Abstract
The efficacy and safety of bevacizumab with modified irinotecan, leucovorin bolus, and 5-fluorouracil intravenous infusion (mIFL) in the first-line treatment of metastatic colorectal cancer (mCRC) has not been well evaluated in randomized clinical trials in Chinese patients. We conducted a phrase III trial in which patients with previously untreated mCRC were randomized 2:1 to the mIFL [irinotecan (125 mg/m2), leucovorin (20 mg/m2) bolus, and 5-fluorouracil intravenous infusion (500 mg/m2) weekly for four weeks every six weeks] plus bevacizumab (5 mg/kg every two weeks) group and the mIFL group, respectively. Co-primary objectives were progression-free survival (PFS) and 6-month PFS rate. In total, 214 patients were enrolled. Our results showed that addition of bevacizumab to mIFL significantly improved median PFS (4.2 months in the mIFL group vs. 8.3 months in the bevacizumab plus mIFL group, P < 0.001), 6-month PFS rate (25.0% vs. 62.6%, P < 0.001), median overall survival (13.4 months vs. 18.7 months, P = 0.014), and response rate (17% vs. 35%, P = 0.013). Grades 3 and 4 adverse events included diarrhea (21% in the mIFL group and 26% in the bevacizumab plus mIFL group) and neutropenia (19% in the mIFL group and 33% in the bevacizumab plus mIFL group). No wound-healing complications or congestive heart failure occurred. Our results suggested that bevacizumab plus mIFL is effective and well tolerated as first-line treatment for Chinese patients with mCRC. Clinical benefit and safety profiles were consistent with those observed in pivotal phase III trials with mainly Caucasian patients.
Keywords: Bevacizumab, chemotherapy, first-line treatment, Chinese patients, metastatic colorectal cancer
In China, colorectal cancer is the fifth most common malignancy in men and the sixth most common malignancy in women[1]. In 2008, colorectal cancer was the fifth most common cause of tumor death in men and women, accounting for 5.6% of the total deaths due to cancer[1]. Current recommended chemotherapy for metastatic colorectal cancer (mCRC) in China is fluoropyrimidine-based chemotherapy, with the addition of oxaliplatin or irinotecan (FOLFOX/FOLFIRI/CapeOx).
Randomized clinical trials have shown that in patients with previously untreated mCRC, bevacizumab improves progression-free survival (PFS) and overall survival (OS) in combination with irinotecan-based[2] or 5-fluorouracil (5-FU)–based chemotherapy[3] and improves PFS in combination with oxaliplatin-based chemotherapy[4]. Furthermore, combination of bevacizumab with 5-FU–based chemotherapy has been shown to improve PFS compared with 5-FU–based chemotherapy alone in the second-line treatment of patients with mCRC[5]. However, there is a paucity of data from large phase III clinical trials on the effect of the combination of bevacizumab with chemotherapy in Chinese patients with mCRC.
Three large observational studies in patients with mCRC have shown that the efficacy and safety profile of bevacizumab in routine clinical practice is consistent with results observed in prospective randomized clinical trials[6]–[9]. However, as with the randomized phase III trials, these observational studies did not include Chinese patients.
This phase III trial—avastin and irinotecon in first-line metastatic colorectal cancer (China ARTIST)— was performed to evaluate whether the benefits observed in Caucasian patients following the addition of bevacizumab to irinotecan-based chemotherapy in a pivotal phase III trial[2] and a phase IV trial[10] could be replicated in Chinese patients with mCRC undergoing first-line therapy with bevacizumab in combination with modified irinotecan, leucovorin bolus, and 5-FU intravenous infusion (mIFL) compared with mIFL alone.
Patients and Methods
Study design
This was a prospective, multicenter, randomized, open-labelled, phase III trial (BO20696; NCT00642577). Patients were eligible for inclusion if they had unresectable, histologically proven, measurable mCRC, were ≥18 years of age, had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, had no previous therapy for metastatic disease [adjuvant or neoadjuvant treatment for non-metastatic (MO) disease was allowed if completed at least 6 months before initiation of study treatment], and had a life expectancy of >3 months. Patients were required to have creatinine clearance >50 mL/min [or serum creatinine ≤1.5 × upper limit of normal (ULN)] and urine dipstick proteinuria ≤2+; those with proteinuria >2+ on dipstick urinalysis at baseline underwent 24-h urine collection and were required to demonstrate ≤1 g protein/24 h. Other inclusion criteria were adequate haematological function (absolute neutrophil count ≥1.5 × 109/L, platelet count ≥100 × 109/L, and hemoglobin ≥90 g/L); international normalized ratio ≤1.5; activated partial thromboplastin time ≤1.5 × ULN; and adequate hepatic function [total bilirubin ≤1.5 × ULN; aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2.5 × ULN in patients without liver metastases; and AST and ALT ≤5 × ULN in patients with liver metastases]. All patients provided written informed consent.
Patients were excluded if they had previously received adjuvant irinotecan or anti-vascular endothelial growth factor (VEGF) therapy or if they had any of the following: untreated brain metastases; history or evidence of central nervous system disease; clinically significant cardiovascular disease, myocardial infarction, unstable angina, congestive heart failure, arrhythmia requiring medication, or uncontrolled hypertension; organ allografts requiring immunosuppressive therapy; evidence of bleeding diathesis or coagulopathy; a serious non-healing wound, ulcer, or bone fracture; or a history of abdominal fistula, gastrointestinal perforation, or intra-abdominal abscess within the six months before enrollment. Ongoing treatment with aspirin (>325 mg/day) or other medications known to predispose patients to gastrointestinal ulceration was prohibited.
Treatment
Patients were assigned by central dynamic randomization in a ratio of 2:1 to two groups and treated with bevacizumab (5 mg/kg) administered intravenously (i.v.) on day 1 every two weeks plus mIFL [irinotecan (125 mg/m2) i.v. on day 1 followed by leucovorin (LV) (20 mg/m2) i.v. and 5-FU (500 mg/m2) i.v. infused over 6–8 h weekly for four weeks] every six weeks or mIFL alone every six weeks, respectively. Treatment was continued until documented progressive disease, death, or unacceptable toxicity.
If chemotherapy was discontinued permanently because of toxicity, the patient was allowed to continue with bevacizumab therapy. Similarly, if bevacizumab was discontinued permanently due to toxicity, the patient was allowed to continue with chemotherapy.
Study endpoints
In this study to evaluate and compare the efficacy of bevacizumab in combination with mIFL to mIFL alone, the co-primary endpoints were PFS rate at six months and duration of PFS. Secondary endpoints were the objective response rate [ORR; according to Response Evaluation Criteria in Solid Tumors (RECIST)], duration of response, OS, and the safety profiles of both treatment arms.
Assessments
Physical examination, ECOG performance status, blood pressure (BP), and routine blood and urine analyses were assessed within 28 days of starting study treatment. During treatment, physical examination, ECOG performance status, BP, and blood and biochemistry analyses were repeated every six weeks for the first 24 weeks and every 12 weeks thereafter. After disease progression or withdrawal, patients were followed up at least every three months until death.
Tumor assessments using abdominal and pelvic spiral computed tomography (CT) with chest X-rays or CT scans were performed at baseline and every six weeks for the first 24 weeks and then every 12 weeks. Objective response and disease progression were determined by the investigator using RECIST. Confirmation of response was performed four weeks after it was first documented. The PFS rate at six months was defined as the proportion of patients not experiencing disease progression or death due to any cause from randomization to six months after that. Patients without tumor assessment or survival information at week 24 for any reason were not regarded as progression-free survival at six months. PFS was defined as the time from the date of randomization to the first date of documented disease progression or death due to any causes. ORR according to RECIST was the best response documented from the start of treatment until disease progression or recurrence. Duration of response was defined as the time from the first documented complete or partial response to disease progression or death due to any cause.
Patients were evaluated for adverse events (AEs) and serious adverse events (SAEs) before each treatment cycle until treatment end. AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.
Statistical analysis
The sample size estimation was based on practical considerations; it had adequate power for detecting the difference in PFS based on the available results from the pivotal global study.
The primary population for all efficacy analyses, full analysis set (FAS) population, was defined as all patients enrolled into the study who received at least one dose of study medication and had at least one tumor assessment after randomization. The safety population was defined as all patients enrolled into the study who received at least one dose of study medication.
PFS rates at six months are presented as the mean and 95% confidence interval (CI) and were compared with a Pearson χ2 test between the two treatment arms. For PFS, the median values are presented as 95% CI and were compared using the Kaplan-Meier method and the log-rank test between the two treatment arms. ORRs are presented as 95% CIs and were analyzed using the Pearson χ2 test. OS and duration of response, including median time to event, were analyzed using the Kaplan-Meier method.
The incidences of AEs, SAEs, deaths, drug-related AEs, grades 3 and 4 AE and AE of special concern to bevacizumab (hypertension, proteinuria, gastrointestinal perforation, wound-healing complications, arterial thromboembolic event, venous thromboembolic event, bleeding and congestive heart failure) are presented descriptively as number of cases and incidence.
Results
Patient population
Between July 20, 2007 and August 7, 2008, 214 patients were enrolled at 12 centers in China, randomized to two groups, and received bevacizumab plus mIFL (n = 142) or mIFL alone (n = 72), respectively. Three patients in the bevacizumab plus mIFL group were excluded because they did not receive study treatment after randomization (n = 1) or did not have tumor assessment or survival information after randomization (n = 2), and eight patients in the mIFL group were excluded because they either did not receive study treatment (n = 2) or did not have tumor assessment or survival information (n = 6). The FAS population, therefore, comprised a total of 203 patients (bevacizumab plus mIFL group, n = 139; mIFL group, n = 64), and the safety population comprised 211 patients (bevacizumab plus mIFL group, n = 141; mIFL group, n = 70). Baseline demographic and clinical characteristics were well balanced between the two groups (Table 1).
Table 1. Baseline characteristics of patients with metastatic colorectal cancer in two groups.
| Characteristic | mIFL group (n = 64) | Bevacizumab plus mIFL group (n = 139) |
| Sexa | ||
| Men | 36 (56.2) | 70 (50.4) |
| Women | 28 (44.8) | 69 (49.6) |
| Age (years)b | 50 (22–72) | 53 (23–77) |
| ECOG performance statusa | ||
| 0 | 23 (35.9) | 66 (47.5) |
| 1 | 41 (64.1) | 73 (52.5) |
| Primary tumor sitea | ||
| Colon | 31 (48.4) | 66 (47.5) |
| Rectum | 32 (50.0) | 66 (47.5) |
| Colorectum | 1 (1.6) | 7 (5.0) |
| Number of metastatic sitesa | ||
| 1 | 23 (35.9) | 58 (41.7) |
| >1 | 41 (64.1) | 81 (58.3) |
| Previous cancer therapya | ||
| Adjuvant chemotherapy | 25 (39.1) | 70 (50.4) |
| Radiotherapy | 12 (18.8) | 17 (12.2) |
ECOG, Eastern Cooperative Oncology Group; mIFL, modified irinotecan, leucovorin bolus, and 5-fluorouracil intravenous infusion. aThe values are presented as number of patients, with the percentage in parentheses. bThe values are median age, with range in the parentheses.
Treatment exposure
The duration of treatment, shown as mean ± standard deviation (SD), for the safety population was (2.3 ± 1.8) months in the mIFL group and (6.2 ± 4.5) months in the bevacizumab plus mIFL group, and the number of treatment cycles was 2.2 ± 1.2 and 4.7 ± 3.0, respectively. The median dose intensity of bevacizumab in the bevacizumab plus mIFL group was 98.7%. The median dose intensity of irinotecan was 79.8% in the mIFL group and 73.6% in the bevacizumab plus mIFL group; for 5-FU, it was 85.4% and 75.5%, respectively; and for LV, it was 95.7% and 88.6%, respectively.
Efficacy
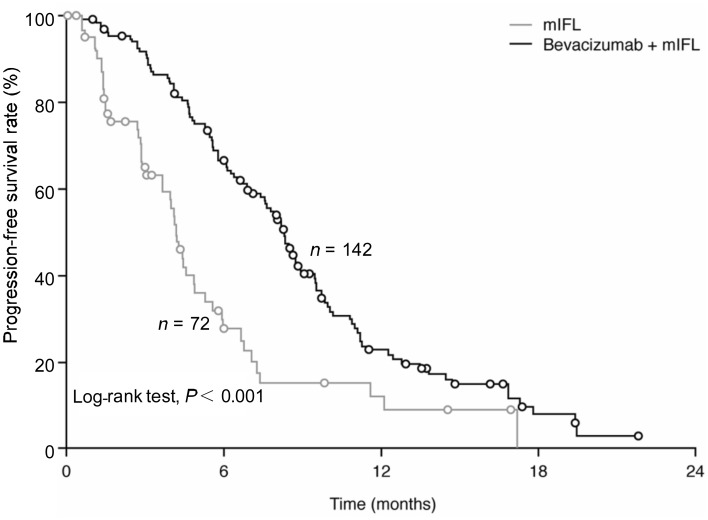
The addition of bevacizumab to chemotherapy as first-line treatment for Chinese patients with mCRC resulted in a significant (P < 0.001) improvement in the co-primary endpoint of PFS rate at six months from 25.0% (95% CI, 14.4%–35.6%) in the mIFL group to 62.6% (95% CI, 54.5%–70.6%) in the bevacizumab plus mIFL group (Figure 1). The addition of bevacizumab to chemotherapy also significantly (P < 0.001) prolonged PFS, with a median PFS of 4.2 months (95% CI, 3.7–4.9 months) in the mIFL group compared to 8.3 months (95% CI, 7.4–8.9 months) in the bevacizumab plus mIFL group (Figure 1). A clinically meaningful reduction in the risk of progression or death of 56% was observed with the addition of bevacizumab to mIFL [hazard ratio (HR), 0.44; 95% CI, 0.31–0.63; P < 0.001].
Figure 1. Progression-free survival (full analysis set) in Chinese patients with metastatic colorectal cancer treated with either bevacizumab plus mIFL or mIFL alone.
mIFL, modified irinotecan, leucovorin bolus, and 5-fluorouracil intravenous infusion.
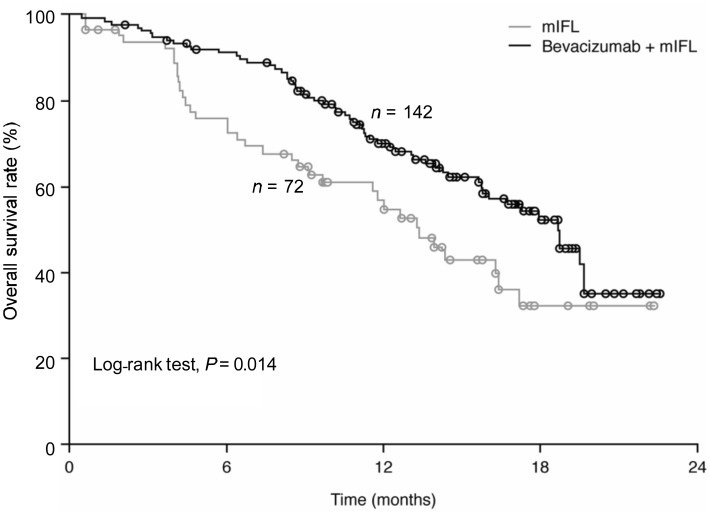
OS was also significantly (P = 0.014) improved with the addition of bevacizumab to mIFL, with the median OS of 13.4 months (95% CI, 9.7–17.2 months) in the mIFL group increasing to 18.7 months (95% CI, 15.8–19.6 months) in the bevacizumab plus mIFL group (Figure 2). Bevacizumab significantly reduced the risk of death by 38% (HR, 0.62; 95% CI, 0.41–0.95; P = 0.014).
Figure 2. Overall survival (full analysis set) In Chinese patients with metastatic colorectal cancer treated with either bevacizumab plus mIFL or mIFL alone.
mIFL, modified irinotecan, leucovorin bolus, and 5-fluorouracil intravenous infusion.
The ORR in the mIFL group was 17% (95% CI, 8.4%–27.7%), and it increased to 35% (95% CI, 27.5%–43.5%; P = 0.013) in the bevacizumab plus mIFL group (Table 2). The median duration of response was 3.5 months (95% CI, 1.8–4.7 months) in the mIFL group and 7.4 months (95% CI, 6.0–8.8 months; P = 0.081) in the bevacizumab plus mIFL group.
Table 2. Overall response rate of patients.
| Outcome | mIFL (n = 64) | Bevacizumab + mIFL (n = 139) | P |
| Overall response rate (%, 95% CI) | 17.2 (8–28) | 35.3 (28–44) | 0.013 |
| Complete responsea | 0 (0) | 4 (2.9) | |
| Partial responsea | 11 (17.2) | 45 (32.4) | |
| Stable diseasea | 33 (51.6) | 81 (58.3) | |
| Progressive diseasea | 13 (20.3) | 3 (2.2) | |
| Not evaluablea | 7 (10.9) | 6 (4.3) |
CI, confidence interval. mIFL, modified irinotecan, leucovorin bolus, and 5-fluorouracil intravenous infusion. aThe values are presented as number of patients, with percentage in the parentheses.
Tolerability
Overall, the addition of bevacizumab to chemotherapy was well tolerated with no new safety signals being observed for bevacizumab. Almost all of the patients experienced at least one AE (mIFL group, 99%; bevacizumab plus mIFL group, 97%). The incidence of grades 3 and 4 AEs, including the most frequent AEs of diarrhea and neutropenia, was comparable between the two groups (mIFL group, 61%; bevacizumab plus mIFL group, 69%) (Table 3). The majority of AEs of special interest to bevacizumab were of grade 1 or 2 severity and medically manageable. The incidence of hypertension (all grades) was 1% in the mIFL group and 16% in bevacizumab plus mIFL group, with 4% of patients in the b evacizumab plus mIFL group experiencing grade 3 hypertension. The incidence of proteinuria was 17% in the bevacizumab plus mIFL group, of which 1 % was of grade 3 severity. There was no proteinuia reported in the mIFL group. The incidence of all bleeding events was higher in the bevacizumab plus mIFL group than in the mIFL group [36% (n = 50) vs. 13% (n = 9)]; however, only 0.7% of patients in the bevacizumab plus mIFL group had a grade 3 bleeding event compared to 1% of patients in the mIFL group. The majority of the bleeding events in the bevacizumab plus mIFL group were mucocutaneous: grades 1 and 2 epistaxis occurred in 30 patients, and grade 1 gingival and vaginal bleeding occurred in four patients each. Two patients in the bevacizumab plus mIFL group reported a grade 3 arterial thromboembolic event, but no grade 3 venous thromboembolic events were observed in either group. Similarly, gastrointestinal perforation was uncommon, and no wound-healing complications or congestive heart failure was observed.
Table 3. Incidence of grades 3 and 4 adverse events during treatment with either mIFL or bevacizumab plus mIFL.
| Grades 3 and/or 4 adverse event | mIFL (n = 70) | Bevacizumab + mIFL (n = 141) |
| Grades 3 and/or 4 adverse event(s) | ||
| Patients with ≥ 1 adverse event | 3 | 8 |
| Diarrhea | 21 | 26 |
| Neutropenia | 19 | 33 |
| Vomiting | 9 | 8 |
| Nausea | 3 | 5 |
| Anemia | 1 | 4 |
| Thrombocytopenia | 4 | 3 |
| Febrile neutropenia | 2 | 2 |
| Events of special interest to bevacizumab | ||
| Hypertension | 0 | 4 |
| Bleeding | 1 | 1 |
| Thromboembolic events-arterial | 0 | 1 |
| Proteinuria | 0 | 1 |
| Gastrointestinal perforation | 0 | 1 |
mIFL, modified irinotecan, leucovorin bolus, and 5-fluorouracil intravenous infusion. The values are presented as percentage.
The proportion of patients who withdrew from all study treatment as a result of an AE was higher in the mIFL group (13/70, 18.6%) than in the bevacizumab plus mIFL group (14/141, 10%), with most of the events being known chemotherapy side effects. The incidence of AEs leading to death was similar in the bevacizumab plus mIFL (2/141, 1%) and mIFL groups (1/70, 1%). There was another death of unknown reason reported as an SAE in the bevacizumab plus mIFL group. The patient died 30 days after withdrawal of informed consent for the study.
Discussion
This phase III ARTIST trial demonstrates that the addition of bevacizumab to mIFL chemotherapy as first-line treatment for Chinese patients with mCRC resulted in a significant increase in PFS rate at six months compared with mIFL alone (62.6% vs. 25.0%, P < 0.001) and significantly prolonged median PFS (8.3 months vs. 4.2 months, P < 0.001), another co-primary endpoint. Evaluation of the secondary efficacy endpoints showed that the addition of bevacizumab to mIFL significantly improved median OS compared to mIFL alone (18.7 months vs. 13.4 months, P = 0.014), increased the ORR from 17.2% to 35.3% (P = 0.013), and increased the duration of response from 3.5 months to 7.4 months (P = 0.081). The improvements in PFS and OS observed following the addition of bevacizumab resulted in a clinically meaningful reduction of 56% in the risk of progression or death and a reduction of 38% in the risk of death.
The efficacy of mIFL chemotherapy alone in this study is comparable with that reported in a phase II trial involving Chinese patients with mCRC receiving mIFL chemotherapy in which the ORR was 17% and the median time to progression was six months[11]. However, the PFS and OS in both groups in the current study were numerically shorter than those observed in the pivotal study by Hurwitz et al.[2]. Besides the ethnic difference of the patients enrolled in the two studies, there are several possible explanations for these differences. First, baseline ECOG performance status was slightly less favorable in this study (48% of patients with ECOG performance status 0) compared to the study by Hurwitz et al.[2] (57% patients with ECOG performance status 0), which suggests that the prognosis of patients in this study might be slightly worse. Also, almost 50% of the patients in this study had rectal cancer; however, only 20% patients with rectal cancer were enrolled in the study by Hurwitz et al.[2]. Despite the above differences between the two studies, the efficacy findings reported here show the same trend toward clinical benefit for bevacizumab in combination with chemotherapy compared to chemotherapy alone in Chinese patients with mCRC as that observed by Hurwitz et al.[2], suggesting that Chinese patients may achieve the same clinical benefit from bevacizumab treatment as Caucasian patients.
The tolerability profile of combination of bevacizumab with mIFL was consistent with that in other studies using this or similar regimens, e.g., the studies by Hurwitz et al.[2] and Huang et al.[11]. Combination of bevacizumab with chemotherapy in Chinese patients with mCRC resulted in a slightly increased incidence of AEs, especially chemotherapy-associated AEs such as neutropenia, diarrhea, and nausea, compared to chemotherapy alone. The incidence of grades 3 and 4 AEs was also increased slightly from 62% to 69% with the addition of bevacizumab to chemotherapy, but treatment discontinuations as a result of AEs were mainly the result of chemotherapy-associated toxicities. Notably, treatment duration was much longer in the bevacizumab plus mIFL group (161 days) than in the mIFL group (64 days), and cumulative doses of chemotherapy were also higher (almost twice as high) in the bevacizumab plus mIFL group than in the mIFL group. Grade 3 hypertension (4% vs. 0%), proteinuria (1% vs. 0%), arterial thromboembolic events (1% vs. 0%), and gastrointestinal perforation (1% vs. 0%) were more commonly observed in the bevacizumab plus mIFL group than in the mIFL group. No grade 3 venous thromboembolic events or wound-healing complications and episodes of congestive heart failure of any grade were observed. No new safety signals were observed with bevacizumab, and the addition of this biological agent to mIFL was well tolerated against a background of irinotecan-containing chemotherapy.
The clinical benefits of adding bevacizumab to first-line chemotherapy for mCRC have been observed across a range of different chemotherapy regimens[2]–[4],[12],[13]. Similar benefits have been observed in the second-line setting for mCRC [5],[14]–[16]. The bevacizumab regimens (investigation of treatment effects and safety, BRiTE) observational study showed that patients with mCRC who continued bevacizumab treatment after first progression had superior median survival beyond progression and median OS (19.2 and 31.8 months, respectively) than did those in whom bevacizumab was discontinued after progression (9.5 and 19.9 months, respectively)[17], indicating that second-line bevacizumab is beneficial even in patients who received this agent in the first-line setting. A separate observational cohort study examined the role of bevacizumab after disease progression in patients who did or did not received first-line bevacizumab and showed a trend towards longer OS in patients who had first- and second-line bevacizumab exposure (median OS, 21.7 months) than in patients who only received bevacizumab after progression (median OS, 17.5 months)[8]. The second-line PFS was similar in both groups (7.6 vs. 8.0 months, respectively)[8]. Those findings, together with the results of the present study, support the use of bevacizumab in combination with chemotherapy as first-line therapy for patients with mCRC.
In summary, this phase III ARTIST trial suggested that the addition of bevacizumab to mIFL chemotherapy in the first-line treatment of Chinese patients with mCRC resulted in a significantly increased PFS rate at six months and improved PFS, OS, and ORR. This clinical benefit was accompanied by a low rate of additional AEs. Furthermore, the clinical benefit and safety profiles of bevacizumab in Chinese patients were consistent with those observed in pivotal phase III multi-national trials.
Acknowledgments
This study was sponsored by Shanghai Roche Pharmaceuticals Ltd. Support for third-party writing assistance for this manuscript, furnished by Miller Medical Communications, was provided by F. Hoffmann-La Roche.
References
- 1.Globocan 2008. Cancer incidence and mortality worldwide in 2008. Available at: http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=160#BOTH. Accessed on 5th August, 2010.
- 2.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer [J] N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 3.Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer [J] J Clin Oncol. 2005;23(16):3706–3712. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study [J] J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. Errata in: J Clin Oncol, 2008,26(18):3110; J Clin Oncol, 2009, 27(4):653. [DOI] [PubMed] [Google Scholar]
- 5.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200 [J] J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 6.Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study [J] Oncologist. 2009;14(9):862–870. doi: 10.1634/theoncologist.2009-0071. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study [J] Ann Oncol. 2009;20(11):1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 8.Bekaii-Saab TS, Bendell JC, Cohn AL, et al. Bevacizumab (BV) plus chemotherapy (CT) in second-line metastatic colorectal cancer (mCRC): Initial results from ARIES, a second BV observational cohort study (OCS) [J] J Clin Oncol. 2010;28(15s):Abstr 3595. Available at: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&conflD=74&abstractlD=48372. [Google Scholar]
- 9.Cohn AL, Bekaii-Saab TS, Bendell JC, et al. Clinical outcomes in bevacizumab (BV)-treated patients (pts) with metastatic colorectal cancer (mCRC): Results from ARIES observational cohort study (OCS) and confirmation of BRiTE data on BV beyond progression (BBP) [J] J Clin Oncol. 2010;28(15s):Abstr 3596. Available at: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&conflD=74&abstractlD=44524. [Google Scholar]
- 10.Sobrero A, Ackland S, Clarke S, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer [J] Oncology. 2009;77(2):113–119. doi: 10.1159/000229787. Errata in: Oncology 2009;77(2):following 119; Oncology, 2009,77(3–4):256. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Qin SK, Qian J, et al. Clinical study of weekly irinotecan in patients with metastatic colorectal cancer [J] Cancer Res Prev Treat. 2005;32:110–112. [in Chinese] [Google Scholar]
- 12.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer [J] J Clin Oncol. 2005;23(15):3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer [J] J Clin Oncol. 2003;21(1):60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 14.Lièvre A, Samalin E, Mitry E, et al. Bevacizumab plus FOLFIRI or FOLFOX in chemotherapy-refractory patients with metastatic colorectal cancer: a retrospective study [J] BMC Cancer. 2009;9:347. doi: 10.1186/1471-2407-9-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincenzi B, Santini D, Russo A, et al. Bevacizumab in association with de Gramont 5-fluorouracil/folinic acid in patients with oxaliplatin-, irinotecan-, and cetuximab-refractory colorectal cancer: a single-center phase 2 trial [J] Cancer. 2009;115(20):4349–4856. doi: 10.1002/cncr.24540. [DOI] [PubMed] [Google Scholar]
- 16.Yildiz R, Buyukberber S, Uner A, et al. Bevacizumab plus irinotecan-based therapy in metastatic colorectal cancer patients previously treated with oxaliplatin-based regimens [J] Cancer Invest. 2010;28(1):33–37. doi: 10.3109/07357900802562996. [DOI] [PubMed] [Google Scholar]
- 17.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) [J] J Clin Oncol. 2008;26(33):5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]