Abstract
Chemotherapy plays an important role in the treatment of metastatic breast cancer. It is important to monitor chemotherapeutic efficacy, to find a simple and efficient tool to guide treatment, and to predict the efficacy of treatment in a timely and accurate manner. This study aimed to detect mucin-1 (MUC1)– positive circulating tumor cells and MUC1 protein in the peripheral blood of patients with metastatic breast cancer and to investigate their relationship to chemotherapeutic efficacy. MUC1 mRNA was detected in the peripheral blood of 34 patients with newly diagnosed metastatic breast cancer by reverse transcription-polymerase chain reaction. The positive rates of MUC1 mRNA were 88.2% before chemotherapy and 70.6% after chemotherapy, without a significant difference (P = 0.564); MUC1 mRNA expression before chemotherapy had no correlation with treatment effectiveness (P = 0.281). The response rate of MUC1 mRNA-negative patients after first-cycle chemotherapy was significantly higher (P = 0.009) and the progression-free survival (PFS) was clearly longer than those of MUC1 mRNA-positive patients (P = 0.095). MUC1 protein in peripheral blood plasma was detected by an ELISA competitive inhibition assay. The patients with decreased MUC1 protein after chemotherapy had a significantly longer PFS than those with elevated MUC1 protein (P = 0.044). These results indicate that the outcomes of MUC1 mRNA-negative patients after chemotherapy are better than those of MUC1 mRNA-positive patients. In addition, patients with decreased expression of MUC1 protein have a better PFS.
Keywords: Breast cancer, circulating tumor cells, MUC1, RT-PCR
Breast cancer is among the most common malignancies of women. The prevention and treatment of breast cancer has become a matter of public health concern in all countries. Improvements in mass screening and diagnosis, as well as the development of systematic and rational treatments, have reduced the mortality of breast cancer[1],[2]. However, approximately 30% of early breast cancer patients still develop distant recurrence[3]. Chemotherapy plays an important role in the treatment of metastatic breast cancer, especially for patients without hormone receptor, resistant to endocrine treatment, unlimited metastasis in the bone or soft tissue, or with symptomatic visceral metastasis. In clinical practice, the evaluation of chemotherapeutic efficacy mainly depends on clinical manifestation, radiological and serological parameters. However, these parameters are not consistently accurate. Therefore, it is important to find a simple and efficient tool to guide treatment and predict chemotherapeutic efficacy timely and accurate manner.
During the ch emotherapeutic treatment for metastatic breast cancer, an evaluation determining efficacy is necessary following two cycles of chemotherapy. Currently, the most widely accepted means of evaluation is a radiological examination. If clinical markers can be found, disclosing chemotherapeutic efficacy after one cycle, ineffective treatment can be avoided. Currently, numerous studies suggest circulating tumor cells (CTC) in breast cancer patients may provide important clues for the evaluation of chemotherapeutic efficacy. Nakamura et al.[4] reported CTC detected in peripheral venous blood could predict radiological evaluation, in which patients with more than 3 CTC in peripheral blood received a poor radiological evaluation. Importantly, CTC detection was earlier than radiological evaluation. Cristofanilli et al. [5] found the progression-free survival (PFS) of patients with more than 5 CTCs/7.5 mL of peripheral venous blood was significantly shorter than that of patients with less than 5 CTCs/7.5 mL (2.7 vs. 7.0 months, P < 0.05). In addition, their study found the overall survival (OS) was reduced, suggesting patients with CTCs may have a poor therapeutic response. Similar results have been observed in other studies[6]. Therefore, CTC detection may provide a means to evaluate the therapeutic efficacy of the metastatic breast cancer in an accurate and efficient manner.
Epithelial mucin-1 (MUC1), an effective marker for breast cancer CTC[7],[8], is located on chromosome 1q21 and encodes the MUC1 protein, whose function is still unclear. Nominal cellular expression of MUC1 is low, with abnormal expression in many epithelial tumors being important for the diagnosis, monitoring of recurrence and metastasis, and prognosis of tumors. Our study aimed to detect CTC by examining MUC1 expression in peripheral blood, and to explore the correlation between CTC and chemotherapeutic efficacy as well as PFS in metastatic breast cancer patients. In addition, our aim was to determine whether CTC could predict chemotherapeutic efficacy earlier than radiological examination, providing a diagnostic basis for therapeutic application and individualized treatment for breast cancer, thus avoiding ineffective treatment.
Patients and Methods
Patients
The 34 patients enrolled in our study were pathologically diagnosed with breast cancer and treated at the Tumor Hospital of Peking University between February 2009 and February 2010. All patients provided written informed consent before enrollment. The patients were female, aged 27–72 years, with a median age of 51.5 years, incurring a chemotherapeutic regimen of Docetaxel combined with Thiotepa: Docetaxel 75 mg/m2 d1, d8, Thiotepa 60 mg/m2 d1, repeated every 3 weeks. The clinicopathologic features are shown in Table 1. Thirty healthy woman volunteers in the medical examination center, aged 31–63 years with a median age of 49.5 years, were enrolled as the control group.
Table 1. Clinical data of 34 patients with metastatic breast cancer.
| Data | Number | Percent |
| Menopausal status | ||
| Postmenopausal | 26 | 76.5% |
| Premenopausal | 8 | 23.5% |
| ECOG | ||
| 0 | 24 | 70.6% |
| 1 | 10 | 29.4% |
| Receptor status | ||
| ER(+/-/unknown) | 14/19/1 | 41.2%/53.8%/5.0% |
| PR(+/-/unknown) | 22/11/1 | 64.7%/32.4%/2.9% |
| HER2(+/-/unknown) | 17/13/4 | 50.0%/38.2%/11.8% |
| Metastatic site | ||
| Liver | 15 | 44.1% |
| Bone | 16 | 47.1% |
| Lymph nodes | 19 | 55.9% |
| Lung | 11 | 32.4% |
| Chest wall | 9 | 26.5% |
| Pleura | 7 | 20.6% |
Enrollment criteria: (1) recurrent metastatic breast cancer with initial treatment; (2) patients with the first-line chemotherapy; (3) patients with lesions measurable by the Response Evaluation Criteria in Solid Tumors (RECIST); (4) patients must have a baseline value for assessment.
Methods
Reagents and equipment
Trizol reagent was purchased from Invitrogen Inc, USA. Chloroform and isopropyl alcohol were purchased from Beijing Chemical Reagent Company. Reverse transcription kit was purchased from Promega, USA. DMEM high glucose medium and heat-inactivated fetal bovine serum were purchased from Invitrogen/GIBCO, USA. MUC1 ELISA kit was purchased from GBD, Canada.
Cell line and cell culture
The breast cancer cell line, MCF-7, was provided by the Chinese Academy of Medical Sciences. Cells were cultured in DMEM high glucose medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C with 5% CO2 and saturated humidity. Medium was changed every 2–3 days. Cells were digested with 1:1 of 0.02% EDTA-2Na and 0.25% trypsin.
Primer design and synthesis
MUC1 and GAPDH primers were designed with Primer-5 software and synthesized by Beijing Aoke Biotechnology Co., Ltd. Primer sequence and size are shown in Table 2.
Table 2. Primers and product size.
| Gene | Primer | Product size |
| MUC1 | Forward: 5′-CTCACCAGCCCAAACAGG-3′ | 312 bp |
| Reverse: 5′-TGCCGCCGAAAGAACTAC-3′ | ||
| GAPDH | Forward: 5′-GTCAACGGATTTGGTCGTATT-3′ | 540 bp |
| Reverse: 5′-AGTCCTCTGGGTGGCAGTGAT-3′ |
MUC1 mRNA expression in peripheral blood was detected by RT-PCR
Collecting venous blood (8 mL, with anticoagulant EDTA treatment) was collected under strict sterile operations. The first tube of blood was discarded to minimize contamination by epithelial cells, with the following tube of blood collected. To prevent RNA degradation, extraction was performed within 30 min after collection. Red blood cells were lysed with red blood cell lysis buffer; following, white blood cells were purified. RNA was extracted with Trizol reagent, and cDNA was synthesized according to manufacturer instruction. cDNA was diluted to 100 µL, and 2 µL cDNA was employed for the polymerase chain reaction (PCR). Sample lacking mRNA was employed as a negative control, with MCF-7 breast cancer cells as the MUC1-positive control. Finally, 5 µL of PCR product was mixed with 2 µL bromophenol blue loading buffer and subjected to 2% agarose gel electrophoresis for product identification.
PCR conditions for GAPDH: pre-denaturation at 95°C for 5 min; 26 cycles of denaturation at 95°C for 1 min, renaturation at 56°C for 1 min, and extension at 72°C for 1 min; followed by a final extension at 72°C for 7 min. PCR conditions for MUC1: pre-denaturation at 95°C for 5 min; 35 cycles of denaturation at 95°C for 45 s, renaturation at 58°C for 45 s, and extension at 72°C for 45 s; followed by a final extension at 72°C for 7 min. To ensure the integrity of result comparison, reagents, laboratory conditions, and experimental procedures were consistent.
MUC1 protein expression in peripheral blood plasma was detected with an ELISA competitive inhibition assay
Assay procedure and data analysis was conducted according to manufacturer instruction. The standard curve was drawn with the CurveExpert 1.4 software. Sample concentration was calculated based on the standard curve.
Therapeutic efficacy evaluation and follow-up
MUC1 mRNA of 34 patients was detected prior to and following chemotherapy. Therapeutic efficacy was evaluated 2 weeks after chemotherapy, based on the RECIST criteria and divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). CR represents all target focuses completely disappeared and maintained for more than 4 weeks. PR represents the sum of long diameters of the baseline focuses reduced by more than 30% and maintained for more than 4 weeks. SD represents the sum of long diameters of the baseline focuses reduced but did not reach PR, or increased but did not reach PD, and maintained for more than 4 weeks. PD represents the sum of long diameters of the baseline focuses increased by more than 20% or new focuses appeared. Clinically, CR and PR were considered effective, while SD and PD were considered ineffective.
After a 12-month follow-up, the correlation between MUC1 mRNA and protein expression, and PFS was clarified.
Statistical analyses
Data was analyzed with SPSS14.0 software. Patient survival was analyzed with the Kaplan-Meier method. Enumeration data were analyzed with the Fisher Chi-square test, and measurement data were analyzed with the paired T test and independent sample T test. P < 0.05 was considered statistically significant.
Results
Sensitivity and specificity of this method
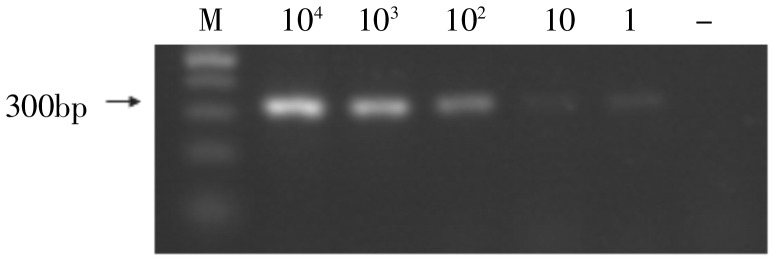
Different amounts (1, 10, 102, 103, and 104) of MCF-7 cells were mixed in 4 mL of blood from healthy volunteers to simulate the CTC of breast cancer. A sensitivity of detecting 1 CTC among 106–107 white blood cells was achieved using RT-PCR to detect MUC1 mRNA (Figure 1) and revealed 1 MUC1-positive case among 30 healthy volunteers with a specificity of 96.8%.
Figure 1. The sensitivity of detecting MUC1 expression by RT-PCR. The expression of amplified MUC1 mRNA (312 bp) in 104, 103, 102, 10, 1, and 0 MCF-7 cells was detected, respectively. MUC1 resolution was directly related to the number of cells assayed. System sensitivity detected MUC1 expression from one tumor cell in 4 mL of blood, equivalent to one CTC/106–107 white blood cells.
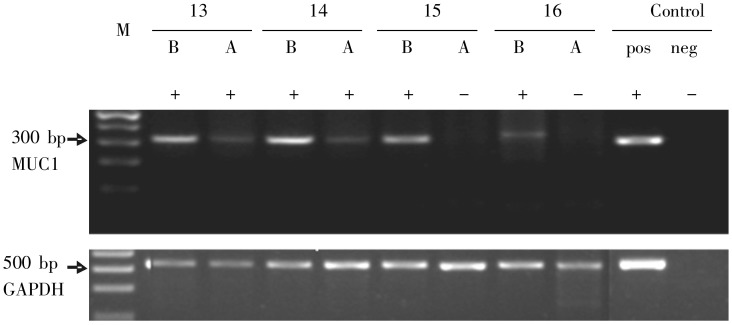
MUC1 expression in peripheral blood
Peripheral blood samples of 34 patients were collected the day before the first and the second cycle of chemotherapy to detect MUC1 mRNA. Figure 2 shows the MUC1 expression of 4 patients (#13–16) before and after chemotherapy. The detection rate of MUC1 was 88.2% before chemotherapy and 70.6% after the first cycle of chemotherapy, without a significant difference (P = 0.564).
Figure 2. Expression of MUC1 in peripheral blood before and after chemotherapy. Patients (#13–16) were assayed for MUC1 expression; GAPDH was employed as an internal control. B, before chemotherapy; A, after chemotherapy; +, MUC1 positive; -, MUC1 negative; pos, positive control; neg, negative control.
Correlation between MUC1 mRNA in peripheral blood and chemotherapeutic efficacy
MUC1 mRNA expression before and after chemotherapy and the chemotherapeutic efficacy
The response rate of patients lacking MUC1 expression before the first cycle of chemotherapy was 50.0% (2 of 4 cases were effective). That of MUC1-positive patients was 23.3% (7 of 30 cases were effective). MUC1 expression before the first cycle of chemotherapy was not correlated with the response rate (P = 0.281).
The response rate of patients lacking MUC1 expression after the first cycle of chemotherapy was 60.0% (6 of 10 cases were effective), and that of MUC1-positive patients was 12.5% (3 of 24 cases were effective) (P = 0.009), indicating that MUC1 expression after the first cycle of chemotherapy may also predict therapeutic efficacy after the second cycle.
MUC1-negative patients before or after chemotherapy had the best therapeutic response with a rate of 100%; MUC1-positive patients before chemotherapy and MUC1-negative after chemotherapy had a response rate of 50%; MUC1-positive patients before or after chemotherapy had a response rate of 13.6%; MUC1-negative patients before chemotherapy and MUC1-positive after chemotherapy had the worst therapeutic response with a rate of 0% (P = 0.013).
Correlation between MUC1 mRNA and PFS before and after chemotherapy
MUC1 mRNA expression was not correlated with PFS before and after chemotherapy (Figure 3A). After chemotherapy, the PFS of MUC1-negative patients was longer than that of MUC1-positive patients, without statistical significance (P = 0.095) (Figure 3B).
Figure 3. Progression-free survival (PFS) curves of 34 breast cancer patients. A, median time-to-progression (TTP) of patients with MUC1 expression before chemotherapy was 8.57 months; that of MUC1-negative patients before chemotherapy was 5.53 months (P = 0.720). B, median TTP of patients with MUC1 expression after chemotherapy was 7.70 months; that of MUC1-negative patients after chemotherapy was >10.9 months (P = 0.095). C, median TTP of patients with increased MUC1 expression after chemotherapy was 7.70 months; that of patients with decreased MUC1 expression after chemotherapy was >10.1 months (P = 0.044).
Correlation between other clinical parameters and chemotherapeutic efficacy as well as PFS of patients
Currently, the clinical parameters used for predicting the chemotherapeutic efficacy included estrogen receptor (ER), progesterone receptor (PR), HER2, physical status of patients, and tumor load. Only PR status was correlated with the clinical therapeutic efficacy (P = 0.034) (Table 3). The response rate of the PR-positive patients was higher than that of PR-negative patients. Parameters were not correlated with PFS.
Table 3. The relationship between clinical factors and chemotherapeutic efficacy in 34 breast cancer patients.
| Factor Status | CR + PR (%) | SD + PD (%) | P |
| ERa | 0.698 | ||
| Negative | 6(31.6) | 13(68.4) | |
| Positive | 3(21.4) | 11(78.6) | |
| PRb | 0.015 | ||
| Negative | 0(0) | 11(100) | |
| Positive | 9(40.9) | 13(49.1) | |
| HER2c | 0.407 | ||
| Negative | 2(15.4) | 11(84.6) | |
| Positive | 6(35.3) | 11(64.7) | |
| ECOG | 0.682 | ||
| 0 | 7(30.4) | 16(69.6) | |
| 1 | 2(18.2) | 9(81.8) | |
| Menopausal status | 0.348 | ||
| Postmenopausal | 6(23.1) | 20(76.9) | |
| Premenopausal | 3(37.5) | 5(62.5) | |
| Lung metastasis | 0.682 | ||
| No | 7(30.4) | 16(69.6) | |
| Yes | 2(18.2) | 9(81.8) | |
| Liver metastasis | 1.000 | ||
| No | 5(26.3) | 14(73.7) | |
| Yes | 4(26.7) | 11(73.3) | |
| Lymph node metastasis | 1.000 | ||
| No | 4(28.6) | 10(71.4) | |
| Yes | 5(25.0) | 15(75.0) | |
| Chest wall metastasis | 1.000 | ||
| No | 7(25.9) | 20(74.1) | |
| Yes | 2(28.6) | 5(71.4) | |
| Bone metastasis | 0.250 | ||
| No | 7(35.0) | 13(65.9) | |
| Yes | 2(14.3) | 12(85.7) | |
| Pleural metastasis | 1.000 | ||
| No | 7(26.9) | 19(73.1) | |
| Yes | 2(25.0) | 6(75.0) |
ER, estrogen receptor; PR, progesterone receptor; HER2, epidermal growth factor 2. aOne patient with unknown ER status, bone with unknown PR status, and cfour with unknown HER2 status were excluded from the analysis.
Correlation between MUC1 protein expression in peripheral blood and chemotherapeutic efficacy as well as PFS
The average concentration of MUC1 protein was 12.43 ng/mL before chemotherapy and 11.69 ng/mL after chemotherapy. MUC1 protein concentration was decreased in 18 patients (52.9%) and increased in 16 patients (47.1%) after chemotherapy; concentration was not significantly changed (P > 0.05). The clinical response rate of patients with decreased MUC1 protein expression was 16.7% (3 of 18 cases were effective); that of patients with increased MUC1 protein expression was 37.5% (6 of 16 cases were effective). Patients with increased MUC1 expression had a higher response rate than patients with decreased MUC1 expression, but without significant difference (P = 0.250).
MUC1 expression was correlated with PFS (P = 0.044). The PFS of the patients with decreased MUC1 expression was longer than that of the patients with increased MUC1 expression (Figure 3C).
Discussion
Breast cancer is among of the most common tumors expressed in women. With the development of early detection, diagnosis, and treatment, breast cancer patient mortality has decreased and prognosis has improved[9]. Recent studies have shown CTC detection in the peripheral blood of patients may provide a simple and efficient means for evaluating the therapeutic efficacy of in the treatment of breast cancer. CTCs, the tumor cells in peripheral blood, may be derived from local or distant metastases of patients with metastatic breast cancer. Currently, the chemotherapeutic approach for tumor patients is intravenous or oral administration. By entering the circulatory system, CTC s come into initial, direct contact with the chemotherapeutic regimen. Hence, if the treatment is effective, the majority of CTCs will be killed or reduced after one cycle of chemotherapy. The existence of CTCs in peripheral blood after chemotherapy indicates the insensitivity or resistance of tumor cells to treatment. Therefore, the chemosensitivity of cells can be determined by detecting CTCs before and after treatment. Andreopoulou et al.[10] reported detecting CTCs could predict therapeutic efficacy and hence, to help design an individual therapeutic regimen.
There are several methods to detect CTC, each with different rates of detection. Among them, RT-PCR is the most sensitive method[6],[11]. Depending on the stage, the detection rates of breast cancer also differ, with a relatively higher rate for advanced breast cancer. It has been reported the positive rate of CTCs in 118 patients with advanced breast cancer was 64.6% by using the Cell Search system[12], with similar results reported in other studies[13]–[15]. We detected MUC1 mRNA, the marker of CTCs, in 34 advanced breast cancer patients before and after chemotherapy. The detection rates of MUC1 mRNA were 88.2% before, and 70.6% after chemotherapy, which were higher than observed in other studies. There are three possible reasons for the discrepancy. First, patients in our study were in an advanced stage of breast cancer with distant metastasis and the tumor load was comparatively larger. Therefore, the probability of CTC existence was higher and the quantity of CTCs was larger. Second, CTC was detected employing RT-PCR, which had a high sensitivity and could detect 1 tumor cell in 4 mL of peripheral blood. Third, we choose MUC1 as the marker of CTC. MUC1 has 7 transcripts after splicing modification. The common part of these 7 transcripts could be amplified with our primers. Therefore, the detection of MUC1 mRNA expression was higher than other reports. In addition, most of the 30 healthy volunteers did not express MUC1 mRNA in our study, which is consistent with the literature[16].
Currently, many studies have reported the existence of CTCs could reflect chemotherapeutic efficacy, to a certain extent. Recently, Liu et al.[17] reported that CTC in peripheral blood could predict chemotherapeutic efficacy. In their study, CTC detected at different time points during chemotherapy correlated with radiological evaluation, suggesting CTC detection could predict therapeutic effect. In our study, we observed that CTC detection before and after the first cycle of chemotherapy predicted therapeutic efficacy. After 2 cycles of chemotherapy, CTC-negative patients after the first cycle of chemotherapy had a better response than CTC-positive positive patients, with a significant difference. However, when analyzing the correlation of ECOG score, ER, PR, HER2, and tumor load to chemotherapeutic effic acy, only PR was correlated to chemotherapeutic efficacy, suggesting CTC can better predict the chemotherapeutic efficacy. These findings are consistent with those of Cristofanilli et al.[18], who found the PFS and OS of metastatic breast cancer patients with more than 5 CTCs/7.5 mL peripheral blood was poorer, and CTC had higher predictive value than the tumor load and tumor type.
We observed a change of CTC before and after the first cycle of chemotherapy. For patients who were CTC-negative before chemotherapy, if CTC was not detected in the peripheral blood after chemotherapy, it suggested these patients had a high response rate and a low possibility of progression. For patients who were CTC-positive before chemotherapy, if CTC was not detected in the peripheral blood after chemotherapy, it suggested chemotherapy had an effect on the tumor cells and that chemotherapeutic efficacy may be good. In contrast, if CTC was still detected in peripheral blood after chemotherapy, it suggested these patients may be insensitive or resistant to chemotherapy, and that chemotherapeutic efficacy may be poor. Our results are consistent with the theory of CTC. However, the sample size of our study was relatively small. Thus, a larger sample size is required to confirm this conclusion.
After 12 months of follow-up, it was found the PFS of patients CTC-negative after chemotherapy was longer than that of the CTC-positive patients, but without a significant difference. These results may differ after a longer follow-up, but still indicate CTC may be able to predict the chemotherapeutic efficacy and PFS of patients.
Petersen et al.[19] reported active CTC could secrete MUC1 protein, whereas apoptotic cells were incapable of secretion. Thus, the activity of CTC could be determined based on the expression of MUC1. To examine whether MUC1 protein was correlated with therapeutic efficacy and PFS, a competitive inhibition ELISA assay was employed to examine MUC1 protein expression. Results showed the PFS of patients with decreased MUC1 expression was longer than that of patients with increased MUC1 expression. However, the clinical response rate of patients with decreased MUC1 expression was lower than that of patients with increased MUC1 expression, suggesting the short-term efficacy of chemotherapeutic drugs on MUC1 expression was low or the reduction in MUC1 protein concentration was slow. However, over an extended time, patients with a decrease in MUC1 protein may have a better prognosis. MUC1 protein could not directly evaluate clinical efficacy, but could predict the PFS of patients.
Our results may provide a basis for the individualized treatment of metastatic breast cancer. After chemotherapy, the detection of CTC tends to predict a poor therapeutic efficacy, necessitating the application of an alternative regimen. In fact, CTC detection has been applied in designing a chemotherapeutic approach. Hayes et al.[20] have detected CTC at different time points in an effort to determine the continuation or modification of a chemotherapeutic treatment. In addition, employing CTC detection as a means of predicting chemotherapeutic efficacy may be more useful in patients with unmeasurable focus, including those with simple osseous metastasis or early breast cancer with postoperative adjuvant chemotherapy. For these patients, therapeutic efficacy could be predicted through CTC detection. Xenidis et al.[21] have reported CTC was not correlated with disease-free survival (DFS) and OS.
In addition to occasional false positive results[22], the method used in our study could not distinguish living from dying tumor cells, due to the presence of apoptotic CTCs, which will interfere with clinic al significance. Mehes et al. [23] observed that some CTCs detected employing the immune method were apoptotic. Clearly, it is inappropriate to classify these cells as viable CTCs, as these cells have no significance toward tumor metastasis, clinical efficacy, and prognosis. Since the mechanism of hematogenous metastasis of breast cancer is not yet fully understood, the release process of CTC into blood may be intermittent. CTCs often occur in clusters; thus, a single time point of sampling may cause certain bias. In addition, CTCs may exist in the peripheral blood of some patients but may not be accurately representative of the whole blood. Therefore, the detection rate and accuracy need to be raised through continuous and multiple sampling or increasing the sample size.
Recent studies have demonstrated the cells causing tumor recurrence and metastasis and affecting the therapeutic efficacy in peripheral blood may not be CTCs. Rather, they may be stem cell-like tumor cells among the disseminated tumor cells/CTCs, which were considered the cells with metastatic and disseminating activity[24]. In addition, Abraham et al.[25] have reported CD44+/CD24− metastatic breast cancer cells in the bone marrow possessing high invasion ability. Thus, further studies are required on cancer stem cells in CTCs.
In summary, our results have shown detection of MUC1-positive CTC may have an important significance for predicting chemotherapeutic efficacy and PFS, with further optimization by detecting CTC-secreted MUC1 protein. These diagnostic tools may provide a rationale for determining the therapeutic regimen in the individualized treatment of breast cancer.
Acknowledgments
This work was supported by Beijing Capital Development Foundation for Medical Sciences (No. 2007-2053).
References
- 1.Veronesi U, Boyle P, Goldhirsch A, et al. Breast cancer [J] Lancet. 2005;365(9472):1727–1741. doi: 10.1016/S0140-6736(05)66546-4. [DOI] [PubMed] [Google Scholar]
- 2.Peto R, Boreham J, Clarke M, et al. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years [J] Lancet. 2000;355(9217):1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 3.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies [J] Lancet. 2007;369(9574):1742–1757. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura S, Yagata H, Ohno S, et al. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer [J] Breast Cancer. 2010;17(3):199–204. doi: 10.1007/s12282-009-0139-3. [DOI] [PubMed] [Google Scholar]
- 5.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer [J] N Engl J Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 6.Ring AE, Zabaglo L, Ormerod MG, et al. Detection of circulating epithelial cells in the blood of patients with breast cancer: comparison of three techniques [J] Br J Cancer. 2005;92(5):906–912. doi: 10.1038/sj.bjc.6602418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felton T, Harris GC, Pinder SE, et al. Identification of carcinoma cells in peripheral blood samples of patients with advanced breast carcinoma using RT-PCR amplification of CK7 and MUC1 [J] Breast. 2004;13(1):35–41. doi: 10.1016/S0960-9776(03)00126-7. [DOI] [PubMed] [Google Scholar]
- 8.de Cremoux P, Extra JM, Denis MG, et al. Detection of MUC1-expressing mammary carcinoma cells in the peripheral blood of breast cancer patients by real-time polymerase chain reaction [J] Clin Cancer Res. 2000;6(8):3117–3122. [PubMed] [Google Scholar]
- 9.Franceschini G, Terribile D, Magno S, et al. Update in the treatment of locally advanced breast cancer: a multidisciplinary approach [J] Eur Rev Med Pharmacol Sci. 2007;11(5):283–289. [PubMed] [Google Scholar]
- 10.Andreopoulou E, Cristofanilli M. Circulating tumor cells as prognostic marker in metastatic breast cancer [J] Expert Rev Anticancer Ther. 2010;10(2):171–177. doi: 10.1586/era.09.105. [DOI] [PubMed] [Google Scholar]
- 11.Bosma AJ, Weigelt B, Lambrechts AC, et al. Detection of circulating breast tumor cells by differential expression of marker genes [J] Clin Cancer Res. 2002;8(6):1871–1877. [PubMed] [Google Scholar]
- 12.Garcia-Saenz JA, Martin M, Maestro ML, et al. Circulating tumour cells in locally advanced breast cancer [J] Clin Transl Oncol. 2009;11(8):544–547. doi: 10.1007/s12094-009-0400-4. [DOI] [PubMed] [Google Scholar]
- 13.Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer [J] Semin Oncol. 2006;33(3 Suppl 9):S9–S14. doi: 10.1053/j.seminoncol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging—predicting overall survival in metastatic breast cancer [J] Clin Cancer Res. 2006;12(21):6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 15.Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer [J] J Clin Oncol. 2005;23(7):1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 16.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases [J] Clin Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 17.Liu MC, Shields PG, Warren RD, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer [J] J Clin Oncol. 2009;27(31):5153–5159. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cristofanilli M, Broglio KR, Guarneri V, et al. Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden [J] Clin Breast Cancer. 2007;7(6):471–479. [PubMed] [Google Scholar]
- 19.Petersen OW, Gudjonsson T, Villadsen R, et al. Epithelial progenitor cell lines as models of normal breast morphogenesis and neoplasia [J] Cell Prolif. 2003;36(Suppl 1):33–44. doi: 10.1046/j.1365-2184.36.s.1.4.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes DF, Smerage J. Is there a role for circulating tumor cells in the management of breast cancer? [J] Clin Cancer Res. 2008;14(12):3646–3650. doi: 10.1158/1078-0432.CCR-07-4481. [DOI] [PubMed] [Google Scholar]
- 21.Xenidis N, Perraki M, Kafousi M, et al. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients [J] J Clin Oncol. 2006;24(23):3756–3762. doi: 10.1200/JCO.2005.04.5948. [DOI] [PubMed] [Google Scholar]
- 22.Kasimir-Bauer S. Circulating tumor cells as markers for cancer risk assessment and treatment monitoring [J] Mol Diagn Ther. 2009;13(4):209–215. doi: 10.1007/BF03256327. [DOI] [PubMed] [Google Scholar]
- 23.Méhes G, Witt A, Kubista E, et al. Circulating breast cancer cells are frequently apoptotic [J] Am J Pathol. 2001;159(1):17–20. doi: 10.1016/S0002-9440(10)61667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype [J] Clin Cancer Res. 2006;12(19):5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 25.Abraham BK, Fritz P, McClellan M, et al. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis [J] Clin Cancer Res. 2005;11(3):1154–1159. [PubMed] [Google Scholar]