Abstract
Hormones coordinate the co-expression of behavioral, physiological, and morphological traits, giving rise to correlations among traits and organisms whose parts work well together. This article considers the implications of these hormonal correlations with respect to the evolution of hormone-mediated traits. Such traits can evolve owing to changes in hormone secretion, hormonal affinity for carrier proteins, rates of degradation and conversion, and interaction with target tissues to name a few. Critically, however, we know very little about whether these changes occur independently or in tandem, and thus whether hormones promote the evolution of tight phenotypic integration or readily allow the parts of the phenotype to evolve independently. For example, when selection favors a change in expression of hormonally mediated characters, is that alteration likely to come about through changes in hormone secretion (signal strength), changes in response to a fixed level of secretion (sensitivity of target tissues), or both? At one extreme, if the phenotype is tightly integrated and only the signal responds via selection's action on one or more hormonally mediated traits, adaptive modification may be constrained by past selection for phenotypic integration. Alternatively, response to selection may be facilitated if multivariate selection favors new combinations that can be easily achieved by a change in signal strength. On the other hand, if individual target tissues readily “unplug” from a hormone signal in response to selection, then the phenotype may be seen as a loose confederation that responds on a trait-by-trait basis, easily allowing adaptive modification, although perhaps more slowly than if signal variation were the primary mode of evolutionary response. Studies reviewed here and questions for future research address the relative importance of integration and independence by comparing sexes, individuals, and populations. Most attention is devoted to the hormone testosterone (T) and a songbird species, the dark-eyed junco (Junco hyemalis).
Introduction
Hormones influence the evolution of sexual dimorphism, alternative phenotypes, trade-offs in life histories, and the development and evolution of complex phenotypes. The objective of this article is to consider how hormonally mediated suites of traits evolve, e.g., one at a time or collectively. The article will be partly a review of existing studies, partly suggestions for future study, and partly speculative.
We begin with terminology. A hormone, acting as a signal, can influence multiple tissues known as targets because the target tissues express protein receptors that bind the hormone. Exposure to the hormone alters the cellular metabolism of the targets, often because the signal–target interaction induces or suppresses gene transcription. Exposure of multiple targets to a signal leads to coordinated expression of a network of traits that may be called a suite or a syndrome. By analogy with genetic processes, the ability of one hormone signal to interact with multiple targets to influence multiple traits has been referred to as hormonal pleiotropy, and the correlations among traits mediated by the same hormone as hormonal correlations (Ketterson and Nolan 1999; Flatt et al. 2005; Zera et al. 2007; Ducrest et al. 2008; McGlothlin and Ketterson 2008; Mills 2008b; Williams 2008).
Over developmental and evolutionary time, hormone signals may vary in their strength or pattern of release. The sensitivity of target tissues to a hormone signal can also vary, e.g., targets may become more or less sensitive or even insensitive if, for example, they no longer express a receptor protein or they produce a gene product constitutively without a hormonal prompt. Such hormonal insensitivity at the target can lead to the loss of phenotypic traits that were formerly coordinated in expression by a hormone signal. This ability of tissues to separate themselves from the influence of a hormone is an endocrine example of independence.
The term phenotypic integration has been used to refer to the network of multivariate relationships among behavioral, physiological, and morphological traits that describe the organism (Pigliucci and Preston 2004). Integration is a matter of degree. It may be tight or loose depending on the relative resistance of the multivariate relationships to change. To emphasize extremes, we use phenotypic integration to imply tight (resistant to change) connections between hormone signals and hormonally mediated traits, and phenotypic independence to refer to connections that are readily uncoupled (Bass and Lester 1983; Conner and Sterling 1996; Conner 2002).
Hormone–target interactions can be far more complex than just portrayed (Ball and Balthazart 2008). Hormone signals are not only secreted at varying levels in varying temporal patterns, they are also broken down at differing rates, transported by carrier proteins in ways that make them unavailable to the target, and often are metabolized into new active forms at the target (Ketterson and Nolan 1999; Nijhout 2003). Some hormones act on many tissues; others are confined to a few, and not all act by influencing gene transcription. Again, the distinction between integration and independence is surely graded, e.g., loss of hormone sensitivity at one target may indicate independence, but to the extent that other targets remain under the influence of the hormone, the hormonal phenotype may still be relatively tightly integrated. Future treatments may address these complexities, but for now we simply acknowledge them and proceed with the analysis.
Adaptation and constraint
Consideration of hormonal integration of the phenotype leads quickly to questions of adaptation and constraint, and the potential for tight integration to limit evolutionary change. The degree to which adaptive divergence may be facilitated or retarded by suites of hormonally correlated characters has become an area of contention among behavioral ecologists and endocrinologists (Sinervo and Svensson 1998; Hau 2007; Adkins-Regan 2008; Lessells 2008; McGlothlin and Ketterson 2008). Indeed Hau (2007) named the alternatives referred to here (integration and independence) as the evolutionary constraint hypothesis and the evolutionary potential hypothesis. To the degree that phenotypic correlations are mediated by hormonal correlations in a manner analogous to genetic correlations, the rate of evolution may be slowed and the directions in which change can occur may be reduced (Pigliucci 2004). Importantly, however, tight correlations may themselves be the product of past selection for an organism whose parts work well together (Schwenk and Wagner 2004; McGlothlin and Ketterson 2008), and they can facilitate future adaptive change (Merilä and Björklund 2004; Agrawal and Stinchcombe 2009). This property of tight hormonal correlations, i.e., their potential to hasten, as well retard, the rate of evolution, is a reason we prefer the term “phenotypic integration” to “evolutionary constraint”, but a shortcoming of both terms is that they attempt to capture the impact of hormonally mediated suites on the evolutionary process with a dichotomy when the variation to be explained is surely quantitative in nature.
We anticipate that tight integration of hormonally mediated traits (Hau's evolutionary constraint hypothesis) may play an important role within a species, both by maintaining stasis and by constraining the directions that evolution may take, but also by permitting rapid evolutionary responses to fluctuating environments. Hormonal independence (Hau's evolutionary potential hypothesis), on the other hand, may play a more important role in permitting new arrangements of hormonally mediated traits during marked environmental change or cladogenesis.
Phenotypic integration and independence of hormonally mediated traits: experimental studies
Several lines of evidence can be brought to bear on the relative importance of integration and independence in accounting for variation in hormonally mediated suites of traits and their evolution. Experimental studies in which the strength of the hormone signal is manipulated, e.g., by using hormone implants to elevate plasma levels of a hormone, provide one approach. Altered phenotypic expression in response to experimentally altered hormone levels is evidence of integration; no change in expression suggests insensitivity to the signal (Lynn et al. 2002, 2005; Lynn 2008) or independence. Fitness measures in altered individuals can also be informative. For example, if the altered phenotype has greater fitness than the norm, it suggests that evolution is constrained in its ability to lead to an optimal phenotype (Ketterson and Nolan 1999).
For groupings of individuals that normally differ in hormonally mediated phenotype, e.g., males and females, an overly simplistic application of strong phenotypic integration would predict that if males and females were to express the same circulating levels of the hormone signal, then their phenotypes would be the same. Past selection for phenotypic independence would predict that similar hormone levels would not lead to similar phenotypic expression. By altering hormone levels experimentally and relating the engineered phenotypes to each other and to fitness, we can ask whether past selection has led to divergence in mechanisms of mediation and whether future selection would act against natural equivalents of the experimentally altered phenotypes.
The hormone testosterone (T) is of particular interest because of its ability to “orchestrate” suites of traits related to male–male competition and sex differences, as well as its role in life history trade-offs (Wingfield et al. 2001). In male birds, the phenotypic effects of experimentally elevated testosterone have been well characterized, as has the likely effect of elevation in T on viability, fecundity, and mating success (Klein et al. 1997; Ketterson et al. 1999; Wingfield et al. 2001; Westneat et al. 2003; Reed et al. 2006). Females also produce testosterone, but until recently its activational effects on the phenotype of adult females have been less well documented (Staub and DeBeer 1997; Van Duyse et al. 2002; Ketterson et al. 2005; Moller et al. 2005; Mank 2007). [The literature on the impact of T on the development of males and females is beyond the scope of this article (Balthazart and Adkins-Regan 2002).] Interest in adult female testosterone has, however, been growing (Gill et al. 2007; Peters 2007; Sandell 2007). With a few notable exceptions (O’Neal et al. 2008; Veiga and Polo 2008), however, studies relating experimentally elevated or natural levels of female testosterone to components of fitness remain to be done.
Phenotypic integration and independence of hormonally mediated traits: studies of natural co-variation
Another line of evidence regarding the relative importance of integration and independence comes from correlations among natural variation in hormone signals, target sensitivity, phenotype, and fitness within and among populations. If signal strength correlates with trait expression, or if signal strength and sensitivity of target tissues are correlated, this can be taken as evidence of integration. If phenotypic variation is achieved via differences in target sensitivity, not signal strength, this can be taken as evidence of independence. In an example of signal and sensitivity working in tandem, the reactivity of the hypothalamo–pituitary–adrenal–axis (HPA) is known to respond to artificial selection (Evans et al. 2006) and to be under natural selection in the wild (Blas et al. 2007; Breuner et al. 2008).
Behavioral ecologists and evolutionary endocrinologists often treat hormones like quantitative traits that co-vary with trait expression (Zera et al. 2007; Fusani 2008; Williams 2008) and are subject to modes of analysis developed for quantitative characters (McGlothlin and Ketterson 2008). This view derives support from studies relating hormone levels to phenotype (Solís and Penna 1997) and to fitness (Comendant et al. 2003; Kempenaers et al. 2008), and to artificial-selection studies in which selection on hormone levels has altered phenotype or vice versa (Gross and Siegel 1985; Zera et al. 2007; Williams 2008). Very few studies have attempted to measure the degree of co-variation among individuals in hormone values and multiple phenotypic traits, much less fitness, and more such studies are needed (McGlothlin and Ketterson 2008). Further, there are very few studies of natural selection on suites of hormone-mediated traits or even on natural variation in hormones themselves (McGlothlin and Ketterson 2008; Mills 2008a).
Figure 1 depicts how traits within a hormone-mediated suite may evolve in response to divergent natural selection. The pattern of co-variation between two traits (z1, z2) within a population is depicted as an ellipse, whose major (long) axis is determined by the degree to which variation in the traits is mediated by common reliance on the strength of a hormonal signal. Residual variation (the minor axis) is mediated by relative individual differences in the hormonal sensitivity of the tissues (e.g., muscle, brain, and epidermis) that underlie the traits. Figure 1A depicts an ancestral population in which two traits co-vary owing to variation in signal strength. Such a population may evolve via a number of different pathways. If evolution occurs primarily by way of a change in the strength of the signal (or less parsimoniously via coordinated change in sensitivity of the target), descendent populations should evolve along the major axis of variation and, among populations, the means of both traits should evolve in the same direction (i.e., by both increasing or decreasing, giving rise to the populations to the right or left of center) (Fig. 1B). Alternatively, evolution could proceed owing to change in only the relative sensitivities of target tissues, which may displace the trait means of populations along the minor axis (i.e., z1 relatively more sensitive in lower right population, z2 relatively more sensitive in upper left population) (Fig. 1C). Yet another possibility is that phenotypic independence could be achieved by an evolutionary loss of sensitivity in one target tissue (loss of sensitivity in z1 in upper left population, loss of sensitivity in z2 in lower right population) (Fig. 1D). This would allow populations to diverge in any direction, as the two traits are no longer correlated by the hormonal signal. Of course, these evolutionary pathways are not mutually exclusive. We know of no examples, as yet, that reveal the relative frequency of these modes of divergence with respect to hormones, but note that others have addressed the nature of selection on complex characters in relation to morphology or morphology and performance (Arnold 1983; Blows 2007; Irschick et al. 2008).
Fig. 1.
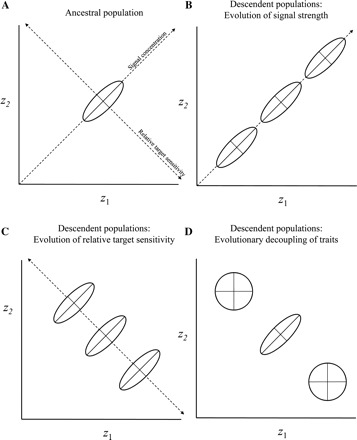
Relationships between two phenotypic variables mediated by the same hormone. In (A) two traits in a hypothetical population, z1 and z2, are correlated, as represented by the ellipse. Mechanistically, the correlation is mediated by variation in a signal (e.g., hormone concentration), which determines the major axis of the ellipse. The minor axis of the ellipse is determined by variation in the relative sensitivity of the target tissues involved in the expression of the trait. In other words, residual variation is mediated by the extent to which the tissues involved in z1 expression differ in sensitivity from those involved in z2 expression. In (B–D), the ancestral population has given rise to two new populations where evolution of the hormonal suite has occurred. In (B), only the strength of the signal has evolved. In each descendant population, the means of z1 and z2 have evolved in the same direction, such that population means are correlated across populations in the same way as are individuals within populations. Note that such a pattern could also occur by coordinated evolution of target sensitivities (i.e., both z1 and z2 tissues become more or less sensitive). In (C), evolution has occurred primarily by adjusting the relative sensitivity of targets. In the right-hand population, the target(s) that affect z1 have become more sensitive while the target(s) that affect z2 have become less sensitive; and in the left-hand population, the target(s) that affect z2 have become more sensitive while the target(s) that affect z1 have become less sensitive. The means of z1 and z2 have evolved in opposite directions. Co-variation within populations is unchanged, but population means are no longer correlated in the same way. In (D), one trait has become dissociated from the hormonal suite altogether, perhaps by loss of sensitivity of the target to the signal. The two traits are no longer correlated in either of the derived populations, and the means of the two traits have evolved in opposite directions.
It is important to note that endocrinologists have generally remained skeptical about the predictive power of hormone signal-phenotype relationships among individuals (Adkins-Regan 2005; Hau 2007; Ball and Balthazart 2008; Fusani 2008; Williams 2008). Hormones are inherently variable enabling plastic responses to changing environments. The prevailing view among endocrinologists has been that plasma hormones are “permissive”. In this view, once hormone concentration exceeds a threshold, further elevation of the hormone does not enhance expression of the trait (Adkins-Regan 2005, 2008; Ball and Balthazart 2008; Williams 2008). Even apparent co-variation between hormone levels and phenotype can be explained by supposing individual variation in thresholds, i.e., sensitivity (Hews and Moore 1997). The skepticism derives support from “negative” data in which hormone levels and phenotype have not been correlated (Adkins-Regan 2005), and from studies of castrates in which the same dose of hormone has elicited very different behavioral responses (Ball and Balthazart 2008). Clearly there is more to learn.
Population comparisons of variation in hormonal responsiveness and in testosterone-mediated characters
Debate surrounds the question of whether adaptive divergence among populations can be retarded by hormonal mediation of suites of characters (Hau 2007; Adkins-Regan 2008; Lessells 2008; McGlothlin and Ketterson 2008). On the one hand, the diversity of hormonal mechanisms in nature is incontrovertible evidence that relationships can be overcome, given enough time (Adkins-Regan 2008). On the other hand, females often express male-typical traits at lower levels, and specific examples, e.g., the shape of the female spotted hyena's enlarged phallus, suggest a degree of inertia when it comes to the evolution of hormone-mediated development (Frank 1997; Drea et al. 1998; Emerson 2000).
Arguments and theory based on quantitative genetics note the retarding effect of strong genetic (and thus perhaps hormonal) correlations on evolutionary outcomes (McGlothlin and Ketterson 2008). In this view, phenotypic correlations that are built-up through correlational selection in one environment may prove at least a temporary impediment to an adaptive evolutionary response to a changed environment. Hau (2007) recommended that we approach these issues by comparing subspecies or populations. Because evolutionary endocrinology is such a young discipline (Zera et al. 2007), relatively few direct comparisons of hormonally mediated characters and their underlying mechanisms have been made across populations, but see Emerson and Hess (1996), Moore et al. (2005) and Sparkman et al. (2009). More such comparisons should prove fruitful.
Variation in sensitivity of target tissues to testosterone in relation to phenotypic integration and independence
Resolution of the relative importance of integration and independence will require more knowledge of sources of variation in the mechanisms underlying variation in target sensitivity at multiple levels (Kabelik et al. 2006, 2008). Recent advances have provided methods, some quantitative, for assessing sensitivity (Ball and Balthazart 2008). Immunocytochemistry (ICC) can, for example, detect variation in gene products and thus in the density of hormone receptors, and in situ hybridization can detect variation in mRNAs and thus in variation of transcripts for hormone receptors and steroid metabolizing enzymes (Silverin et al. 2004; Forlano et al. 2006; Ball and Balthazart 2008).
Two recent studies have used in situ hybridization to compare seasonal and sexual differences in steroid sensitivity in key nuclei of the brain (Canoine et al. 2007; Voigt and Goymann 2007). Spotted antbirds are aggressive during both the breeding and nonbreeding season, despite nonbreeding season declines in testosterone. Apparently sensitivity of the brain increases in ways that compensate: androgen receptor (AR) transcript in the nucleus taeniae (nT), a brain nucleus associated with aggression, increases during the nonbreeding season. Similarly, in black coucals, a polyandrous African bird species that exhibits “sex role reversal”, females are more aggressive and less parental than are males. Importantly, males have higher circulating levels of testosterone, whereas females exhibit greater AR mRNA expression in the (nT) (Voigt and Goymann 2007), further apparent evidence of phenotypic independence.
Most studies investigating the mechanistic links between hormone signals and target phenotypes, to date, have used ICC or in situ hybridization to compare variation among groups (i.e., between sexes or between breeding versus nonbreeding individuals). Characterizing individual variation in the mechanistic links along hormone-signaling pathways should be an important next step in understanding the evolutionary significance of hormone–phenotype relationships. For example, one question discussed in greater detail below is whether signal concentrations and receptor densities consistently co-vary within individuals (integration) or whether signal and receptors may respond independently to modulate the expression of a performance trait (independence).
Relationship of past and present junco research to phenotypic integration and independence
We study an abundant north-temperate songbird, the dark-eyed junco (Junco hyemalis) that has received intensive study (e.g., Deviche et al. 2000; Nolan et al. 2002; Meddle et al. 2006; Mila et al. 2007). Juncos are quantitatively dimorphic in wing length and amount of white in the outer tail feathers (tail white), a trait that when manipulated enhances male but not female attractiveness and social status in winter (Holberton et al. 1989; Hill et al. 1999; Wolf et al. 2004; McGlothlin et al. 2005). The sexes differ qualitatively in vocal behavior (typically only males sing) and parental behavior (only females incubate). Juncos are territorial when breeding and form socially monogamous bonds, but they frequently produce young via extra-pair fertilizations (∼24%) (Raouf et al. 1997; Ketterson et al. 1998; Reed et al. 2006; Price et al. 2008). Females build the nest (clutch size, 3–4; brood number, 1–3); both sexes care for nestlings and fledglings. Nest predation is common in most years; site fidelity is nearly complete among males, less so among females, and least among offspring (∼15%) (Nolan et al. 2002).
Recent molecular data suggest that the six to eight sub-species of junco have radiated from a common ancestor during only the past 10,000 years; yet juncos exhibit marked geographic divergence in plumage coloration, morphometric measures, and life-history traits such as duration of the breeding season and migratory behavior (see Fig. 2) (Nolan et al. 2002; Mila et al. 2007).
Fig. 2.
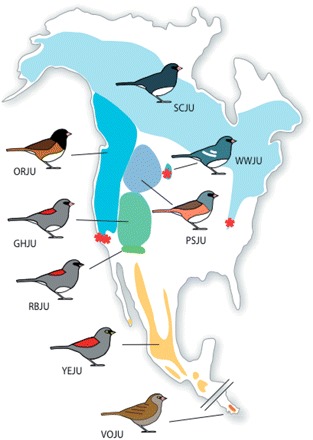
Phenotypic variation across the geographic range of Junco. The colored areas on the map represent breeding ranges of eight genetically differentiated groups of juncos abbreviated as follows (clockwise from the south): VOJU, volcano junco, dark-orange; YEJU, yellow-eyed junco, yellow-orange; RBJU, red-backed junco, green; GHJU, grey-headed junco, green; ORJU, Oregon junco, bright blue; SCJU, slate-colored junco, light blue; WWJU, white winged junco, medium blue; PSJU, pink-sided junco, grey-blue (after Mila et al. 2007) (© 2007 Royal Society). Asterisks indicate populations studied by our research group in CA, SD, and VA.
We have studied the Carolina subspecies of the slate-colored junco, J. hyemalis carolinensis, in Virginia (VA) for many years, and have recently begun to study junco populations in California (CA, J. hyemalis thurberi) and South Dakota (SD, J. hyemalis aikini). In CA, we focus on two populations of the Oregon junco that have been the subject of recent and important studies by Price and colleagues (Rasner et al. 2004; Yeh 2004; Yeh and Price 2004; Price et al. 2008). In SD, we study the white-winged junco, an endemic to the Black Hills, which is distinctive in being the largest sub-species of junco and also the one that has the most white in the tail (Nolan et al. 2002; Fig. 2).
Our research has progressed in three stages. The first stage involved experimental manipulation of male phenotypes using testosterone (T) implants; the second stage involved similar studies on females; and the third stage related individual variation in male testosterone to phenotypic characters identified as hormone-sensitive in the first stage. All stages were directed toward increasing understanding of how hormone-mediated characters evolve.
Studies of juncos involving experimental elevation of testosterone
Experimental elevation of T in male juncos led to the conclusion that testosterone plays a potentially key role in trade-offs between mating effort and parental effort and between reproductive effort and self-maintenance. This work has been summarized a number of times and we will not repeat details here (Ketterson et al. 1996, 2001, 2005; Ketterson and Nolan 1999). Briefly these manipulations indicated that male juncos with higher-than-normal levels of testosterone should out-compete those with typical levels owing to greater extra-pair mating success that outweighed the cost of greater mortality (Reed et al. 2006). The fact that higher T-levels have not evolved led to the “constraint hypothesis”, which posited that phenotypic, and potentially genetic, correlations between the sexes might retard the evolution of higher testosterone in males (Clotfelter et al. 2004).
To address this alternative, we elevated testosterone experimentally in females and asked whether T-treated females exhibited male-typical traits or trait values, and, if so, whether these traits put them at a fitness disadvantage. The implanting phase of the research, now completed, has shown that T enhances female aggression, suppresses immune function, and elevates corticosterone and corticosterone-binding globulin (Zysling et al. 2006). T had no detectable effect on incubation or defense of eggs against predators, initially suggesting that unlike in males, parental behavior in females is insensitive to experimentally elevated T (Clotfelter et al. 2004). Recently, however, we found that while T does not suppress feeding of nestlings, it does suppress defense of nests during the nestling period (O’Neal et al. 2008).
With respect to fitness of females, the strongest determinants of fecundity are likely to be whether a female initiates a nest and how successful she is at escaping nest predators. To date, females treated with testosterone initiated fewer nests than did females treated as controls (49% of 80 T-females versus 66% of 97 C-females, chi-square, P < 0.003, ED Ketterson, unpublished data), an observation which suggests that treatment with T suppresses initiation of reproduction. However, reproduction was not suppressed in all females. This observation is highly relevant to issues raised by integration and independence because it suggests variation in females’ sensitivity to treatment with T.
For those females that laid eggs, clutch size did not differ between treatments (ED Ketterson, unpublished data). Significantly, however, nest success was suppressed by testosterone treatment (O’Neal et al. 2008). Of females that nested, the percentage of females producing at least one fledgling from a nest was greater in controls (C-females) than in T-treated females (49% of 92 C-females 30% of 63 T-females). Finally with respect to viability, T does not appear to influence survival (annual return, T-females 32% of 81 T-females, 34% or 95 C-females), but analysis of data is incomplete. Data on extra-pair parentage are still forthcoming.
To return to our stated goal, which was to see whether experimental elevation of T in females supported integration or independence, and thus whether the sexes were free to evolve independently, we compared the traits that we studied in both sexes (Table 1). The comparison revealed traits that were similarly affected in both males and females (defense of offspring, immune function, corticosterone, and molt), as well as traits that were not (provisioning of offspring).
Table 1.
Comparing sexes for sensitivity/insensitivity to experimentally elevated T (+, treatment enhanced mean trait value, −, treatment reduced mean trait value)
| Trait | Males | Females | Source |
|---|---|---|---|
| Attractiveness | + | − | (Enstrom et al. 1997; Ketterson et al. 2005) |
| Song | + | n/aa | (Ketterson et al. 1992; n/a) |
| Incubate eggs | n/a | nddb | (n/a; Clotfelter et al. 2004) |
| Feed offspring | − | ndd | (Ketterson et al. 1992; Schoech et al. 1998; Clotfelter et al. 2004; O’Neal et al. 2008) |
| Defend offspring | − | − | (Cawthorn et al. 1998; O’Neal et al. 2008) |
| Immune function | − | − | (Casto et al. 2001; Zysling et al. 2006) |
| Corticosterone, CBGc | + | + | (Klukowski et al. 1997; Zysling et al. 2006) |
| Molt | − | − | (Nolan et al. 1992; Clotfelter et al. 2004) |
an/a, not applicable because trait is typically expressed in only one sex; T-treated females occasionally sang but frequency was not quantified; males do not incubate; bndd, no (statistically) detectable difference; cCBG, corticosteroid binding globulin.
The combined studies of implanted males and females provide evidence for phenotypic integration of the functional relationship between T and immune function, stress physiology, molt and nest-defense behavior. However, the data also provide evidence for phenotypic independence with regard to the relationship between T and feeding of offspring, as well as morphology and behavior associated with attractiveness to mates. The studies also suggest that prolonged natural elevation of T in females might reduce fecundity by suppressing reproduction or by increasing nest predation, but that not all females are equally affected.
We conclude that the evidence is consistent with both integration and independence, but the impact may be one of constraint in that evolution of testosterone-mediated characters in male juncos may well be restricted by the potential impact that this might have on phenotype and fitness in females.
Studies of juncos addressing natural variation in signal strength
There are, of course, limitations to the conclusions that can be drawn from studies employing implants (Zera et al. 2007; Fusani 2008). Implants can interfere with homeostatic mechanisms that normally regulate hormone secretion and with normal interactions among hormones. This is particularly true if implanting animals creates distributions of variation in hormone signal or target traits that lie outside the normal range of variation. Hence a fuller understanding of the maintenance of variation in male and female testosterone requires that we assess natural variation in circulating testosterone and relate that variation to survival and reproductive success.
To assess natural variation in testosterone in male juncos, we chose to measure the responsiveness of the hypothalamo-pituitary-gonadal (HPG) axis, using methods described by Jawor et al. (2006). We challenged males and females with GnRH, which stimulates release of luteinizing hormone (LH), which in turn stimulates release of T by the gonads. Briefly, we collected an initial blood sample (initial T), injected the pectoral muscle with a solution of GnRH-I, and exactly 30 min after the injection took a second blood sample to measure post-challenge T. The difference between initial T and post-challenge T was computed as the rise in T in response to GnRH (Jawor et al. 2006). Importantly, testosterone levels produced after a GnRH challenge are repeatable (Jawor et al. 2006) and are correlated with natural increases in testosterone produced in response to a simulated territorial intrusion (McGlothlin et al. 2008).
Published results on juncos in VA reveal significant among-male covariation in testosterone and testosterone-mediated characters. Specifically, males that readily elevate testosterone in response to physiological or social stimulation are more aggressive, more ornamented, and less parental. Conversely, males that less readily elevate testosterone are less aggressive, less ornamented, and more parental (Fig. 3). Further, males with higher initial levels of testosterone have less robust immune function (Fig. 3). Note that the relationship of the phenotypic characters to the measures of T varies. In some cases the tightest correlation was with initial T, in others with post-challenge T, and in still others with rise in T in response to GnRH. These variables are highly inter-correlated, and we do not yet know what meaning to ascribe to the variability in which correlation with phenotype was the strongest.
Fig. 3.
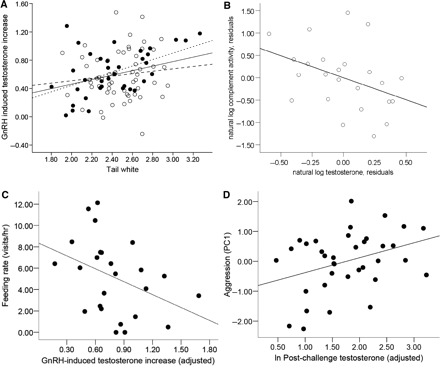
Individual covariation between testosterone and associated traits in male slate-colored (Carolina) juncos from VA. Testosterone in response to a GnRH challenge co-varied positively with tail white (A; McGlothlin et al. 2008) (© 2007 European Society of Evolutionary Biology), negatively with complement immune activity (B; Grieves et al. 2006) (© 2006 British Ecological Society), positively with aggressive behavior (C; McGlothlin et al. 2007) (© 2007 by the University of Chicago) and negatively with parental behavior (D; McGlothlin et al. 2007) (© 2007 by The University of Chicago).
Importantly, these results provide surprisingly strong qualitative validation of the conclusions drawn from the experimental implant studies, which revealed that T enhances phenotypic traits related to mating effort at the cost of traits related to parental effort and self-maintenance. The newer results also leave unanswered questions.
One critical unanswered question is the nature of the relationship between T in response to GnRH and fitness as measured by survival, mating success, and fecundity. Results of our implant studies (Reed et al. 2006), which showed reduced survivorship in T-treated males, would predict directional viability selection opposing males with high levels of T in response to GnRH. However, because we have no experimental data on males with lower-than-average circulating levels of testosterone, an alternative and equally proper prediction would be that viability selection on T in response to GnRH should be stabilizing. The experimental data revealing greater mating success of T-treated males would predict positive directional sexual selection on T in response to GnRH (Reed et al. 2006). Data analyzed thus far from two breeding seasons indicate that survival selection on the change in T in response to GnRH is indeed stabilizing, acting to maintain this response at intermediate values (J.W. McGlothlin et al., manuscript in preparation; Fig. 4). Analyses of viability and fecundity selection on T in response to GnRH are still underway (J.W. McGlothlin et al., manuscript in preparation).
Fig. 4.
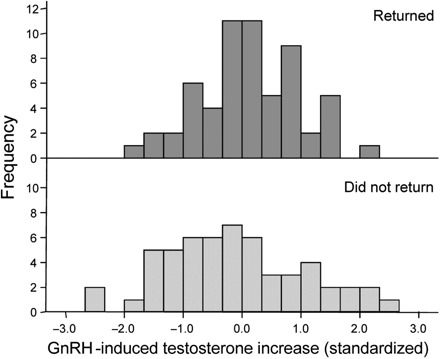
Frequency distributions represent T in response to GnRH (values normalized to the mean) for individual males that returned in the following year (“returned”, top) to those that failed to return (“did not return”, bottom). Variance in the distribution of nonreturners was greater, and selection was stabilizing (significance of stabilizing selection gradients was tested using mixed model with binomial errors; J.W. McGlothlin et al., manuscript in preparation).
Comparing junco populations for variation in T in response to GnRH and testosterone-mediated characters, preliminary findings
A goal of our recent and largely preliminary research has been to determine whether patterns of natural variation in T observed within one population will generalize to other populations of the junco and in so doing to ask whether the results provide support for phenotypic integration or independence. The working hypothesis under phenotypic integration is that hormone–phenotype relationships will be stable across populations and can be used to predict among-population variation in the levels of expression of T-mediated traits. Alternatively, under independence, hormone–phenotype relationships will vary among populations. Integration would be supported by situations in which a change in signal strength indicates a change in phenotype; independence would be supported by a situation in which the same signal strength induces different phenotypic values across populations (Fig. 1). These predictions can be stated in terms more particular to the system being studied.
Prediction 1: Under integration, the pattern of co-variation between T in response to GnRH and phenotype documented in VA predicts similar relations in other populations, i.e., males with stronger responses to GnRH will have whiter tails, be more aggressive and less parental. Under independence, T in response to GnRH need not co-vary with phenotype as observed in VA.
The CA populations are particularly interesting because one of them colonized an urban environment only very recently (∼1980), and based on common garden results has undergone rapid evolution in the direction of reduced amount of white in the tail and of smaller body size when compared to a nearby ancestral population (Yeh 2004). The CA populations also differ in aggressiveness and vocal behavior (Newman et al. 2006, 2008), breeding phenology (Price et al. 2008) and parental behavior (J.W. Atwell et al., manuscript in preparation). In fact, the complex of traits expressed in the urban CA population that colonized the University of California San Diego is just what would be predicted if the suite of character changes from the ancestral population were achieved by a dialing down of T in response to GnRH, as would be predicted by phenotypic integration. This leads to Prediction 2.
Prediction 2: Within-population relationships between T in response to GnRH and amount of white on the tail in VA may be used to predict that among populations, rise in T should be greater in populations with greater extent of tail white and less in populations with lesser amount of white. Similarly, aggression should be higher in the population with more white in the tail (SD) and less in the population with the lesser amount (CA-colonist). Conversely, based on the VA relationship between tail white and T in response to GnRH, parental behavior should be lower in SD and higher in the CA-colonist (see Table 2).
Table 2.
Mean values for tail white, parental behavior, and aggression across populations and in relation to T in response to GnRH
| Trait | VA | CA-A | CA-C | SD |
|---|---|---|---|---|
| Tail white (sum) | 2.29a | 2.64f | 2.21f | 3.57h |
| Tail white (%) | 38a | 44f | 37f | 60h |
| Parental behavior (visits/h) | 4.3b | 2.53g | 3.82g | – |
| Aggression (latency in sec, songs/10 min) | 115c, 54c | – | – | – |
| T, rise spring (ng/ml) | 4.2d | 7.2g | 6.1g | 5.9i |
| T, post STI (ng/ml) | 8.3e | 6.0g | 2.5g | – |
Data from VA are published; data for CA and SD are still preliminary and are currently under analysis by J.W. Atwell, C. Bergeon Burns, and K. Cain.
VA (a–e): a(Wolf et al. 2004); b(Ketterson et al. 1992);c(McGlothlin et al. 2007); d(Jawor et al. 2006); e(McGlothlin et al. 2008). CA-A and CA-C: (f–g); f(Yeh 2004); g(Atwell et al. in preparation, data still preliminary; SD (h–i); h(Ketterson and Nolan, unpubl.); i(Bergeon, Cain, et al. unpubl., data quite preliminary).
Tail white (sum) = proportion of tail feathers that are white, summed over right side of tail; for CA we took published percentage times 6, then converted to a proportion. Tail white = computed percentage of each feather that is white, summed over right side, divided by 6; for VA we took published sum of proportions, converted to a percentage, divided by 6. Parental behavior = feeding nestlings; aggressive behavior = behavioral response after an STI; T, rise spring = increase in T after GnRH challenge; T, post STI = T upon capture after an STI.
Thus far we have challenged more than 150 males with GnRH in CA and more than 80 males in SD. We have yet to determine whether T in response to GnRH co-varies with traits, as would be predicted from relationships in VA (Prediction 1), although preliminary data from SD indicates that tail white is greater in males with greater increases in T (Bergeon Burns and Cain, preliminary data). We report this not as conclusive but as indicative of the kinds of specific predictions that will be tested.
Information regarding Prediction 2 is presented in Table 2. The data from the two California populations support Prediction 2. T in response to GnRH and T after a simulated territorial intrusion (STI) are greater in CA-A than CA-C, as is tail white, while parental behavior is lower. Comparing SD to VA, T in response to GnRH and tail white are both higher in SD, as would be predicted. However, if we rank all four populations with respect to tail white, the match with T in response to GnRH is not in the predicted rank order. More observations will provide greater confidence in any patterns, as some of these data are quite preliminary; but to date we see support for both integration and independence.
Future studies of juncos assessing variation in sensitivity of target tissues to testosterone
We have repeatedly made the point that hormonally mediated characters can differ in expression owing to differences in signal strength and/or target sensitivity, but we know surprisingly little about which occurs more frequently in nature (but see Silverin et al. 2004). Future research on the junco will attempt to determine how individuals shown to be strong or weak responders to GnRH vary along each level of the HPG. That is, are strong responders at the level of the pituitary, also strong responders at the level of the gonad (integration), or are they not (independence)? Said another way, where along the HPG axis does individual variation reside, e.g., do individuals vary in the pituitary's response to GnRH (as measured by LH output in response to GnRH) or in the gonad's response to LH (as measured by T output in response to LH), or both? Under phenotypic integration, we would predict a correlation between secretion of LH in response to GnRH and secretion of T in response to LH; under phenotypic independence we predict no necessary association or even a negative correlation.
A second question we propose to ask is whether strong responders as measured by T after injection with GnRH are also more sensitive to T, as measured by density or transcription of hormone receptors in key target tissues in the brain (integration), or is there no predictable relationship between hormone signal and receptor density (independence)? Future research will compare neural sensitivity of strong and weak responders to GnRH by visualizing and quantifying neurons containing receptor proteins using immunocytochemistry and in situ hybridization. Co-variation between signal (T in response to GnRH) and target (number of target cells and intensity of hybridization of receptor/enzyme) will be taken as evidence for the existence of strong and weak responders “system-wide”. That is, individuals producing higher levels of T in response to GnRH would also be transcribing higher quantities of androgen receptor (AR) or mRNA for AR, i.e., they would have both strong signal and high sensitivity. On the assumption that within-population comparisons of strong and weak male responders are revealing, future studies could compare males to females within populations, and males to males from closely related populations.
An unresolved question is whether conversion of a circulating signal to an active signal constitutes integration or independence. That is, if strong responders are found to have higher levels of estrogen receptor or aromatase in target tissues, thereby indicating effective conversion of testosterone to estradiol, should that be taken as evidence of integration (more testosterone from the gonad promotes higher phenotypic values) or independence (the target tissue's response is induced by a different hormone)? Again, these are questions for future research and discussion and genomic approaches may prove extremely helpful (Mank et al. 2008).
Conclusions
This article has attempted to contrast the evolutionary implications of tight hormone signal-driven integration, which can constrain or facilitate adaptive evolution, with target-based independent evolution of hormone-mediated traits, which can be beneficial by allowing individual traits to evolve separately from a network of traits, but also detrimental because building an organism one part at a time can be slow and inefficient. Both modes of change (signal-driven, target-based) have disadvantages and advantages in promoting adaptation, depending on whether the organism will benefit from change in groups of traits or one trait at a time.
The research described here compared resemblance between male and female dark-eyed juncos in their phenotypic sensitivity to experimentally elevated testosterone. Results provided insight into the potential for both direct and correlated responses to selection. Data relating natural variation in T to phenotype and fitness provided further insight into how hormonally mediated characters can be expected to evolve and into the role they play in phenotypic integration and independence. Comparisons across populations have the potential to reveal whether hormonal traits are tightly integrated in their expression or are more independent. Mechanistic studies relating signal and target will also increase our understanding of the relative importance of integration and independence in evolutionary change.
Funding
The National Science Foundation supported this research (NSF BSC-05-19211 to E.D.K., DEB-0808284 to J.W.A., DEB-0508692 to J.W.M.), as did the National Institutes of Health (NICHD T32-HD-49339) and the Indiana University Faculty Research Support Program (FRSP).
Acknowledgments
We thank the Society for Integrative and Comparative Biology, especially the Divisions of Animal Behavior, Comparative Endocrinology, and Vertebrate Morphology, for providing logistical and financial support. We also thank the National Science Foundation for providing financial support of the symposium. The research was conducted in compliance with Indiana University IACUC protocol 06-242. The authors thank the many people whose ideas, editing, and help in the field were crucial. In particular, we are indebted to Christy Bergeon Burns, Gonçalo Cardoso, Amy Dapper, Nicki Gerlach, Dawn O’Neal, and Eric Snajdr. For critical readings we thank Creagh Breuner, Ela Hau, Jerry Husak, Duncan Irschick, Sharon Lynn, Trevor Price, and an anonymous reviewer; for figure 2 we thank Borja Mila. In particular we thank Ela Hau for stimulating our thinking.
References
- Adkins-Regan E. Hormones and animal social behavior. Princeton: Princeton University Press; 2005. [Google Scholar]
- Adkins-Regan E. Do hormonal control systems produce evolutionary inertia? Philos Trans R Soc B: Biol Sci. 2008;363:1599–609. doi: 10.1098/rstb.2007.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal AF, Stinchcombe JR. How much do genetic covariances alter the rate of adaptation? Proc R Soc B: Biol Sci. 2009;276:1183–91. doi: 10.1098/rspb.2008.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S. Morphology. Perform Fitness. Am Zool. 1983;23:347–61. [Google Scholar]
- Ball GF, Balthazart J. Individual variation and the endocrine regulation of behaviour and physiology of birds: a cellular/molecular perspective. Philos Trans R Soc B: Biol Sci. 2008;363:1699–710. doi: 10.1098/rstb.2007.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Adkins-Regan. E. Hormones, brain and behavior. London: Academic Press; 2002. Sexual differentiation of brain and behavior in birds; pp. 223–301. [Google Scholar]
- Bass MB, Lester D. Genetic-analysis of sensitivity to ethanol-induced depression of motor-activity and impairment of swimming in rats. Behav Genet. 1983;13:77–89. doi: 10.1007/BF01071745. [DOI] [PubMed] [Google Scholar]
- Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA. Stress response during development predicts fitness in a wild, long lived vertebrate. Proc Natl Acad Sci USA. 2007;104:8880–4. doi: 10.1073/pnas.0700232104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blows MW. A tale of two matrices: multivariate approaches in evolutionary biology. J Evol Biol. 2007;20:1–8. doi: 10.1111/j.1420-9101.2006.01164.x. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Patterson SH, Hahn TP. In search of relationships between acute adrenocortical response and fitness. Gen Comp Endocrinol. 2008;157:288–95. doi: 10.1016/j.ygcen.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Canoine V, Fusani L, Schlinger BA, Hau M. Low sex steroids, high steroid receptors: increasing the sensitivity of the nonreproductive brain. Dev Neurobiol. 2007;67:57–67. doi: 10.1002/dneu.20296. [DOI] [PubMed] [Google Scholar]
- Cawthorn JM, Morris D, Ketterson ED, Nolan V., Jr Influence of elevated testosterone on nest defence in dark-eyed juncos. Anim Behav. 1998;56:617–621. doi: 10.1006/anbe.1998.0849. [DOI] [PubMed] [Google Scholar]
- Casto JM, Nolan V, Jr, Ketterson ED. Steroid hormones and immune function: experimental studies in wild and captive dark-eyed juncos (Junco hyemalis) American Naturalist. 2001;157:408–420. doi: 10.1086/319318. [DOI] [PubMed] [Google Scholar]
- Clotfelter ED, O’Neal DM, Gaudioso JM, Casto JM, Parker-Renga IM, Snajdr EA, Duffy DL, Nolan V, Jr, Ketterson ED. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm Behav. 2004;46:171–9. doi: 10.1016/j.yhbeh.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Comendant T, Sinervo B, Svensson EI, Wingfield JC. Social competition, corticosterone and survival in female lizard morphs. J Evol Biol. 2003;16:948–55. doi: 10.1046/j.1420-9101.2003.00598.x. [DOI] [PubMed] [Google Scholar]
- Conner JK. Genetic mechanisms of floral trait correlations in a natural population. Nature. 2002;420:407–10. doi: 10.1038/nature01105. [DOI] [PubMed] [Google Scholar]
- Conner JK, Sterling A. Selection for independence of floral and vegetative traits: evidence from correlation patterns in five species. Can J Bot-Rev Can Bot. 1996;74:642–4. [Google Scholar]
- Deviche P, Wingfield JC, Sharp PJ. Year-class differences in the reproductive system, plasma prolactin and corticosterone concentrations, and onset of prebasic molt in male dark-eyed juncos (Junco hyemalis) during the breeding period. Gen Comp Endocrinol. 2000;118:425–35. doi: 10.1006/gcen.2000.7478. [DOI] [PubMed] [Google Scholar]
- Drea CM, Weldele ML, Forger NG, Coscia EM, Frank LG, Licht P, Glickman SE. Androgens and masculization of genitalia in the spotted hyaena (Crocuta crocuta). 2. Effects of prenatal anti-androgens. J Reprod Fertil. 1998;113:117–27. doi: 10.1530/jrf.0.1130117. [DOI] [PubMed] [Google Scholar]
- Ducrest A-L, Keller L, Roulin A. Pleiotropy in the melanocortin system, coloration, and behavioural syndromes. Trends Ecol Evol. 2008;23:502–10. doi: 10.1016/j.tree.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Emerson SB. Vertebrate secondary sexual characteristics—physiological mechanisms and evolutionary patterns. Am Nat. 2000;156:184–91. doi: 10.1086/303370. [DOI] [PubMed] [Google Scholar]
- Emerson SB, Hess DL. The role of androgens in opportunistic breeding, tropical frogs. Gen Comp Endocrinol. 1996;103:220–30. doi: 10.1006/gcen.1996.0113. [DOI] [PubMed] [Google Scholar]
- Enstrom DA, Ketterson ED, Nolan V., Jr Testosterone and mate choice in the dark-eyed junco. Anim Behav. 1997;54:1135–1146. [Google Scholar]
- Evans MR, Roberts ML, Buchanan KL, Goldsmith AR. Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. J Evol Biol. 2006;19:343–52. doi: 10.1111/j.1420-9101.2005.01034.x. [DOI] [PubMed] [Google Scholar]
- Flatt T, Tu M-P, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Schlinger BA, Bass AH. Brain aromatase: new lessons from non-mammalian model systems. Front Neuroendocrinol. 2006;27:247–74. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Frank LG. Evolution of genital masculinization: why do female hyaenas have such a large ‘penis?’. Trends Ecol Evol. 1997;12:58–62. doi: 10.1016/s0169-5347(96)10063-x. [DOI] [PubMed] [Google Scholar]
- Fusani L. Endocrinology in field studies: problems and solutions for the experimental design. Gen Comp Endocrinol. 2008;157:249–53. doi: 10.1016/j.ygcen.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Gill SA, Alfson ED, Hau M. Context matters: female aggression and testosterone in a year-round territorial neotropical songbird (Thryothorus leucotis) Proc R Soc B. 2007;274:2187–94. doi: 10.1098/rspb.2007.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greives TJ, McGlothlin JW, Jawor JM, Demas GE, Ketterson ED. Testosterone and innate immune function inversely covary in a wild population of breeding Dark-Eyed Juncos (Junco hyemalis) Func Ecol. 2006;205:812–818. [Google Scholar]
- Gross W, Siegel PB. Selective breeding of chickens for corticosterone response to social stress. Poultry Sci. 1985;64:2230–33. doi: 10.3382/ps.0642230. [DOI] [PubMed] [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays. 2007;29:133–44. doi: 10.1002/bies.20524. [DOI] [PubMed] [Google Scholar]
- Hews DK, Moore MC. Hormones and sex-specific traits: critical questions. In: Beckage N, editor. Parasites and pathogens: effects on host hormones and behavior. New York: Chapman & Hall; 1997. pp. 277–92. [Google Scholar]
- Hill JA, Enstrom DA, Ketterson ED, Nolan V, Jr, Ziegenfus C. Mate choice based on static versus dynamic secondary sexual traits in the dark-eyed junco. Behav Ecol. 1999;101:91–96. [Google Scholar]
- Holberton RL, Able KP, Wingfield JC. Status signaling in dark-yyed juncos, Junco hyemalis – plumage manipulations and hormonal correlates of dominance. Anim Behav. 1989;37:681–689. [Google Scholar]
- Irschick DJ, Meyers JJ, Husak JF, Le Galliard JF. How does selection operate on whole-organism functional performance capacities? A review and synthesis. Evol Ecol Res. 2008;10:177–96. [Google Scholar]
- Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE, Ketterson ED. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis) Gen Comp Endocrinol. 2006;149:182–9. doi: 10.1016/j.ygcen.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Kabelik D, Weiss SL, Moore MC. Steroid hormone mediation of limbic brain plasticity and aggression in free-living tree lizards, Urosaurus ornatus. Horm Behav. 2006;49:587–97. doi: 10.1016/j.yhbeh.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kabelik D, Weiss SL, Moore MC. Steroid hormones alter neuroanatomy and aggression independently in the tree lizard. Physiol Behav. 2008;93:492–501. doi: 10.1016/j.physbeh.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempenaers B, Peters A, Foerster K. Sources of individual variation in plasma testosterone levels. Philos Trans R Soc B. 2008;363:1711–23. doi: 10.1098/rstb.2007.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V., Jr Adaptation, exaptation, and constraint: a hormonal perspective. Am Nat. 1999;154:S4–25. doi: 10.1086/303280. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Jr, Cawthorn MJ, Parker PG, Ziegenfus C. Phenotypic engineering: using hormones to explore the mechanistic and functional bases of phenotypic variation in nature. Ibis. 1996;138:70–86. [Google Scholar]
- Ketterson ED, Parker PG, Raouf SA, Nolan V, Jr, Ziegenfus C, Chandler CR. The relative impact of extra-pair fertilizations on variation in male and female reproductive success in dark-eyed juncos (Junco hyemalis) Ornithol Monogr. 1998;49:81–101. [Google Scholar]
- Ketterson ED, Casto JM, Nolan V., Jr Elevated testosterone suppresses cell-mediated and humoral immunity in male dark-eyed juncos. Am Zool. 1999;39:28A. [Google Scholar]
- Ketterson ED, Nolan V, Jr, Casto JM, Buerkle CA, Clotfelter E, Grindstaff JL, Jones KJ, Lipar JL, McNabb FMA, Neudorf DL, Parker-Renga I, Schoech SJ, Snajdr E. Testosterone, phenotype, and fitness: a research program in evolutionary behavioral endocrinology. In: Dawson A, Chaturvedi C, editors. Avian Endocrinology. New Delhi, India: Narosa Publishing House; 2001. pp. 19–40. [Google Scholar]
- Ketterson ED, Nolan V, Jr, Sandell M. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am Nat. 2005;166:S85–98. doi: 10.1086/444602. [DOI] [PubMed] [Google Scholar]
- Klein SL, Hairston JE, DeVries AC, Nelson RJ. Social environment and steroid hormones affect species and sex differences in immune function among voles. Horm Behav. 1997;32:30–9. doi: 10.1006/hbeh.1997.1402. [DOI] [PubMed] [Google Scholar]
- Klukowski L, Cawthorn JM, Ketterson ED, Nolan V., Jr Effects of testosterone on corticosterone and corticosterone binding globulin in captive dark-eyed juncos (Junco hyemalis) Gen Comp Endocrin. 1997;108:141–151. doi: 10.1006/gcen.1997.6956. [DOI] [PubMed] [Google Scholar]
- Lessells CM. Neuroendocrine control of life histories: what do we need to know to understand the evolution of phenotypic plasticity. Philos Trans R Soc. 2008;363:1589–98. doi: 10.1098/rstb.2007.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn SE. Behavioral insensitivity to testosterone: why and how does testosterone alter paternal and aggressive behavior in some avian species but not others? Gen Comp Endocrinol. 2008;157:233–40. doi: 10.1016/j.ygcen.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Lynn SE, Hayward LS, Benowitz-Fredericks ZM, Wingfield JC. Behavioural insensitivity to supplementary testosterone during the parental phase in the chestnut-collared longspur, Calcarius ornatus. Anim Behav. 2002;63:795–803. [Google Scholar]
- Lynn SE, Walker BG, Wingfield JC. A phylogenetically controlled test of hypotheses for behavioral insensitivity to testosterone in birds. Horm Behav. 2005;47:170–7. doi: 10.1016/j.yhbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Mank JE. The evolution of sexually selected traits and antagonistic androgen expression in actinopterygiian fishes. Am Nat. 2007;169:142–9. doi: 10.1086/510103. [DOI] [PubMed] [Google Scholar]
- Mank J, Hultin-Rosenberg L, Zwahlen M, Ellegren H. Pleiotropic constraint hampers the resolution of sexual antagonism in vertebrate gene expression. Am Nat. 2008;171:35–43. doi: 10.1086/523954. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Ketterson ED. Hormone-mediated suites as adaptations and evolutionary constraints. Philos Trans R Soc B. 2008;363:1611–20. doi: 10.1098/rstb.2007.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin JW, Parker PG, Nolan V, Jr, Ketterson ED. Correlational selection leads to genetic integration of body size and an attractive plumage trait in dark-eyed juncos. Evolution. 2005;593:658–671. [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. American Naturalist. 2007;170:864–875. doi: 10.1086/522838. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Greives TJ, Casto JM, Phillips JL, Ketterson ED. Hormones and honest signals: males with larger ornaments elevate testosterone more when challenged. J Evol Biol. 2008;21:39–48. doi: 10.1111/j.1420-9101.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Wingfield JC, Millar RP, Deviche PJ. Hypothalamic GnRH-I and its precursor during photorefractoriness onset in free-living male dark-eyed Juncos (Junco hyemalis) of different year classes. Gen Comp Endocrinol. 2006;145:148–56. doi: 10.1016/j.ygcen.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Merilä J, Björklund M. Phenotypic integration as a constraint and adaptation. In: Pigliucci M, Preston K, editors. Phenotypic integration: studying the ecology and evolution of complex phenotypes. New York: Oxford University Press; 2004. pp. 107–29. [Google Scholar]
- Mila B, McCormack JE, Castaneda G, Waye RK, Smith TB. Recent postglacial range expansion drives the rapid diversification of a songbird lineage in the genus Junco. Proc R Soc B. 2007;274:2653–60. doi: 10.1098/rspb.2007.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. Gonadotropin hormone modulation of testosterone, immune function, performance, and behavioral trade-offs among male morphs of the lizard Uta stansburiana. Am Nat. 2008;171:339–57. doi: 10.1086/527520. [DOI] [PubMed] [Google Scholar]
- Mills S, Hazard L, Lancaster L, Mappes T, Miles D, Oksanen TA, Sinervo B. Gonadotropin hormone modulation of testosterone, immune function, performance, and behavioral trade offs among male morphs of the lizard Uta stansburiana. Am Nat. 2008;171:339–57. doi: 10.1086/527520. [DOI] [PubMed] [Google Scholar]
- Moller AP, Garamszegi LZ, Gil D, Eens M. Correlated evolution of male and female testosterone profiles in birds and its consequences. Behav Ecol Sociobiol. 2005;58:534–44. [Google Scholar]
- Moore IT, Bonier F, Wingfield JC. Reproductive asynchrony and population divergence between two tropical bird populations. Behav Ecol. 2005;16:755–62. [Google Scholar]
- Newman MM, Yeh PJ, Price TD. Reduced territorial responses in dark-eyed juncos following population establishment in a climatically mild environment. Anim Behav. 2006;71:893–9. [Google Scholar]
- Newman MM, Yeh PJ, Price TD. Song variation in a recently founded population of the dark-eyed juncos (Junco hyemalis) Ethology. 2008;114:164–73. [Google Scholar]
- Nijhout HF. Development and evolution of adaptive polyphenisms. Evol Dev. 2003;5:9–18. doi: 10.1046/j.1525-142x.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- Nolan V, Jr, Ketterson ED, Ziegenfus C, Chandler CR, Cullen DP. Testosterone and avian life histories: effects of experimentally elevated testosterone on molt and survival in male dark-eyed juncos. Condor. 1992;94:364–370. [Google Scholar]
- Nolan V, Jr, Ketterson ED, Cristol D, Rogers C, Clotfelter ED, Titus RC, Schoech S, Snajdr E. Dark-eyed Junco (Junco hyemalis) In: Poole A, Gill F, editors. The birds of North America. Vol. 716. Philadelphia, PA: The Birds of North America, Inc; 2002. [Google Scholar]
- O’Neal DM, Reichard DG, Pavilis K, Ketterson ED. Experimentally-elevated testosterone, female parental care, and reproductive success in a songbird, the dark-eyed Junco (Junco hyemalis) Horm Behav. 2008;54:571–8. doi: 10.1016/j.yhbeh.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Peters A. Testosterone treatment of female Superb Fairy-wrens (Malurus cyaneus) induces a male-like prenuptial moult, but no coloured plumage. Horm Behav. 2007;149:121–7. [Google Scholar]
- Pigliucci M. Studying mutational effects on G-matrices. In: Pigliucci M, Preston K, editors. Phenotypic integration: studying the ecology and evolution of complex phenotypes. New York: Oxford University Press; 2004. pp. 231–48. [Google Scholar]
- Pigliucci M, Preston K. Phenotypic integration: studying the ecology and evolution of complex phenotypes. Oxford University Press; 2004. [Google Scholar]
- Price TD, Yeh PJ, Harr B. Phenotypic plasticity and the evolution of a socially selected trait following colonization of a novel environment. Am Nat. 2008;172:S49–62. doi: 10.1086/588257. [DOI] [PubMed] [Google Scholar]
- Raouf SA, Parker PG, Ketterson ED, Nolan V, Jr, Ziegenfus C. Testosterone affects reproductive success by influencing extra-pair fertilizations in male dark-eyed juncos (Aves: Junco hyemalis) Proc R Soc B. 1997;264:1599–603. [Google Scholar]
- Rasner CA, Yeh P, Eggert LS, Hunt KE, Woodruff DS, Price TD. Genetic and morphological evolution following a founder event in the dark-eyed junco (Junco hyemalis thurberi) Mol Ecol. 2004;13:671–81. doi: 10.1046/j.1365-294x.2004.02104.x. [DOI] [PubMed] [Google Scholar]
- Reed WL, Clark ME, Parker PG, Raouf SA, Arguedas N, Monk DS, Snajdr E, Nolan V, Jr, Ketterson ED. Physiological effects on demography: a long-term experimental study of testosterone's effect on fitness. Am Nat. 2006;167:667–83. doi: 10.1086/503054. [DOI] [PubMed] [Google Scholar]
- Sandell MI. Exogenous testosterone increases female aggression in the European starling (Sturnus vulgaris) Behav Ecol Sociobiol. 2007;62:255–62. [Google Scholar]
- Schoech SJ, Ketterson ED, Nolan V, Jr, Sharp PJ, Buntin JD. The effect of exogenous testosterone on parental behavior, plasma prolactin, and prolactin binding sites in dark-eyed juncos. Horm Behav. 1998;341:1–10. doi: 10.1006/hbeh.1998.1455. [DOI] [PubMed] [Google Scholar]
- Schwenk K, Wagner GP. The relativism of constraints on phenotypic evolution. In: Pigliucci M, Preston K, editors. Phenotypic integration: studying the ecology and evolution of complex phenotypes. New York: Oxford University Press; 2004. pp. 390–408. [Google Scholar]
- Silverin B, Baillien M, Balthazart J. Territorial aggression, circulating levels of testosterone, and brain aromatase activity in free-living pied flycatchers. Horm Behav. 2004;45:225–34. doi: 10.1016/j.yhbeh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Sinervo B, Svensson E. Mechanistic and selective causes of life history trade-offs and plasticity. Oikos. 1998;83:432–42. [Google Scholar]
- Solís R, Penna M. Testosterone levels and evoked vocal responses in a natural population of the frog (Batrachyla taeniata) Horm Behav. 1997;31:101–9. doi: 10.1006/hbeh.1997.1366. [DOI] [PubMed] [Google Scholar]
- Sparkman AM, Vleck CM, Bronikowski AB. Evolutionary ecology of endocrine-mediated life-history variation in the garter snake Thamnophis elegans. Ecology. 2009;90:720–8. doi: 10.1890/08-0850.1. [DOI] [PubMed] [Google Scholar]
- Staub NL, DeBeer M. The role of androgens in female vertebrates. Gen Comp Endocrinol. 1997;108:1–24. doi: 10.1006/gcen.1997.6962. [DOI] [PubMed] [Google Scholar]
- Van Duyse E, Pinxten R, Eens M. Effects of testosterone on song, aggression, and nestling feeding behavior in male great tits, Parus major. Horm Behav. 2002;41:178–86. doi: 10.1006/hbeh.2001.1747. [DOI] [PubMed] [Google Scholar]
- Veiga JP, Polo V. Fitness consequences of increased testosterone levels in female spotless starlings. Am Nat. 2008;172:42–53. doi: 10.1086/587850. [DOI] [PubMed] [Google Scholar]
- Voigt C, Goymann W. Sex-role reversal is reflected in the brain of African Black Coucals (Centropus grillii) Dev Neurobiol. 2007;67:1560–73. doi: 10.1002/dneu.20528. [DOI] [PubMed] [Google Scholar]
- Westneat DF, Hasselquist D, Wingfield JC. Tests of association between the humoral immune response of red-winged blackbirds (Agelaius phoeniceus) and male plumage, testosterone, or reproductive success. Behav Ecol Sociobiol. 2003;53:315–23. [Google Scholar]
- Williams TD. Individual variation in endocrine systems: moving beyond the ‘tyrrany of the golden mean’. Philos Trans R Soci B: Biol Sci. 2008;363:1687–98. doi: 10.1098/rstb.2007.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield JC, Lynn SE, Soma KK. Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain Behav Evol. 2001;57:239–51. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- Wolf WL, Casto JM, Nolan V, Jr, Ketterson ED. Female ornamentation and male mate choice in dark-eyed juncos. Anim Behav. 2004;67:93–102. [Google Scholar]
- Yeh PJ. Rapid evolution of a sexually selected trait following population establishment in a novel habitat. Evolution. 2004;58:166–74. doi: 10.1111/j.0014-3820.2004.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Yeh PJ, Price TD. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am Nat. 2004;164:531–42. doi: 10.1086/423825. [DOI] [PubMed] [Google Scholar]
- Zera AJ, Harshman LG, Williams TD. Evolutionary endocrinology: the developing synthesis between endocrinology and evolutionary genetics. Annu Rev Ecol Evol Syst. 2007;38:793–817. [Google Scholar]
- Zysling DA, Greives TJ, Breuner CW, Casto JM, Demas GE, Ketterson ED. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis) Horm Behav. 2006;50:200–7. doi: 10.1016/j.yhbeh.2006.03.004. [DOI] [PubMed] [Google Scholar]


