverse sections up to approximately 1 cm from the ectocervix. The remaining 1 cm of distal cervix together with vaginal cuff was sectioned longitudinally (usually in the sagittal plane centrally and the coronal plane laterally). All slices (each 3 to 4 mm thick) thus obtained were processed and embedded in paraffin. Two to three micron sections (obtained from the paraffin blocks on to glass slides) were deparaffinised and stained with haematoxylin and eosin. According to the thickness of the slice obtained at macroscopy, each histology slide was separated from the next by 3 to 4 mm.
Tumour volumes were calculated on trachelectomy histopathology specimens using a technique similar to that used for imaging sections. Slides that contained tumour had tumour outlined by a gynaecological pathologist (AA or SH). The distance from the superior aspect of the tumour to the proximal resection margin was measured and recorded. Slides then were scanned or photographed at high resolution with an overlying set scale (Canon CanoscanLiDE 200 MP Navigator EX 2.0 or Nikon D100) and the images transferred to a computer. Images were viewed in Adobe Photoshop (CS3 Extended) and the area of the tumour ROI (mm2) on each slice where it was visible was multiplied by the slide interval measurement (4 mm) to obtain a volume in cm3 (Fig. 3).
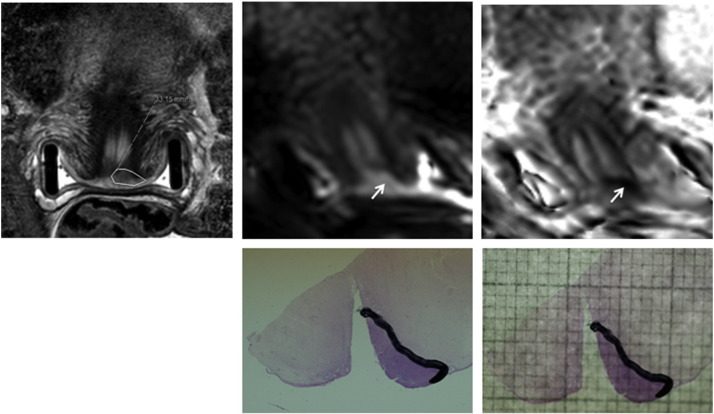
Fig. 3.
Assessing tumour volume on histology. Coronal T2-W (top left), b-800 s/mm2 diffusion-weighted (top middle) and ADC map (top right) images through the mid cervix. Tumour is outlined in T2-W with reference to diffusion-weighted images (arrow). The trachelectomy specimen with the tumour delineated (bottom left) is photographed with an overlying millimetre grid (bottom right) and the area in mm2 calculated.
Statistical analysis
The sensitivity, specificity, positive and negative predictive values for determining tumour in a population referred for fertility-sparing surgery were determined. Maximum dimension of tumour, tumour volume and the tumour to internal os distance on MRI measured by independent observers were correlated between observers and between each observer and histology measurements using a Pearson's correlation coefficient where zero indicates no linear relationship between the readings and 1.0 reflects a perfect linear relationship. Differences between imaging and histology measurements were recorded using a paired t-test. A p value of less than 0.05 was used to indicate significance.
Results
Of the 55 patients referred for consideration of fertility sparing treatment, 25 patients had a radical trachelectomy (1 after a further knife cone), 19 patients had extended cone biopsy, 1 had a LLETZ, 3 had hysterectomy (2 lesion too large for trachelectomy, 1 endometriosis) and 7 went for chemoradiotherapy (6 staged as 2b, 1 neuroendocrine histology).
Of the 45 patients that underwent fertility-sparing surgery, 15 patients had residual tumour (13 at trachelectomy (Fig. 4), 1 at cone biopsy after MRI that was followed by a negative trachelectomy and 1 on extended cone biopsy only). Of these, 2 had no visible tumour on MRI and tumour volume was recorded as zero. Six patients had tumour recorded at MRI by observer 1 and 3 by observer 2, who were negative on histology. Sensitivity and specificity for detecting tumour in the cohort treated with fertility sparing surgery were 86.7% and 80.0%, respectively (PPV 68.4%, NPV 92.3%) for observer 1 and 86.7% and 90% respectively (PPV 81.0%, NPV 93.1%) for observer 2.
Fig. 4.
Transverse T2-W (top left), b-800 s/mm2 (top right) and ADC map (bottom left) showing a small tumour in the posterior endocervix (arrows) that correlates with that shown on the trachelectomy specimen (bottom right).
Tumour size
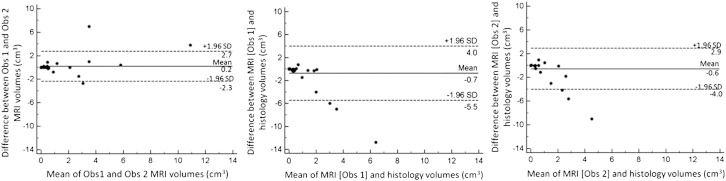
In patients treated with fertility sparing surgery, maximum tumour dimensions on histology in the 15 patients positive for tumour (13 true positive [TP], 2 false negative [FN]) ranged from 1.5 mm to 30.0 mm (mean ± SD 11.3 ± 7.6 mm, median 10.0 mm, IQR 6.1–14.8 mm). Corresponding MRI measurements were 0–38 mm (mean 14.2 ± 10.5 mm, median 14.0 mm, IQR 8.5–18.5 mm) (observer 1) and 0 mm to 30.0 mm (mean ± SD = 13.3 ± 8.7 mm, median 12.0 mm, IQR 7.8–18.9 mm) (observer 2). Detailed data were provided in Supplemental material Table 1. For the whole cohort, the Pearson's correlation coefficient for maximum dimension of lesion between observers was 0.96. The correlation between MRI and histology was 0.91 (observer 1) and 0.93 (observer 2) (p = < 0.0001). Bland–Altman plots comparing differences in tumour maximal dimension between observers, and each observer with histology are given in Fig. 5. Paired t-tests indicated no significant differences between measurements by observer 1 and histology (p = 0.09) or observer 2 and histology (p = 0.15).
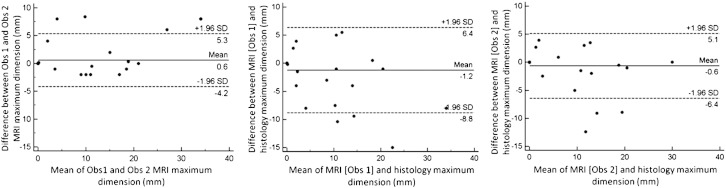
Fig. 5.
Bland–Altman plots of variability in assessment of maximal tumour dimension between observers (left), between observer 1 and histology (middle) and between observer 2 and histology (right). Dashed lines show the limits of agreement (95% confidence intervals).
Tumour volumetry
In the 15 cases positive for tumour (13 TP, 2FN), volumetry on histology was possible in 13 (as 1 trachelectomy and 1 patient with knife cone were operated elsewhere and the specimens were not available for analysis). In these 13 cases tumour volumes on histology ranged from 0.012 cm3 to 2.0 cm3 (mean ± SD 0.62 ± 0.67 cm3, median 0.28 cm3, IQR = 0.14–1.06 cm3). Corresponding MRI measurements were 0–2.1 cm3 (mean ± SD 0.75 ± 0.72 cm3, median 0.5 cm3, IQR = 0.3–1.2 cm3) (observer 1) and 0 cm3 to 4.4 cm3 (mean ± SD 0.97 ± 1.3 cm3, median 0.35 cm3, IQR 0.13–1.04 cm3) (observer 2). Detailed data were provided in Supplemental material Table 1. For the whole cohort, the Pearson's correlation coefficient for tumour volume was 0.84 between observers p = < 0.0001. Although there was no significant difference between the volume measurements by either observer and those on histology (paired t-test, p = 0.2 for both observers), there was no significant correlation demonstrated between MRI measured volumes and those on histology (r = 0.08, p = 0.6, observer 1 and r = 0.21, p = 0.16, observer 2). Bland–Altman plots comparing differences in tumour volume between observers, and each observer with histology are given in Fig. 6.
Fig. 6.
Bland–Altman plots of variability in assessment of tumour volume between observers (left), between observer 1 and histology (middle) and between observer 2 and histology (right). Dashed lines show the limits of agreement (95% confidence intervals).
Assessment of endocervical canal
Comparison between MRI and histology estimates of endocervical canal length was done in 19 cases who underwent trachelectomy and in whom tumour was identified on MRI or histology (12 TP, 2 FN, 5 false positive [FP]); in one TP and 1 FP case who underwent knife cone, this measurement could not be made. In true negative cases the clearance of margins was not an issue. In these 19 cases, the length of the endocervical canal (distance from the proximal aspect of the tumour to the internal os) ranged from 8.0 to 32.0 mm (mean ± SD 19.0 ± 5.7 mm, median 20.0 mm, IQR 15.5–22.0 mm) (observer 1) and 6.0 mm to 27.0 mm (mean ± SD 18.9 ± 5.6 mm, median 19.6 mm, IQR 17.1–21.6 mm) (observer 2). Measurements on histopathology specimens for these patients from superior aspect of tumour to resection margin ranged from 4.0 mm to 34.0 mm (mean ± SD 15.5 ± 8.5 mm, median 17.5 mm, IQR 6.5–21.0 mm). There was no significant difference between MRI and histology measurements for length of endocervical canal (paired t-test p = 0.1 for both observers). Detailed data provided in Supplemental material Table 2.
Discussion
This study shows that measurements of tumour size and volume in subcentimetre cervical cancers made on endovaginal T2-W images in conjunction with DW images agree well between observers and with the measurements made on the pathological specimen. This technique is therefore ideal for pre-operative planning in patients with stage Ia2/Ib1 cervical cancer where fertility sparing procedures are being considered.
The excellent correlation in maximum tumour dimensions on MRI with subsequent histopathological examination is in keeping with previous studies where external pelvic array MRI has measured tumours of larger size (70–93% accuracy to within 0.5 cm) in cohorts of patients undergoing hysterectomy [18,19]. The increased signal-to-noise ratio provided by an endovaginal receiver coil enables improved resolution in order to identify and evaluate subcentimetre lesions. Similarly, the volume at MRI also correlated significantly with histopathology for both observers in the 13 TP and 2 FN cases. In all but 3 cases tumour volumes were larger in size and volume on endovaginal MRI than at histology; 2 of these cases were falsely negative. In the third case where the histological volume was larger than on MRI, the time interval from MRI to surgery was 59 days. It is remarkable that, in the remaining 12, despite a mean time interval between endovaginal MRI and surgery of 41 days (range 9–89 days), tumour size/volume on histopathology did not increase to greater than that measured on MRI.
The overestimation of tumour diameter on MRI compared to pathology in previous studies (up to 19%) has been partly attributed to specimen shrinkage on formalin fixation [20,21]. Ex vivo volume change in surgical specimens is complicated. In the first instance, loss in blood volume occurs. It also is known that resected tissue specimens containing non-small cell lung cancer, breast cancer, vulvar and head and neck cancers shrink post formalin fixation [22–24] as do normal colorectal tissue specimens. It has been shown however that lateral resection margins in resected rectal cancer specimens increase in diameter and normal muscles of porcine models expand after formalin fixation whilst the fat of the same models shrink [25]. In the normal fibro-muscular cervix there are ex vivo changes both due to formalin fixation leading to dehydration (indeed retraction pockets of histopathological ‘retraction artefact’ were demonstrated in some tumours at histopathological assessment where the stroma had contracted away from the epithelium) but also due to the process of paraffin wax embedding, section cutting and section mounting. Boonstra et al. found that there was shrinkage of 2.7% in specimens following formalin fixation and a change from original dimensions by 12.6% in specimens following paraffin wax embedding, cutting and mounting. It is postulated that the second value is not due to shrinkage per se but deformation caused by pressure on the tissue during sectioning. The dimensions decreased in the cutting direction and increased in a direction that was perpendicular to this [26].
Another explanation for the discrepancy between MRI and histological findings is the inability to differentiate tumour from peritumoural inflammatory tissue that is often increased following a diagnostic cone biopsy [16]. However, the inclusion of inflammatory tissue on estimates of lesion size/volume can be problematic on both MRI and histopathology estimates. The addition of diffusion-weighted to T2-weighted images should enable distinction between post biopsy change (such as oedema, granulation tissue and fibrosis) and tumour, because in the former case, tissue does not show diffusion restriction, whereas restriction of water diffusion is characteristic of tumour tissue [27,28]. Nevertheless, the median percentage difference between MRI and histology in lesion size (26.7%) and volume (17.6%) in our cohort for observer 1 was similar to previously reported data, although observer 2 did considerably better (median 5% and 5% respectively).
Involvement of the internal os on MRI is poorly documented in previous literature [29]. In our study, tumour was not documented beyond the internal os on MRI in any case and these findings were confirmed at histology. The exact distance in millimetres from the proximal aspect of the tumour to the internal os at endovaginal MRI evaluation did not however correlate well with subsequent histology because surgical resection aimed to leave a rim of normal cervix in situ where possible to reduce the risk of miscarriage of subsequent pregnancies. The amount of this rim was variable and dependent on patient anatomy. Although comparisons of endocervical canal length would best be made on hysterectomy specimens in order to accurately assess the cervical–endometrial junction on histology, tumours in this population are larger, easier to define on external coil MRI and often extend to the internal os, making them an unsuitable population for endovaginal MRI measurements. Alternatively, validation of measurement of endocervical canal length on endovaginal MRI could be done in patients with benign disease due for hysterectomy by comparison with macroscopic histologic sections.
A major limitation to this study was that there were small patient numbers with residual disease. In many cases, most (if not all) of the tumour was removed at previous cone biopsy. Previous reports indicate that 65% of patients do not have any residual cancer in trachelectomy specimens after diagnostic cone biopsies [4,30]. Of the 45 patients referred for trachelectomy who had subsequent surgery (and therefore histopathology for assessment) in this cohort, 15 patients (33%) had visible tumour on MRI. Whilst the negative predictive value is also crucial for management decisions, it means that to achieve a particular cohort size with residual disease, three times as many cases would need to be studied.
This study demonstrates that in the assessment of subcentimetre cervical cancers, endovaginal MRI using T2-W and diffusion-weighted imaging has a high sensitivity and specificity for experienced as well as inexperienced observers with good agreement between them for measurements of lesion size and volume. Correlation between MRI and histology is similar to that of other studies with larger tumours that do not use endovaginal imaging. Endovaginal MRI to define tumour size and volume is likely to be of major utility in the selection of candidates for fertility sparing treatment.
Conflict of interest statement
None of the authors have any financial relationships or conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary tables.
References
- 1.Fagotti A., Gagliardi M.L., Moruzzi C., Carone V., Scambia G., Fanfani F. Excisional cone as fertility-sparing treatment in early-stage cervical cancer. Fertil Steril. 2011;95:1109–1112. doi: 10.1016/j.fertnstert.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Morris M., Mitchell M.F., Silva E.G., Copeland L.J., Gershenson D.M. Cervical conization as definitive therapy for early invasive squamous carcinoma of the cervix. Gynecol Oncol. 1993;51:193–196. doi: 10.1006/gyno.1993.1271. [DOI] [PubMed] [Google Scholar]
- 3.Dargent B., Paillart C., Carlier E., Alcaraz G., Martin-Eauclaire M.F., Couraud F. Sodium channel internalization in developing neurons. Neuron. 1994;13:683–690. doi: 10.1016/0896-6273(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd J.H., Spencer C., Herod J., Ind T.E. Radical vaginal trachelectomy as a fertility-sparing procedure in women with early-stage cervical cancer-cumulative pregnancy rate in a series of 123 women. BJOG. 2006;113:719–724. doi: 10.1111/j.1471-0528.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 5.Beiner M.E., Hauspy J., Rosen B., Murphy J., Laframboise S., Nofech-Mozes S. Radical vaginal trachelectomy vs. radical hysterectomy for small early stage cervical cancer: a matched case–control study. Gynecol Oncol. 2008;110:168–171. doi: 10.1016/j.ygyno.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Covens A., Shaw P., Murphy J., DePetrillo D., Lickrish G., Laframboise S. Is radical trachelectomy a safe alternative to radical hysterectomy for patients with stage IA-B carcinoma of the cervix? Cancer. 1999;86:2273–2279. doi: 10.1002/(sici)1097-0142(19991201)86:11<2273::aid-cncr15>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Marchiole P., Benchaib M., Buenerd A., Lazlo E., Dargent D., Mathevet P. Oncological safety of laparoscopic-assisted vaginal radical trachelectomy (LARVT or Dargent's operation): a comparative study with laparoscopic-assisted vaginal radical hysterectomy (LARVH) Gynecol Oncol. 2007;106:132–141. doi: 10.1016/j.ygyno.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Rob L., Skapa P., Robova H. Fertility-sparing surgery in patients with cervical cancer. Lancet Oncol. 2011;12:192–200. doi: 10.1016/S1470-2045(10)70084-X. [DOI] [PubMed] [Google Scholar]
- 9.Lakhman Y., Akin O., Park K.J., Sarasohn D.M., Zheng J., Goldman D.A. Stage IB1 cervical cancer: role of preoperative MR imaging in selection of patients for fertility-sparing radical trachelectomy. Radiology. 2013;269:149–158. doi: 10.1148/radiol.13121746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milliken D.A., Shepherd J.H. Fertility preserving surgery for carcinoma of the cervix. Curr Opin Oncol. 2008;20:575–580. doi: 10.1097/CCO.0b013e32830b0dc2. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd J.H. Cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2012;26:293–309. doi: 10.1016/j.bpobgyn.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 12.deSouza N.M., Dina R., McIndoe G.A., Soutter W.P. Cervical cancer: value of an endovaginal coil magnetic resonance imaging technique in detecting small volume disease and assessing parametrial extension. Gynecol Oncol. 2006;102:80–85. doi: 10.1016/j.ygyno.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 13.deSouza N.M., McIndoe G.A., Soutter W.P., Krausz T., Chui K.M., Hughes C. Value of magnetic resonance imaging with an endovaginal receiver coil in the pre-operative assessment of stage I and IIa cervical neoplasia. Br J Obstet Gynaecol. 1998;105:500–507. doi: 10.1111/j.1471-0528.1998.tb10149.x. [DOI] [PubMed] [Google Scholar]
- 14.deSouza N.M., Scoones D., Krausz T., Gilderdale D.J., Soutter W.P. High-resolution MR imaging of stage I cervical neoplasia with a dedicated transvaginal coil: MR features and correlation of imaging and pathologic findings. AJR Am J Roentgenol. 1996;166:553–559. doi: 10.2214/ajr.166.3.8623627. [DOI] [PubMed] [Google Scholar]
- 15.deSouza N.M., Whittle M., Williams A.D., Sohail M., Krausz T., Gilderdale D.J. Magnetic resonance imaging of the primary site in stage I cervical carcinoma: a comparison of endovaginal coil with external phased array coil techniques at 0.5 T. J Magn Reson Imaging. 2000;12:1020–1026. doi: 10.1002/1522-2586(200012)12:6<1020::aid-jmri30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Charles-Edwards E., Morgan V., Attygalle A.D., Giles S.L., Ind T.E., Davis M. Endovaginal magnetic resonance imaging of stage 1A/1B cervical cancer with A T2- and diffusion-weighted magnetic resonance technique: effect of lesion size and previous cone biopsy on tumor detectability. Gynecol Oncol. 2011;120:368–373. doi: 10.1016/j.ygyno.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Charles-Edwards E.M., Messiou C., Morgan V.A., De Silva S.S., McWhinney N.A., Katesmark M. Diffusion-weighted imaging in cervical cancer with an endovaginal technique: potential value for improving tumor detection in stage Ia and Ib1 disease. Radiology. 2008;249:541–550. doi: 10.1148/radiol.2491072165. [DOI] [PubMed] [Google Scholar]
- 18.Hricak H., Lacey C.G., Sandles L.G., Chang Y.C., Winkler M.L., Stern J.L. Invasive cervical carcinoma: comparison of MR imaging and surgical findings. Radiology. 1988;166:623–631. doi: 10.1148/radiology.166.3.3340756. [DOI] [PubMed] [Google Scholar]
- 19.Subak L.L., Hricak H., Powell C.B., Azizi L., Stern J.L. Cervical carcinoma: computed tomography and magnetic resonance imaging for preoperative staging. Obstet Gynecol. 1995;86:43–50. doi: 10.1016/0029-7844(95)00109-5. [DOI] [PubMed] [Google Scholar]
- 20.Ebner F., Tamussino K., Kressel H.Y. Magnetic resonance imaging in cervical carcinoma: diagnosis, staging, and follow-up. Magn Reson Q. 1994;10:22–42. [PubMed] [Google Scholar]
- 21.Hawnaur J.M., Johnson R.J., Buckley C.H., Tindall V., Isherwood I. Staging, volume estimation and assessment of nodal status in carcinoma of the cervix: comparison of magnetic resonance imaging with surgical findings. Clin Radiol. 1994;49:443–452. doi: 10.1016/s0009-9260(05)81738-6. [DOI] [PubMed] [Google Scholar]
- 22.Docquier P.L., Paul L., Cartiaux O., Lecouvet F., Dufrane D., Delloye C. Formalin fixation could interfere with the clinical assessment of the tumor-free margin in tumor surgery: magnetic resonance imaging-based study. Oncology. 2010;78:115–124. doi: 10.1159/000306140. [DOI] [PubMed] [Google Scholar]
- 23.Hsu P.K., Huang H.C., Hsieh C.C., Hsu H.S., Wu Y.C., Huang M.H. Effect of formalin fixation on tumor size determination in stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:1825–1829. doi: 10.1016/j.athoracsur.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Schned A.R., Wheeler K.J., Hodorowski C.A., Heaney J.A., Ernstoff M.S., Amdur R.J. Tissue-shrinkage correction factor in the calculation of prostate cancer volume. Am J Surg Pathol. 1996;20:1501–1506. doi: 10.1097/00000478-199612000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Eid I., El-Muhtaseb M.S., Mukherjee R., Renwick R., Gardiner D.S., Macdonald A. Histological processing variability in the determination of lateral resection margins in rectal cancer. J Clin Pathol. 2007;60:593–595. doi: 10.1136/jcp.2006.040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boonstra H., Oosterhuis J.W., Oosterhuis A.M., Fleuren G.J. Cervical tissue shrinkage by formaldehyde fixation, paraffin wax embedding, section cutting and mounting. Virchows Arch A Pathol Anat Histopathol. 1983;402:195–201. doi: 10.1007/BF00695061. [DOI] [PubMed] [Google Scholar]
- 27.Charles-Edwards E.M., deSouza N.M. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging. 2006;6:135–143. doi: 10.1102/1470-7330.2006.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malayeri A.A., El Khouli R.H., Zaheer A., Jacobs M.A., Corona-Villalobos C.P., Kamel I.R. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics. 2011;31:1773–1791. doi: 10.1148/rg.316115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Boer P., Adam J.A., Buist M.R., van de Vijver M.J., Rasch C.R., Stoker J. Role of MRI in detecting involvement of the uterine internal os in uterine cervical cancer: systematic review of diagnostic test accuracy. Eur J Radiol. 2013;82:e422–e428. doi: 10.1016/j.ejrad.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Plante M., Renaud M.C., Francois H., Roy M. Vaginal radical trachelectomy: an oncologically safe fertility-preserving surgery. An updated series of 72 cases and review of the literature. Gynecol Oncol. 2004;94:614–623. doi: 10.1016/j.ygyno.2004.05.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.