Abstract
Nuclear factor of activated T cells (NFAT) is an important family of transcription factors that can be activated by calmodulin and calcineurin in human cells. To investigate the expression and clinical significance of NFAT isoforms and calcineurin in non-small cell lung cancer (NSCLC), we collected tumor and adjacent normal tissues from 159 NSCLC patients and assembled them in a tissue microarray. Protein levels of NFAT1, NFAT2, NFAT3, NFAT4, and calcineurin were determined using immunohistochemistry. Correlations between NFAT and calcineurin expression and clinicopathologic characteristics were analyzed. We found that the positive rates of NFAT1 (52.8%, 84/159), NFAT2 (11.3%, 18/159), NFAT3 (28.3%, 45/ 159), NFAT4 (47.2%, 75/159), and calcineurin (47.8%, 76/159) expression were significantly higher in tumor tissues than in adjacent normal lung tissues (P < 0.001), respectively. The positive rate of NFAT1 expression was significantly higher in patients with adenocarcinoma (63.5%, 47/74) than in those with squamous cell carcinoma (43.5%, 37/85) (χ2 = 6.340, P = 0.012); with lymph node metastasis (61.6%, 53/ 86) than without lymph node metastasis (42.5%, 31/73) (χ2 = 5.818, P = 0.016); and with stage-ll and -III diseases (61.8%, 55/89) than with stage-I disease (41.4%, 29/70) (χ2 = 6.524, P = 0.011). Moreover, the overexpression of NFAT1 was associated with poor survival of NSCLC patients (χ2 = 5.006, P = 0.025). The positive rate of NFAT4 was significantly higher in patients with squamous carcinoma (57.6%, 49/85) than in those with adenocarcinoma (35.1%, 26/74) (χ2 = 8.045, P = 0.005) and with high and moderate differentiation (54.9%, 61/111) than with low differentiation (29.2%, 14/48) (χ2 = 8.943, P = 0.003). Calcineurin overexpression was significantly associated with histologic type (higher in squamous carcinoma than in adenocarcinoma, χ2 = 8.897, P = 0.003), differentiation grade (higher in high-moderation grade than in low grade, χ2 = 9.566, P = 0.002) and gender (higher in male than in female, χ2 = 5.766, P = 0.016). Furthermore, calcineurin expression was significantly correlated with NFAT4 level (r = 0.429, P < 0.001). These results suggest that NFAT1 expression is associated with lung adenocarcinoma progression, and NFAT4 expression, which was higher in squamous lung cancer, is associated with calcineurin expression and differentiation grade.
Keywords: Lung neoplasms, NFATC transcription factors, calcineurin
Nuclear factor of activated T cells (NFAT) is a family of common cellular transcription factors that were initially identified in T cells. The NFAT family includes five members: NFAT1, NFAT2, NFAT3, NFAT4, and NFAT5. NFAT1–4 proteins are comprised of three domains: NHR (NFAT homology domain), RHR (Rel homology domain), and a C-terminal domain. The NHR domain contains multiple serine phosphorylation sites and is involved in transcription initiation and calcineurin (CaN) binding. The RHR domain contains binding sites for target DNA sequences and other transcription factors. The activity of four of five NFAT isoforms, NFAT1, NFAT2, NFAT3 and NFAT4, depends on calcium channels. After external stimuli trigger an increase in intracellular calcium levels, phospholipase C activates calmodulin, which then activates CaN. Activated CaN dephosphorylates NFAT, thereby exposing nuclear localization sequences that promote its nuclear translocation. Once inside the nucleus, NFAT exerts its transcriptional functions. In most cases, NFAT forms heterodimers with other transcription factors, including AP1, MEF2, GATA, and HDAC, and interacts with specific DNA binding sequences to initiate downstream gene transcription[1].
The role of NFAT as a transcription factor in the immune system has been explored extensively and confirmed after its identification in T cells in 1988. In recent years, several studies demonstrated that NFAT also plays important roles in cancer cells. More specifically, NFAT regulates cell cycle, proliferation, apoptosis, invasion, and angiogenesis in a variety of cancer cells, including colon cancer, breast cancer, pancreatic cancer, endometrial cancer, and melanoma. NFAT1 and NFAT2 are expressed in colon cancer cell lines such as Caco-2, HT116 and HT29. When NFAT1 and NFAT2 are activated and translocated into nucleus, they initiate the transcription of downstream gene cyclooxygenase-2 (COX2) and enhance cancer cell invasion[2],[3]. In breast cancer, NFAT regulates cell motility and invasion by several different mechanisms. The transcriptional activity of NFAT1 and NFAT5 in breast cancer cells is increased by α6β4 integrin, resulting in enhanced cell migration[4]. NFAT1 promotes breast cancer cell invasion by inducing COX2 and prostaglandin endoperoxide 2 (PGE2) expression[5]. Furthermore, Akt-mediated inhibition of breast cancer cell motility and invasion is due to activation of downstream Ubiquitin ligase HDM2, which leads to ubiquitin-mediated degradation of NFAT and loss of its transcriptional activity[6]. Pancreatic cancer cells overexpress NFAT2, which enhances c-myc transcription and promotes cancer cell proliferation and malignancy[7]. A more recent study shows that calcium- activated NFAT recruits the transcription factor ELK by inducing chromatin acetylation, resulting in formation of NFAT/ELK complex. This complex activates the expression of early transcription factor c-myc and induces pancreatic cancer cell proliferation[8]. In endometrial cancer cells, cooperative interactions between calcium-activated NFAT and activator protein 1 (AP-1) activate the expression of cytokine CLCX-8 and promote cancer cell proliferation[9]. In malignant melanoma cells, NFAT is activated by the BRAF/MEK/ERK signaling pathway, which regulates the transcription of COX-2 to promote tumor cell proliferation[10]. NFAT is also involved in tumorigenesis of Wilms tumor[11]. Despite this knowledge, the role of NFAT in cancer cells has not been fully investigated. In particular, its regulation of downstream target genes and function in other cancer types are still unclear.
Several studies have shown that NFAT regulates lung development in mice. NFAT is expressed in mouse lung epithelial cells and can regulate the expression of pulmonary surfactant[12]. NFAT4, in particular, is an important transcription factor in perinatal lung development and maturation [13]. Moreover, the NFAT-mediated signaling pathway plays an important role in lung cancer caused by nickel or arsenic exposure. After exposure to arsenite, normal human bronchial epithelial Beas-2B cells were highly resistant to cell apoptosis, which is activated by NFAT through COX2 transcription[14]. In addition to NFAT's role in lung development, our preliminary studies have shown that NFAT also participates in lung cancer development. More specifically, in non–small cell lung cancer (NSCLC), the total expression level of NFAT is up-regulated and related to cell differentiation, tissue type, and other phenotypes[5].
Based on our preliminary data, we sought to further investigate the role of NFAT in NSCLC. In this study, we used isoform-specific antibodies to measure the expression of NFAT1–4 and CaN, a key regulator of NFAT activity, in NSCLC tissues. The expression patterns were correlated with clinical characteristics to identify calcium-regulated NFAT isoforms that play a major role in NSCLC, as well as the relationship between NFAT and CaN in NSCLC.
Material and Methods
Patients and their clinical data
The clinical data of 159 cases of primary NSCLC were collected. Each case had detailed clinical and five-year follow-up data. All patients had undergone surgical treatment at the Cancer Institute and Hospital, Chinese Academy of Medical Sciences, between 1998 and 2001, but no one received preoperative radiotherapy or chemotherapy. The patients' ages ranged from 31 to 80, with a median age of 63. Of 159 cases, 111 were male and 48 were female; 74 were adenocarcinoma and 85 were squamous cell carcinoma; 8 were highly differentiated, 103 were moderately differentiated, and 48 were poorly differentiated; and 70 were at stage I, while 89 were at stages II and III.
Samples and reagents
Haematoxylin and eosin (HE)–stained paraffin specimens were re-examined by pathologists. For each patient, two different cancer tissue spots and two different normal tissue spots (> 5 cm apart from the cancer tissue s pots) were used for tissue microarray. The diameter of each tissue spot was 1 mm, and each case included 4 spots. Continuous 4 µm–thick slices were sectioned. Mouse anti-human NFAT1 and rabbit anti-human NFAT3 antibodies were purchased from the British Abcam, Inc. Mouse anti-human NFAT2 and mouse anti-human NFAT4 antibodies were purchased from Santa Cruz, Inc. Rabbit anti-human antibodies were purchased from Canadian Chemicom International, Inc. PV9000 and DAB reagents were purchased from Beijing Zhongshan Goldenbridge Biotechnology, Ltd.
Immunohistochemistry
Slides were dewaxed with xylene and hydrated with gradient ethanol. Slides were then microwaved at high level for 5 min, followed by 15 min microwave at middle-low levels for heat antigen retrieval. After treatment with 3% hydrogen peroxide/80% methanol at room temperature for 30 min to quench endogenous peroxidase activity, slides were incubated with primary antibody at 4°C overnight. Slides were washed with PBS, incubated with PV9000 at room temperature, and developed with DAB staining. Slides were counter-stained with hematoxylin as follows: slides were differentiated in 1% hydrochloric acid/75% ethanol, treated with 1% ammonia, dehydrated with gradient ethanol, and then sealed. For negative controls, primary antibody was substituted with PBS.
Slides were independently examined by two pathologists from Cancer Institute and Hospital, Chinese Academy of Medical Sciences. The expressions of NFAT and CaN were scored as 0, 1, 2, 3, or 4 according to staining intensity, and scored from 0 to 4 based on the percentage of positive cells: score 0 (≤ 5%), 1 (6% –25%), 2 (26%–50%), 3 (51%–75%), 4 (76%–100%). Five high power fields (×200) were randomly selected. Final scores were determined according to the sum of both of the scores described above. A final score of zero was defined as negative; scores ranging from one to four were defined as weak positive; and scores higher than four defined as strong positive.
Statistical analyses
Statistical analyses were performed using SPSS13.0 software. The Chi-square (χ2) test was used to analyze differences between different groups. Correlation of protein expression levels was analyzed using Spearman rank correlation analysis. Survival analysis was obtained by the Kaplan-Meier analysis, log-rank test, and Cox multivariate regression analysis. P < 0.05 was considered as significantly different.
Results
Expression profile of NFAT proteins in NSCLC
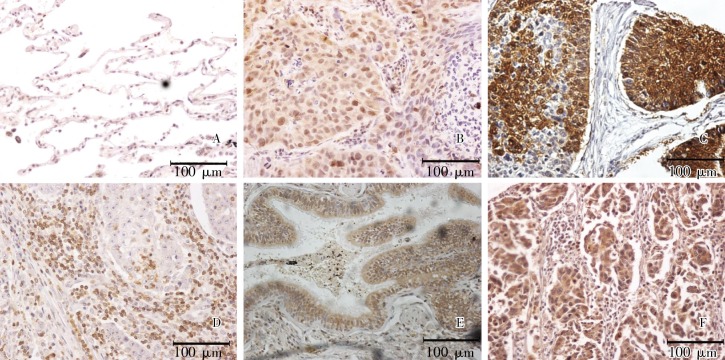
The specificities of four NFAT antibodies were detected by Western blot analysis. These antibodies specifically recognized NFAT1, NFAT2, NFAT3, and NFAT4 without cross-reactivity. We detected the expression levels of these four NFAT isoforms using immunohistochemistry in 159 cases of NSCLC and adjacent normal lung tissues. The results showed that NFAT proteins were positively stained in both the cytoplasm and nucleus (Figure 1). Among the four NFAT isoforms studied, NFAT1 was most highly expressed in NSCLC tissues, with positive staining in 52.8% (84/159) samples, whereas only 8.8% (14/159) showed positive staining in normal lung tissue. Of the NFAT1-positive NSCLC samples, 42.8% (36/84) showed nuclear staining. The positive rate of NFAT4 in tumor tissue was 47.2% (75/159), with nuclear staining in 29.3% (22/75) of samples, whereas only 3.1% (5/159) of normal lung tissues were positive. The positive rate of NFAT3 was 28.3% (45/159) in tumor tissues, with nuclear staining in 24.4% (11/45) of samples, whereas only two cases of normal lung tissues were positive. The expression level of NFAT2 was the lowest, with 11.3% (18/159) positive cases, including 22.2% (4/18) that showed nuclear staining. All normal tissues were negative for NFAT2. For each NFAT isoform, the differential expression between tumor and adjacent normal lung tissues was statistically significant (P < 0.001).
Figure 1. Expression of NFAT proteins and CaN in non–small cell lung cancer (immunohistochemistry). A, negative staining of NFAT1; B–E, positive staining of NFAT1, NFAT2, NFAT3, and NFAT4, respectively; F, positive staining of CaN. Bars, 100 µm.
Clinical significance of NFAT expression
Immunohistochemistry results showed that 63.5% (47/74) of adenocarcinoma cases expressed NFAT1, which was significantly more than squamous cell carcinoma cases (43.5%, 37/85) (χ2 = 6.340, P = 0.012). The positive rate of NFAT1 in patients with lymph node metastasis was 61.6% (53/86), which was significantly higher than that in patients with non-lymph node metastasis (42.5%, 31/73) (χ2 = 5.818, P = 0.016). The positive rate of NFAT1 in stage-II and -III cases was 61.8% (55/89), and that was higher than in the stage-I group (41.4%, 29/70) (χ2 = 6.524, P = 0.011). The positive rate of NFAT4 was 54.9% (61/111) in highly differentiated group, which was significantly higher than that in the poorly differentiated group (29.2%, 14/48) (χ2 = 8.943, P = 0.003). This suggests that NFAT4 expression level is related to differentiation. In addition, 57.6% (49/85) of squamous carcinomas were positive for NFAT4, which is significantly more than adenocarcinomas (35.1%, 26/74) (χ2 = 8.045, P = 0.005). The expressions of NFAT1 and NFAT4 in NSCLC patients were not correlated with age, sex, and smoking condition (Table 1). The expression levels of NFAT2 and NFAT3 were low and not significantly related to various clinical factors.
Table 1. Association between NFAT1 and NFAT4 expression and clinicopathologic characteristics in 159 patients with non–small cell lung cancer.
| Clinicopathologic characteristic | Patient No. | Expression of NFAT1 |
χ2 | P | Expression of NFAT4 |
χ2 | P | ||
| Number | Rate (%) | Number | Rate (%) | ||||||
| Age (years) | 0.014 | 0.904 | 0.192 | 0.661 | |||||
| ≤ 63 | 84 | 44 | 52.4 | 41 | 48.9 | ||||
| > 63 | 75 | 40 | 53.3 | 34 | 45.3 | ||||
| Gender | 0.049 | 0.824 | 0.836 | 0.361 | |||||
| Male | 111 | 58 | 52.3 | 55 | 49.5 | ||||
| Female | 48 | 26 | 54.2 | 20 | 41.2 | ||||
| Smoking history | 3.250 | 0.071 | 2.194 | 0.139 | |||||
| No | 67 | 41 | 61.2 | 27 | 40.3 | ||||
| Yes | 92 | 43 | 46.7 | 48 | 52.2 | ||||
| Differentiation | 0.221 | 0.638 | 8.943 | 0.003 | |||||
| High–moderate | 111 | 60 | 54.1 | 61 | 54.9 | ||||
| Low | 48 | 24 | 50.0 | 14 | 29.2 | ||||
| Histologic type | 6.340 | 0.012 | 8.045 | 0.005 | |||||
| Adenocarcinoma | 74 | 47 | 63.5 | 26 | 35.1 | ||||
| Squamous cell carcinoma | 85 | 37 | 43.5 | 49 | 57.6 | ||||
| Lymph node metastasis | 5.818 | 0.016 | 1.988 | 0.157 | |||||
| No | 73 | 31 | 42.5 | 30 | 41.1 | ||||
| Yes | 86 | 53 | 61.6 | 45 | 52.3 | ||||
| TNM staging | 6.524 | 0.011 | 2.580 | 0.108 | |||||
| I | 70 | 29 | 41.4 | 28 | 40.0 | ||||
| II + III | 89 | 55 | 61.8 | 47 | 52.8 | ||||
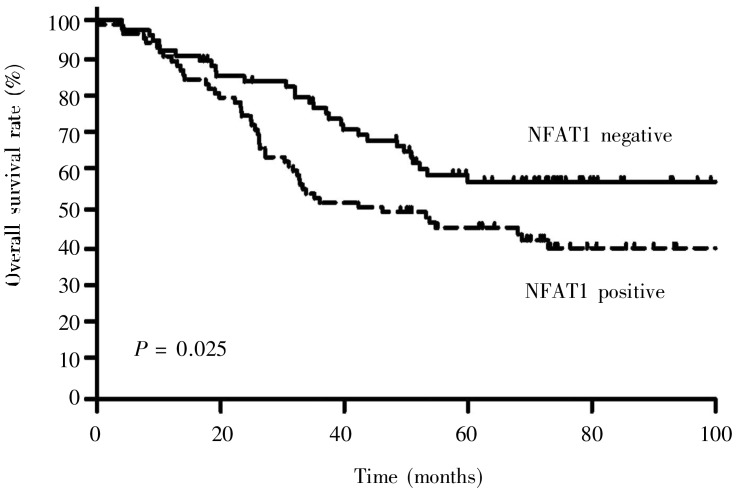
Log-rank analysis showed that the survival rate of patients with high NFAT1 expression (5-year overall survival rate, 45.3%) was significantly lower than that of patients with low NFAT1 expression (5-year overall survival rate, 57.4%) (χ2 = 5.006, P = 0.025) (Figure 2). Univariate Cox analysis showed that NFAT1 expression, TNM stage, lymph node metastasis, and differentiation were related to patient survival. The patients with high NFAT1 expression had a higher relative risk of poor prognosis than did those with low NFAT1 (OR = 1.675, 95% confidential interval = 1.061–2.644). However, multivariate Cox regression analysis showed that only TNM stage (P = 0.009) and degree of differentiation (P = 0.027) were independent prognostic factors, whereas NFAT1 overexpression was not an independent prognostic factor (P = 0.079) (Table 2).
Figure 2. Kaplan-Meier overall survival curves for patients with non–small cell lung cancer. A total of 84 patients were NAFT1 positive and 75 patients were NFAT1 negative. Log-rank test was used to analyze the difference between the two groups.
Table 2. Correlation of overall survival of patients with non–small cell lung cancer with clinicopathologic characteristics and NFAT1 expression.
| Variable | Subgroups | Univariate analysis |
Multivariate analysis |
||
| P | Hazard ratio (95% CI) | P | Hazard ratio (95% Cl) | ||
| Differentiation | Low/High–Moderate | 0.003 | 2.002 (1.267–3.164) | 0.027 | 1.699 (1.061–2.720) |
| Lymph node metastasis | Yes/No | 0.005 | 1.931 (1.218–3.060) | 0.578 | – |
| TNM staging | III + II/I | 0.001 | 2.155 (1.343–3.457) | 0.009 | 1.916 (1.178–3.117) |
| NFAT1 | Positive/Negative | 0.027 | 1.675 (1.061–2.644) | 0.079 | – |
CI, confidential interval.
The expression of CaN in NSCLC and its relationship with NFAT
As revealed by immunohistochemistry assay (Figure 1), the overall positive rate of CaN was 47.8% (76/159). The CaN positive rate was significantly higher in squamous cell carcinoma (58.8%, 50/85) than in adenocarcinoma (35.1%, 26/74) (χ2 = 8.897, P = 0.003). CaN expression was also significantly higher in the highly differentiated group than in the poorly differentiated group (χ2 = 9.566, P = 0.002) and was significantly higher in men than in women (χ2 = 5.766, P = 0.016) (Table 3). However, CaN expression was not significantly correlated with patient survival (P = 0.225). Spearman rank correlation analysis showed that in lung cancer tissues, CaN expression correlated well with the expression of NFAT4 (r = 0.429, P < 0.001) (Table 4).
Table 3. Association between expression of CaN and clinicopathologic characteristics of patients with non–small cell lung cancer.
| Clinicopathologic characteristic | Number | Expression of CaN |
χ2 | P | |
| Positive | Negative | ||||
| Age (years) | 1.499 | 0.221 | |||
| ≤ 63 | 84 | 44 | 40 | ||
| > 63 | 75 | 32 | 43 | ||
| Gender | 5.766 | 0.016 | |||
| Male | 111 | 60 | 51 | ||
| Female | 48 | 16 | 32 | ||
| Smoking history | 0.946 | 0.331 | |||
| No | 67 | 29 | 38 | ||
| Yes | 92 | 47 | 45 | ||
| Differentiation | 9.566 | 0.002 | |||
| High–moderate | 111 | 62 | 49 | ||
| Low | 48 | 14 | 34 | ||
| Histologic type | 8.897 | 0.003 | |||
| Adenocarcinoma | 74 | 26 | 48 | ||
| Squamous cell carcinoma | 85 | 50 | 35 | ||
| Lymph node metastasis | 0.081 | 0.776 | |||
| No | 73 | 34 | 39 | ||
| Yes | 86 | 42 | 44 | ||
| TNM staging | 0.619 | 0.432 | |||
| I | 70 | 31 | 39 | ||
| II + III | 89 | 45 | 44 | ||
The values are presented as number of cases.
Table 4. Correlation between the expressions of NFAT4 and CaN in non–small cell lung cancer.
| CaN expression | NFAT4 expression |
r | P | ||
| Negative | Weak positive | Strong positive | |||
| Negative | 58 | 12 | 13 | 0.429 | < 0.001 |
| Weak positive | 21 | 15 | 20 | ||
| Strong positive | 5 | 7 | 8 | ||
The values are presented as number of cases.
Discussion
The five different NFAT isoforms play different roles. NFAT1, NFAT2, NFAT3, and NFAT4 are activated by calcium, while NFAT5 is mainly regulated by osmotic pressure. Previous studies using knockout mice have shown that different NFAT isoforms have isoform-specific roles in mice development. NFAT1 knockout mice showed decreased expression of cytokines and Fas ligand, which led to increased production of splenic B cells and T cells[16]. Heart development in NFAT2 knockout mice is affected[17], and NFAT4 mainly influences the development of muscle and thymus[18]. Therefore, different NFAT isoforms play unique roles depending on cell type. For example, NFAT2 is expressed in approximately 70% of pancreatic cancers, but both NFAT2 and CaN levels are very low in normal pancreatic or pancreatitis tissues[7]. In the present study, we found that NFAT1 (52.8% ) and NFAT4 (47.2%) were highly expressed in NSCLC tissues, but the expressions of NFAT2 and NFAT3 were low.
NFAT1 and NFAT2 have been shown to play opposite roles in fibroblasts and knockout mice. NFAT1 induces cell cycle arrest and apoptosis in NIH 3T3 fibroblasts, whereas NFAT2 leads to increased proliferation capacity and cell transformation [19]. Our results indicate that NFAT1 and NFAT4 play different roles in lung cancer. NFAT1 expression in patients with lymph node metastasis was significantly higher than that in patients without lymph node metastasis. In addition, the later the TNM stage, the higher the positive expression rate of NFAT1, indicating that NFAT1 acts as an Oncogene in lung cancer. The post-operative survival rate of patients with high NFAT1 expression was also lower than that of patients with low NFAT1 expression, indicating that NFAT1 promotes lung cancer recurrence and metastasis by serving as an Oncogene. In contrast, the expression levels of NFAT4 in highly and moderately differentiated groups was significantly higher than that in the poorly differentiated group, indicating NFAT4 may act as a tumor suppressor. NFAT4 was highly expressed in squamous cell carcinoma and NFAT1 was highly expressed in adenocarcinoma, suggesting that these two NFAT isoforms play a major role in squamous cell carcinoma and adenocarcinoma, respectively.
CaN is a key protein that regulates NFAT activity. It was highly expressed in almost half of the lung cancer tissues analyzed, and its expression level was significantly related to NFAT4 expression. Similar to the expression of NFAT4, CaN expression was higher in highly differentiated lung cancers and in squamous cell carcinoma. These results suggest that NFAT4 plays a major protective role against squamous cell carcinoma, and its activity is regulated by CaN.
Lung cancer is the primary cause of cancer-related deaths worldwide. Therefore, studies to understand the molecular mechanisms of lung cancer development are highly significant. NFAT proteins are important transcription factors, and our study revealed that two NFAT isoforms, NFAT1 and NFAT4, have opposing roles in lung cancer. Our results, thus, provide new molecular markers for lung cancer. Further studies are needed to identify the downstream targets and molecular mechanisms of NFAT proteins.
Acknowledgments
We thank Su-Sheng Shi and Xiao-Li Feng for their pathological examination.
This work was supported by Hi-Tech Research and Development Program of China (No. 2006AA020707).
References
- 1.Mancini M, Toker A. NFAT proteins: emerging roles in cancer progression [J] Nat Rev Cancer. 2009;9(11):810–820. doi: 10.1038/nrc2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duque J, Fresno M, Iniguez MA. Expression and function of the nuclear factor of activated T cells in colon carcinoma cells: involvement in the regulation of cyclooxygenase-2 [J] J Biol Chem. 2005;280(10):8686–8693. doi: 10.1074/jbc.M413076200. [DOI] [PubMed] [Google Scholar]
- 3.Corral RS, Iniguez MA, Duque J, et al. Bombesin induces cyclooxygenase-2 expression through the activation of the nuclear factor of activated T cells and enhances cell migration in Caco-2 colon carcinoma cells [J] Oncogene. 2007;26(7):958–969. doi: 10.1038/sj.onc.1209856. [DOI] [PubMed] [Google Scholar]
- 4.Jauliac S, Lopez-Rodriguez C, Shaw LM, et al. The role of NFAT transcription factors in integrin-mediated carcinoma invasion [J] Nat Cell Biol. 2002;4(7):540–544. doi: 10.1038/ncb816. [DOI] [PubMed] [Google Scholar]
- 5.Yiu GK, Toker A. NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2 [J] J Biol Chem. 2006;281(18):12210–12217. doi: 10.1074/jbc.M600184200. [DOI] [PubMed] [Google Scholar]
- 6.Yoeli-Lerner M, Yiu GK, Rabinovitz I, et al. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT [J] Mol Cell. 2005;20(4):539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Buchholz M, Schatz A, Wagner M, et al. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway [J] Embo J. 2006;25(15):3714–3724. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig A, Linhart T, Schlengemann K, et al. NFAT-induced histone acetylation relay switch promotes c-Myc–dependent growth in pancreatic cancer cells [J] Gastroenterology. 2009;138(3):1189–1199. doi: 10.1053/j.gastro.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sales KJ, Maldonado-Perez D, Grant V, et al. Prostaglandin F (2alpha)-F-prostanoid receptor regulates CXCL8 expression in endometrial adenocarcinoma cells via the calcium-calcineurin-NFAT pathway [J] Biochim Biophys Acta. 2009;1793(12):1917–1928. doi: 10.1016/j.bbamcr.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flockhart RJ, Armstrong JL, Reynolds NJ, et al. NFAT signalling is a novel target of oncogenic BRAF in metastatic melanoma [J] Br J Cancer. 2009;101(8):1448–1455. doi: 10.1038/sj.bjc.6605277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen AH, Beland M, Gaitan Y, et al. Calcineurin a-binding protein, a novel modulator of the calcineurin-nuclear factor of activated T-cell signaling pathway, is overexpressed in wilms' tumors and promotes cell migration [J] Mol Cancer Res. 2009;7(6):821–831. doi: 10.1158/1541-7786.MCR-08-0402. [DOI] [PubMed] [Google Scholar]
- 12.Dave V, Childs T, Xu Y, et al. Calcineurin/Nfat signaling is required for perinatal lung maturation and function [J] J Clin Invest. 2006;116(10):2597–2609. doi: 10.1172/JCI27331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dave V, Childs T, Whitsett JA. Nuclear factor of activated T cells regulates transcription of the surfactant protein D gene (Sftpd) via direct interaction with thyroid transcription factor-1 in lung epithelial cells [J] J Biol Chem. 2004;279(33):34578–34588. doi: 10.1074/jbc.M404296200. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Li J, Xue C, et al. Cyclooxygenase-2 induction by arsenite is through a nuclear factor of activated T-cell-dependent pathway and plays an antiapoptotic role in Beas-2B cells [J] J Biol Chem. 2006;281(34):24405–24413. doi: 10.1074/jbc.M600751200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang K, Li N, Chen Z, et al. High expression of nuclear factor of activated T cells in Chinese primary non-small cell lung cancer tissues [J] Int J Biol Markers. 2007;22(3):221–225. doi: 10.1177/172460080702200310. [DOI] [PubMed] [Google Scholar]
- 16.Masuda ES, Imamura R, Amasaki Y, et al. Signalling into the T-cell nucleus: NFAT regulation [J] Cell Signal. 1998;10(9):599–611. doi: 10.1016/s0898-6568(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 17.Horsley V, Pavlath GK. NFAT: ubiquitous regulator of cell differentiation and adaptation [J] J Cell Biol. 2002;156(5):771–774. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amasaki Y, Adachi S, Ishida Y, et al. A constitutively nuclear form of NFATx shows efficient transactivation activity and induces differentiation of CD4(+)CD8(+) T cells [J] J Biol Chem. 2002;277(28):25640–25648. doi: 10.1074/jbc.M201860200. [DOI] [PubMed] [Google Scholar]
- 19.Robbs BK, Cruz AL, Werneck MB, et al. Dual roles for NFAT transcription factor genes as oncogenes and tumor suppressors [J] Mol Cell Biol. 2008;28(23):7168–7181. doi: 10.1128/MCB.00256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]