Abstract
Although the anti-malaria drug chloroquine (CQ) has been shown to enhance chemotherapy and radiation sensitivity in clinical trials, the potential mechanisms underlying this enhancement are still unclear. Here, we examined the relevant mechanisms by which the multipotent CQ enhanced the cytotoxicity of topotecan (TPT). The lung cancer cell line A549 was treated with TPT alone or TPT combined with CQ at non-cytotoxic concentrations. Cell viability was assessed using the MTT assay. The percentage of apoptotic cells and the presence of a side population of cells were both determined by flow Cytometry. Autophagy and the expression of Bcl-2 family proteins were examined by Western blotting. The accumulation of YFP-LC3 dots and the formation of acidic vesicular organelles were examined by confocal microscopy. CQ sensitized A549 cells to TPT and enhanced TPT-induced apoptosis in a Bcl-2 family protein-independent fashion. CQ inhibited TPT-induced autophagy, which modified the cytotoxicity of TPT. However, CQ failed to modify the transfer of TPT across the cytoplasmic membrane and did not increase lysosomal permeability. This study showed that CQ at non-cytotoxic concentrations potentiated the cytotoxicity of TPT by interfering with autophagy, implying that CQ has significant potential as a chemotherapeutic enhancer.
Keywords: Chloroquine, chemotherapy, autophagy, lung cancer
Chemotherapy shows therapeutic efficacy mainly by inducing apoptosis of cancer cells[1]. Autophagy, a dynamic process in which intracellular membrane structures sequester proteins and organelles for degradation and turnover, has the potential to promote or delay apoptosis[2]. At the beginning of autophagy, portions of the cytoplasm and intracellular organelles are sequestered in double-membrane-bound structures known as autophagosomes. These autophagosomes fuse with lysosomes to form autolysosomes, and the sequestered contents are degraded by lysosomal hydrolases and recycled. Autophagy provides cells with energy and materials for biosynthesis[3]–[7]. Multiple chemotherapeutic agents, including topotecan (TPT), doxorubicin, etoposide, cyclophosphamide, cisplatin, temozolomide, gemcitabine, paclitaxel analogs, and 5-fluorouranial, have shown the potential to induce autophagy in the context of different cancer cells[8]–[16].
Chloroquine (7-chloro-4- [4-diethylamino-1-methyl-butylamino] quinoline; CQ) is commonly sold as malaria medication and is used as an anti-inflammation drug for rheumatoid arthritis, discoid lupus erythematosus, and amoebic hepatitis[17],[18]. In 1992, Djordevic et al.[19] reported that treating mouse melanoma cells with CQ potentiated radiation-induced cell killing. Subsequently, CQ was reported to have direct cytotoxicity or to enhance the cytotoxicity of radiotherapy and targeted therapy. The cytotoxicity of CQ has been examined in cell lines derived from breast, lung, colon, hepatic, pancreatic, and ovarian cancers as well as glioma[20]–[25]. Recently, a randomized, double-blind, placebo-controlled trial demonstrated that CQ may improve mid-term survival when given in addition to conventional chemotherapy and radiotherapy for glioblastoma multiforme[26],[27]. Presently, more than 10 trials have been initiated to evaluate the effect of CQ combined with radiotherapy, chemotherapy, and targeted therapy[28].
There may be multiple means by which CQ exerts its antitumor effects. First, CQ increases lysosomal permeability and cell death. The lysosomotropic properties of CQ promote its accumulation in several intracellular organelles and raise intravesicular pH. Consequently, both lysosomal volume and permeability increase[29],[30]. Second, CQ can disrupt lysosomal function and prevent completion of autophagy at the final step, resulting in an accumulation of LC3-II[27]. Third, CQ blocks ABC transporters in Plasmodium and tumor cells, resulting in increased intracellular drug availability and cell damage[31]–[33]. Finally, CQ can directly interfere with DNA, resulting in defective DNA synthesis and repair[33]. Nevertheless, the relevant mechanisms of the enhanced effect of CQ are still unclear.
If CQ can enhance the efficacy of chemotherapy at non-cytotoxic concentrations, CQ and its analogs might be developed as chemotherapy sensitizers because of their safety and low cost. Therefore, to evaluate the potential of CQ as a chemotherapy sensitizer, we examined the mechanisms by which CQ enhanced the efficacy of chemotherapy. Specifically, we analyzed the ability of non-cytotoxic doses of CQ to enhance the efficacy of TPT, a semisynthetic derivative of camptothecin that specifically targets topoisomerase I.
Materials and Methods
Drugs and reagents
Chloroquine diphosphate (CQ), which was dissolved in PBS at a concentration of 100 mmol/L and stored at 4°C, Hoechst 33342, propidium iodide, acridine orange, RPMI-1640 medium, sodium dodecyl sulfate (SDS), and 6-diamidino-2-phenylindole (DAPI), were purchased from Sigma-Aldrich (St. Louis, MO, USA). TPT was purchased from Merck (Darmstadt, Germany).
Cell lines, cell culture, and cell viability
The human lung carcinoma cell line A549, provided by the Department of Experimental Research of Sun Yat-sen University Cancer Center, was cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum, penicillin (50 U/mL), and streptomycin (50 µg/mL). The cells were kept at 37°C in a humidified incubator with 5% CO2. For cell viability assays using TPT and CQ, the cells were seeded into 96-well culture plates at a density of 8000 cells/well and allowed to adhere overnight. The next day, cells were treated with TPT (0.39, 0.783, 1.563, 3.125, 6.25 µg/mL), CQ (1.95, 3.9, 7.8, 15.625, 31.25 µg/mL), or TPT combined with CQ (0.39/1.95, 0.783/3.9, 1.563/7.8, 3.125/15.625, 6.25/31.25 µg/mL). Cell viability after treatment was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-nyltetrazolium bromide (MTT) assay (In vitro Toxicology Assay Kit; Sigma-Aldrich, St. Louis, MO, USA).
The combination index (CI)–isobologram equation was used for the quantitative determination of drug interactions, where CI < 1 indicates synergism, CI = 1 indicates additive effect, and CI > 1 indicates antagonism[33].
Cell cycle analysis by flow Cytometry
A549 cells were treated with 2 µg/mL TPT, 10 µg/mL CQ, or both for 48 h in a 6-well plate. For cell cycle analysis, cells were collected, washed with PBS, and fixed in 70% alcohol overnight. Cells were then resuspended in 1 mL propidium iodide (50 µg/mL), placed on ice in dark, and immediately analyzed by flow Cytometry (FC500; Beckman-Coulter, CA, USA) at a wavelength of 625 nm.
Detection of apoptotic cells by flow Cytometry
TPT- and CQ-induced cell death was evaluated using an annexin V–FITC apoptosis assay. Cells were cultured in 6-well plates and exposed to drugs for 48 h, as described in the subsection of cell culture. Staining was then performed using the annexin V–fluorescein isothiocyanate apoptosis detection kit (Calbiochem, Darmstadt, Germany) according to the manufacturer's instruction. Apoptosis was detected by flow Cytometry (FC500; Beckman-Coulter, CA, USA) at a wavelength of 625 nm.
Detection of side population (SP) cells by flow Cytometry
Cells were cultured in 60-mm plates and exposed to drugs for 12 h. The cells were then collected, resuspended in ice-cold RPMI-1640 media (supplemented with 2% FBS) at a concentration of 1 × 106 cells/mL, and placed in an incubator at 37°C with 5% CO2 for 10 min. Hoechst 33342, a DNA-binding dye, was then added at a final concentration of 10 µg/mL, and the cells were incubated for 90 min in the dark with intermittent mixing (every 10 min). After two washes with PBS, 1 µg/mL propidium iodide was added, and the cells were kept at 4°C in the dark prior to dual wavelength FACS analysis (Beckman Coulter, Fullerton, California, USA). Because Hoechst 33342 would be expelled from cells via verapamil–sensitive ATP-binding cassette (ABC) transporters, a subset of the cells were incubated with 50 µmol/L verapamil for 30 min at 37°C before adding Hoechst 33342 to block the efflux of fluorescent dye from SP cells within the A549 population.
Plasmids and transfection
A549 cells were seeded in 6-well plates the day before transfection. The activated plasmid pYFP-LC3 or empty plasmid pYFP-N1 was transfected into cells using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer's instructions. After 48 h of transfection, the positive clones were selected using G418 (500 µg/mL).
Confocal microscopy for LC3
A549 cells overexpressing pYFP-LC3 were grown on glass coverslips. After treatment with 2 µg/mL TPT for 12 h, the cells were fixed with 4% paraformaldehyde at room temperature for 20 min. After three PBS washes, cells were incubated in DAPI (1 µg/mL) for 5 min at room temperature and washed three more times with PBS. Cells were observed using an Olympus FV 1000 confocal microscope.
Confocal microscopy for acridine orange staining
Cells were plated at 50% confluence on glass coverslips and treated with 2 µg/mL TPT for 12 h. Thereafter, the cells were incubated with 1 mg/mL acridine orange for 15 min. After three PBS washes, cells were incubated in DAPI (1 µg/mL) for 5 min at room temperature and washed three more times with PBS. Cells were then fixed and dried at room temperature. Cells were immediately observed using an Olympus FV 1000 confocal microscope.
Western blot analysis
Protein extraction and Western blotting were performed as described previously[34]. Anti - LC3 was purchased from Novus (Littleton, CO, USA). Anti-GAPDH, anti-Bax, anti–Caspase-3, anti-PARP, anti–Bcl-2, anti-P62, and corresponding second antibodies were purchased from Santa Cruz (Santa Cruz, CA, USA). Anti-Atg5, anti-Bid, and anti-Bim antibodies were purchased from Cell Signal Technology (Danvers, MA, USA).
Retroviral infection and RNA interference
A549 cells stably expressing shATG5 were selected as previously described[34]. Cells were then seeded at a density of 8000 per well into 96-well culture plates for further experiments.
Statistical analyses
Data were analyzed by t-test using SPSS11.0 software, are expressed as mean ± standard deviation (SD), and are accompanied by the number of experiments independently performed. Differences at P < 0.05 were considered statistically significant.
Results
CQ sensitized A549 cells to TPT
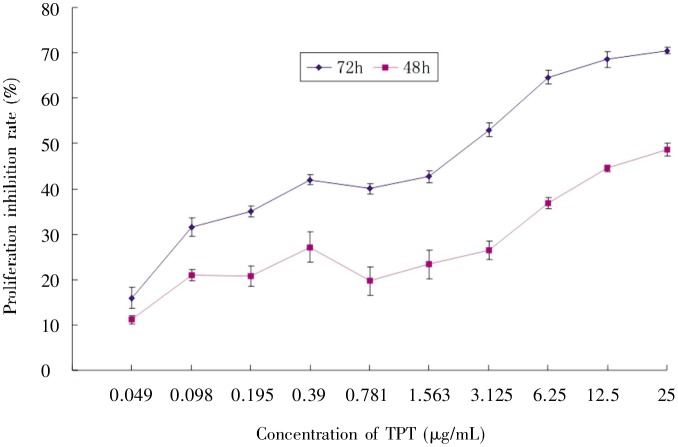
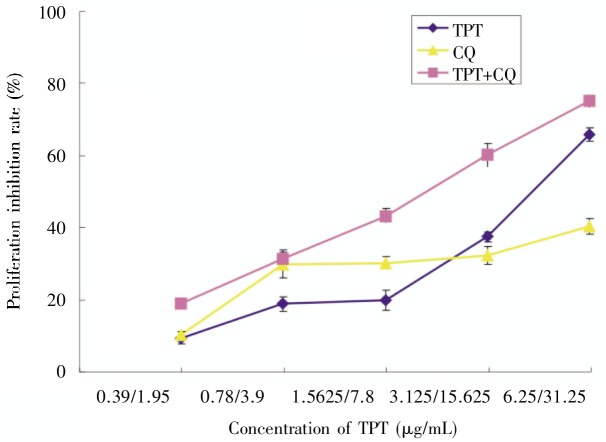
Using the MTT assay, we determined the effect of TPT alone or in combination with CQ on the proliferation of the human lung cancer cell line A549. A549 cells were exposed to 0–25 µg/mL TPT for 48 and 72 h, which resulted in inhibition of cell viability (Figure 1). At 72 h, the IC50 value was 1.776 ± 0.25 µg/mL A549 cells were also exposed to 0.39–6.25 µg/mL TPT along with 1.95–31.25 µg/mL CQ for 72 h. Within this range of concentration, CQ showed a weak cytotoxic effect; whereas TPT combined with CQ caused a much more pronounced inhibition of cell viability than either compound alone (Figure 2). Moreover, the combination of TPT and CQ produced a combination index (CI) of <1 in A549 cells (Table 1). These data indicated that CQ could sensitize A549 cells to TPT in vitro.
Figure 1. Topotecan (TPT) suppressed A549 cell proliferation.
Cells were cultured at 8000 cells per well in a 96-well plate, exposed to the indicated concentrations of TPT, and incubated for 48 or 72 h. Cell proliferation was detected using an MTT assay. Data points are the average of three experiments. Bars represent the standard deviation (SD).
Figure 2. Inhibitory effects of TPT and Chloroquine (CQ) on A549 cell proliferation.
Cells were cultured at 8000 cells per well in a 96-well plate and exposed to the indicated concentrations of TPT and CQ at a fixed ratio (1:5) for 72 h. The proliferation inhibition was detected using an MTT assay. The results are representative of three experiments.
Table 1. Inhibitory effects of chloroquine diphosphate (CQ) combined with topotecan (TPT) on the growth of A549 cells.
| Concontration of TPT (µg/mL) | Concontration of CQ (µg/mL) | Fa | CI |
| 0.39 | 1.95 | 0.190 | 0.984 |
| 0.78 | 3.9 | 0.315 | 0.729 |
| 1.5625 | 7.8 | 0.433 | 0.728 |
| 3.125 | 15.625 | 0.601 | 0.615 |
| 6.25 | 31.25 | 0.750 | 0.545 |
Fa, fraction affected, equals to the inhibition rate of the combination; CI, combination index, determined using CalcuSyn software.
CQ increased apoptosis induced by TPT
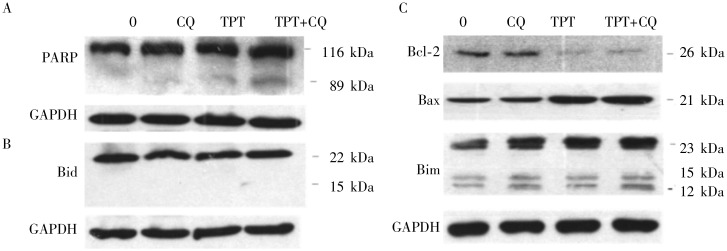
To study whether the inhibition of A549 cell proliferation caused by the two drugs was due to cell growth arrest or apoptosis, flow Cytometry and Western blot analyses were used to detect levels of apoptosis. The percentage of early apoptotic cells (annexin V-positive) was 17.0% when treated with 2 µg/mL TPT alone, and increased to 32.8% when treated with TPT combined with 10 µg/mL CQ (Figure 3A). The sub-G1 DNA content of A549 cells increased from 11.4% when treated with 2 µg/mL TPT alone to 23.1% when treated with TPT combined with 10 µg/mL CQ (Figure 3B). Western blot results demonstrated that PARP was cleaved to produce an 89-kDa fragment after TPT treatment, and the levels of cleaved PARP were increased in cells treated with CQ combined with TPT (Figure 4A). These data indicated that TPT induced apoptosis and that an additional treatment with CQ enhanced this effect.
Figure 3. Effect of CQ on TPT-induced apoptosis determined by flow Cytometry.
A549 cells were treated with 2 µg/mL TPT, 10 µg/mL CQ, or both for 48 h. A, Annexin-V and propidium iodide (PI) staining shows that combined treatment increased the percentages of early and late apoptotic cells. B, sub-G1 phase analysis shows that combined treatment increased the sub-G1 DNA content in A549 cells.
Figure 4. Effect of TPT and CQ on the expression of PARP and Bcl-2 family proteins.
A549 cells were treated with 2 µg/mL TPT, 10 µg/mL CQ, or both for 48 h. GAPDH was used as an internal control. A, cleavage of PARP induced by TPT and CQ in A549 cells. B and C, the expression levels of Bcl-2 family proteins were similar between A549 cells treated with TPT and CQ alone or in combination.
CQ enhanced TPT-induced apoptosis independently of Bcl-2 family proteins
As TPT induces apoptosis via the mitochondrial pathway, we examined whether CQ interfered with Bcl-2 family proteins. The expression levels of the pro-apoptotic protein Bax and the anti-apoptotic proteins Bcl-2 and Bim were determined by Western blot analysis. No change in expression of these Bcl-2 family proteins was observed when A549 cells were treated with CQ alone or in combination with TPT (Figure 4B and 4C). CQ can interfere with lysosome permeabilization, which initiates cell death via proteolytic activation of Bid. However, this study indicated that CQ treatment did not modify Bid expression (Figure 4B). These data suggested that CQ enhanced the cytotoxicity of TPT independently of Bcl-2 family proteins and that, at concentrations of less than 10 µg/mL, CQ failed to increase lysosomal permeabilization.
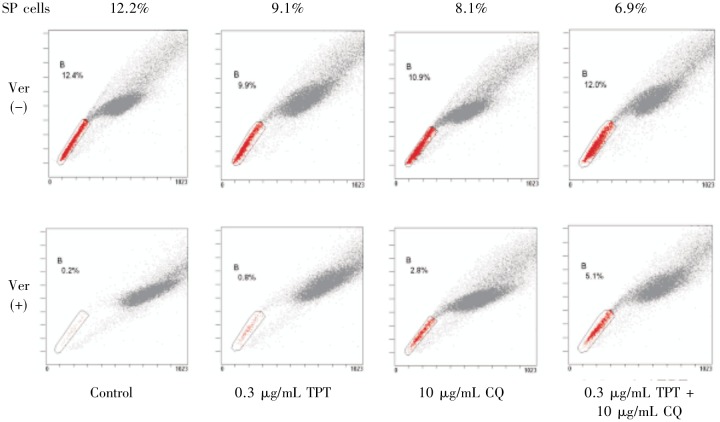
CQ decreased the ratio of SP cells
Stem-like cancer cells can be isolated by their ability to efflux Hoechst 33342 dye and are referred to as the “side population” (SP). TPT can be transferred in the same way that Hoechst 33342 dye is effluxed. Because CQ has the potential to inhibit this type of transfer, CQ might enhance the effect of TPT by preventing efflux, thus increasing the intracellular accumulation of TPT and promoting SP cell death. Therefore, we examined the variation in the fraction of SP cells after treatment with TPT and CQ. The results showed that treatment with either TPT or CQ each slightly decreased the ratio of SP cells (Figure 5). However, treatment with both drugs did not further enhance the effect of TPT, indicating that CQ did not interfere with the transfer of TPT.
Figure 5. Detection of side population (SP) cells among A549 cells.
A549 cells were treated with 0.3 µg/mL TPT, 5 µg/mL CQ, or both for 48 h, then stained with Hoechst 33342 in the presence (lower) or absence (upper) of 50 µmol/L verapamil (Ver) and analyzed by flow Cytometry. The SP cells, which disappear in the presence of verapamil, were gated and are shown as a percentage of the whole viable cell population. The percentage of SP cells among untreated A549 cells was 12.2%. The percentage decreased to 8.1% after treatment with CQ and to 9.1% after treatment with topotecan for 12 h. When A549 cells were treated with topotecan combined with CQ, the percentage of SP cells was slightly decreased to 6.9%.
TPT induced autophagy
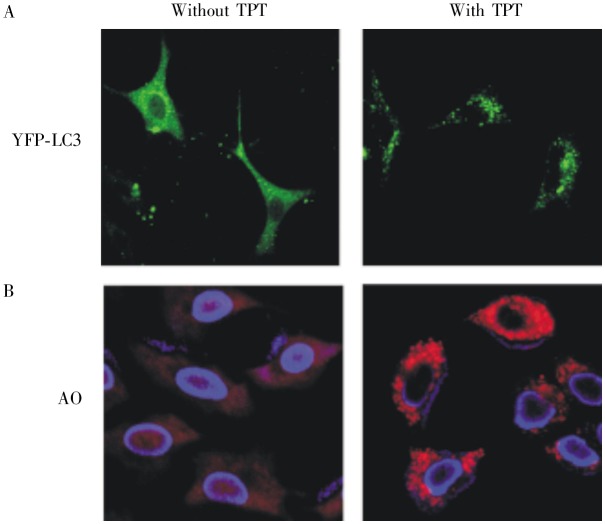
A previous study indicated that TPT could induce autophagy in Hep3B cells[13]. In the current study, we examined whether TPT could also induce autophagy in A549 cells. First, A549 cells stably expressing pYFP-LC3 were examined by fluorescence microscopy. In control cells, YFP-LC3 was evenly distributed throughout the cytoplasm. After exposure to 2 µg/mL TPT, ring-shaped structures were detected in the cytosol, indicating the association of YFP-LC3 with autophagosomal membranes and the induction of autophagy (Figure 6A). Additionally, A549 cells were stained with acridine orange, which labels acidic vacuoles and lysosomes and can be used as an indirect marker for autophagosome formation. The results showed abundant cytoplasmic acidic vesicular organelle formation in TPT-treated cells (Figure 6B). To further confirm that autophagy is induced by TPT, we detected the autophagy markers LC3-II and P62, a marker of autophagic activity which is degraded in the autolysosomes, by Western blotting. The results revealed that TPT induced autophagy in A549 cells in a time- and dose-dependent fashion (Figure 7A and 7B). Thus, taken together, these data indicated that TPT induced autophagy in A549 cells.
Figure 6. TPT induced autophagy in A549 cells.
A, TPT treatment caused accumulation of YFP-LC3 dots. A549 cells overexpressing pYFP-LC3 were treated with or without 2 µg/mL TPT for 12 h, and then visualized under a fluorescent microscope. Puncta represent autophagosome formation. B, TPT treatment caused formation of cytoplasmic acidic vesicular organelle. A549 cells were treated with or without 2 µg/mL TPT for 12 h, stained with acridine orange (1 µg/mL), and then visualized under a confocal microscope.
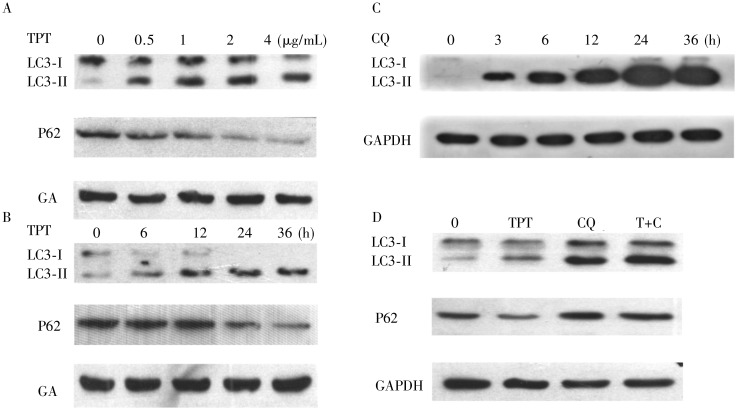
Figure 7. CQ inhibited autophagy induced by TPT.
A, detection of LC3-I, LC3-II, and P62 by Western blotting. A549 cells were treated with TPT at the indicated concentrations for 24 h. B, time course following treatment of A549 cells with 2 µg/mL TPT. C, treatment with 10 µg/mL CQ alone induced the accumulation of LC3-II. D, effect of 2 µg/mL TPT and 10 µg/mL CQ on LC3 and P62 processing.
CQ enhanced the cytotoxicity of TPT by inhibiting autophagy
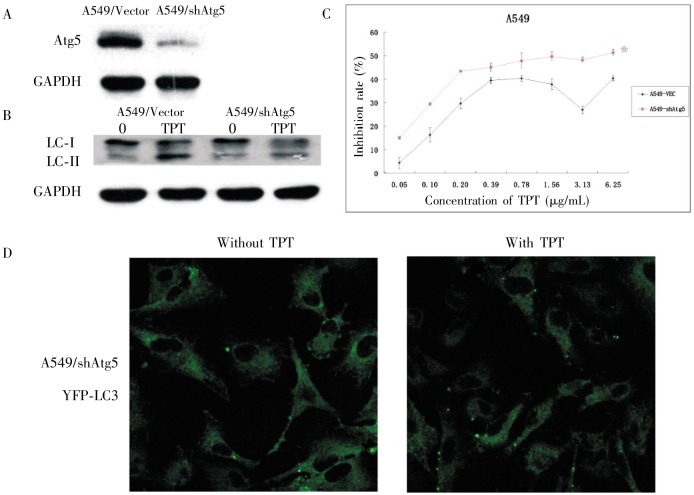
Because autophagy has the potential to promote or delay cell death, we examined whether the enhanced cytotoxicity of TPT when combined with CQ could be due to the inhibition of autophagy by CQ. When A549 cells were treated with CQ alone, LC3-II accumulated (Figure 7C) because CQ prevented fusion of the autophagosome with the lysosome. When CQ was combined with TPT, LC3-II and P62 accumulated (Figure 7D), indicating that CQ inhibited autophagy induced by TPT at the final stage. A549 cells stably expressing shATG5 (Figure 8A) were used to mimic inhibition of autophagy at the stage of autophagosome formation. Knockdown of Atg5 blocked the TPT-induced increase in LC3-II levels (Figure 8B) and the accumulation of YFP-LC3 dots (Figure 8D), and reduced proliferation due to treatment with TPT at concentrations between 0.05–6.25 µg/mL (Figure 8C). Furthermore, we used the MTT assay to determine the effect of TPT on the proliferation of the A549/shATG5 h7 cells. Notably, co-treatment with TPT and CQ produced a CI >1 in A549/shATG5 h7 cells (Table 2), suggesting an antagonistic relationship. These data showed that CQ did not further enhance cell death following TPT treatment in Atg5 knockdown cells. Taken together, these results indicated that the inhibition of autophagy enhanced the cytotoxic effect of TPT on A549 cells.
Figure 8. Effects of Atg5 knockdown on cytotoxicity of TPT.
A, Western blot analysis of Atg5 from lysates of A549/vector cells and A549/shATG5 h7 cells. GAPDH was used as a loading control. B, A549/vector cells and A549/shATG5 h7 cells were treated with 2 µg/mL TPT for 24 h and then harvested. LC3-II expression was detected by Western blotting. GAPDH served as a loading control. C, A549/vector cells and A549/shATG5 h7 cells were cultured at 6000 cells per well in a 96-well plate and exposed to TPT at concentrations from 0.05 to 6.25 µg/mL for 72 h. Growth inhibition was detected using MTT assays. Reported values are mean ± SD of triplicate samples from a representative experiment. *P < 0.05 as compared with cells treated in the same way but without transfection with the shRNA. D, when A549/shATG5 h7 cells overexpressing pYFP-LC3 were treated with 2 µg/mL TPT for 12 h, YFP-LC3 accumulation cannot be visualized under the fluorescent microscope.
Table 2. Inhibitory effects of CQ, TPT, or both on the growth of A549/shATG5 h7 cells.
| Concontration of TPT (µg/mL) | Concontration of CQ (µg/mL) | Fa | CI |
| 0.39 | 1.95 | 0.151 | 1.054 |
| 0.78 | 3.9 | 0.232 | 1.019 |
| 1.5625 | 7.8 | 0.327 | 1.134 |
| 3.125 | 15.625 | 0.434 | 1.365 |
| 6.25 | 31.25 | 0.680 | 1.013 |
Footnotes as in Table 1.
Discussion
This study demonstrated that non-cytotoxic concentrations of CQ potentiated the cytotoxicity of TPT in lung cancer cells by inhibiting autophagy induced by TPT, supporting the view that CQ and its analogues have potential as chemotherapy sensitizers.
Previous studies have indicated that the biological effect of CQ is concentration-dependent. For example, at low concentrations (<32 µmol/L), CQ inhibits growth of the lung cancer cell line A549 and induces vacuolation with increased acidic compartments. At higher concentrations, or over longer periods of time, CQ directly induces apoptosis and necrosis[34]. In this study, CQ showed a weak cytotoxic effect at the concentration of 10 µg/mL (Figure 2). Therefore, the concentration we used for this research did not have a direct cytotoxic effect on A549 cells.
The pleiotropic effects of CQ made it difficult to identify the mechanisms underlying the CQ-mediated enhancement of TPT cytotoxicity. SP cells have been isolated from several solid tumors. They are enriched for tumor initiating capacity, express stem-like genes, and are resistant to chemotherapeutic drugs. As TPT is transferred out of cells by ABCG2 and CQ may block these transporters and thereby increase intracellular drug availability[31], we examined whether the combination of CQ with TPT interfered with the ratio of SP cells. The results showed that although TPT and CQ alone slightly decreased the ratio of SP cells, the combination of CQ and TPT did not decrease the ratio of SP cells further. Thus, these data suggest that CQ could not enhance the efficacy of TPT by inhibiting its transfer out of the cells.
Another potential mechanism by which CQ might enhance the pro-apoptotic effect of TPT is by interfering with autophagy. To determine whether autophagy contributed to the cell death induced by TPT, we first examined the effect of CQ and TPT on autophagy. The results based on Western blotting and fluorescence microscopy indicated that TPT induced autophagy and that CQ inhibited the fusion between autophagic vacuoles and lysosomes. Furthermore, the effect of CQ on tumor cells was compared with the effect of genetic inhibition of autophagy. Knockdown of the autophagic gene Atg5 using shRNA (shATG5) blocks autophagy at a proximal step by preventing the formation of the Atg5-Atg12 complex, which is required for the generation of autophagosomes[35]. The results demonstrated that knockdown of Atg5 also enhanced the cytotoxicity of TPT, confirming that the antineoplastic effect of CQ observed in this study resulted from the ability of CQ to inhibit autophagy-based survival.
How autophagy impacts cell death is still unknown. In this study, we examined the role of Bcl-2 family proteins in cell death induced by the combination of CQ and TPT. No variations in expression of Bcl-2 family members, including Bcl-2, Bax, and Bim, were observed, implying that CQ promoted cell death induced by TPT in a Bcl-2 family-independent manner, consistent with previous observations in mouse models of lymphomagenesis[36],[37]. Additionally, CQ can alter lysosomal membrane permeabilization[38], which is a potentially lethal event because lysosomal proteases in the cytosol can digest vital proteins and activate additional hydrolases, including caspases. The latter process usually causes the proteolytic activation of Bid, resulting in cytochrome c release and apoptosome-dependent Caspase activation[39],[40]. However, in this study, CQ failed to modify the activation of Bid, indicating that the lysosome-mitochondria axis is not involved. Therefore, the mechanism by which CQ promotes cell death induced by TPT is still not known.
Previous studies have demonstrated that CQ sensitizes cells to radiation by destabilizing the lysosomal membrane, leading to induction of cell death by necrosis[41]. CQ also sensitizes cells to Akt inhibitors by promoting the accumulation of abnormal autophagolysosomes and reactive oxygen species, leading to tumor cell-specific death[42]. The current study has revealed that CQ enhanced cytotoxicity induced by chemotherapy by inhibiting the autophagy pathway. These data indicate that within different contexts, the mechanisms of CQ-mediated enhancement of cytotoxicity might vary. Because CQ can potentiate the effects of TPT at non-cytotoxic concentrations, CQ and its analogs have potential as chemotherapy and radiation sensitizers without significant side effects.
Acknowledgments
This study was supported by research grants from the National Natural Science Foundation of China (No. 30972882) and the Natural Science Foundation of Guangdong Province, China (No. 9151008901000149).
References
- 1.de Bruin EC, Medema JP. Apoptosis and non-apoptotic deaths in cancer development and treatment response [J] Cancer Treat Rev. 2008;34(8):737–749. doi: 10.1016/j.ctrv.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Xu CX, Jin H, Cho MH. Apoptosis and apoptosis-based therapy in lung cancer [J] Anticancer Agents Med Chem. 2009;9(9):952–957. doi: 10.2174/187152009789377682. [DOI] [PubMed] [Google Scholar]
- 3.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer [J] Nat Rev Cancer. 2007;7(12):961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Ranganathan R. Autophagy: Snapshot of the network [J] Nature. 2010;466(7302):38–40. doi: 10.1038/466038a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang RC, Levine B. Autophagy in cellular growth control [J] FEBS Lett. 2010;584(7):1417–1426. doi: 10.1016/j.febslet.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carew JS, Nawrocki ST, Cleveland JL. Modulating autophagy for therapeutic benefit [J] Autophagy. 2007;3(5):464–467. doi: 10.4161/auto.4311. [DOI] [PubMed] [Google Scholar]
- 7.Scarlatti F, Granata R, Meijer AJ, et al. Does autophagy have a license to kill mammalian cells [J] Cell Death Differ. 2009;16(1):12–20. doi: 10.1038/cdd.2008.101. [DOI] [PubMed] [Google Scholar]
- 8.Oehadian A, Koide N, Hassan F, et al. Differential expression of autophagy in Hodgkin lymphoma cells treated with various anti-cancer drugs [J] Acta Med Indones. 2007;39(4):153–156. [PubMed] [Google Scholar]
- 9.Lambert LA, Qiao N, Hunt KK, et al. Autophagy: a novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model [J] Cancer Res. 2008;68(19):7966–7974. doi: 10.1158/0008-5472.CAN-08-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaushal GP, Kaushal V, Herzog C, et al. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity [J] Autophagy. 2008;4(5):710–712. doi: 10.4161/auto.6309. [DOI] [PubMed] [Google Scholar]
- 11.Górka M, Daniewski WM, Gajkowska B, et al. Autophagy is the dominant type of programmed cell death in breast cancer MCF-7 cells exposed to AGS 115 and EFDAC, new sesquiterpene analogs of paclitaxel [J] Anticancer Drugs. 2005;16(7):777–788. doi: 10.1097/01.cad.0000171514.50310.85. [DOI] [PubMed] [Google Scholar]
- 12.Amaravadi RK, Yu D, Lum JJ, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma [J] J Clin Invest. 2007;117(2):326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li DD, Wang LL, Deng R, et al. The pivotal role of c-Jun NH2terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells [J] Oncogene. 2009;28(6):886–898. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- 14.Kanzawa T, Germano IM, Komata T, et al. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells [J] Cell Death Differ. 2004;11(4):448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 15.Lee SB, Tong SY, Kim JJ, et al. Caspase-independent autophagic cytotoxicity in etoposide-treated CaSki cervical carcinoma cells [J] DNA Cell Biol. 2007;26(10):713–720. doi: 10.1089/dna.2007.0577. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Hou N, Faried A, et al. Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells [J] Ann Surg Oncol. 2009;16(3):761–771. doi: 10.1245/s10434-008-0260-0. [DOI] [PubMed] [Google Scholar]
- 17.Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies [J] Eur J Pharmacol. 2009;625(1–3):220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Solomon VR, Hu C, et al. Synthesis and in vitro cytotoxicity evaluation of 4-aminoquinoline derivatives [J] Biomed Pharmacother. 2008;62(2):65–69. doi: 10.1016/j.biopha.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djordevic B, Lange CS, Rotman M. Potentiation of radiation lethality in mouse melanoma cells by mild hyperthermia and chloroquine [J] Melanoma Res. 1992;2(5–6):321–326. doi: 10.1097/00008390-199212000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki K, Tsuno NH, Sunami E, et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells [J] BMC Cancer. 2010;10:370. doi: 10.1186/1471-2407-10-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seiler N, Chaabi M, Roussi S, et al. Synergism between apple procyanidins and lysosomotropic drugs: Potential in chemoprevention [J] Anti-cancer Res. 2006;26(5A):3381–3385. [PubMed] [Google Scholar]
- 22.Degtyarev M, De Maziere A, Orr C, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents [J] J Cell Biol. 2008;183(1):101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milano V, Piao Y, LaFortune T, et al. Dasatinib-induced autophagy is enhanced in combination with temozolomide in glioma [J] Mol Cancer Ther. 2009;8(2):394–406. doi: 10.1158/1535-7163.MCT-08-0669. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, Luo RZ, Lu Y, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells [J] J Clin Invest. 2008;118(12):3917–3929. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang PD, Zhao YL, Shi W, et al. Cell growth inhibition, G2/M cell cycle arrest, and apoptosis induced by chloroquine in human breast cancer cell line Bcap-37 [J] Cell Physiol Biochem. 2008;22(5–6):431–440. doi: 10.1159/000185488. [DOI] [PubMed] [Google Scholar]
- 26.Briceño E, Calderon A, Sotelo J. Institutional experience with chloroquine as an adjuvant to the therapy for glioblastoma multiforme [J] Surg Neurol. 2007;67(4):388–391. doi: 10.1016/j.surneu.2006.08.080. [DOI] [PubMed] [Google Scholar]
- 27.Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies? [J] Autophagy. 2009;5(4):442–450. doi: 10.4161/auto.5.4.7667. [DOI] [PubMed] [Google Scholar]
- 28.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer [J] Clin Cancer Res. 2009;15(17):5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function [J] Nat Rev Mol Cell Biol. 2009;10(9):623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 30.González-Pons M, Szeto AC, González-Méndez R, et al. Identification and bioinformatic characterization of a multidrug resistance associated protein (ABCC) gene in Plasmodium berghei [J] Malar J. 2009;8:1. doi: 10.1186/1475-2875-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato W, Fukazawa N, Nakanishi O, et al. Reversal of multidrug resistance by a novel quinoline derivative, MS-209 [J] Cancer Chemother Pharmacol. 1995;35(4):271–277. doi: 10.1007/BF00689444. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee T, Muhkopadhyay A, Khan KA, et al. Comparative mutagenic and genotoxic effects of three antimalarial drugs, chloroquine, primaquine and amodiaquine [J] Mutagenesis. 1998;13(6):619–624. doi: 10.1093/mutage/13.6.619. [DOI] [PubMed] [Google Scholar]
- 33.Haworth S, Lawlor T, Mortelmans K, et al. Salmonella mutagenicity test results for 250 chemicals [J] Environ Mutagen. 1983;5(Suppl 1):1–142. [PubMed] [Google Scholar]
- 34.Fan C, Wang W, Zhao B, et al. Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells [J] Bioorg Med Chem. 2006;14(9):3218–3222. doi: 10.1016/j.bmc.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 35.Mehrpour M, Esclatine A, Beau I, et al. Overview of macroautophagy regulation in mammalian cells [J] Cell Res. 2010;20(7):748–762. doi: 10.1038/cr.2010.82. [DOI] [PubMed] [Google Scholar]
- 36.Amaravadi RK, Yu D, Lum JJ, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma [J] J Clin Invest. 2007;117(2):326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maclean KH, Dorsey FC, Cleveland JL, et al. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis [J] J Clin Invest. 2008;118(1):79–88. doi: 10.1172/JCI33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkegaard T, Jäättelä M. Lysosomal involvement in cell death and cancer [J] Biochim Biophys Acta. 2009;1793(4):746–754. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Conus S, Simon HU. Cathepsins: key modulators of cell death and inflammatory responses [J] Biochem Pharmacol. 2008;76(11):1374–1382. doi: 10.1016/j.bcp.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 40.Di X, Shiu RP, Newsham IF, et al. Apoptosis, autophagy, accelerated senescence and reactive oxygen in the response of human breast tumor cells to adriamycin [J] Biochem Pharmacol. 2009;77(7):1139–1150. doi: 10.1016/j.bcp.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, Cai Y, Santi S, et al. Chloroquine-mediated radiosensitization is due to the destabilization of the lysosomal membrane and subsequent induction of cell death by necrosis [J] Radiat Res. 2005;164(3):250–257. doi: 10.1667/rr3436.1. [DOI] [PubMed] [Google Scholar]
- 42.Degtyarev M, De Mazière A, Orr C, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents [J] J Cell Biol. 2008;183(1):101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]