Abstract
Objective
To estimate 24-month continuation rates of all reversible contraceptive methods for women enrolled in the Contraceptive CHOICE Project.
Methods
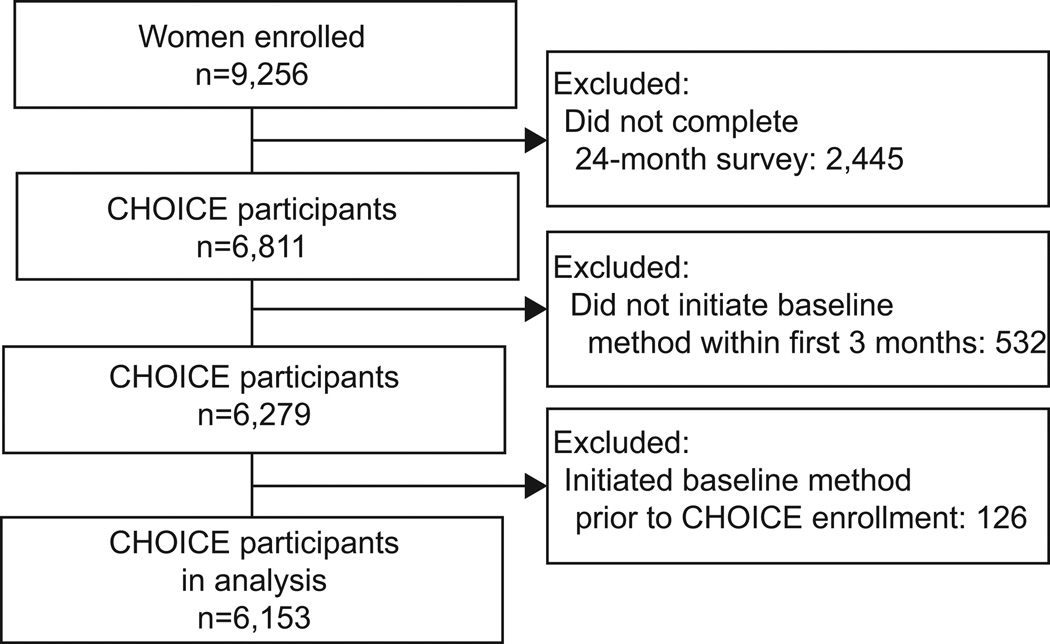
We analyzed 24-month data from the 9,256 participants enrolled in the Contraceptive CHOICE Project, a prospective observational cohort study that provides no-cost contraception to women in the St. Louis region. The project promoted the use of long-acting reversible contraception (LARC; intrauterine devices (IUDs) and implants) in an effort to reduce the rates of unintended pregnancy. This analysis includes participants who received their baseline contraceptive method within 3 months of enrollment and who completed a 24-month follow-up survey (n=6,153).
Results
Twenty-four month continuation rates for LARC and non-LARC methods were 77% and 41%, respectively. Continuation rates for the levonorgestrel and the copper IUDs were similar (79% versus 77%), whereas the implant continuation rate was significantly lower (69%, p<0.001) compared to IUDs at 24 months. There was no statistically significant difference in 24-month continuation rates among the four non-LARC methods (oral contraceptive pill 43%, patch 40%, ring 41%, depot medroxyprogesterone acetate (DMPA) 38%, p=0.72). Participants who chose a LARC method at enrollment were at significantly lower risk of contraceptive method discontinuation (adjusted hazard ratio=0.29, 95% confidence interval 0.26, 0.32) compared with women who selected a non-LARC method.
Conclusion
IUDs and the implant have the highest rates of continuation at 24-months. Given their effectiveness and high continuation rates, IUDs and implants should be first-line contraceptive options and shorter-acting methods such as OCPs, patch, ring, and DMPA should be second tier.
INTRODUCTION
Unintended pregnancies are a major public health problem in the United States. These pregnancies account for 9 out of 10 abortions, and among women that continue their pregnancy, unintended pregnancies are associated with higher rates of adverse maternal and infant outcomes (1). It has been shown that when cost and access barriers to contraception are removed, and the most effective contraceptive methods (e.g., intrauterine devices and implant) are promoted, unintended pregnancy is reduced (2). These long-acting reversible contraceptive (LARC) methods are over 20-fold more effective at preventing unintended pregnancy than the commonly used oral contraceptive pill (OCP), contraceptive vaginal ring or patch (3).
There is a paucity of data on the long-term continuation rates of LARC methods. In fact, most studies fail to assess continuation beyond 12 months of use (4–18). While some studies assess method side effects, little is known about risk factors for discontinuation of LARC methods. Most studies that have examined LARC continuation were retrospective, focused on an individual method, and were conducted outside the United States.
The purpose of this analysis is to estimate the 24-month continuation rates of LARC methods among women enrolled in the Contraceptive CHOICE Project. Our hypothesis was that females using LARC methods would have higher continuation rates than women using OCPs, contraceptive patch, ring, and depot medroxyprogesterone acetate (DMPA). We also examined risk factors for discontinuation of contraceptive methods. We hypothesized that adolescents and women of lower socioeconomic status would be more likely to discontinue their contraceptive method.
METHODS
The Contraceptive CHOICE Project (CHOICE) is an observational cohort study that provided no-cost contraception to adolescents and women within the St. Louis region in an effort to reduce the rates of unintended pregnancies. The project promoted the use of LARC methods: the implant and intrauterine devices (IUDs). CHOICE eliminated the financial barriers for all contraceptive methods and educated participants on the safety and effectiveness of all FDA-approved contraceptive methods through comprehensive contraceptive counseling. A prior publication describes in detail the research methods utilized in CHOICE (19). The following is a brief description of CHOICE and the analytic methods employed for this particular analysis.
CHOICE participants were recruited from St. Louis City and County between 2007 and 2011. Potential participants learned of CHOICE through health care provider referral, posted flyers, and word of mouth. We recruited females from local clinics, two centers providing abortion services, and at a university-affiliated clinical research site. Prior to participant recruitment and enrollment, the Washington University in St. Louis Human Research Protection Office approved the study protocol.
CHOICE participants were required to meet the following inclusion criteria: 1) 14–45 years of age; 2) currently using no contraceptive method or willing to initiate a new reversible contraceptive method; 3) no desire to conceive within 12 months; 4) sexually active with a male partner (or intent to be within the next 6 months); 5) residing in or receiving clinical services at designated recruitment sites in the region; and 6) able to consent in English or Spanish. Adolescents and women with a previous hysterectomy or permanent sterilization were excluded. Participants selected for this analysis met the following additional criteria: 1) received and initiated their baseline chosen method of contraception within 3 months of enrollment; and 2) reached the time point for the 24-month follow-up survey or other data sources were available to verify their continuation status at 24-months. The rates of follow-up in CHOICE were 94% at 12 months, 91% at 18 months, and 87% at 24 months.
All potential participants were read a standardized script regarding LARC methods (19), and those that enrolled received additional contraceptive counseling. The counseling session included a review of all available reversible contraceptive methods in order of effectiveness, and briefly discussed common side effects, and the risks and benefits of each method (20). Participants completed a comprehensive baseline interview, were screened for sexually transmitted infections (STIs), and provided no-cost, reversible contraception for 2–3 years (depending on the date of enrollment). Participants were followed with structured telephone interviews at 3, 6, and every 6 months for the duration of participation, and received a $10 gift card for every completed follow-up survey. Clinical research staff collected and recorded participant complaints, complications, side effects, method expulsions and removals, pregnancies and outcomes from any contraceptive-related problem visit or phone call.
Method continuation was assessed at each follow-up telephone survey. We defined method continuation as continuous use of the baseline method at each survey time point without a period of discontinuation greater than one month in duration. Conversely, we defined method discontinuation as the absence of using the baseline method at any of the follow-up surveys, or a temporary discontinuation of the method for one month or longer. If continuation could not be assessed through participant survey responses, research logs for any reported IUD or implant removal and pharmacy refill records were reviewed to confirm continuation status. We offered IUD replacement to women who experienced an expulsion (4%). If the participant proceeded with replacement of the same type of IUD, we documented this as continuation. Conversely, if she declined replacement with the same type of IUD, we considered this discontinuation. In our analysis, we calculated 24-month continuation rates for each contraceptive method. Because OCPs are the most commonly used method of reversible contraception in the U.S. (21), all other methods were compared to this referent group. Additionally, we compared LARC users to non-LARC users.
To describe the demographic characteristics of the study participants we used frequencies, percentages, means, and standard deviations. Chi-square tests were performed to compare baseline categorical variables among different method users, while Student t-tests were used to compare continuous normally distributed variables. A histogram was used to assess normality. We constructed Kaplan-Meier survival curves to estimate the continuation rates and used the log-rank test to compare continuation among different method users. Cox proportional hazard models were used to estimate the hazard ratios (HR) for the rate of discontinuation among methods, adjust for important confounding variables, and determine predictors of discontinuation. Participants lost to follow-up were censored at their last completed survey date or known device removal date; these participants contributed data up until the point of last contact. If participants discontinued a method because they conceived or were trying to conceive, they were censored at the time they discontinued the method. We evaluated age (14–19 years and 20+) and parity for effect modification by including an interaction term between the method and the covariate of interest in the model. Confounding factors were defined as those factors associated with both the exposure (contraceptive method) and the primary outcome (method discontinuation), and also changed the HR estimate for the rate of discontinuation by 10% or more compared to the estimate from a model without the potential confounding factor included. We considered the variables listed in Table 1 as potential confounders. All variables determined to be confounders were included in the final multivariable model. To evaluate risk factors for discontinuation, we initially examined the crude association between each individual factor and discontinuation. We included all the risk factors in the model using a stepwise selection approach to create the final model. STATA 11 (StataCorp LP, College Station, TX) was used for all analyses. The alpha for all analyses was set at 0.05.
TABLE 1.
Baseline Characteristics of Analytic Sample Stratified by Contraceptive Method and Age STI, sexually transmitted infection.
| Adolescents (14–19 years) | Adults (20–45 years) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (n=6153) |
Non- LARC (n=1730) |
LARC (n=4423) |
P | Non- LARC (n=289) |
LARC (n=611) |
P | Non- LARC (n=1441) |
LARC (n=3812) |
P | |||
| Mean | SD | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | |||||
| Age | 25.2 | 5.8 | 24.1(5.1) | 25.6(6.0) | <0.001 | 18.6(1.3) | 17.6(1.4) | <0.001 | 25.3(4.7) | 26.9(5.5) | <0.001 | |
| n | % | % | % | % | % | % | % | |||||
| Race | 0.243 | 0.062 | 0.184 | |||||||||
| Black | 3017 | 49.0 | 50.3 | 48.5 | 56.7 | 62.8 | 49.0 | 46.2 | ||||
| White | 2661 | 43.3 | 41.6 | 43.9 | 32.9 | 30.8 | 43.3 | 46.0 | ||||
| Others | 474 | 7.7 | 8.1 | 7.6 | 10.4 | 6.4 | 7.6 | 7.7 | ||||
| Education | <0.001 | <0.001 | <0.001 | |||||||||
| Less than high school or high school | 2154 | 35.0 | 31.1 | 36.6 | 63.0 | 75.8 | 24.7 | 30.3 | ||||
| Some college | 2601 | 42.3 | 44.2 | 41.6 | 36.7 | 24.1 | 45.7 | 44.4 | ||||
| College/grad | 1395 | 22.7 | 24.8 | 21.9 | 0.3 | 0.2 | 29.7 | 25.4 | ||||
| Income | <0.001 | 0.201 | 0.001 | |||||||||
| None | 1161 | 19.2 | 17.4 | 20.0 | 37.9 | 40.7 | 13.4 | 16.7 | ||||
| $1–800 | 1933 | 32.0 | 35.6 | 30.7 | 54.0 | 48.2 | 32.1 | 27.9 | ||||
| $801–1600 | 1757 | 29.1 | 29.3 | 29.1 | 6.6 | 10.2 | 33.7 | 32.1 | ||||
| $1601+ | 1181 | 19.6 | 17.7 | 20.3 | 1.5 | 1.0 | 20.8 | 23.4 | ||||
| Body mass index | <0.001 | <0.001 | <0.001 | |||||||||
| Underweight (<18.5) | 178 | 2.9 | 4.8 | 2.1 | 7.6 | 2.8 | 4.2 | 2.0 | ||||
| Normal (18.5–24.9) | 2406 | 39.1 | 46.6 | 36.2 | 61.9 | 48.1 | 43.6 | 34.2 | ||||
| Overweight (25.0–29.9) | 1582 | 25.7 | 22.7 | 26.9 | 14.2 | 26.0 | 24.4 | 27.0 | ||||
| Obese (≥30.0) | 1987 | 32.3 | 25.9 | 34.8 | 16.3 | 23.1 | 27.8 | 36.7 | ||||
| Receiving public assistance | <0.001 | <0.001 | <0.001 | |||||||||
| No | 3937 | 64.0 | 73.9 | 60.2 | 81.7 | 69.0 | 72.3 | 58.8 | ||||
| Yes | 2210 | 36.0 | 26.1 | 39.8 | 18.3 | 31.0 | 27.7 | 41.2 | ||||
| Trouble paying at baseline | 0.050 | 0.365 | 0.005 | |||||||||
| No | 3712 | 60.4 | 58.5 | 61.2 | 76.4 | 73.6 | 54.9 | 59.2 | ||||
| Yes | 2430 | 39.6 | 41.5 | 38.8 | 23.6 | 26.4 | 45.1 | 40.8 | ||||
| Insurance | <0.001 | <0.001 | <0.001 | |||||||||
| None | 2603 | 42.6 | 48.1 | 40.5 | 36.0 | 29.6 | 50.4 | 42.2 | ||||
| Private | 2640 | 43.2 | 45.0 | 42.5 | 48.4 | 39.5 | 44.4 | 43.0 | ||||
| Public | 865 | 14.2 | 6.9 | 17.0 | 15.6 | 30.9 | 5.2 | 14.8 | ||||
| Gravidity | <0.001 | <0.001 | <0.001 | |||||||||
| 0 | 1775 | 28.8 | 42.5 | 23.5 | 60.9 | 42.6 | 38.9 | 20.4 | ||||
| 1 | 1342 | 21.8 | 23.3 | 21.2 | 28.7 | 39.6 | 22.2 | 18.3 | ||||
| 2 | 1105 | 18.0 | 14.6 | 19.3 | 8.0 | 13.4 | 15.9 | 20.2 | ||||
| 3+ | 1931 | 31.4 | 19.6 | 36.0 | 2.4 | 4.4 | 23.0 | 41.1 | ||||
| Parity | <0.001 | <0.001 | <0.001 | |||||||||
| 0 | 2878 | 46.8 | 64.6 | 39.8 | 88.2 | 72.7 | 59.8 | 34.5 | ||||
| 1 | 1531 | 24.9 | 19.5 | 27.0 | 11.4 | 22.9 | 21.2 | 27.6 | ||||
| 2 | 1068 | 17.4 | 10.3 | 20.1 | 0.3 | 3.8 | 12.4 | 22.7 | ||||
| 3+ | 676 | 11.0 | 5.5 | 13.1 | 0.0 | 0.7 | 6.7 | 15.1 | ||||
| Unintended pregnancies | <0.001 | <0.001 | <0.001 | |||||||||
| 0 | 2153 | 35.1 | 47.3 | 30.3 | 63.1 | 45.5 | 44.1 | 27.8 | ||||
| 1 | 1681 | 27.4 | 27.1 | 27.5 | 27.5 | 39.3 | 27.0 | 25.6 | ||||
| 2 | 1038 | 16.9 | 12.7 | 18.5 | 7.3 | 12.1 | 13.8 | 19.6 | ||||
| 3+ | 1269 | 20.7 | 12.9 | 23.7 | 2.1 | 3.1 | 15.1 | 27.0 | ||||
| Ever abortion at baseline | 0.019 | 0.603 | 0.023 | |||||||||
| No | 3859 | 62.7 | 65.0 | 61.8 | 78.2 | 79.7 | 62.4 | 58.9 | ||||
| Yes | 2294 | 37.3 | 35.0 | 38.2 | 21.8 | 20.3 | 37.6 | 41.1 | ||||
| History of STI | 0.019 | 0.043 | 0.167 | |||||||||
| No | 3730 | 60.7 | 63.0 | 59.7 | 79.2 | 73.0 | 59.7 | 57.6 | ||||
| Yes | 2420 | 39.3 | 37.0 | 40.3 | 20.8 | 27.0 | 40.3 | 42.4 | ||||
| Any STI at baseline | 0.850 | 0.886 | 0.819 | |||||||||
| No | 5585 | 93.4 | 93.3 | 93.4 | 92.8 | 92.5 | 93.4 | 93.5 | ||||
| Yes | 397 | 6.6 | 6.7 | 6.6 | 7.2 | 7.5 | 6.6 | 6.5 | ||||
RESULTS
Of the 9,256 adolescents and women enrolled in CHOICE, 6,153 participants met inclusion criteria for this analysis. Figure 1 illustrates the exclusion of participants for this analysis. Adolescents and women included in the analysis were similar to the entire CHOICE cohort in terms of demographic and reproductive characteristics (22). The mean age was 25 years; 49% were black, 35% had a high school education or less, 51% earned less than $800 per month in income, and 43% had no health insurance. Nearly half (47%) were nulliparous, 65% reported at least one unintended pregnancy, and 37% had had an abortion (Table 1).
Figure 1.
Contraceptive CHOICE Project Participants Eligible for 1 Examination of 24 Month Continuation
Table 1 also compares the sample stratified by LARC versus non-LARC users and by LARC use within 2 age categories (14–19 years, “adolescents” compared to 20–45 years, “adults”). Compared to participants using non-LARC methods, LARC users had a higher BMI, were less educated, more likely to be dependent on public assistance, and have public insurance. LARC users were also more likely to have higher parity and at least one prior unintended pregnancy. These findings were consistent across age groups. Among the adolescent group, LARC users reported a younger mean age, whereas among women 20 and older LARC users reported an older mean age compared to non-LARC users.
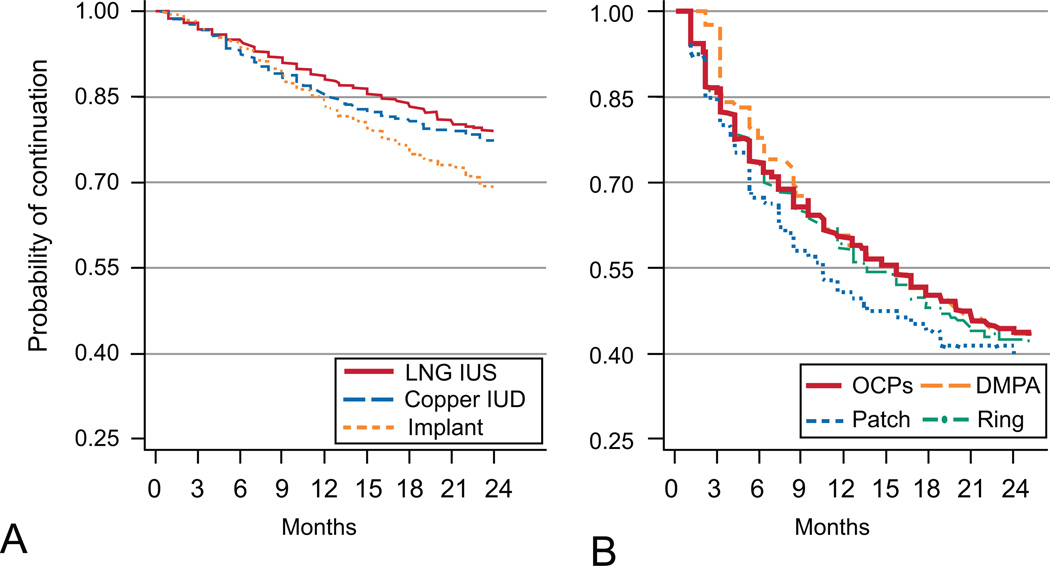
Table 2 presents 12- and 24-month continuation data by contraceptive method. We also present LARC versus non-LARC continuation among adolescent and adult women. Twenty-four month continuation rates for LARC and non-LARC methods were 77% and 41%, respectively. Continuation at 24-months for the levonorgestrel intrauterine system (LNG-IUS) and the copper IUD were the highest reported and similar: 79%, and 77%, respectively. The 24-month continuation of the implant was 69% and significantly lower than the IUDs (Figure 2, p<0.001). Continuation at 24 months for each of the non-LARC methods were similar and all below 45% (DMPA 38%, OCPs 43%, Ring 41%, Patch 40%). (Figure 2, p=0.72).
TABLE 2.
Twelve-Month and 24-Month Continuation of all Contraceptive Methods
| 12 Month | 24 Month | |
|---|---|---|
| % | % | |
| Overall | 78.7 | 67.0 |
| LNG-IUS | 88.1 | 78.9 |
| Copper IUD | 85.1 | 77.3 |
| Implant | 83.4 | 68.5 |
| DMPA | 57.5 | 38.0 |
| OCP | 59.0 | 43.1 |
| Ring | 56.0 | 41.1 |
| Patch | 49.6 | 39.9 |
| LARC | 86.7 | 76.6 |
| Non-LARC | 57.1 | 40.9 |
| Adolescents 14–19 years | ||
| LARC | 81.8 | 66.5 |
| Non-LARC | 48.8 | 36.6 |
| Adults 20–45 years | ||
| LARC | 87.4 | 78.2 |
| Non-LARC | 58.8 | 41.8 |
LNG-IUS, levonorgestrel intrauterine system; IUD, intrauterine device; DMPA, depot medroxyprogesterone acetate; OCP, oral contraceptive pill, LARC, long-acting reversible contraception.
Figure 2.
Continuation Over 24 Months for long-acting reversible contraceptive LARC (A) and non-long acting reversible contraceptive non-LARC(B) methods. Log rank P-value=0.72. Levonorgestrel intrauterine system (LNG IUS; copper intrauterine system (IUD); oral contraceptive pill (OCP); depot medroxyprogesterone acetate (DMPA).
Eighty-seven percent of LARC users were using their method at 12 months and 77% at 24 months. In comparison, 12- and 24-month continuation among non-LARC users was 57% and 41%, respectively. Adjusting for potential confounding factors listed in Table 3, LARC users were significantly less likely to discontinue their method at 24 months (HRadj=0.29, 95% CI: 0.26, 0.32) than non-LARC users. In fact, each of the LARC methods had a lower hazard of discontinuation at 24 months (LNG-IUS: HRadj=0.26, 95% CI 0.23, 0.30; copper IUD: HRadj=0.30, 95% CI 0.25, 0.37; implant: HRadj=0.35, 95% CI 0.29, 0.42) when compared to OCPs. Greater discontinuation of non-LARC methods was consistent when stratified by age. Among adolescents, 33% of LARC users discontinued their method compared to 63% of non-LARC users (HRadj=0.34, 95% CI: 0.27, 0.44). Among adult women, 22% of LARC users and 58% of non-LARC users discontinued their method (HRadj=0.27, 95% CI: 0.24, 0.30).
TABLE 3.
Crude and Adjusted Hazard Ratios for Risk of Discontinuation of Baseline Method at 24 Months
| Crude Model | Adjusted Model | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| Contraceptive Method | |||||||
| LNG-IUS | 0.26 | 0.23 | 0.30 | 0.26 | 0.23 | 0.30 | |
| Copper IUD | 0.29 | 0.24 | 0.36 | 0.30 | 0.25 | 0.37 | |
| Implant | 0.40 | 0.33 | 0.47 | 0.35 | 0.29 | 0.42 | |
| DMPA | 1.02 | 0.86 | 1.20 | 0.94 | 0.79 | 1.13 | |
| OCP | Reference | Reference | |||||
| Ring | 1.04 | 0.88 | 1.22 | 1.03 | 0.87 | 1.23 | |
| Patch | 1.18 | 0.89 | 1.55 | 1.10 | 0.83 | 1.48 | |
| Age | |||||||
| 14–19 | 1.47 | 1.31 | 1.66 | 1.40 | 1.22 | 1.60 | |
| 20+ | Reference | Reference | |||||
| Race | |||||||
| Black | 1.22 | 1.10 | 1.34 | 1.16 | 1.03 | 1.30 | |
| White | Reference | Reference | |||||
| Others | 1.30 | 1.09 | 1.55 | 1.26 | 1.04 | 1.51 | |
| Education | |||||||
| Less than high school or high school | Reference | - | |||||
| Some college | 0.98 | 0.88 | 1.09 | - | |||
| College/grad | 0.90 | 0.79 | 1.02 | - | |||
| Income | |||||||
| None | Reference | - | |||||
| $1–800 | 0.96 | 0.84 | 1.10 | - | |||
| $801–1600 | 0.84 | 0.74 | 0.97 | - | |||
| $1601+ | 0.89 | 0.77 | 1.03 | - | |||
| Body mass index | |||||||
| Underweight (<18.5) | 1.06 | 0.81 | 1.38 | 0.89 | 0.67 | 1.19 | |
| Normal (18.5–24.9) | Reference | Reference | |||||
| Overweight (25.0–29.9) | 0.92 | 0.82 | 1.04 | 1.04 | 0.92 | 1.17 | |
| Obese (≥30.0) | 0.74 | 0.66 | 0.83 | 0.84 | 0.74 | 0.95 | |
| Receiving public assistance | |||||||
| No | Reference | Reference | |||||
| Yes | 0.93 | 0.85 | 1.03 | 1.13 | 1.00 | 1.26 | |
| Trouble paying at baseline | |||||||
| No | Reference | - | |||||
| Yes | 1.07 | 0.97 | 1.17 | - | |||
| Insurance | |||||||
| None | 1.06 | 0.96 | 1.17 | - | |||
| Private | Ref | - | |||||
| Public | 0.93 | 0.80 | 1.08 | - | |||
| Gravidity | |||||||
| 0 | Reference | Reference | |||||
| 1+ | 0.73 | 0.67 | 0.81 | 0.87 | 0.77 | 0.98 | |
| Parity | |||||||
| 0 | Reference | - | |||||
| 1+ | 0.73 | 0.67 | 0.80 | - | |||
| Unintended pregnancies | |||||||
| 0 | Reference | - | |||||
| 1+ | 0.81 | 0.73 | 0.89 | - | |||
| Ever abortion at baseline | |||||||
| No | Reference | - | |||||
| Yes | 0.96 | 0.87 | 1.05 | - | |||
| History of STI | |||||||
| No | Reference | Reference | |||||
| Yes | 1.16 | 1.05 | 1.27 | 1.28 | 1.16 | 1.42 | |
| Any STI at Baseline | |||||||
| No | Reference | - | |||||
| Yes | 0.99 | 0.82 | 1.20 | - | |||
HR, hazard ratio; CI, confidence interval; LNG-IUS, levonorgestrel intrauterine system; ; IUD, intrauterine device; DMPA, depot medroxyprogesterone acetate; OCP, oral contraceptive pill; STI, sexually transmitted infection.
Table 3 presents significant demographic and reproductive risk factors for discontinuation adjusted for contraceptive method. Females aged 14–19 years were at higher risk of discontinuation than those 20 years and older (HRadj=1.40, 95% CI 1.22, 1.60), as were those who identified themselves as black or as a race other than black or white (black HRadj=1.16, 95% CI 1.03, 1.30; other race HRadj=1. 26, 95% CI 1.04, 1.51). Women and adolescents with at least one prior pregnancy had a lower risk of discontinuation (HRadj=0.87, 95% CI 0.77, 0.98). Finally, participants with a history of prior STI were at increased risk of method discontinuation (HRadj=1.28, 95% CI 1.16, 1.42).
DISCUSSION
Despite LARC methods being greater than 20 times more effective than non-LARC methods at preventing unintended pregnancy; there is a paucity of data on long-term LARC continuation rates and risk factors for discontinuation among LARC users. Among the first 6,153 women with 24-months of follow-up in the Contraceptive CHOICE Project, we found that LARC users have higher rates of contraceptive continuation compared with women using non-LARC methods. This pattern was consistent among both adolescent and adult women. At 24 months, continuation rates for both the LNG-IUS and copper IUD were similar (> 75%), while continuation rates for the implant were high, but somewhat lower (69%). Previous retrospective studies estimated the 24-month continuation rates for the implant between 50–75% (4–6), similar to the rate among our study population. Importantly, while the 24-month continuation rate for the implant was the lowest among all LARC methods, it was still significantly higher than any of the non-LARC methods (range 38–43%).
Among our participants, nearly four times as many women chose the LNG-IUS (N=2,825) than the copper IUD (N=705). We previously postulated that this difference was due to a number of factors: beneficial side effects, direct-to-consumer advertising, and provider bias (19). While the LNG-IUS is a more popular choice than the copper IUD among our participants, the 24-month continuation rates were not significantly different. The 24-month continuation rates of the LNG-IUS and earlier forms of the copper IUD (Nova T and copper-T) in previous studies ranged from 57–66% for the LNG-IUS and 68–72% for the copper IUD (15–18). These rates are slightly lower than those observed among our study population at 24 months. Several of these studies randomized participants to either IUD, while our study population was allowed to choose their desired method (15–17). Randomization may adversely affect continuation rates compared to individual choice.
Few studies have examined the long-term continuation rates of non-LARC methods beyond 12 months of use. In our study, the continuation rate for all combined non-LARC methods (OCPs, patch, contraceptive ring, and DMPA) was markedly lower than that for all LARC methods. This is not surprising, given the continuous adherence required by non-LARC methods. Moreover, the continuation rates of non-LARC methods may be an overestimation, as method “continuation” was defined as use of the baseline method at each survey time point without a period of discontinuation greater than one month in duration. This allows for those participants who discontinue their method for less than one month to be considered a “continuer.”
We also aimed to identify risk factors for discontinuation of reversible contraception at 24 months. Women choosing a LARC method were at significantly lower risk of discontinuation. This was true of all of the LARC methods compared to all non-LARC methods. There are minimal requirements of the participant to adhere to LARC method use, and removal of LARC methods requires seeking medical care and clinician intervention for discontinuation. Although adolescents were 40% more likely to discontinue their baseline method at 24 months than adult women, two-thirds of adolescent LARC users were still using their method at 2 years compared to one-third of non-LARC users. In a prior analysis, CHOICE participants 14–19 years were more likely to discontinue non-LARC methods at 12 months compared to women aged 26 and older, and were less likely to be satisfied with non-LARC methods than women over 25 years of age (7). In that same analysis, satisfaction rates for LARC methods among those aged 14–19 years were similar to those among women over 25 years.
The risk of discontinuation was lower among participants with a history of at least one prior pregnancy and women with at least one prior unintended pregnancy. These adolescents and women, as well as those with a history of abortion, were more likely to choose a LARC method at enrollment and may be more motivated to avoid a future pregnancy (23). Finally, adolescents and women with previous history of STI were at higher risk of method discontinuation. STI history may be a marker for high-risk behaviors, which may include having unprotected intercourse and lack of adherence to reliable contraception.
One of the strengths of our study is the relatively long duration of follow-up compared to other studies; there are a small number of published reports that estimate continuation rates of both LARC and non-LARC methods beyond 12 months. Of those studies that do examine long-term continuation, rarely do they focus on more than one contraceptive method. Additional strengths of this analysis include its prospective design, large sample size, and high rate of follow-up at 24 months. The main limitations include use of a convenience sample, and the requirement of our participants to be starting a new method of contraception or be interested in switching from their present method of contraception at enrollment. If a participant changed from a method they were particularly satisfied with at study enrollment, this may have resulted in higher discontinuation rates. Our goal in this design was to create a setting wherein participants were allowed to choose their contraceptive method without limitations of knowledge, affordability, or access. This design also sought to minimize discontinuation due to randomization and cohort effects where existing users may have had greater overall satisfaction than new users. For example, we did not want to compare the contraceptive continuation of an OCP user who has been satisfied with her method for the past 3 years to a participant starting an implant or IUD. Finally, there is always concern regarding external validity: the CHOICE population may not be generalizable to other populations.
Despite the high up-front cost of LARC, a recent analysis of the cost-effectiveness of LARC compared to shorter-acting contraceptive methods has shown that a shift from shorter-acting methods toward greater use of LARC will generate significant cost savings in under 2 years (24). Our study has shown that both adolescent and adult LARC users are much more likely to continue their method at 2 years. Given their efficacy, high continuation rates, and their cost-effectiveness (25), LARC methods should be first-line contraceptive options for all females, and non-LARC methods should be second tier.
Acknowledgments
The Contraceptive CHOICE Project is funded by the Susan T. Buffett Foundation. This research was also supported in part by a Midcareer Investigator Award in Women’s Health Research (K24 HD01298), by a Clinical and Translational Science Award (UL1RR024992), by award number K23HD070979 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD); and by Grant Number KL2RR024994 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Dr. Peipert receives research funding from Bayer and Merck, and is on advisory boards for Teva and Watson. Dr. Madden receives research funding from Merck & Co, Inc and honoraria for serving on an advisory board for Bayer Healthcare Pharmaceuticals.
Footnotes
Financial Disclosure
The other authors did not report any potential conflicts of interest.
REFERENCES
- 1.Hellerstedt WL, Pirie PL, Lando HA, et al. Differences in preconceptional and prenatal behaviors in women with intended and unintended pregnancies. Am J Public Health. 1998 Apr;88(4):663–666. doi: 10.2105/ajph.88.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peipert JF, Madden T, Allsworth JE, Secura GM. Preventing unintended pregnancies by providing no-cost contraception. Obstet Gynecol. 2012 Dec;120(6):1291–1297. doi: 10.1097/aog.0b013e318273eb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, Secura GM. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012 May 24;366(21):1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- 4.Lakha F, Glasier AF. Continuation rates of Implanon in the UK: data from an observational study in a clinical setting. Contraception. 2006;74:287–289. doi: 10.1016/j.contraception.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 5.Harvey C, Seib C, Lucke J. Continuation rates and reasons for removal among Implanon users accessing two family planning clinics in Queensland, Australia. Contraception. 2009 Dec;80(6):527–532. doi: 10.1016/j.contraception.2009.05.132. [DOI] [PubMed] [Google Scholar]
- 6.Arribas-Mir L, Rueda-Lozano D, Agrela-Cardona M, Cedeño-Benavides T, Olvera-Porcel C, Bueno-Cavanillas A. Insertion and 3-year follow-up experience of 372 etonogestrel subdermal contraceptive implants by family physicians in Granada, Spain. Contraception. 2009 Nov;80(5):457–462. doi: 10.1016/j.contraception.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Rosenstock JR, Peipert JF, Madden T, Zhao Q, Secura GM. Continuation of reversible contraception in teenagers and young women. Obstet Gynecol. 2012 Dec;120(6):1298–1305. doi: 10.1097/aog.0b013e31827499bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming D, Davie J, Glasier A. Continuation Rates of Long-Acting Methods of Contraception: A Comparative Study of Norplant Implants and Intrauterine Devices. Contraception. 1998;57:19–21. doi: 10.1016/s0010-7824(97)00202-3. [DOI] [PubMed] [Google Scholar]
- 9.Jenabi E, Alizade SM, Baga RI. Continuation rates and reasons for discontinuing TCu380A IUD use in Tabriz, Iran. Contraception. 2006;74:483–486. doi: 10.1016/j.contraception.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Alton TM, Brock GN, Yang D, Wilking DA, Hertweck SP, Loveless MB. Retrospective review of intrauterine device in adolescent and young women. J Pediatr Adolesc Gynecol. 2012 Jun;25(3):195–200. doi: 10.1016/j.jpag.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Teal SB, Sheeder J. IUD use in adolescent mothers: retention, failure and reasons for discontinuation. Contraception. 2012 Mar;85(3):270–274. doi: 10.1016/j.contraception.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Khademloo M, Ghasemian R, Yasari M. Continuation rates and reasons for discontinuing TCu380A IUD use in Sari, Iran. Pak J Biol Sci. 2008 Jun 1;11(11):1514–1516. doi: 10.3923/pjbs.2008.1514.1516. [DOI] [PubMed] [Google Scholar]
- 13.Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception. 2002 Feb;65(2):129–132. doi: 10.1016/s0010-7824(01)00302-x. [DOI] [PubMed] [Google Scholar]
- 14.Backman T, Huhtala S, Blom T, Luoto R, Rauramo I, Koskenvuo M. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: a nation-wide study of 17,360 users. BJOG. 2000 Mar;107(3):335–339. doi: 10.1111/j.1471-0528.2000.tb13228.x. [DOI] [PubMed] [Google Scholar]
- 15.Sivin I, Stern J, Diaz J, Diaz MM, Faundes A, el Mahgoub S, Diaz S, Pavez M, Coutinho E, Mattos CE, et al. Two years of intrauterine contraception with levonorgestrel and with copper: a randomized comparison of the TCu 380Ag and levonorgestrel 20 mcg/day devices. Contraception. 1987 Mar;35(3):245–255. doi: 10.1016/0010-7824(87)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Luukkainen T, Allonen H, Haukkamaa M, Lähteenmäki P, Nilsson C, Toivonen J. Five years’ experience with levonorgestrel-releasing IUDs. Contraception. 1986;33:139–148. doi: 10.1016/0010-7824(86)90080-6. [DOI] [PubMed] [Google Scholar]
- 17.Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56–72. doi: 10.1016/0010-7824(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 18.Cox M, Tripp J, Blacksell S. Clinical performance of the levonorgestrel intrauterine system in routine use by the UK Family Planning and Reproductive Health Research Network: 5-year report. Journal of Family Planning and Reproductive Health Care. 2002;28(2):73–77. doi: 10.1783/147118902101196225. [DOI] [PubMed] [Google Scholar]
- 19.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010 Aug;203(2):115.e1–117.e1. doi: 10.1016/j.ajog.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madden T, Mullersman JL, Omvig KJ, Secura GM, Peipert JF. Structured contraceptive counseling provided by the Contraceptive CHOICE Project. Contraception. 2012 Sep 5; doi: 10.1016/j.contraception.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones J, Mosher W, Daniels K. Current Contraceptive Use in the United States, 2006–2010, and Changes in Patterns of Use Since 1995. National Health Statistics Reports. 2012 Oct;(No. 60) [PubMed] [Google Scholar]
- 22.Peipert JF, Madden T, Allsworth JE, Secura GM. Preventing unintended pregnancies by providing no-cost contraception. Obstet Gynecol. 2012;120:1291–1297. doi: 10.1097/aog.0b013e318273eb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madden T, Secura GM, Allsworth JE, Peipert JF. Comparison of Contraceptive Method Chosen by Women with and without a Recent History of Induced Abortion. Contraception. 2011 Dec;84(6):571–577. doi: 10.1016/j.contraception.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of Unintended Pregnancy in the United States: Potential Savings with Increased use of Long-Acting Reversible Contraception. Contraception. 2013 Feb;87(2):154–161. doi: 10.1016/j.contraception.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Stewart FH, Kowal D, editors. Contraceptive technology, 19th revised edition. New York: Ardent Media; 2007. p. 759. [Google Scholar]