Abstract
Familial adenomatous polyposis (FAP) is an autosomally dominant disease characterized by the early development of colorectal adenomas and carcinoma in untreated patients. Patients with FAP may develop rectal cancer at their initial presentation (primary) or after prophylactic surgery (secondary). Controversies exist regarding which surgical procedure represents the best first-line treatment. The options for FAP are ileorectal anastomosis (IRA) or a restorative proctocolectomy (RPC) with either a handsewn or a stapled ileal pouch-anal anastomosis (IPAA), with or without mucosectomy. The purpose of these surgeries is to stop progression to an adenoma-cancer sequence by eradicating the colon, a disease prone organ. Unfortunately, these surgical procedures, which excise the entire colon and rectum while maintaining transanal fecal continence, do not guarantee that patients still won't develop adenomas. Based on the available literature, we therefore reviewed reported incidences of pouch-related adenomas that occurred post prophylactic surgery for FAP. The review consists of a collection of case, descriptive, prospective and retrospective reports.
Objectives
To provide available data on the natural history of subsequent adenomas after prophylactic surgery (by type) for FAP.
Methods
A review was conducted of existing case, descriptive, prospective and retrospective reports for patients undergoing prophylactic surgery for FAP (1975 – August, 2013). In each case, the adenomas were clearly diagnosed in one of the following: the ileal pouch mucosa (above the ileorectal anastomosis), within the anorectal segment (ARS) below the ileorectal anastomosis, or in the afferent ileal loop.
Results
A total of 515 (36%) patients with pouch-related adenomas have been reported. Two hundred and eleven (211) patients had adenomas in the ileal pouch mucosa, 295 had them in the ARS and in 9 were in the afferent ileal loop. Patients with pouch adenomas without dysplasia or cancer were either endoscopically polypectomized or were treated with a coagulation modality using either a Nd:Yag laser or argon plasma coagulation (as indicated). Patients with dysplastic pouch adenomas or pouch adenomas with cancer had their pouch excised (pouchectomy).
Conclusion
In patients with FAP treated with IRA or RPC with IPAA, the formation of adenomas in the pouch-body mucosa or ARS/anastomosis and in the afferent ileal loop is apparent. Because of risks for adenoma recurrence, a life time endoscopic pouch-surveillance is warranted.
Keywords: Familial-adenomatous polyposis, restorative proctocolectomy, ileal-anal pouch anastomosis, ileorectal anastomosis, adenomas
Background
Familial adenomatous polyposis (FAP) is an inherited autosomal dominant disease caused by mutations in the adenomatous polyposis coli (APC) gene located on chromosome band 5q 21-q22.1–4 The APC gene is a tumor suppressor and has been shown to play a part in metaphase chromosome alignment.5 The normal APC protein promotes apoptosis in colonic cells. Its most important function may be to sequester the growth stimulatory effects of β-catenin, a protein that transcriptionally activates growth-associated genes in conjunction with tissue-coding factors. Mutations of the APC gene result in a truncated/nonfunctional protein. The resultant loss of APC function prevents apoptosis and allows β-catenin to accumulate intracellularly and to stimulate cell growth with the consequent development of adenomas. As the clonal expansion of cells that lack APC function occurs, their rapid growth increases the possibility for other growth-advantageous genetic events to also occur. This causes alterations in the expression of a variety of genes, thereby affecting the proliferation, differentiation, migration and apoptosis of cells. Ultimately, enough genetic events can happen which allow the adenomatous polyps to become malignant in patients with FAP. This process is similar to that which occurs in sporadic adenomas. As a result, APC is considered the gatekeeper of colonic neoplasia. Its mutation/inactivation is the initial step in the development of colorectal cancer (CRC) in patients with FAP.6,7
The reported incidence of FAP is one in 7,000 to 12,000 live births.8,9 The disease is characterized by the presence of hundreds of colorectal adenomas (CRA) leading to a 100% lifetime chance of transformation to CRC if the colon is not removed.8,10–12 A prophylactic colectomy is therefore advocated for such patients to prevent CRC.13 Four surgical options are available for patients with FAP:14,15 colectomy with ileorectal anastomosis (IRA); restorative proctocolectomy (RPC) with ileal pouch-anal anastomosis (IPAA); total proctocolectomy (TPC) with ileostomy; and TPC with continent ileostomy (Kock). The first two approaches are currently the most popular techniques. While colectomy with IRA provides superior functional results (because it leaves the rectum intact), patients have a higher probability of developing adenomas, compared to those receiving RPC with IPAA.16,17 An RPC with IPAA reduces anal rectal mucosal volume, preserves transanal defecation and is used an alternative procedure to IRA; however small mucosal residual remnants may remain. These mucosal residuals are potential sites for the development of an adenoma.17–23 Surgical treatment via TPC (excision of the entire colon and rectum) with mucosectomy to the dentate line significantly reduces the incidence of adenomas in the ARS, although adenomas still have been reported after mucosectomy.24–27 In addition, because FAP patients have a germline mutation, all cells carry the APC gene, therefore a negative pathology report may not mean that the patient will be permanently free of developing an adenoma with the potential of neoplastic transformation.1–4 The major reasons for indicating IPAA in patients with FAP remain the risk of secondary rectal neoplasia after IRA and the development of adenomas within the distal ileum or ileal pouch after IRA,22,28,29 IPAA,18–21,23,30–33 and ileostomy.34–37
This review summarizes the incidence, macroscopic/histologic patterns, location and degree of severity of adenomas developing in the ileal pouch mucosa (above the ileorectal anastomosis), within the anorectal segment (ARS) below the ileorectal anastomosis, or in the afferent ileal loop after colectomy in patients with FAP. It also seeks to correlate patient characteristics with the type of surgical intervention used, which may guide the reader to develop the appropriate follow-up surveillance.
Methods
Based on the available literature, we reviewed the reported incidences of subsequent adenomas arising from the ileal pouch mucosa, the ARS mucosa and the afferent ileal loop in patients following preventive surgery for FAP. The review consists of case, prospective and retrospective studies published between 1975 and August, 2013.
The US National Library of Medicine database (MEDLINE), the Excerpta Medica database (EMBASE), the Cochran Library and the Google® search engine were searched for published articles on “familial adenomatous polyposis”, “colectomy”, “restorative proctocolectomy”, “ileoanal anastomosis”, “ileal pouches”, “villous adenoma”, “dysplasia”, “pouch dysplasia”, “pelvic pouch” and “pouch neoplasia”.
The search excluded non-English languages and non-human studies as well as five editorials. Additional articles were identified by cross-referencing papers retrieved in the initial search. Papers were included on the basis of the most recently available evidence for each specific point of interest. Final and conclusive agreement was assessed using the k-statistic, determined during title and abstract reviews. If the k-value was ≥ 0.6 for titles, they were divided into two sets; each set was reviewed by only one of two reviewers. If the k-value was < 0.6, discrepancies were discussed, followed by assessments of agreement. A similar process for abstract reviews was done with an increased k-value of 0.7 for acceptance.
Results
Surgical Treatment of FAP
The aim of surgical treatment of FAP is to intervene in the polyp-cancer transition by removing the adenomas before the transformation to malignancy occurs.24,38,39 Currently there are no standardized guidelines as to which of the four surgical options should be offered to patients40 and there is no consensus about which procedure is the best first-line treatment.41 Proctocolectomy, however, is universally indicated for patients with profuse polyposis (>20 rectal adenomas and >1,000 colonic adenomas) and in some centers it is a routine operation in all FAP patients.42,43 In addition, molecular genetic testing has been proposed as a guide to the surgical management of patients with FAP.18,44 Thus, it has been suggested that those patients with an APC mutation before codon 1250 have a lower probability of developing rectal adenomas and should undergo a colectomy and IRA. Wu et al.18 observed that among 31 IRA patients with mutations on the APC gene outside of codons 1309 and 1328, only one patient required a secondary proctectomy because of rectal polyp proliferation. However, there are many factors to be considered in the surgical decision process. The options, advantages and disadvantages, indications, contraindications and timing for surgery are depicted in Table 1.
Table 1.
Indications, contraindications, advantages and disadvantages of surgical options for patients with FAP. Reproduced with permission of the authors: Smith et al., J Cancer Ther 2013;4:260–270.27
| Option | Indications | Contraindications | Advantages | Disadvantages |
|---|---|---|---|---|
| IRA | *< 20 rectal adenomas *< 1000 colonic adenomas31,53,54,70 |
*Severe dysplasia in the rectum *Cancer anywhere in large bowel *Large (>3cm) rectal adenomas |
*Avoiding pelvic dissection20 *Simple surgery *Lower complications *Good functional results *No stoma55 |
*Retained rectum may need to be removed later *Possibility of rectal cancer if patient is not compliant with follow-up |
| RPC with IPAA ■ Stapled or ■ Mucosectomv |
*> 20 rectal adenomas, *>1000 colonic adenomas53 *Severe dysplasia in the rectum *Cancer anywhere in large bowel *Large (>3cm) rectal adenomas *ATZ clear of adenomas |
*Incompetent sphincters *Rectal cancer invading sphincters *Pouch won't reach anus |
Avoid permanent stoma *Good function in most patients 74 |
*Higher complication rate *May provoke desmoids *Decreased ability to conceive in women.80,87 *Retained anal and lower rectal mucosa may develop neoplasia (28%)42 |
| TPC & IL | *> 20 rectal adenomas, *>1000 colonic adenomas53 *Severe dysplasia in the rectum *Cancer anywhere in large bowel *Large (>3cm) rectal adenomas *ATZ clear of adenomas *Incompetent sphincters *Rectal cancer invading sphincters *Pouch won't reach anus |
* Avoids permanent stoma *Reasonable function in most patients. No residual anal mucosa (although neoplasia can still occur)26,74 |
*Avoids permanent stoma *Reasonable function in most patients. No residual anal mucosa (although neoplasia can still occur)26,74 |
*Higher complication rate *May provoke desmoids *Decreased ability to conceive in women.80,87 *Retained anal and lower rectal mucosa may develop neoplasia (28%) *Frequent seepage *Night time incontinence.74 *Anal neoplasia in 14%.42 |
| TPC with CIL (Kock) | *> 20 rectal adenomas, >1000 colonic adenomas53 *Severe dysplasia in the rectum *Cancer anywhere in large bowel *Large (>3cm) rectal adenomas *ATZ clear of adenomas *Incompetent sphincters *Rectal cancer invading sphincters *Pouch won't reach anus |
*Competent sphincters *No rectal cancer *Pouch reaches anus |
*Lower complication rate *Lower chance of reoperation *No anal incontinence |
*Permanent stoma |
Abbreviation:
IRA = lleorectal anastomosis; RPC = Restorative proctocolectomy; IPAA = Meal pouch-anal anastomosis; TPC & IL = Proctocolectomy and lleostomy; TPC with CIL = Proctocolectomy with continent ileostomy (Kock); ATZ = anal transit zone
In the last three decades the two attractive criterion surgical options for FAP have been colectomy with IRA and RPC with IPAA.
Colectomy with IRA
IRA is advocated as a preferred option in patients with a low risk of rectal cancer, particularly in those female patients who wish to have children.45 A colectomy with IRA can be defined as removal of the entire colon, leaving 15 cm of rectum for optimal bowel function.43,46 Triaging the fate of the rectum according to the number, size and histological interpretation of rectal adenomas is effective in minimizing the need for a future proctectomy. If there are fewer than 20 adenomas, none larger than 1 cm and none severely dysplastic, the rectum may be retained.43 The IRA preserves excellent bowel function, is simple, and can be done with major benefits to the lifestyles of the patients.46
RPC with IPAA
This approach demands removal of the entire colon and rectum down to the pelvic floor (dentine line) thereby achieving significant prevention of both colon and rectal adenomas but requiring the construction of an ileal pouch.14 An anastomosis between an ileal pouch and the upper anus is performed. Two techniques are currently used to construct an ileal pouch-anal anastomosis: (1) a double-stapled anastomosis between the pouch and the anal canal and (2) a mucosectomy with a hand-sewn ileoanal anastomosis at the dentine line. This procedure is thought to diminish the risk of colorectal adenomas. There are three options that affect the conduct of the operation: the type of pouch, the type of anastomosis and the construction of a diverting loop ileostomy.
Type of Pouch
There are four different pouch conformations (J-, S-, W- and H- shaped).14 The most common and easiest pouch to make is the J-shaped pouch.47 Limbs are 15 to 20 cm long but the main factor determining length is the position of the apex of the superior mesenteric artery.14
Type of anastomosis
It is important to differentiate between the concept of an anal transit zone (ATZ) and a rectal cuff (RC). The ATZ is the area where the squamous and columnar epitheliums from the rectum, transition close to the pectinel/dentine line. The mean length of this zone in adults is 4.5 mm, and the anastomosis is sewn at this site when a mucosectomy is performed.48 The columnar cuff is that area where the entire columnar epithelium of the rectum is left behind, and involves the region from the anastomosis to the ATZ. This rectal cuff can vary in length (1.0 to 2.5 cm, but can be longer).24,49 The simpler type of anastomosis is a double-stapled end-of-pouch to anus (1–2 cm above pectinel line) anastomosis.50 The rectum is stapled distally at the level of the pelvic floor, a purse string suture is inserted into the open end of the pouch and used to tie in the anvil of the stapler; the anastomosis is completed by transanal insertion of the stapler cartridge, uniting the cartridge with the anvil and firing the stapler. The residual ATZ is often less than 1.0 cm, as the stapler removes from 0.5 to 1.0 cm of bowel. Alternatively, the ARS is mucosectomized and the pouch pulled into the anus and anastomosed transanally to the dentate line, by hand. The stripping (mucosectomy) and handsewn anastomosis takes longer and in some studies is associated with more complications and a poorer functionality than the stapled anastomosis, but its putative advantage is removal of most of the anal transitional and rectal epithelium, with a more complete prevention of anal transitional adenomas.41,51 However, ARS adenomas have been described even after mucosectomy.25
Diverting loop ileostomy
Patients with FAP are at low risk for an anastomotic leak or fistula because they are generally healthy, are not taking immunosuppressive medications and have a normal bowel except for the presence of adenomas. Although an ileostomy creates the need for another surgery for closure (and has its own risks of postoperative complications), an undiverted pouch is at a higher risk of anastomotic leak.52 Therefore, in most patients a “safety first” approach is better and the postoperative course is smoother. To our knowledge, to date there are no comparable published data examining the incidence of adenoma development of the pouch or ARS in patients who received a diverting loop ileostomy versus those who did not.
Surveillance, Diagnosis and Treatment of Subsequent Adenomas
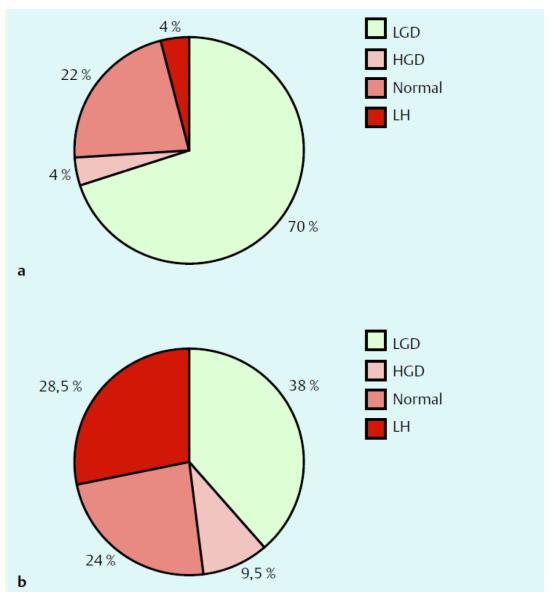
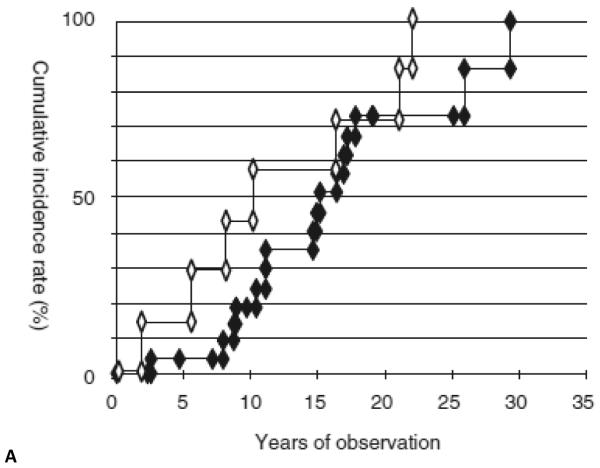
Moussata et al.53 used a pie chart to report the histologies of visible types of adenomas detected in the ileal mucosa at chromoendoscopy in a total of 116 FAP patients who had an IPAA of which 102 had an IRA surgery performed as an initial procedure (Fig. 1). The patients with IPAA had a median age of 37 ± 13 years (mean ± SD; range 16 to 63 years) at index examination, with a mean time since surgery of 7.9 ± 3.9 years, and a mean duration of ileal pouch endoscopic follow-up of 5.4 ± 2.6 years (range, 1 – 11 years). The mean number of endoscopic sessions per patient was 5.2 ± 3 (range, 2 – 10). Among these patients, 78% had visible polyps after indigo carmine chromoendoscopy, and 22% had no visible polyp. Feinberg et al.54 had similar observations. Prost et al.55 demonstrated the cumulative incidence rate of adenomas in the ileal pouch after proctocolectomy with Kock and IPAA and that of rectal adenoma after colectomy with IRA (Fig. 2A). Boostrom et al.65 followed 117 patients who underwent pouch surgery with a median age of 26, 52 were male. Ileal reservoirs included J-pouch (n = 104), Kock pouch (n = 9), S-pouch (n = 3), and W-pouch (n = 1). Median follow-up was 125 months. Polyps were biopsied in 33 patients: non-dysplastic polyps (n = 2), low-grade dysplasia (n = 30), and adenocarcinoma (n = 1). No patients had high-grade dysplasia. Median time to development of dysplasia was 149 months. Adenocarcinoma developed in one patient after 284 months. Risk of dysplasia at 10, 20, and 25 years was 17, 45, and 69 %, respectively (Fig. 2B).
Figure 1.
Pie chart indicating the histology results of visible polyps detected in ileal mucosal at chromoendoscopy in patients with an IPAA (a) or an IRA (b). Abbreviations: LGD=low grade dysplasia; HGD=high grade dysplasia; and LH=lymphoid (nodular) hyperplasia. Reproduced with permission of the publisher: Feinberg et al., Dis Colon Rectum 1988;31:169–175.54
Figure 2A.
The cumulative incidence rate of adenomas in the ileal pouch after proctocolectomy with Kock and IPAA (closed diamond) and that of rectal adenomas after colectomy with IRA (open diamond). Reproduced with permission of the publisher: Prost et al., Gastrointest Endosc 2004;59:929–932.55
Figure 2B.
The risk of dysplasia over time in a total of 117 subjects. The risk of dysplasia at 10, 20 and 25 years was 17, 45 and 69 percent respectively. Reproduced with permission of the publisher: Boostrom et al., J Gastrointest Surg 2013;17:1804–8.65
Pouch surgical specialists developed guidelines at St. Mark's Hospital in London, England56 in order to provide consistent evidence-based care. Pouch adenomas are typically diagnosed by surveillance pouchoscopy and/or by incidentally detecting them on a diagnostic pouchoscopy.18,25 The pouch mucosa always should be considered as having the potential to form an adenoma. Small adenomas 1–3 mm in size with high-grade dysplasia may be detected and practicing physicians should remain vigilant. Because most adenomas are located at the ARS, digital examination of this area may suggest areas harboring adenomas. A full examination under anesthesia in the operating room may be warranted. Irrespective of pathological findings, a regular personalized pouch surveillance needs to be carried out early to detect abnormal lesions.57–60
To provide an effective adenoma screening program, the use of surveillance endoscopy is of utmost importance.84–86 The incidence of developing adenomas appears to be is dependent not only on the type of surgery but the time elapsed post surgery.18 Patients who have had an IRA, need a proctoscopy in 6 months to a year following surgery, to monitor the rectum, while patients who have had an IPAA need lifelong endoscopic surveillance.12,61 It is noteworthy that mucosectomy does not guarantee complete excision of rectal epithelium and adenomas may still occur in these patients.2,8 Unfortunately, this has not been emphasized enough, despite concerns regarding the risk of retained rectal mucosal tissue following the procedure.2,12,61,62
In all reported studies, patients were followed for an average period of 5.8 (1.5 to 46.4) years. The mean duration of pouch endoscopic follow-up was 6.2 ± 4.1 years. Fewer than 20% (in China) to 37.1−54.5% (in the UK) had regular postoperative follow-up visits.25,63 The failure of follow-up surveillance in some countries has been largely attributed to patients not following the recommended visits due to patient education, economical constraints and/or the cultural stigma associated with the condition.25,63 Although, the median age (data not shown) and the median follow-up duration of IRA patients (13.5 years) was longer than that of the IPAA patients (10.3 years), there was no statistically significant difference in either measure.
Therapeutic modalities
Table 2 summarizes treatments of those pouch-related adenomas found post FAP surgery based on the available referenced literature. When rectal or pouch adenoma is diagnosed, the continued role of the IPAA is uncertain, because it may compromise oncologic therapy and oncologic therapy may compromise the function of the IPAA. The management of adenomas, however, is related to the number, size and histological evaluation of the adenomas. In the presence of small adenomas (< 5 mm) without any kind of dysplasia, simple monitoring with careful follow-up may be suggested.13,54 When larger adenomatous formations (> 5 mm) are diagnosed in the pouch, endoscopic resection with free margins is recommended.64 Alternatively, a transanal approach or an abdominal approach, which may require a complete and difficult mobilization of the pouch from the pelvis, may be indicated.13 Unfortunately in such a situation, this appears to be the only option that preserves the pouch from excision (pouchectomy) (Fig. 3).2,17,55,65 It is also important to realize that patients who have undergone an endoscopic removal of an adenoma are at increased risk for a recurrence of the adenoma.83–86 Endoscopic mucosal resection (EMR) is a major therapeutic advance in the treatment of sessile and flat colorectal polyps.66 Following a cohort of 78 referred patients with polyps, Carvalhol et al.66 observed recurrence of adenomas in 22.2% of patients at 3 months, 11.1% at 12 months and 0% at 36 months. By logistic regression, a location near the pectinate line (OR 26.13)66 and a previous history of polypectomy (OR 7.70) became independent risk factors related to recurrence.66,67 Arebi et al.68 found no statistically significant relationship between site and recurrence of adenomas but observed in their study that the recurrence was significantly related to polyp size (p< 0.001). Some studies have reported that the use of nonsteroid anti-inflammatory drugs (NSAIDs) was effective in suppressing ileal pouch adenomas.31,69 Chemoprophylaxis with Sulindac or Celebrex may be used to minimize the growth of small adenomas but they will not necessarily prevent neoplastic transformation.70,71 Chemoprophylaxis can also be used for patients with a significant polyp burden but who are not ready or suitable for proctectomy. Phillips and Spigelman72 proposed delaying RPC until after IRA, as an alternative approach. Some patients have also been treated endoscopically within the ileal mucosa, using argon plasma coagulation.53,55
Table 2.
Depicts options available for the diagnosis, management, surveillance and care of patients with pouch-related adenomas after FAP preventive surgery based on a literature review.
| Option/Diagnosis | Management | Note |
|---|---|---|
| Follow-up Surveillance Life-term endoscopic surveillance of all FAP patients after IPAA or IRA surgery along with evaluation of potential therapeutic options for pouch adenomas. |
To fulgurate new and recurrent polyps and screen for the development of cancer Lifelong proctoscopy every 6 months to a year. Lifelong endoscopic pouch surveillance. Pouchoscopy is well tolerated without sedation in the clinic setting; thus recommend annual surveillance for the first 5 years following pouch construction. Endoscopy can then be done less frequently (every 3 years) in patients with no polyps or low polyp burden on initial pouchoscopies.77 |
The incidence of developing subsequent adenomas is time-dependent from surgery Patients who have an IRA, need a proctoscopy following surgery, to monitor the rectum Pouchoscopy is recommended to be done yearly for life, initially to look for anastomotic adenomas and then later to check for pouch adenomas. Once neoplasia is seen, appropriate treatment needs to be determined. Risk for the development of adenomas in the ATZ is higher after a stapled IPAA than after a mucosectomy with handsewn anastomosis. However, control of ATZ neoplasia results in a similar risk of cancer development. Because the stapled procedure is associated with better long-term functional outcomes than a mucosectomy, stapled IPAA is the preferable procedure for most patients with FAP.92 |
| Adenomas < 5 mm without dysplasia | Simple monitoring with careful follow-up13,62 Nonsteroid anti-inflammatory drugs (NSAID) Chemoprophylaxis • Sulindacor • Celebrax |
Both prophylactic surgical options do NOT cure FAP, and multiple polyps can occur in the ileal pouch mucosa, ARS and afferent ileal loop. The role of NSAID to suppress ileal pouch adenomas in FAP has been established31,68,85–90 Chemoprophylax may be used to minimize small adenoma growth but will not necessarily prevent neoplastic transformation.70,71 |
| Adenomas > 5 mm with or without dysplasia Adenomas larger than 1 cm and/ or showing high-grad dysplasia |
Endoscopic resection (polypectomy) with free margins.52 Alternatively a transanal excision of residual, adenoma-bearing ATZ, or abdominal approach may be indicated.2,13,17 Transanal polypectomy or coagulation modalities using Nd:YAG laser (25 W).90 or Argon plasma coagulation using 40 W to 50 W power setting and 1 L/min gas flow.53,64,90 |
Endoscopic mucosal resection (EMR) is a major therapeutic advance in the treatment of sessile and flat colorectal polyps.66,68 These patients however are at increased risk for adenoma recurrence,27,86–90 particularly there is a higher risk of development of adenomas at the anastomotic site after a bouble-stapled anastomosis.73 |
| Uncontrollable adenomas with or without high-grade dysplasia/ or adenocarcinoma | Surgery, pouch excision (pouchectomy)65,73 | Endoscopic treatment of pouch related adenomas is likely to be difficult because of the thin ileal mucosa and the way it is tethered to the submucosa and underlying muscle, reducing the options for their control to excising the entire pouch or chemoprevention.2,73 The median interval between RPC and pouch excision was 0.6 years (range, 0.2–11)73 |
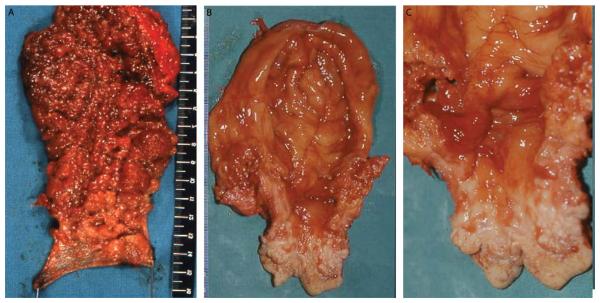
Fig. 3.
3A, Macroscopic picture of proctectomy in a 29-year-old man who underwent ileorectal anastomosis 10 years earlier. The mucosa surface was affected by a diffuse polyposis without areas free of neoplastic growth. The mucosectomy specimen is continuous, and the submucosal dissection plane defines the completeness of its removal from the anal transitional zone area. No carcinoma was found in the rectum, but there was 1 adenoma with high-grade dysplasia.
3B and 3C, Four years later, the patient underwent pouch excision and definitive ileostomy; the ileal pouch mucosa presented a right lateral elevated mass of 2 cm over a firm basis, located 4 cm from the anal margin and extending cranially for 2 cm. Histological analysis showed an advanced mucinous adenocarcinoma (T3, N0). Reproduced with permission of the publisher: Tonelli et al., Dis Colon Rectum 2012;55:322–329.17
Adenomas Following Surgery for FAP
In the literature reviewed, there were 1,412 patients presented that were followed-up and endoscopically surveyed; of these 515 patients (36%) developed pouch-related adenomas following prophylactic surgery for FAP. Two hundred and eleven (211) adenomas were found in the pouch-body mucosa, 295 in the ARS (and anastomosis) and 9 in the afferent ileal loop. The results underscore the importance of the intervention, given the fact that surgery is likely provided to thousands of patients worldwide.
The natural history of ileal pouches and their development into ARS adenomas is not known. However the incidences we found of adenomas in both the ileal pouch and/or ARS of FAP patients after undergoing prophylactic surgery are depicted in Table 3. The data from a recently published article from St. Mark's Hospital25 suggests that the risk of developing adenomas in pouches after RPC for FAP, increases as a function of increasing age of the patient and time post surgery.25 They analyzed 140 out of 260 patients who were seen for an endoscopic follow-up (median of 10.3 years) after RPC. Several patients were identified with multiple adenomas or large adenomas, or were found to have long history adenomas which might predispose them to a malignancy in future. Fifty-two patients (37%) developed adenomas in the ARS, with a cumulative risk at 10 years of 22.6% post mucosectomy with handsewn anastomosis, and 51.1% after a stapled IAA (ileal anal anastomosis) (p< 0.001). The median time to the first adenoma formation was longer after mucosectomy with handsewn anastomosis than after stapled IAA (10.1 versus. 6.5 years, p< 0.001). At a 15 year follow-up, the difference was even greater (28.8% versus. 85.2%, p< 0.001).25 By multivariate analysis, a stapled IAA [hazard ratio (HR) = 3.45, 95% confidence interval = 1.01–4.98)] and an age at RPC of > 40 years (HR = 2.20. 95% confidence interval = 1.01–4.89) were significantly associated with an increased risk of adenoma formation, p< 0.049 (Table 4). Nine patients (6.4%) developed large (> 10 cm) adenomas. Eight patients required polypectomy in the handsewn mucosectomy group compared to 12 in the stapled group (p< 0.018). One patient with a handsewn ileoanal anastomosis developed adenomas in the anorectal mucosa at 13 years and required pouchectomy.25 Two patients in the mucosectomy group compared to five patients in the stapled group developed recurrent adenoma in the ARS requiring repeated polypectomy, p = 0.098. Sixteen patients with Kock and IPAA were reported to have developed adenomas and all patients with IRA developed adenomas in the rectal mucosa. Only one patient with Kock showed an adenoma in the prepouch area. Other researchers have also reported adenomas at the ARS and found that the risk was observed to be twice as high with a stapled anastomosis.24,73,74
Table 3.
Summary of published data on the incidence of adenomas following prophylactic surgery for FAP. This table underscores the fact that mucosectomy does not necessarily prevent the development of adenomas in the ATZ.
| Author | Nature of study | Age at FAP diaqnosis/vears | Operatio technique | Interval, surgery to adenoma/vears | Age at adenoma diagnosis | Number of patients operated, followed and studied (n=1412) | Number of patients developed adenomas (n=515, (36%) | Location | Histology |
|---|---|---|---|---|---|---|---|---|---|
| Hamilton, 197929 | Prospective series | IRA | 2 and 26 | Not reported | 9 | Pouch mucosa and ARS | Adenomas | ||
| Beart, 198233 | Case report | 22 | KP | 16 | 48 | 1 | 1 | Kock continent ileostomy | Tubulovillous adenoma |
| Wolfstein, 198294 | Retrospective | IAA | 3 | 2 | 2 | Rectal mucosa | Adenomas 8 and 12 cm | ||
| Jarvinen, 198395 | Retrospective | IRA | Not reported | 5 | 1 | ARS | Adenomas | ||
| Burt,198496 | Retrospective | IRA | 11 | 6 | ARS | Adenomas | |||
| Shepherd, 198719 | Retrospective | RPC | 1 | 12 | 2 | Pouch mucosa | Adenomas (LGD) | ||
| Stryker, 198737 | Case report | KP | 12 | 33 | 1 | 1 | Reservoir adenomas | Tubulovillous adenomas | |
| Myrhoj, 198923 | Case report | IAA | 25 | 12 | 1 | 1 | IAA | Adenomas to 12 cm (LGD) | |
| Nugent, 199262 | Retrospective | 26 | IRA | 30 | 56 | 222 | 5 | Pouch mucosa | Adenomas (LGD) |
| Nugent, 199330 | Retrospective | RPC | 0.1, 0.25 and 1 | Mean 28 (range, 12–65) | 50 | 38 | Pouch mucosa | Adenomas | |
| Bertoni, 199528 | Retrospective | IRA | Not reported | 17 | 9 | ARS | Adenomas | ||
| Church, 199631 | Case report | RPC | Not reported | 1 | 1 | Pelvic pouch mucosa | Adenomas | ||
| Wu, 199879 | Retrospective | RPC | Not reported | 26 | 11 | Pouch mucosa, gut above pouch & ATZ | Adenomas | ||
| Van Duijvendijk, 199973 | Retrospective | Not reported | RPC (6 mucosectomized & 7 stapled anastomoses | 38 (range 22–60) | Median, 35(16–60) | 126 | 13 | Anastomotic site | Adenomas, 4 moderate dysplasia, 4 mild dysplasia and 4 severe dysplasia |
| Valle, 200197 | Retrospective | RPC | 5 | Not reported | 1 | 1 | Diffuse | Adenomas | |
| Thompson-Fawcett, 200120 | Retrospective | 5 KP, 28 RPC | 7 | 33 | 21 | Pouch mucosa | Adenomas 1 to 3 mm | ||
| Pare, 200121 | Retrospective | RPC initial procedure, in 19 converted IRA to a RPC | Mean ± SDE, 7.1 ± 4.5) | 27 (range,9–67; mean ± SDE, 28±12) | 85 | 30 | Pouch mucosa | Adenomas (in 28 grossly vissible (one HGD, 21 LGD) <->5mm,and in 2 microadenomas) | |
| Polese, 200398 | Retrospective series | Not reported | RPC | 0.3 to 12 | 9 and 11 | 46 (9 handsewn / 37 stapled IPAA) | 2 | Pouch mucosa & at the margin | Adenomas |
| Beveridge, 200432 | Case report | Median 35 (range, 32–38) | RPC | 4 and 10 | 32 and 38 | 2 | 2 | Lower pouch 2 cm of terminal cuff - behind a fold in the upper pouch. Terminal ileum & rectal glandular mucosa from incomplete MU | Adenoma 3 cm × 2 cm & 2 cm × 2 cm large. Tubulovillous adenomas Microadenomas |
| Groves, 200522 | Retrospective | Median, 25 (range, 13 to 45) | IRA | Mean 12 (range 0 to 39) | 18 to 65 | 30 IPAA and 30 conversional IRA | 34 | ARS | Adenomas (5 patients had > 1 cm) |
| Von Roon, 200774 and 201125 | Retrospective and Retrospective | Mean, 32.6±10.8 and 32.6±11.9 (range 17–63) | RPC (121(64%) were hand- sewn and 54 (29%) stapled)). W-pouch, n=57; J-pouch, 102; and S-pouch, n=10) and RPC + MUC |
Mean 10.4 (mucosectomy group) and 7.2 (stapled group) and 7,7,8, 12, 13, 11, 11, 12, 23&13 | Mean 10 (range, 2 to 23) and 28.7±12 (mean±SD) | 91 of 189 and 189 of 189 (out of 1,652) | 24 and 96 | Handsewn vs. stapled (19% vs. 38%; p= 0.047) | Adenomas and adenomas (9 patients had > 10 mm) Adenomas: 18/23 (78%)(1 HGD & 16 LGD) |
| Moussata, 200853 | Retrospective | 42.6±11.7 (mean±SD) | IPAA (23) IRA(n=21) |
4.7 ±3.3 (mean ±SD) 16.4 (mean ± SD) |
28.7±12 (mean±SD) | 44 | 18 10 |
Pouch mucosa ARS |
Adenomas: 16/21 (77%)(2 HGD & 8 LGD) & lymphoid nodular hyperplasis in 6 |
| Tonellie, 201217 | Prospective | 32.6±11.9 (range 17–63) | RPC+MUC(51); IRA (18, later RPC) | 11 (range 1–24). Mean endoscopic session was 9, range, 3–22 | the number of adenomas ranged 1–47 per patient | 69 | 25 | All adenomas found in Pouch-body mucosa | Adenomas |
| Pommaret, 201382 | Retrospective | Not reported | IPAA 118, IRA 13, 8 DI | Median 15 | 25 (range, 9–61) | 139 | 85 | Pouch mucosa, ARS, afferent ileal loop, DI | Adenomas (7 HGD) |
| Wasmuth, 201349 | Retrospective | Mean 20 (range, 10–49) | IPAA (61); IPAA+MUC (39); IPAA-MUC (22) (15 stapled and 7 handsewn)) | MUC and without MUC Mean 15.5 and 13.7 |
15.5 for mucosectomy and 13.7 for without mucosectomy | 61 | 28 | Anastomotic site | Adenomas and in 1 patient without mucosectomy had cancer (Dukes A) |
| Boostrom, 201365 | Retrospective | Median, 26 (range, 4–60) | RPC(J-pouch, n=104; Kock pouch, n=9; S-pouch, n=3 & W-pouch, n=1) | Range, 11–59 | Median, 11 (range, 2–35.3) | 117 | 39 | 14 pouch, 2 pre-pouch ileum, 3 anastomotic site, 1 Kock nipple valve, 6 pouch and anastomosis, 2 pouch and pre-pouch ileum and 9 not described. | Tubular adenoma (n=22) and tubulovillous adenoma (n=8). Low-grade-dysplasia (n=30), non-dysplastic (n=2) and adenocarcinoma (n=1) |
Abbreviations:
HGD = High-grade dysplasia, LGD = Low-grade dysplasia, ARS= Anorectal segment, IRA = lleorectal anastomosis, RPC = Restorative proctocolectomy, IPAA = ileal pouch-anal anastomosis, MUC = Mucosectomy, IPAA+MUC = ileal pouch-anal anastomosis with mucosectomy; IPAA-MUC = ileal pouch-anal anastomosis without mucosectomy; ARS = Anorectal segment, DI=Definite ileostomy.
Table 4.
Multivariate analysis of the risk of adenoma formation in the anorectal segment. Stapled IAA and age at RPC > 40 years were independent predictors of adenoma formation in ARS. There was no significant association between the APC mutation position or preoperative colonic polyp density and adenoma risk. Reproduced with permission of the publisher: Weston-Petrides et al., Arch Surg 2008;143:406–412.52
| Preoperative Factors | Hazard Ratio | 95% Confidence Interval | P < |
|---|---|---|---|
| Gender | |||
| Female | 1 | ||
| Male | 0.80 | 0.47–1.43 | 0.820 |
|
| |||
| Age at restorative proctocolectomy, years | |||
| <20 | 1 | ||
| 21–39 | 1.31 | 0.67–2.56 | 0.426 |
| ≥40 | 2.20 | 1.01–4.89 | 0.049 |
|
| |||
| Ileoanal anastomosis | |||
| Handsewn and mucosectomy | 1 | ||
| Stapled | 3.45 | 1.87–6.39 | <0.001 |
|
| |||
| APC mutation (codon) | |||
| 1309 | 1 | ||
| <1250 | 1.96 | 0.79–4.82 | 0.079 |
| 1250–1464 (excluding 1309) | 0.34 | 0.07–1.72 | 0.194 |
| >1464 | 1.49 | 0.18–12.53 | 0.289 |
|
| |||
| Preoperative colonic polyposis density | |||
| <1000 | 1 | ||
| 1000–4999 | 1.07 | 0.55–2.10 | 0.837 |
| >5000 | 0.94 | 0.27–3.24 | 0.925 |
Parc et al.13 assessed the effects of an ileal pouch-anal anastomosis on health quality of life (HQoL) in 48 teenagers operated for FAP between 1981 and 1998 and also in 167 adult patients21 likely overlap of pts who had operations between January 1984 and December 1996. The purposes were to determine the prevalence of adenomas in ileal pouches and whether there was a correlation between the presence of pouch adenomas and the site of the adenomatous polyposis coli gene mutation. They reported that adenomas were frequently found in the ileal pouch of patients after RPC for FAP and that there was no correlation between adenoma development and the site of the adenomatous polyposis coli mutation.
After 10 years of prospective endoscopic follow-up of 69 patients treated for FAP with RPC with mucosectomy, Tonelli et al.,17 found that 64.9% of the patients had ileal pouch adenomas (as depicted in Figures 4A and B). Colonic metaplasia was implicated as a possible reason for the development of ileal adenomas in the pouch and in fact, this diagnosis was frequently reported in the earlier descriptions of changes observed in the ileal pouch mucosa. Some considered it an adaptive response of the pouch to its new role as a neorectum and could be partly due to a combination of fecal stasis and a rapid epithelial turnover rate.19,26,35,75,77,78,79,80,81
Figure 4.
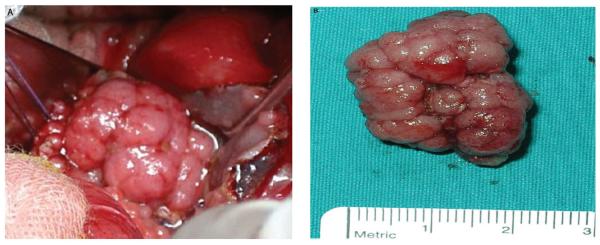
Spherical sessile adenoma 2 cm in diameter surgically removed from the pouch (A), possessing a lobulated configuration with a smooth surface, broken into lobules by interconnecting clefts (B). Reproduced with permission of the publisher: Tonelli et al., Dis Colon Rectum 2012;55:322–329.17
A recent study from France82 analyzed 442 pouch-endoscopies for 139 (118 IPAA, 13 IRA and 8 ileostomy) patients. Among the 118 IPAA patients, 57 (48.3%) had pouch adenomas when evaluated at a median of 15 years after surgery. The risk factors were considered to be delays in the surgery, the duration between the initial surgery and the endoscopy [odds ratio (OR), 1.11; p = 0.016)] and the presence of advanced duodenal adenomas prior to surgery (OR, 4.35; p = 0.011). Seven of these patients had pouch adenomas with high-grade dysplasia. Nine patients (6.5%) had adenomas in the afferent ileal loop. According to this study,82 the only significant risk factor for ileal adenomas was the presence of pouch adenomas (OR, 2.16: p = 0.007).
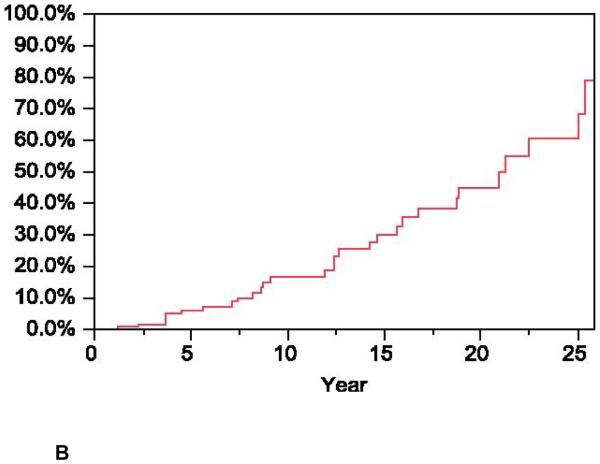
Another new study from Scandinavia49 (from the Norwegian Polyposis Registry and The Cancer Registry of Norway Database83) retrospectively examined the fate of 61 patients for a period of 20 years (range 10–49 years) following their primary surgery. The mean observational time for IPAA patients with mucosectomy was 15.5 (SD, 6.6) years and 13.7 (SD, 6.8) years for those without mucosectomy (p = 0.34). By the end of the study, the mean age was 42.2 years and 38.2 years (p = 0.14), respectively. Four of 39 patients (10%) with mucosectomy developed adenomas at the anastomotic site in comparison with 14 of 22 patients (64%) with a rectal cuff (p< 0.0001). Feinberg et al.54 estimated cumulative rate of adenomas postoperatively and found that 17% of patients with mucosectomy (mean, 28 years) and 75% without mucosectomy (mean, 15 years) (p< 0.0001)), developed adenomas, Fig. 5. They also found that there was no difference in the rate of adenomas in the ileal pouch between patients who had a mucosectomy and those who had a rectal mucosa remnant (8/39 vs. 6/22; p = 0.57). The estimated cumulative rate of initial adenoma diagnoses was 38% of patients during the observational time of 20 years, regardless of the surgical technique used (p = 0.10). Among patients with ileal pouch adenomas, 8 patients also had adenomas at the anastomotic site; 5 of these patients had a rectal cuff, and 3 had undergone mucosectomy.49 They summarized the data as follows: for FAP patients who underwent IPAA, adenoma formation at the anastomotic site was significantly reduced after mucosectomy; thus recommended mucosectomy as a preferable procedure to prevent adenomas at the anastomotic site.49,54 Although some researchers investigated APC gene mutations in pouch patients, they did not find any apparent correlation between the presence of a particular phenotype and the development of ileal adenomas.13,18,20–22,44,53,64,84 It is therefore impossible at this time to predict who is at risk and who is not at risk, as all FAP pouch recipients appear vulnerable for developing subsequent adenomas.18–22 However, because the incidence of pouch adenomas increases steadily as a function of the period of time post surgery, it seems that the age of the pouch (or patient) is important in the development of ileal adenomas. It implies that most, if not all, of these patients are destined to develop adenomas after two decades of follow-up.18,20,64
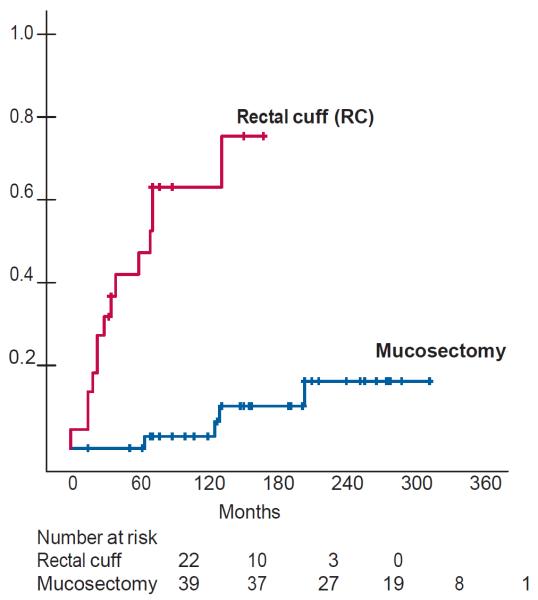
Figure 5.
Kaplan Meir curves showing the estimated rate of adenoma formation at the anastomotic site after IPAA with or without (rectal cuff) mucosectomy. Reproduced with permission of the publisher: Reproduced with permission of the publisher: Feinberg et al., Dis Colon Rectum 1988;31:169–175.54 The figure demonstrates data that underscores the importance of mucosectomy to reducing adenomas incidence compared to those patients who had their rectal cuff retained after IPAA or IRA.
There are also studies reporting that the prevalence of adenomas is in the range of 13%–15% at a median follow-up of four to six years post surgery.18,22,45 Groves et al.22 estimated that the prevalence of adenomas in the ileal pouch increased by 6.6 % per year of age, in 20% of follow-up patients. Parc et al.21 showed that the risk of adenoma development in the ileal pouch was 7%, 35% and 75% at 5, 10 and 15 years follow-up, respectively. Tajika et al.64 showed that the incidence of ileal adenoma was as high as 50% in Kock and 75% in IPAA at a median follow-up of 14.7 years after surgery. The risk of adenoma in the pouch was 13%, 43% and 72% at 5, 10 and 20 years of follow-up. The risk of rectal adenoma after colectomy with IRA was 14%, 57% and 85% at 5, 10 and 20 years of follow-up, respectively. There was no significant difference in the cumulative prevalence of ileal pouch adenomas and rectal adenomas. Moussata et al.53 showed a high prevalence of ileal pouch adenomas (17 of 23, or 74%) in FAP patients with IPAA at a median interval of eight years of follow-up after surgery. They emphasized the importance of chromoendoscopy using indigo carmine, which can be used as an aid to identify flat adenomas. Development of adenomas in the prepouch ileal segment immediately above the IPAA has also been reported.18 Prepouch adenomas were reported in 10 of 26 (38%) patients by Wu et al..18 Groves et al.22 also reported two of 20 (10%) patients with prepouch adenomas, in one of 24 (4%) patients by Thompson-Fawcett et al.20 and in one of 24 (4%) pouch patients by Tajika et al.64 at a median follow-up of 15.1 years after surgery.
Subsequent Adenomas: IPAA vs. IRA
The incidence of subsequent rectal adenomas was significantly different in the IPAA versus the IRA group of patients.53 The prevalence of ileal adenoma was significantly higher in IPAA patients (especially in the pouch mucosa) as compared to IRA patients (p< 0.002) and there was a statistically significant correlation between the number of ileal adenomas and the time since pouch surgery in IPAA patients (p< 0.02). A logistic regression model confirmed that there were significant associations between the increasing age of the patient and the presence of pouch adenoma (p< 0.02) as well as the length of follow-up since pouch surgery (p< 0.05). This suggests that older patients should have shorter intervals between follow-up surveillances.
The overall risk of developing a high-risk adenoma in the ARS was 6.4%. On multivariate analysis, multiple adenomas were found more frequently in elderly patients. The stapled IAA and an age at RPC > 40 years were independent predictors of adenoma formation in the ARS, p< 0.001 and p< 0.049 (Table 4).52 Patients without adenomas were significantly younger (26.2 ± 8.4 years) than those with adenomas (32.6 ± 11.9 years) (p = 0.02). There was no association between the development of adenomas and the type of pouch and or the diverting loop ileostomy construction. However, the number of colonic adenomas observed at the time of colectomy influenced the occurrence of pouch adenomas: all patients with less than 200 colonic polyps had no adenomas at the follow-up where as 47.5% of patients with more than 1000 colonic polyps had adenomas at follow-up. Only 25% of patients with 200 to 1000 colonic polyps had adenomas.
The frequency of ileal adenomas was 74% in the IPAA group versus 47.5% in the IRA group (p= 0.07) (Fig. 1). The frequency of advanced ileal adenomas reported is, to date, 17.4% in the IPAA group versus 9.5% in the IRA group (p= 0.075). In contrast, the mean time from surgery to development of adenomas was significantly longer in the IRA group than in the IPAA group (16.4 ± 8.5 vs. 4.76 ± 3.3 years, p< 0.0001).53 The incidences of adenomas in the ARS in mucosectomized, handsewn vs. stapled IPAA in patients with FAP is depicted in Table 5. Out of 1,049 patients who were followed-up, 373 patients (36%) were diagnosed with adenomas. These observations underscore the importance of regular endoscopic surveillance of these patients postoperatively.
Table 5.
Summary of available data on the incidence of adenomas in the ARS in mucosectomized, handsewn IPAA and stapled IPAA in patients surgically treated for FAP.
| Incidence of Adenoma in the ARS in mucosectomized, Handsewn IPAA and Stapled IPAA in Patients operated for FAP | ||||||
|---|---|---|---|---|---|---|
| Author | Follow-up-yrs | Number of patients followed-up in the study | Number of patients developed neoplastic transformation | IPAA with mucosectomy that developed neoplastic transformation | IRA Stapled that developed neoplastic transformation | P-value |
| Van Duijvendijk et al, 199973 | Median 5.5, range1–1.7) | 126 | 13 | Handsewn with mucosectom: 6 (of 13) (46%)) vs. Double-stapled without mucosectomy: 7 of 13) (54%)) |
N/A | 0.01 |
| Remzietal. 200124 | 5.8 vs. 3.6 | 119 | 44 (58%) | 9 (of 42) (21%)) in the pouch and 6 of 42 had it in mucosectomized ARS | 21 (of 76) (28%)) in ARS and 8 (11%) had adenomas in the pouch body mucosa | N/A |
| Moussata et al.53 | IPAA (4.76±3.3) IRA(16.4±8.5) |
44 | 24 (55%) | 0.0001 | ||
| Friederich et al. 200893 | 6.8 (range 0.4–20.3) | 212 | 74 (35%) | 29% | 64% | 0.0004 |
| Von Roon et al, 200774 and 201125 | 5 (range 0.1–24.75) and 10.3 (median) | (91 and 49 =)140 | 24 (26%) and 52 (37%) | 11 (19%) Handswen vs. stapled anastomosis (19 vs. 38%) and 22.6% | 13 (38)% and 51.1% | 0.047 and 0.001 |
| Pommaret et al, 201382 | Median 15 | 139 | 85 (65%) | 73 of 118 (62%) patients had adenomas in pouch mucosa, 57 (48.3%) had advanced adenomas in 15 patients (12.7%). Transformation of IRA to IPAA was done for 36 patients). In 8 cases (16.3%) to treat rectal cancer | 12 of 13 (92.3%) had rectal adenomas, including one patient with advanced rectal rectal adenoma, treated by endoscopic mucosal resection | 0.0001 |
| Wasmuth et al, 201349 | 20 (range, 10–49) | 61 | 18(30%) | Mucosectomized: 4 (of 39) (10%)) vs. None-mucosectomized: 14 (of 22) (64%)) |
N/A | The estimated rates was 17% vs. 75%. 0.0001 |
| Boostrom et al.65 | Median 26 (range, 4–16) | 117 | 39 (33%) | Handsewn with mucosectomy (31) (26%) vs. Stapled without mucosectomy (8) (7%) | N/A | N/A |
Risk factors for pouch adenomas as presented in the multivariate analysis were,82 the time delay since construction of the ileal pouch [OR, 1.11; 95% confidence interval (CI), 1.02–1.22; p = 0.016] and the presence of an advanced duodenal adenomas (OR, 4.35; 95% CI, 1.35–13.98; p = 0.0011). Both were the only significant independent risk factors for the development of pouch adenomas. While the risk factors for afferent ileum adenomas in the univariate analysis, pouch adenomas (p = 0.003) and IPAA as the first surgery (p = 0.03) were significant risk factors, with a nonsignificant trend toward an increasing risk of ileal adenomas over time (p = 0.08). In the multivariate analysis, the unique risk factor for ileal adenoma occurrence was the presence of pouch adenomas (OR, 2.16; 95% CI, 0.17–26.98; p = 0.007).82
Summary
Despite significant advances in the surgical treatment options to treat FAP and to minimize the risk of adenomas and subseqent malignant transformation, the choice of IPAA versus IRA is still a matter of debate, although IPAA remains the alternative to IRA. The incidence of adenomas in the ARS and ileal pouches, in the prepouch ileum and ileal mucosa, noted above, of FAP patients surgically treated using IRA is apparent. When there are adenomas encroaching on the pectinel line, a mucosectomy should be the preferred option, but it is also noteworthy that this does not guarantee elimination of the risk of development of a subsequent adenoma. It seems likely that most, if not all, FAP-IRA and/or FAP-IPAA patients are destined to developing adenomas postoperatively. Formation of adenomas in the ileal pouch itself does not seem to be influenced by the two surgical procedures. Managing non-dysplastic/cancerous adenomas by endoscopic mucosal resection/ polypectomy is largely satisfactory; however recurrence of adenomas is not uncommon. Most important, regardless of the anastomotic technique used, careful regular endoscopic surveillance of all patients surgically treated for FAP who have a retained functionally acceptable pouches, is critical. Management strategies for pouch adenomas, though satisfactory, clearly need further evaluation.
Conclusion
After prophylactic colectomy for FAP, using IRA or an RPC approach with an IPAA procedure, a recurrence of adenomas is frequently diagnosed in the pouch, ARS and afferent ileal loop, with an increasing risk over time. Adenomas larger than five millimeters should be removed by endoscopy or surgery. Mucosectomy is necessary to attempt completely eradication all of the rectal columnar epithelium, but some microscopic residual mucosal tissue is inevitably retained which may subsequently develop adenomas that can transform into dysplasia or cancer at a later time. The most important point we found in this review is that patients who have undergone IPAA or IRA in the setting of FAP are clearly at risk of developing subsequent adenomas. Therefore, regardless of the anastomotic procedure and term post surgery, conventional endoscopic assessment and novel adjunctive endoscopic technologies (e.g. magnification endoscopy and confocal endomicroscopy) are recommended to improve surveillance, diagnostic and therapeutic management of patients.
Acknowledgements
We acknowledge all physicians, surgeons and scientists who made contributions to the areas of research reviewed but were not cited due to space constraints. I am grateful to Jeremy N. Myers, Ph.D, Diana Marver, Ph.D., and Naji N. Abumrad, M.D., for critical reading of the manuscript.
Source of support: NIH/NIDDK 1R21DK095186-01A1; 3U54CA09140809S1; 5U54RR026140-03/ 8U54MD007593-04; Research Foundation, American Society of Colon and Rectal Surgeons (ASCRS)-LPG-086; and Vanderbilt CTSA 1 UL1 RR024975/NCRR/NIH
Footnotes
The authors contributed not only to the conception and design but also participated in the acquisition, analysis and interpretation of data and drafting of the manuscript.
Competing Interests Statement The authors have no conflict of interest or financial ties to disclose.
References
- 1.Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 2.Church J. Ileoanal pouch neoplasia in familial adenomatous polyposis: an underestimated threat. Dis Colon Rectum. 2005;48:1708–13. doi: 10.1007/s10350-005-0057-1. [DOI] [PubMed] [Google Scholar]
- 3.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 4.Linehan G, Cahill RA, Kalimuthu SN, O'Connell F, Redmond HP, Kirwan WO. Adenocarcinoma arising in the ileoanal pouch after restorative proctocolectomy for familial adenomatous polyposis. Int J Colorectal Dis. 2008;23:329–30. doi: 10.1007/s00384-007-0400-1. [DOI] [PubMed] [Google Scholar]
- 5.Nieuwenhuis MH, De Vos Tot Nederveen Cappel W, Botma A, et al. Desmoid tumors in a dutch cohort of patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:215–9. doi: 10.1016/j.cgh.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Will OC, Hansmann A, Phillips RK, et al. Adrenal incidentaloma in familial adenomatous polyposis: a long-term follow-up study and schema for management. Dis Colon Rectum. 2009;52:1637–44. doi: 10.1007/DCR.0b013e3181a876d6. [DOI] [PubMed] [Google Scholar]
- 7.Duncan RE, Gillam L, Savulescu J, Williamson R, Rogers JG, Delatycki MB. The challenge of developmentally appropriate care: predictive genetic testing in young people for familial adenomatous polyposis. Fam Cancer. 2010;9:27–35. doi: 10.1007/s10689-009-9294-0. [DOI] [PubMed] [Google Scholar]
- 8.Bussey HJ, Veale AM, Morson BC. Genetics of gastrointestinal polyposis. Gastroenterology. 1978;74:1325–30. [PubMed] [Google Scholar]
- 9.Church J. Familial adenomatous polyposis. Surg Oncol Clin N Am. 2009;18:585–98. doi: 10.1016/j.soc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Baglioni S, Genuardi M. Simple and complex genetics of colorectal cancer susceptibility. Am J Med Genet C Semin Med Genet. 2004;129C:35–43. doi: 10.1002/ajmg.c.30023. [DOI] [PubMed] [Google Scholar]
- 11.de Campos FG, Perez RO, Imperiale AR, Seid VE, Nahas SC, Cecconello I. Evaluating causes of death in familial adenomatous polyposis. J Gastrointest Surg. 2010;14:1943–9. doi: 10.1007/s11605-010-1288-6. [DOI] [PubMed] [Google Scholar]
- 12.Campos FG, Habr-Gama A, Kiss DR, et al. Adenocarcinoma after ileoanal anastomosis for familial adenomatous polyposis: review of risk factors and current surveillance apropos of a case. J Gastrointest Surg. 2005;9:695–702. doi: 10.1016/j.gassur.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Parc YR, Moslein G, Dozois RR, Pemberton JH, Wolff BG, King JE. Familial adenomatous polyposis: results after ileal pouch-anal anastomosis in teenagers. Dis Colon Rectum. 2000;43:893–8. doi: 10.1007/BF02237346. [DOI] [PubMed] [Google Scholar]
- 14.M'Koma AE, Wise PE, Muldoon RL, Schwartz DA, Washington MK, Herline AJ. Evolution of the restorative proctocolectomy and its effects on gastrointestinal hormones. Int J Colorectal Dis. 2007;22:1143–63. doi: 10.1007/s00384-007-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziv Y, Church JM, Oakley JR, McGannon E, Schroeder TK, Fazio VF. Results after restorative proctocolectomy and ileal pouch-anal anastomosis in patients with familial adenomatous polyposis and coexisting colorectal cancer. Br J Surg. 1996;83:1578–80. doi: 10.1002/bjs.1800831128. [DOI] [PubMed] [Google Scholar]
- 16.Newton CR, Baker WN. Comparison of bowel function after ileorectal anastomosis for ulcerative colitis and colonic polyposis. Gut. 1975;16:785–91. doi: 10.1136/gut.16.10.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonelli F, Ficari F, Bargellini T, Valanzano R. Ileal pouch adenomas and carcinomas after restorative proctocolectomy for familial adenomatous polyposis. Dis Colon Rectum. 2012;55:322–9. doi: 10.1097/DCR.0b013e318241e6f2. [DOI] [PubMed] [Google Scholar]
- 18.Wu JS, McGannon EA, Church JM. Incidence of neoplastic polyps in the ileal pouch of patients with familial adenomatous polyposis after restorative proctocolectomy. Dis Colon Rectum. 1998;41:552–6. doi: 10.1007/BF02235258. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd NA, Jass JR, Duval I, Moskowitz RL, Nicholls RJ, Morson BC. Restorative proctocolectomy with ileal reservoir: pathological and histochemical study of mucosal biopsy specimens. J Clin Pathol. 1987;40:601–7. doi: 10.1136/jcp.40.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson-Fawcett MW, Marcus VA, Redston M, Cohen Z, McLeod RS. Adenomatous polyps develop commonly in the ileal pouch of patients with familial adenomatous polyposis. Dis Colon Rectum. 2001;44:347–53. doi: 10.1007/BF02234731. [DOI] [PubMed] [Google Scholar]
- 21.Parc YR, Olschwang S, Desaint B, Schmitt G, Parc RG, Tiret E. Familial adenomatous polyposis: prevalence of adenomas in the ileal pouch after restorative proctocolectomy. Ann Surg. 2001;233:360–4. doi: 10.1097/00000658-200103000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groves CJ, Beveridge G, Swain DJ, et al. Prevalence and morphology of pouch and ileal adenomas in familial adenomatous polyposis. Dis Colon Rectum. 2005;48:816–23. doi: 10.1007/s10350-004-0835-1. [DOI] [PubMed] [Google Scholar]
- 23.Myrhoj T, Bulow S, Mogensen AM. Multiple adenomas in terminal ileum 25 years after restorative proctocolectomy for familial adenomatous polyposis. Report of a case. Dis Colon Rectum. 1989;32:618–20. doi: 10.1007/BF02554184. [DOI] [PubMed] [Google Scholar]
- 24.Remzi FH, Church JM, Bast J, et al. Mucosectomy vs. stapled ileal pouch-anal anastomosis in patients with familial adenomatous polyposis: functional outcome and neoplasia control. Dis Colon Rectum. 2001;44:1590–6. doi: 10.1007/BF02234377. [DOI] [PubMed] [Google Scholar]
- 25.von Roon AC, Will OC, Man RF, et al. Mucosectomy with handsewn anastomosis reduces the risk of adenoma formation in the anorectal segment after restorative proctocolectomy for familial adenomatous polyposis. Ann Surg. 2011;253:314–7. doi: 10.1097/SLA.0b013e318f3f498. [DOI] [PubMed] [Google Scholar]
- 26.Will OC, Robinson J, Gunther T, Phillips RK, Clark SK, Tomlinson I. APC mutation spectrum in ileoanal pouch polyps resembles that of colorectal polyps. Br J Surg. 2008;95:765–9. doi: 10.1002/bjs.6110. [DOI] [PubMed] [Google Scholar]
- 27.Smith JC, Schaffer MW, Ballard BR, et al. Adenocarcinomas After Prophylactic Surgery For Familial Adenomatous Polyposis. J Cancer Ther. 2013;4:260–70. doi: 10.4236/jct.2013.41033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertoni G, Sassatelli R, Nigrisoli E, et al. First observation of microadenomas in the ileal mucosa of patients with familial adenomatous polyposis and colectomies. Gastroenterology. 1995;109:374–80. doi: 10.1016/0016-5085(95)90323-2. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton SR, Bussey HJ, Mendelsohn G, et al. Ileal adenomas after colectomy in nine patients with adenomatous polyposis coli/Gardner's syndrome. Gastroenterology. 1979;77:1252–7. [PubMed] [Google Scholar]
- 30.Nugent KP, Spigelman AD, Nicholls RJ, Talbot IC, Neale K, Phillips RK. Pouch adenomas in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1620. doi: 10.1002/bjs.1800801245. [DOI] [PubMed] [Google Scholar]
- 31.Church JM, Oakley JR, Wu JS. Pouch polyposis after ileal pouch-anal anastomosis for familial adenomatous polyposis: report of a case. Dis Colon Rectum. 1996;39:584–6. doi: 10.1007/BF02058717. [DOI] [PubMed] [Google Scholar]
- 32.Beveridge IG, Swain DJ, Groves CJ, et al. Large villous adenomas arising in ileal pouches in familial adenomatous polyposis: report of two cases. Dis Colon Rectum. 2004;47:123–6. doi: 10.1007/s10350-003-0020-y. [DOI] [PubMed] [Google Scholar]
- 33.Beart RW, Jr., Fleming CR, Banks PM. Tubulovillous adenomas in a continent ileostomy after proctocolectomy for familial polyposis. Dig Dis Sci. 1982;27:553–6. doi: 10.1007/BF01296737. [DOI] [PubMed] [Google Scholar]
- 34.Iida M, Itoh H, Matsui T, Mibu R, Iwashita A, Fujishima M. Ileal adenomas in postcolectomy patients with familial adenomatosis coli/Gardner's syndrome. Incidence and endoscopic appearance. Dis Colon Rectum. 1989;32:1034–8. doi: 10.1007/BF02553876. [DOI] [PubMed] [Google Scholar]
- 35.Johnson CD, White H. Colonic metaplasia with colonic-type polyps on an ileostomy stoma in polyposis coli. Report of a case. Dis Colon Rectum. 1988;31:405–7. doi: 10.1007/BF02564900. [DOI] [PubMed] [Google Scholar]
- 36.Nakahara S, Itoh H, Iida M, Iwashita A, Ohsato K. Ileal adenomas in familial polyposis coli. Differences before and after colectomy. Dis Colon Rectum. 1985;28:875–7. doi: 10.1007/BF02555497. [DOI] [PubMed] [Google Scholar]
- 37.Stryker SJ, Carney JA, Dozois RR. Multiple adenomatous polyps arising in a continent reservoir ileostomy. Int J Colorectal Dis. 1987;2:43–5. doi: 10.1007/BF01648998. [DOI] [PubMed] [Google Scholar]
- 38.Smith KD, Rodriguez-Bigas MA. Role of surgery in familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer (Lynch syndrome) Surg Oncol Clin N Am. 2009;18:705–15. doi: 10.1016/j.soc.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Lockhart-Mummery JP. The Causes and Treatment of Pruritus Ani. Postgrad Med J. 1934;10:429–34. doi: 10.1136/pgmj.10.110.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitellaro M, Ferrari A, Trencheva K, et al. Is laparoscopic surgery an option to support prophylactic colectomy in adolescent patients with Familial Adenomatous Polyposis (FAP)? Pediatr Blood Cancer. 2012;59:1223–8. doi: 10.1002/pbc.24113. [DOI] [PubMed] [Google Scholar]
- 41.Aziz O, Athanasiou T, Fazio VW, et al. Meta-analysis of observational studies of ileorectal versus ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg. 2006;93:407–17. doi: 10.1002/bjs.5276. [DOI] [PubMed] [Google Scholar]
- 42.Kartheuser AH, Parc R, Penna CP, et al. Ileal pouch-anal anastomosis as the first choice operation in patients with familial adenomatous polyposis: a ten-year experience. Surgery. 1996;119:615–23. doi: 10.1016/s0039-6060(96)80185-1. [DOI] [PubMed] [Google Scholar]
- 43.Church J, Burke C, McGannon E, Pastean O, Clark B. Predicting polyposis severity by proctoscopy: how reliable is it? Dis Colon Rectum. 2001;44:1249–54. doi: 10.1007/BF02234779. [DOI] [PubMed] [Google Scholar]
- 44.Vasen HF, van der Luijt RB, Slors JF, et al. Molecular genetic tests as a guide to surgical management of familial adenomatous polyposis. Lancet. 1996;348:433–5. doi: 10.1016/s0140-6736(96)01340-2. [DOI] [PubMed] [Google Scholar]
- 45.Bulow C, Vasen H, Jarvinen H, Bjork J, Bisgaard ML, Bulow S. Ileorectal anastomosis is appropriate for a subset of patients with familial adenomatous polyposis. Gastroenterology. 2000;119:1454–60. doi: 10.1053/gast.2000.20180. [DOI] [PubMed] [Google Scholar]
- 46.Church JM, Fazio VW, Lavery IC, Oakley JR, Milsom J, McGannon E. Quality of life after prophylactic colectomy and ileorectal anastomosis in patients with familial adenomatous polyposis. Dis Colon Rectum. 1996;39:1404–8. doi: 10.1007/BF02054529. [DOI] [PubMed] [Google Scholar]
- 47.Utsunomiya J, Iwama T, Imajo M, et al. Total colectomy, mucosal proctectomy, and ileoanal anastomosis. Dis Colon Rectum. 1980;23:459–66. doi: 10.1007/BF02987076. [DOI] [PubMed] [Google Scholar]
- 48.Thompson-Fawcett MW, Warren BF, Mortensen NJ. A new look at the anal transitional zone with reference to restorative proctocolectomy and the columnar cuff. Br J Surg. 1998;85:1517–21. doi: 10.1046/j.1365-2168.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 49.Wasmuth HH, Trano G, Myrvold HE, Aabakken L, Bakka A. Adenoma formation and malignancy after restorative proctocolectomy with or without mucosectomy in patients with familial adenomatous polyposis. Dis Colon Rectum. 2013;56:288–94. doi: 10.1097/DCR.0b013e31827c970f. [DOI] [PubMed] [Google Scholar]
- 50.Utsunomiya J, Oota M, Iwama T. Recent trends in ileoanal anastomosis. Ann Chir Gynaecol. 1986;75:56–62. [PubMed] [Google Scholar]
- 51.Ziv Y, Fazio VW, Church JM, Lavery IC, King TM, Ambrosetti P. Stapled ileal pouch anal anastomoses are safer than handsewn anastomoses in patients with ulcerative colitis. Am J Surg. 1996;171:320–3. doi: 10.1016/S0002-9610(97)89634-1. [DOI] [PubMed] [Google Scholar]
- 52.Weston-Petrides GK, Lovegrove RE, Tilney HS, et al. Comparison of outcomes after restorative proctocolectomy with or without defunctioning ileostomy. Arch Surg. 2008;143:406–12. doi: 10.1001/archsurg.143.4.406. [DOI] [PubMed] [Google Scholar]
- 53.Moussata D, Nancey S, Lapalus MG, et al. Frequency and severity of ileal adenomas in familial adenomatous polyposis after colectomy. Endoscopy. 2008;40:120–5. doi: 10.1055/s-2007-995363. [DOI] [PubMed] [Google Scholar]
- 54.Feinberg SM, Jagelman DG, Sarre RG, et al. Spontaneous resolution of rectal polyps in patients with familial polyposis following abdominal colectomy and ileorectal anastomosis. Dis Colon Rectum. 1988;31:169–75. doi: 10.1007/BF02552541. [DOI] [PubMed] [Google Scholar]
- 55.Prost B, Poncet G, Scoazec JY, Saurin JC. Unusual complications of argon plasma coagulation. Gastrointest Endosc. 2004;59:929–32. doi: 10.1016/s0016-5107(04)01268-4. [DOI] [PubMed] [Google Scholar]
- 56.McLaughlin SD, Clark SK, Tekkis PP, Ciclitira PJ, Nicholls RJ. Review article: restorative proctocolectomy, indications, management of complications and follow-up--a guide for gastroenterologists. Aliment Pharmacol Ther. 2008;27:895–909. doi: 10.1111/j.1365-2036.2008.03643.x. [DOI] [PubMed] [Google Scholar]
- 57.Saurin JC, Napoleon B, Gay G, et al. Endoscopic management of patients with familial adenomatous polyposis (FAP) following a colectomy. Endoscopy. 2005;37:499–501. doi: 10.1055/s-2005-861295. [DOI] [PubMed] [Google Scholar]
- 58.Chambers WM, Mc CMNJ. Should ileal pouch-anal anastomosis include mucosectomy? Colorectal Dis. 2007;9:384–92. doi: 10.1111/j.1463-1318.2007.01211.x. [DOI] [PubMed] [Google Scholar]
- 59.Delaini GG, Scaglia M, Colucci G, Hulten L. The ileoanal pouch procedure in the long-term perspective: a critical review. Tech Coloproctol. 2005;9:187–92. doi: 10.1007/s10151-005-0225-2. [DOI] [PubMed] [Google Scholar]
- 60.McLaughlin SD, Clark SK, Thomas-Gibson S, Tekkis PP, Ciclitira PJ, Nicholls RJ. Guide to endoscopy of the ileo-anal pouch following restorative proctocolectomy with ileal pouch-anal anastomosis; indications, technique, and management of common findings. Inflamm Bowel Dis. 2009;15:1256–63. doi: 10.1002/ibd.20874. [DOI] [PubMed] [Google Scholar]
- 61.Kartheuser A, Stangherlin P, Brandt D, Remue C, Sempoux C. Restorative proctocolectomy and ileal pouch-anal anastomosis for familial adenomatous polyposis revisited. Familial cancer. 2006;5:241–60. doi: 10.1007/s10689-005-5672-4. [DOI] [PubMed] [Google Scholar]
- 62.Nugent KP, Phillips RK. Rectal cancer risk in older patients with familial adenomatous polyposis and an ileorectal anastomosis: a cause for concern. Br J Surg. 1992;79:1204–6. doi: 10.1002/bjs.1800791136. [DOI] [PubMed] [Google Scholar]
- 63.Yan Z, Liao G, Pei H. Surgical treatment of familial adenomatous polyposis: Experience from a single institution in China. Asia Pac J Clin Oncol. 2012;8:e23–8. doi: 10.1111/j.1743-7563.2011.01488.x. [DOI] [PubMed] [Google Scholar]
- 64.Tajika M, Nakamura T, Nakahara O, et al. Prevalence of adenomas and carcinomas in the ileal pouch after proctocolectomy in patients with familial adenomatous polyposis. J Gastrointest Surg. 2009;13:1266–73. doi: 10.1007/s11605-009-0871-1. [DOI] [PubMed] [Google Scholar]
- 65.Boostrom SY, Mathis KL, Pendlimari R, Cima RR, Larson DW, Dozois EJ. Risk of Neoplastic Change in Ileal Pouches in Familial Adenomatous Polyposis. J Gastrointest Surg. 2013;17:1804–8. doi: 10.1007/s11605-013-2319-x. [DOI] [PubMed] [Google Scholar]
- 66.Carvalho R, Areia M, Brito D, Saraiva S, Alves S, Cadime AT. Endoscopic mucosal resection of large colorectal polyps: prospective evaluation of recurrence and complications. Acta gastroenterol Belg. 2013;76:225–30. [PubMed] [Google Scholar]
- 67.Luigiano C, Consolo P, Scaffidi MG, et al. Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: a single-center experience with long-term follow-up. Endoscopy. 2009;41:829–35. doi: 10.1055/s-0029-1215091. [DOI] [PubMed] [Google Scholar]
- 68.Arebi N, Swain D, Suzuki N, Fraser C, Price A, Saunders BP. Endoscopic mucosal resection of 161 cases of large sessile or flat colorectal polyps. Scand J Gastroenterol. 2007;42:859–66. doi: 10.1080/00365520601137280. [DOI] [PubMed] [Google Scholar]
- 69.Schulz AC, Bojarski C, Buhr HJ, Kroesen AJ. Occurrence of adenomas in the pouch and small intestine of FAP patients after proctocolectomy with ileoanal pouch construction. Int J Colorectal Dis. 2008;23:437–41. doi: 10.1007/s00384-007-0422-8. [DOI] [PubMed] [Google Scholar]
- 70.Lynch HT, Thorson AG, Smyrk T. Rectal cancer after prolonged sulindac chemoprevention. A case report. Cancer. 1995;75:936–8. doi: 10.1002/1097-0142(19950215)75:4<936::aid-cncr2820750407>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 71.Spagnesi MT, Tonelli F, Dolara P, et al. Rectal proliferation and polyp occurrence in patients with familial adenomatous polyposis after sulindac treatment. Gastroenterology. 1994;106:362–6. doi: 10.1016/0016-5085(94)90593-2. [DOI] [PubMed] [Google Scholar]
- 72.Phillips RK, Spigelman AD. Can we safely delay or avoid prophylactic colectomy in familial adenomatous polyposis? Br J Surg. 1996;83:769–70. doi: 10.1002/bjs.1800830613. [DOI] [PubMed] [Google Scholar]
- 73.van Duijvendijk P, Vasen HF, Bertario L, et al. Cumulative risk of developing polyps or malignancy at the ileal pouch-anal anastomosis in patients with familial adenomatous polyposis. J Gastrointest Sur. 1999;3:325–30. doi: 10.1016/s1091-255x(99)80075-4. [DOI] [PubMed] [Google Scholar]
- 74.von Roon AC, Tekkis PP, Clark SK, et al. The impact of technical factors on outcome of restorative proctocolectomy for familial adenomatous polyposis. Dis Colon Rectum. 2007;50:952–61. doi: 10.1007/s10350-006-0872-z. [DOI] [PubMed] [Google Scholar]
- 75.Corfield AP, Warren BF, Bartolo DC, Wagner SA, Clamp JR. Mucin changes in ileoanal pouches monitored by metabolic labelling and histochemistry. Br J Surg. 1992;79:1209–12. doi: 10.1002/bjs.1800791139. [DOI] [PubMed] [Google Scholar]
- 76.de Silva HJ, Millard PR, Kettlewell M, Mortensen NJ, Prince C, Jewell DP. Mucosal characteristics of pelvic ileal pouches. Gut. 1991;32:61–5. doi: 10.1136/gut.32.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson JA, 3rd, Talton DS, Poole GV. Adenocarcinoma of a Brooke ileostomy for adenomatous polyposis coli. Am J Gastroenterol. 1993;88:1122–4. [PubMed] [Google Scholar]
- 78.de Silva HJ, Millard PR, Soper N, Kettlewell M, Mortensen N, Jewell DP. Effects of the faecal stream and stasis on the ileal pouch mucosa. Gut. 1991;32:1166–9. doi: 10.1136/gut.32.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu JS, Paul P, McGannon EA, Church JM. APC genotype, polyp number, and surgical options in familial adenomatous polyposis. Ann Surg. 1998;227:57–62. doi: 10.1097/00000658-199801000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veress B, Reinholt FP, Lindquist K, Liljeqvist L. Different types of mucosal adaptation in the ileal reservoir after restorative proctocolectomy. A two-year follow-up study. APMIS. 1990;98:786–96. doi: 10.1111/j.1699-0463.1990.tb04999.x. [DOI] [PubMed] [Google Scholar]
- 81.Veress B, Reinholt FP, Lindquist K, Liljeqvist L. Prospective studies of the mucosa of the ileoanal pouch. Gastroenterology. 1995;108:953–4. doi: 10.1016/0016-5085(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 82.Pommaret E, Vienne A, Lefevre JH, et al. Prevalence and risk factors for adenomas in the ileal pouch and the afferent loop after restorative proctocolectomy for patients with familial adenomatous polyposis. Surg Endosc. 2013;27:3816–22. doi: 10.1007/s00464-013-2980-x. [DOI] [PubMed] [Google Scholar]
- 83.Ahlquist T. New doctorial cancer research:novel genetic and epigenetic alterations in colorectal tumors and their potential as biomarkers. Crit Rev Oncog. 2009;15:81–83. [Google Scholar]
- 84.Bertario L, Russo A, Radice P, et al. Genotype and phenotype factors as determinants for rectal stump cancer in patients with familial adenomatous polyposis. Hereditary Colorectal Tumors Registry. Ann Surg. 2000;231:538–43. doi: 10.1097/00000658-200004000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 86.Cruz-Correa M, Hylind LM, Romans KE, et al. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122:641–645. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- 87.Tonelli F, Valanzano R, Messerini L, Ficari F. Long-term treatment with sulindac in familial adenomatous polyposis: is there an actual efficacy in prevention of rectal cancer? J Surg Oncol. 2000;74:15–20. doi: 10.1002/1096-9098(200005)74:1<15::aid-jso4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 88.Winde G, Schmid KW, Schlegel W, et al. Complete reversion and prevention of rectal adenomas in colectomized patients with familial adenomatous polyposis by rectal low-dose sulindac maintenance treatment. Advantages of a low-dose nonsteroidal anti-inflammatory drug regimen in reversing adenomas exceeding 33 months. Dis Colon Rectum. 1995;38:813–830. doi: 10.1007/BF02049838. [DOI] [PubMed] [Google Scholar]
- 89.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 90.Slim R, Ponchon T, Chavaillon A, et al. Follow-up and laser Nd-Yag treatment of adenomas in the rectal stump of familial adenomotous polyposis patients: long term results [abstract] Gastrointest Endosc. 2000;51:151. AB. [Google Scholar]
- 91.Madden MV, Neale KF, Nicholls RJ, et al. Comparison of morbidity and function after colectomy with ileorectal anastomosis or restorative proctocolectomy for familial adenomatous polyposis. Br J Surg. 1991;78:789–792. doi: 10.1002/bjs.1800780708. [DOI] [PubMed] [Google Scholar]
- 92.Ozdemir Y, Kalady MF, Aytac E, et al. Anal transitional zone neoplasia in patients with familial adenomatous polyposis after restorative proctocolectomy and IPAA: incidence, management, and oncologic and functional outcomes. Dis Colon Rectum. 2013;56:808–814. doi: 10.1097/DCR.0b013e31829005db. [DOI] [PubMed] [Google Scholar]
- 93.Friederich P, de Jong AE, Mathus-Vliegen LM, et al. Risk of developing adenomas and carcinomas in the ileal pouch in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:1237–1242. doi: 10.1016/j.cgh.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 94.Wolfstein IH, Bat L, Neumann G. Regeneration of rectal mucosa and recurrent polyposis coli after total colectomy and ileoanal anastomosis. Arch Surg. 1982;117:1241–1242. doi: 10.1001/archsurg.1982.01380330095024. [DOI] [PubMed] [Google Scholar]
- 95.Järvinen H, Nyberg M, Peltokallio P. Upper gastrointestinal tract polyps in familial adenomatosis coli. Gut. 1983;24:333–339. doi: 10.1136/gut.24.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burt RW, Berenson MM, Lee RG, et al. Upper gastrointestinal polyps in Gardner's syndrome. Gastroenterology. 1984;86:295–301. [PubMed] [Google Scholar]
- 97.Valle RD, de'Angelis GL. Pouch adenomas after ileal pouch-anal anastomosis for familial adenomatous polyposis. Dis Colon Rectum. 2001;44:456–458. doi: 10.1007/BF02234750. [DOI] [PubMed] [Google Scholar]