Abstract
A low plasma 25-OH vitamin D3 level is a universal risk factor for a wide range of diseases and has also been implicated in late-life depression. It is currently unknown whether the biologically active form of vitamin D, that is, 1,25-(OH)2 vitamin D3, is also decreased in late-life depression, or whether vitamin D levels correlate with specific depression characteristics. We determined plasma 25-OH vitamin D3, 1,25-(OH)2 vitamin D3 and parathormone levels in 355 depressed older persons and 124 non-depressed comparison subjects (age⩾60 years). Psychopathology was established with the Composite International Diagnostic Interview 2.1, together with potential confounders and depression characteristics (severity, symptom profile, age of onset, recurrence, chronicity and antidepressant drug use). Adjusted for confounders, depressed patients had significantly lower levels of 25-OH vitamin D33 (Cohen's d =0.28 (95% confidence interval: 0.07–0.49), P=0.033) as well as 1,25-(OH)2 vitamin D3 (Cohen's d =0.48 (95% confidence interval: 0.27–0.70), P<0.001) than comparison subjects. Of all depression characteristics tested, only the use of tricyclic antidepressants (TCAs) was significantly correlated with lower 1,25-(OH)2 vitamin D3 levels (Cohen's d =0.86 (95% confidence interval: 0.53–1.19), P<0.001), but not its often measured precursor 25-OH vitamin D3. As vitamin D levels were significantly lower after adjustment for confounders, vitamin D might have an aetiological role in late-life depression. Differences between depressed and non-depressed subjects were largest for the biologically active form of vitamin D. The differential impact of TCAs on 25-OH vitamin D3 and 1,25-(OH)2 vitamin D3 levels suggests modulation of 1-α-hydroxylase and/or 24-hydroxylase, which may in turn have clinical implications for biological ageing mechanisms in late-life depression.
Introduction
Depressive disorders commonly occur in older adults, with prevalence rates ranging between 1.8 and 9.3%.1,2 The prospective association between depression and the development and prognosis of somatic diseases as well as mortality has led to the postulation of a causal role of ageing-related mechanisms in late-life depression.3,4 In this respect, 25-(OH) vitamin D3 (or calcidiol) is of considerable interest. Vitamin D insufficiency, defined as a 25-(OH) vitamin D3 level less than 50 nmol l−1, is a universal risk factor for a range of multifactorial disorders, particularly those related to aging5,6 like chronic kidney disease, cardiovascular disease, diabetes, frailty, autoimmune conditions, multiple sclerosis and even all-cause mortality.7, 8, 9, 10
Recently, 25-(OH) vitamin D3 insufficiency has also been implicated in the pathogenesis of late-life depression.11,12 This hypothesis, however, is primarily based on cross-sectional studies showing decreased 25-(OH) vitamin D3 levels in depressed older persons.11,13,14 Calcitriol (1,25-(OH)2vitamin D3) is the biologically active form of vitamin D and acts as a neurosteroid on the vitamin D receptors within the mood regulation brain circuitries.15 1,25-(OH)2 Vitamin D3 activates gene expression of tyrosine hydroxylase, the enzyme responsible for catalysing the conversion of L-tyrosine to L-DOPA, the precursor of dopamine.15,16 Animal models of depression clearly point towards a role for the dopamine system in depression17,18 as well as to a regulatory role on dopamine-related neurotrophic growth factors for 1,25-(OH)2 vitamin D3. In humans, disturbed dopamine transmission correlates with the symptoms of apathy and anhedonia in depression.19 Finally, 1,25-(OH)2 vitamin D3 protects the brain from neurotoxicity by enhancing gluthathion function.15,20
Although an aetiological role of 25-(OH) vitamin D3 has been postulated in late-life depression, results showing lowered 25-(OH) vitamin D3 levels in depression may easily be confounded by decreased sunlight exposure owing to less outdoor activities, different clothing habits and season of measurement, malnutrition, low socioeconomic status, dark skin and comorbid somatic diseases.21, 22, 23 A recent meta-analyses identified an inverse association between depression and serum vitamin D levels.24 Subgroup analyses by age, however, showed that the inverse association was only significant in persons younger than 60 years and not in those aged 60 years and over. Negative results could be explained in one study by correction for self-reported health status,13 whereas in another study the association between vitamin D and depressive symptoms was primarily confined to persons with a history of depression.12 Moreover, one of the first studies on vitamin D levels in (late-life) depression was not included in the meta-analyses despite meeting the (somewhat vaguely described) inclusion criteria.11 This cohort study was well controlled for confounders and found a significant inverse association between serum vitamin D levels and depression.11 Also, not included by having measured vitamin D intake by food instead of serum levels, was the Women's Health Initiative observational study, in which lower vitamin D intake appeared to be associated with depressive symptoms.25 Finally, studies published after conclusion of the search for the meta-analyses showed that lower vitamin D levels were associated with depression among 1618 older primary care patients,26 whereas a small nested case–control study of the Survey in Europe on Nutrition and the Elder, a Concerted Action study27 as well as a nursing home study did not.28 Except for the study that took a history of depression into account,12 characteristics of depression like age of onset or specific symptom profiles have not been evaluated systematically in relation to 25-(OH) vitamin D3 levels. Moreover, no study has evaluated the biologically active form of vitamin D, that is, 1,25-(OH)2 vitamin D3, in depression.
The objectives of the present study are therefore twofold. First, to compare vitamin D, both 25-(OH) vitamin D3 and 1,25-(OH)2 vitamin D3 levels, between depressed and non-depressed older persons. The second objective is to examine the association of vitamin D levels with specific depression characteristics (severity, symptom profile of depressive symptoms, comorbid anxiety disorders, age of onset, recurrence, chronicity and antidepressant drug use).
Materials and methods
Sample
For the present study, we used the baseline assessment of the Netherlands Study of Depression in Older people.29 The Netherlands Study of Depression in Older people is an on-going cohort study designed to examine the (determinants of the) course and consequences of depressive disorders in older 378 depressed and 132 non-depressed persons aged 60 through 93 years. Recruitment of depressed older persons took place in five regions in the Netherlands from both mental health care institutes and general practitioners in order to include persons with late-life depression in various developmental and severity stages. Persons with a primary diagnosis of dementia, a Mini Mental State Examination Score under 18 (out of 30 points), and insufficient command of the Dutch language were excluded. Non-depressed controls were recruited from general practitioners. Inclusion criteria for non-depressed controls were the following: no lifetime diagnosis of depression, dementia or other serious psychiatric disorders, and good command of the Dutch language.
Data collection of the baseline assessment started in 2007 and was finished in September 2010. The baseline assessment included written questionnaires, interviews and physical assessments. Interviews were audio taped to control the quality of the data. The ethical review boards of the participating institutes have approved this study. All participants gave informed consent after oral and written information about the study.
For the present study, we excluded 14 persons owing to the missing of a vitamin D blood sample (no blood withdrawal, n=12; vitamin D assessment failed, n=2) and 17 because they used vitamin D supplementation. Vitamin D supplementation included vitamin A with vitamin D, vitamin D and analogue compounds, ergocalciferol, alfacalcidol, calcitriol, colecalciferol and calcium with colecalciferol.
This resulted in a final study sample of 355 depressed older patients and 124 non-depressed controls. Excluded subjects did not differ from included subjects with respect to age, sex, cognitive functioning (Mini Mental State Examination Score) or depression status (all P-values>0.36).
Depression diagnoses
The past 6-month diagnosis of depression and dysthymia according to Diagnostic and Statistical Manual of Mental Disorders-IV-R criteria30 were assessed with the Composite International Diagnostic Interview (WHO version 2.1; 12-month version). The Composite International Diagnostic Interview is a structured clinical interview that is designed for use in research settings and has high validity for depressive and anxiety disorders. As in the Netherlands Study of Depression in Adults,31 we added questions to determine the research Diagnostic and Statistical Manual of Mental Disorders-IV diagnosis of current minor depression.29
Depression characteristics
Among the depressed sample, 339 (95.5%) met criteria for a major depressive disorder with the past 6 months, 17 (3.8%) for a minor depression and 93 (26.2%) for dysthymia. Owing to double diagnoses, numbers do not add up to 100%. Within the group suffering from a past 6-month major depressive disorder diagnosis, 78 persons did not meet the criteria for a major depressive disorder within the past month and were classified as ‘recently remitted'.
Based on data from the Composite International Diagnostic Interview, we assessed the age of onset of the depression (age of the participant at the time of the first depressive episode) and recurrence (presence of depressive episode before the current episode). Severity of depression was measured by the 30-item self-rating Inventory of Depressive Symptomatology, which has adequate psychometric properties.32 To examine symptom profiles of late-life depression, three subscales of the Inventory of Depressive Symptomatology were used: a mood, a motivation and a somatic subscale.33 These three homogenous subscales yielded a good fit with exploratory and confirmatory factor analysis in the Netherlands Study of Depression in Older people study.33 Comorbid anxiety was also taken into account. The Composite International Diagnostic Interview anxiety diagnosis was used to determine the presence of an anxiety disorder in the past year that is, generalised anxiety disorders, panic disorder, agoraphobia or social phobia. The Beck Anxiety Inventory was used to assess the severity of anxiety symptoms.34 The score on the Beck Anxiety Inventory was used as a continuous variable. Neuroticism was assessed using the subscale of the NEO-Five-Factor Inventory.35 Medication use was assessed on drug container inspection of all drugs used in the past month and classified according to the World Health Organization Anatomical Therapeutic Chemical classification.36 Medication was only considered when taken on a regular basis (at least 50% of the time). Antidepressant medication was classified in selective serotonin reuptake inhibitors (N06AB), tricyclic antidepressants (TCAs; N06AA) and other antidepressants (N06AX16, N06AX21, N06AX03, N06AX05 and N06AX11).
Vitamin D status
Plasma 25-(OH) vitamin D3 levels were determined using isotope dilution-online solid-phase extraction liquid chromatography–tandem mass spectrometry, as described previously.37,38 Method characteristics are the following: limit of quantitation 4.0 nmol l−1; intra-assay coefficient of variation<7.2% and inter-assay coefficient of variation<14.1% for three concentrations between 20 and 150 nmol l−1; recovery ranges from 93 to 98% and linearity was acceptable (r2=0.9972). The accuracy of 25-(OH) vitamin D3 levels was established using (the National Institute of Standards & Technology, Gaithersburg, MD, USA) reference material to establish true values for calibration standards. Calibration standards, quality control samples and patient samples were stable for 6 days at 6 °C (coefficient of variation<11%). Samples were stable for at least three freeze-thaw cycles (coefficient of variation<3%). The plasma 1,25-(OH)2 D3 was measured by radioimmunoassay. Standard laboratory measurements were performed as described previously.39 Subsequently, 25-(OH) vitamin D3 levels were classified in categories, that is, severe deficient (<10 nmol l−1), deficient (10–24 nmol l−1), insufficient (25–49 nmol l−1), hypovitaminosis (50–79) and optimal (⩾80 nmol l−1) plasma levels.39,40
The plasma parathyroid hormone concentration was determined by using a tube sandwich chemiluminescence assay technic (Independent Lubricant Manufacturers Association) at VU Medical Center Amsterdam, a highly sensitive method. As summarised in a review on vitamin D, parathyroid hormone levels are inversely associated with 25-(OH) vitamin D3 levels until the latter reach 75–100 nmol l−1, at which point parathyroid hormone levels begin to level off (at their nadir).22
Covariates
In addition to demographic variables (age, sex, partner status, years of education) and astronomical season of blood withdrawal (defined as winter (21 November-20 February), spring (21 February-20 May), summer (21 May-20 August), autumn (21 August-20 November)), the following confounders were a priori considered based on their relationship with depressive symptoms and vitamin D level.11, 12, 13
The first set of potential confounders were lifestyle factors and included smoking, use of alcohol and physical activity. Smoking was defined as currently smoking (yes/no). On the basis of first two questions of the Alcohol Use Disorder Identification Test,41 we classified alcohol consumption into three categories, that is, no drinking, moderate alcohol use and problematic alcohol use. Problematic alcohol use was defined as taking 5–10 units on a typical drinking day irrespective of the frequency of drinking or 3 or 4 units on a typical drinking day at least 4 or more days a week. Moderate alcohol use was defined as any alcohol use not being problematic use. Physical activity in the past week was measured with the short form (eight items) of the International Physical Activities Questionnaire.42 Psychometric properties of the long and short version of the International Physical Activities Questionnaire were acceptable.
The second set of confounders consisted of parameters of physical and cognitive functioning and included parathyroid hormone (pmol l−1), renal function (estimated glomerular filtration rate estimated by the MDRD (Modification of Diet in Renal Disease Study) formula in ml min−1), waist circumference (cm) and number of chronic diseases. The number of chronic diseases was assessed with previously used self-report questions about the presence of the following chronic diseases or disease events: cardiac disease (including myocardial infarction), peripheral atherosclerosis, stroke, diabetes mellitus, chronic obstructive pulmonary disease (asthma, chronic bronchitis or pulmonary emphysema), arthritis (rheumatoid arthritis or osteoarthritis) and cancer. The accuracy of self-reports of these diseases was shown to be adequate and independent of cognitive impairment compared with data obtained from general practitioners.43 Global cognitive functioning was measured by the Mini Mental State Examination.44 The Mini Mental State Examination Score ranges from 0 to 30, with higher scores indicating better cognitive functioning.
Statistical analysis
In order to obtain a normal distribution of plasma 1,25-(OH)2vitamin D3 and parathormone (PTH) levels, respectively, two positive outliers were trimmed at the mean level plus 3 s.d., resulting in a skewness of 0.76 (s.e.=0.11) and kurtosis of 1.35 (s.e.=0.22) for 1,25-(OH)2 vitamin D3 and skewness of 2.02 (s.e.=0.13) and kurtosis of 7.31 (s.e.=0.26) for PTH. The latter was log transformed in order to get a normal distribution. All other continuous variables had a normal distribution.
Potential determinants of 25-(OH) vitamin D3, 1,25-(OH)2 vitamin D3 and PTH levels were first compared between depressed and non-depressed persons with Student's t-tests for continuous variables and Pearson's χ2-tests for categorical variables. Thereafter, these potential determinants were entered as covariates in the analysis of covariance with plasma 25-(OH) vitamin D3, 1,25-(OH)2 vitamin D3 and PTH levels as dependent variables. Cohen's d, defined as the difference between two means divided by the pooled s.d. of the two means, was used to evaluate the effect size. A Cohen's d of >0.2 is considered a small effect, >0.5 a medium effect and >0.8 a large effect.45
Finally, we examined the relationship between several characteristics of depressive disorder as independent variables in multiple linear regression analyses with 25-(OH) vitamin D3 and 1,25-(OH)2vitamin D3 levels as the dependent variables within the depressed subgroup, adjusted for all covariates described above.
All analyses were carried out using the Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM SPSS, Armonk, NY, USA).
Results
The 355 depressed older patients (34.1% male) had a mean (s.d.) age of 70.7 (7.4) years; the 124 non-depressed persons (35.5% male) had a mean (s.d.) age of 69.9 (7.3) years. Table 1 presents the other characteristics of the study population by depression status. As shown, depressed and non-depressed groups significantly differed with respect to educational level, use of alcohol, smoking, physical activity, waist circumference, renal function, number of chronic diseases and cognitive functioning.
Table 1. Baseline characteristics of study sample by depression status (n=479).
| Characteristic | Depressed patients (n=355) | Non-depressed controls (n=124) | Statistics |
|---|---|---|---|
| Sociodemographics | |||
| Age, mean (s.d.) | 70.7 (7.4) | 69.9 (7.2) | t=−0.967, df=477, P=0.334 |
| Male sex, n (%) | 121 (34.1) | 49 (39.5) | X2=1.18, df=1, P=0.276 |
| Educational level (years), mean (s.d.) | 10.5 (3.4) | 12.4 (3.5) | t=5.44, df=477, P=0.000 |
| Seasons of measurement | |||
| Spring, n (%) | 113 (31.8) | 41(33.1) | X2=7.09, df=3, P=0.069 |
| Summer, n (%) | 94 (26.5) | 46 (37.1) | |
| Autumn, n (%) | 74 (20.8) | 17 (13.7) | |
| Winter, n (%) | 74 (20.8) | 20 (16.1) | |
| Lifestyle | |||
| Use of alcohol | |||
| No alcohol use, n (%) | 140 (40.0) | 17 (14.2) | X2=31.21, df=2, P=0.000 |
| Moderate alcohol use, n (%) | 178 (50.9) | 78 (65.0) | |
| Severe alcohol use, n (%) | 32 (9.1) | 25 (20.8) | |
| Smoking (yes), n (%) | 91 (25.9) | 10 (8.1) | X2=17.36, df=1, P=0.000 |
| Physical activity | |||
| Low, n (%) | 107 (31.1) | 22 (18.3) | X2=7.5, df=2, P=0.024 |
| Moderate, n (%) | 130 (37,8) | 51 (42,5) | |
| High, n (%) | 107 (31.1) | 47 (39,2) | |
| Waist circumference (cm), mean (s.d.) | 93.51 (12.81) | 98.99 (15.26) | t=3.88, df=474, P=0.000 |
| Physical functioning | |||
| No. of chronic diseases, mean (s.d.) | 2.09 (1.44) | 1.47 (1.11) | t=−4.39, df=476, P=0.000 |
| Cognitive functioning (MMSE), mean (s.d.) | 27.69 (2.00) | 28.32 (1.58) | t=3.08, df=476, P=0.002 |
| Renal function (eGFR, ml min−1), mean (s.d.) | 75.1 (18.04) | 78.8 (16.67) | t=1.99, df=477, P=0.048 |
Abbreviations: eGFR, estimated glomerulate filtration rate; MMSE, Mini Mental State Examination.
The distribution of the 25-(OH) vitamin D3 levels over the five predefined categories showed that among depressed older persons no one had a severe deficiency (<10 nmol l−1 or 4.0 ng ml−1), 36 (10.1%) a deficiency (10–24 nmol l−1 or 4.0–10.0 ng ml−1), 149 (42.0%) insufficiency (25–49 nmol l−1 or 10.0–20.0 ng ml−1), 127 (35.8%) hypovitaminosis D (50–79 nmol l−1 or 20.0–32.0 ng ml−1) and 43 (12.1%) had optimal levels (⩾80 nmol l−1 or 32.0 ng ml−1). Cross tabulation showed that the distribution of the five categories was significantly different between the depressed group versus the control group (χ2=16.3, df=3, P=0.001) with a higher frequency of the lower categories in the depressed group.
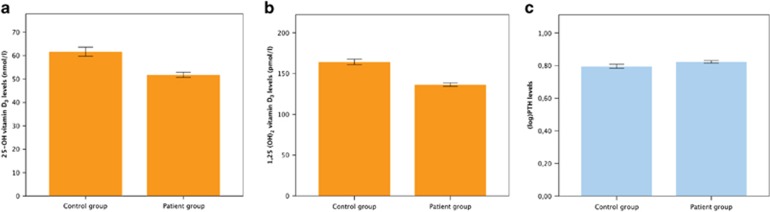
As shown in Table 2, mean (s.d.) levels of 25-(OH) vitamin D3 as well as 1,25-(OH)2 vitamin D3 differed significantly between depressed and non-depressed older persons, whereas PTH levels did not. After adjustment for potential confounders (see notes in Table 2), the differences in vitamin D levels remained significant (see Table 2 and Figure 1). Moreover, results did not change substantially after additional correction for PTH levels: the estimated marginal means (s.e.) for depressed and non-depressed persons were 53.0 (1.2) and 58.2 (2.1) nmol l−1 for 25-OH vitamin D3 (F=4.2; df=1,433; P=0.040) and 137.9 (2.4) and 160.0 (4.4) pmol l−1 for 1,25-(OH)2 vitamin D3 (F=17.6; df=1,433; P=0.039), respectively.
Table 2. Unadjusted and adjusted mean (s.e.) vitamin D and PTH levels by depression status.
| Variable | Depressed patients (n=355) | Non-depressed controls (n=124) | Statistics | Cohen's d (95% CI) |
|---|---|---|---|---|
| Unadjusted analyses | ||||
| 25-OH-vitamin D3 (nmol l−1) | 51.9 (1.2) | 62.0 (2.1) | F=17.66, df=1, P<0.001 | 0.45 (0.24–0.67) |
| 1,25-diOH-vitamin D3 (pmol l−1) | 136.7 (2.5) | 163.7 (3.9) | F=31.27, df=1, P<0.001 | 0.61 (0.39–0.83) |
| Parathormone (PTH) (pmol l−1)a | 0.82 (0.19) | 0.80 (0.19) | F=1.55, df=1, P=0.213 | Not relevant |
| Adjusted for covariatesb | ||||
| 25-OH vitamin D3 (nmol l−1) | 52.9 (1.2) | 58.3 (2.1) | F=4.59, df=1, P=0.033 | 0.28 (0.07–0.49) |
| 1,25-(OH)2 vitamin D3 (pmol l−1) | 138.0 (2.5) | 159.6 (4.4) | F=16.93, df=1, P<0.001 | 0.48 (0.27–0.70) |
| Parathormone (PTH) (pmol l−1)a | 0.82 (0.10) | 0.81 (0.18) | F=0.181, df=1, P=0.670 | Not relevant |
Abbreviation: CI, confidence interval.
Analyses based on log-transformed values.
Adjusted for age, sex, educational level, season of measurement, use of alcohol, smoking, physical activity, waist circumference, number of chronic diseases, cognitive functioning and renal function (see Table 1 for units and/or categories of the covariates).
Figure 1.
(a–c) Adjusted marginal mean values (with error bars representing the s.e.m.) of 25-(OH) vitamin D3, 1,25-(OH)2 vitamin D3 and parathormone (PTH) levels in depressed versus non-depressed persons.
Subsequently, we checked whether we could identify characteristics of depression that were specifically associated with vitamin D levels within the depressed group. As shown in Table 3, none of the characteristics correlate with the 25-(OH) vitamin D3 levels. Only the use of antidepressants was significantly correlated with the level of 1,25-(OH)2 vitamin D3.
Table 3. Association between vitamin D levels and specific characteristics of depression by separate linear regression adjusted for confoundersa within the depressed group.
| Characteristic |
Mean (s.d.) |
Calcidiol (25-(OH)D3) |
Calcitriol (1,25-(OH)2D3) |
||||
|---|---|---|---|---|---|---|---|
| or n (%) | B (s.e.) | β | P | B (s.e.) | β | P | |
| Depression severity (IDS) | 30.1 (13.0) | −0.14 (0.10) | −0.077 | 0.174 | −0.10 (0.21) | −0.026 | 0.631 |
| IDS mood subscale | 9.0 (5.2) | −0.15 (0.25) | −0.034 | 0.540 | −0.10 (0.51) | −0.011 | 0.837 |
| IDS motivation subscale | 5.0 (3.1) | −0.25 (0.41) | −0.034 | 0.537 | −0.22 (0.83) | −0.014 | 0.789 |
| IDS somatic subscale | 9.7 (4.2) | −0.40 (0.30) | −0.071 | 0.204 | 0.03 (0.63) | 0.003 | 0.962 |
| Age of onset, continuous (years) | 48.6 (20.4) | 0.04 (0.06) | 0.032 | 0.556 | 0.14 (0.13) | 0.058 | 0.280 |
| Late onset depression (>60 years) | 117 (33.5) | −2.07 (2.63) | −0.043 | 0.432 | −2.77 (5.46) | −0.027 | 0.612 |
| Recurrent depression | 162 (45.6) | 2.02 (2.49) | 0.044 | 0.417 | 3.51 (4.97) | 0.037 | 0.480 |
| Recently remitted depression | 94 (26.5) | −3.50 (2.87) | −0.064 | 0.224 | −2.66 (5.42) | −0.03 | 0.625 |
| Double diagnosesb (y/n) | 93 (26.2) | 0.04 (0.06) | 0.032 | 0.556 | −6.79 (5.44) | −0.063 | 0.213 |
| Use of antidepressants (y/n) | 257 (72.4) | −3.40 (2.63) | −0.067 | 0.197 | −13.72 (5.31) | −0.130 | 0.010 |
| Comorbid anxiety disorder (any) | 140 (39.4) | −1.43 (2.49) | −0.031 | 0.567 | 3.55 (5.06) | 0.036 | 0.484 |
| Anxiety severity (BAI) | 17.4 (11.3) | −0.08 (0.11) | −0.040 | 0.472 | 0.15 (0.23) | 0.037 | 0.496 |
| Neuroticism | 39.0 (7.1) | −0.03 (0.18) | −0.009 | 0.867 | −0.14 (0.35) | −0.021 | 0.695 |
Abbreviations: BAI, Beck Anxiety Inventory; IDS, Inventory of Depressive Symptoms; MDD, major depressive disorder; y/n, yes/no.
Adjusted for age, sex, educational level, season of measurement, use of alcohol, smoking, physical activity, waist circumference, number of chronic diseases, cognitive functioning and renal function (see Table 1 for units and/or categories of the covariates).
MDD and dysthymia.
Of the 257/355 (72.4%) patients using antidepressants, 99 patients used a selective serotonin reuptake inhibitor, 75 patients a TCA and 100 patients another antidepressant (numbers do not add to 257 as 17 patients used antidepressants from more than one class). Further exploration of the association between antidepressant drug use and lower levels of 1,25-(OH)2 vitamin D3 showed that the impact of antidepressant drug use was driven by TCAs (see Table 4). As these findings may be confounded by indication, we additionally adjusted the analyses for the severity of depressive symptoms as well as for inversus outpatient status. These additional adjustments did not change the results.
Table 4. Association between vitamin D levels, severity of depressive symptoms and use of antidepressants in one, fully adjusted linear regression model (n=355).
| Characteristic |
Calcidiol (25-(OH)D3) |
Calcitriol (1,25-(OH)2D3) |
||||
|---|---|---|---|---|---|---|
| B (s.e.) | β | P | B (s.e.) | β | P | |
| Model 1 (n=355) | ||||||
| Use of a SSRI (1=yes) | −1.73 (2.93) | −0.03 | 0.553 | −7.84 (5.78) | −0.07 | 0.176 |
| Use of a TCA (1=yes) | −3.65 (3.37) | −0.07 | 0.280 | −31.78 (6.64) | −0.27 | <0.001 |
| Use of other antidepressants (1=yes) | −3.84 (2.89) | −0.08 | 0.184 | −2.23 (5.69) | −0.02 | 0.695 |
| Model 2 (n=355) | ||||||
| Use of a SSRI (1=yes) | −1.01 (2.95) | −0.02 | 0.732 | −7.03 (5.82) | −0.07 | 0.228 |
| Use of a TCA (1=yes) | −2.08 (3.49) | −0.04 | 0.553 | −28.13 (6.89) | −0.24 | <0.001 |
| Use of other antidepressants (1=yes) | −3.69 (2.88) | −0.07 | 0.201 | −1.79 (5.68) | −0.02 | 0.753 |
Abbreviations: eGFR, estimated glomerular filtration rate; MMSE, Mini Mental State Examination; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressants.
Model 1: Adjusted for age, sex, level of education, smoking status (yes/no), alcohol use (no, moderate, severe), physical activity (low, medium, large), waist circumference, renal function (eGFR), chronic comorbid diseases and global cognitive functioning (MMSE).
Model 2: Similar to model 1, but additionally adjusted for depressive symptom severity and inversus outpatient status.
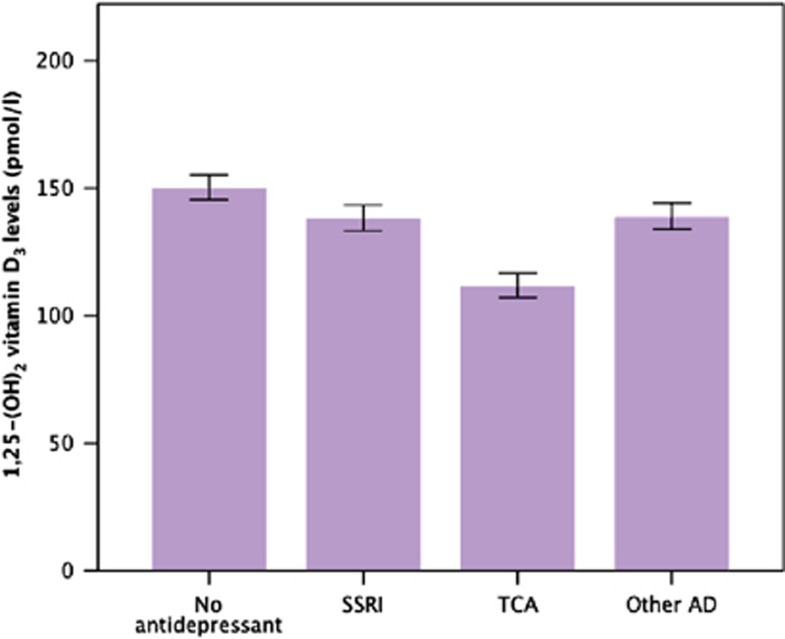
Figure 2 presents the estimated marginal means adjusted for confounders for depressed persons not using any antidepressants and those using SSRIs, TCAs or other antidepressants (after having excluded 17 persons who used two or more types of antidepressants).
Figure 2.
Marginal mean values (with error bars representing the s.e.m.) of 1,25-(OH)2 vitamin D3 levels by antidepressant class. Significant pairwise comparisons: (i) tricyclic antidepressants (TCA) versus no antidepressant: Cohen's d=0.86 (95% confidence interval (CI): 0.53–1.19; P<0.001); (ii) TCA versus selective serotonin reuptake inhibitor (SSRI): Cohen's d=0.53 (95% CI: 0.20–0.86; P=0.005); (iii) TCA versus other antidepressant: Cohen's d=0.71 (95% CI: 0.38–1.05; P=0.001); (iv) SSRI versus no antidepressant: Cohen's d=0.37 (95% CI: 0.07–0.67; P=0.027).
Discussion
Main findings
Adjusted for potential confounders, depressed older persons had significantly lower 25-OH vitamin D3 and 1,25-(OH)2 vitamin D3 compared with their non-depressed counterparts, with a small and medium effect size, respectively. Of the 355 depressed older patients, only 12.1% had optimal (>80 nmol l−1 or 32 ng ml−1) 25-OH vitamin D3 levels, whereas 52.1% had insufficient (<50 nmol l−1 or 20 ng ml−1) levels and even more than 10% a deficiency (<25 nmol l−1 or 10 ng ml−1). None of the characteristics of depression was associated with 25-(OH) vitamin D3 levels, whereas the use of antidepressants was significantly associated with lower 1,25-(OH)2 vitamin D3 levels only. In particular, persons using tricylic antidepressants (TCA) had lower 1,25-(OH)2 vitamin D3 levels, which could neither be explained by depression severity nor by an inpatient setting.
Comparison with previous findings
To our knowledge, this is the first study comparing the biologically active vitamin D form between depressed and non-depressed persons in a sufficiently large sample and adequate confounder control. One older pilot study also found lower vitamin D levels, including 1,25-(OH)2 vitamin D3 levels, in 25 depressed compared with 31 non-depressed middle-aged persons.46 Interpretation of that study is hampered, because only unadjusted results are presented and no information is given on the assays used. Interestingly, the differences between depressed and non-depressed persons were largest for the biologically active form of vitamin D, suggesting that the usually measured inactive form may underestimate the hypothesised pathophysiological role of vitamin D in late-life depression.
Our findings of lowered levels of 25-OH vitamin D3 levels in depression extends previous population-based studies showing reduced levels of 25-OH vitamin D3 levels in community-dwelling older persons with depressive symptoms or disorders,11, 12, 13, 47 and are in line with much smaller controlled46 and uncontrolled48 hospital-basedstudies.
In line with most previous studies on the association between depression and vitamin D, we did not find any association between increased PTH levels and depression.49,50 Nonetheless, some studies did find this association.11 The lack of a consistent association between depression and PTH level may point to specific effects for vitamin D in depression, but may also be simply explained by PTH being a less sensitive marker for depression compared with vitamin D, as PTH is essential for minute-to-minute regulation serum-ionised calcium, whereas vitamin D effects are more delayed.50,51
So far, three prospective studies are available, which found a two times higher risk to develop a depression when 25-OH vitamin D3 levels are below 50 nmol l−1 (refs. 52, 53, 54) and even tripled risk when levels are smaller than 15 nmol l−1.52 Nonetheless, results of intervention studies using vitamin D3 supplementation are inconsistent. Positive effects on the improvement of depressive symptoms have been reported for vitamin D3 supplements55 and for vitamin D3 as adjunct to fluoxetine,56 whereas others did not find any effect.57, 58, 59, 60
Impact of antidepressant drug use
None of the tested depression characteristics were associated with 25-OH vitamin D3 levels, whereas TCA usage was associated with lower levels of 1,25-(OH)2 vitamin D3 in depressed persons. This association seems puzzling at first glance. Post hoc analyses with additional adjustment for depression severity and an inpatient setting in order to attempt to control for confounding by indication did not explain the association.61,62 Our results therefore may point to a true effect of TCAs on 1,25-(OH)2 vitamin D3 levels. Hydroxylation of 25-OH vitamin D3 to the biologically active compound 1,25-(OH)2 vitamin D3 is catalysed by 1-α-hydroxylase (CYP27B1). 1-α-Hydroxylase is strongly expressed in the kidney and strictly regulated by calcium, PTH and phosphate levels. Interestingly, 1 α-hydroxylase is also expressed in the brain tissue, but in contrast to the kidney, not regulated by calcium-related pathways. Moreover, 1,25-(OH)2 vitamin D3 can be inactivated by 1,25-(OH)2 vitamin D3 24-hydroxylase (CYP24A1), which is also present in the brain. Vitamin D3 signalling may thus involve autocrine and paracrine pathways in the brain, as the biologically active form can be synthesised and eliminated. We hypothesise that TCAs may dampen 1-α-hydroxylase activity and induce the activity of 1,25-(OH)2 vitamin D3 24-hydroxylase.
For a detailed overview of vitamin D physiology in the brain, we refer to a recent review by Eyles et al.15 In brief, vitamin D has a neuroprotective role in the brain by reducing harmful cellular processes by functioning like an immunosuppressor, stimulating neurogenesis and clearance of amyloid beta and regulating the synthesis of neurotrophic factors, important for cell differentiation and survival.63,64 It has been hypothesised that the regulatory effect of 25-OH and 1,25-(OH)2vitamin D3 on the vitamin D receptor in the degenerating brain might fail and induce ageing mechanisms, like genomic instability, neuroendocrine dysfunction, production of oxidative compounds, altered calcium metabolism and inflammatory neuron damage.5 Whether TCAs may accelerate biological ageing mechanisms in depressed older persons through modulation of 1-α-hydroxylase and/or 24-hydroxylase activity should be addressed in future studies.
Methodological considerations
Although this study was conducted within a large and well-phenotyped cohort of depressed and non-depressed older persons, two limitations deserve attention. First, vitamin D3 levels were measured peripherally giving no information about the actual level in the brain. Nonetheless, it is assumed that the free fraction of 25-(OH) and 1,25-(OH)2 vitamin D3 freely crosses the blood–brain barrier being small and lipophilic molecules via energy-independent passive mechanisms.15 Furthermore, the free fraction of 25-OH vitamin D3 and 1,25-(OH)2 vitamin D3 enters cells via energy-independent passive mechanisms, but its level of penetration remains unknown.15 Second, owing to its cross-sectional design no causal inferences can be made, as residual confounding can never be fully excluded. Residual confounding due to ethnicity or skin tone seems unlikely as only 26 participants (7 controls, 19 patients) were born outside Western Europe, but residual confounding, for example, because of sunscreen use cannot be excluded. Similarly, we can definitely rule out that the association with TCAs depends on confounding by indication as depression severity and setting are not the sole indicators for prescribing TCAs.
Future implications
First, experimental studies should be set up to test the effect of different classes of antidepressant drugs on vitamin D metabolism. If proven, the clinical impact of antidepressant drug use on the contribution to biological ageing mechanism should be explored. Unravelling these underlying mechanisms may shed more light on mixed results of randomised controlled trials on the efficacy of vitamin D supplementation57, 58, 59, 60 with respect to the prevention and treatment of depression.
Acknowledgments
We thank the lab of Professor Dr I Kema for performing the plasma 25-(OH) vitamin D3 and the plasma 1,25-(OH)2 vitmin D3 levels. The infrastructure for the Netherlands Study of Depression in Older people is funded through the Fonds NutsOhra, Stichting tot Steun VCVGZ, NARSAD The Brain and Behaviour Research Fund and the participating universities and mental health care organisations (VU University Medical Center, Leiden University Medical Center, University Medical Center Groningen, Radboud University Nijmegen Medical Center, and GGZ inGeest, GGNet, GGZ Nijmegen, GGZ Rivierduinen, Lentis and Parnassia). MH de Borst is supported by personal development grants from the Dutch Kidney foundation (KJPB.08.07 and NIGRAM consortium), University Medical Center Groningen (Mandema stipend) and the Netherlands Organisation for Scientific Research (VENI grant), and by a consortium grant from the Dutch Kidney Foundation (NIGRAM Consortium, grant no CP10.11).
DISCLAIMER
The authors maintained full independence in the conduct of this work. The sponsors had no role in design, methods, subject recruitment, data collections, analysis or preparation of the manuscript.
AUTHOR CONTRIBUTIONS
All authors had substantial contributions to conception and design or analysis and interpretation of the data. All authors had substantial contributions to drafting the article and revising it critically for important intellectual content. All authors approved the final version.
The authors declare no conflict of interest.
References
- Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307–311. doi: 10.1192/bjp.174.4.307. [DOI] [PubMed] [Google Scholar]
- Luppa M, Sikorski C, Luck T, Ehreke L, Konnopka A, Wiese B, et al. Age- and gender-specific prevalence of depression in latest-life – Systematic review and meta-analysis. J Affect Disord. 2012;136:212–221. doi: 10.1016/j.jad.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Byers AL, Covinsky KE, Barnes DE, Yaffe K. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am J Geriatr Psychiatry. 2012;20:664–672. doi: 10.1097/JGP.0b013e31822001c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Sibille E. The age-by-disease interaction hypothesis of late-life depression. Am J Geriatr Psychiatry. 2013;21:418–432. doi: 10.1016/j.jagp.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohimaa P, Keisala T, Minasyan A, Cachat J, Kalueff A. Vitamin D, nervous system and aging. Psychoneuroendocrinology. 2009;34:S278–S286. doi: 10.1016/j.psyneuen.2009.07.003. [DOI] [PubMed] [Google Scholar]
- de Borst MH, de Boer RA, Stolk RP, Slaets JP, Wolffenbuttel BH, Navis G. Vitamin D deficiency: universal risk factor for multifactorial diseases. Curr Drug Targets. 2011;12:97–106. doi: 10.2174/138945011793591590. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Souberbielle JC. Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis. Brain. 2010;133:1869–1888. doi: 10.1093/brain/awq147. [DOI] [PubMed] [Google Scholar]
- Shardell M, D'Adamo C, Alley DE, Miller RR, Hicks GE, Milaneschi Y, et al. Serum 25-hydroxyvitamin D, transitions between frailty states, and mortality in older adults: the Invecchiare in Chianti Study. J Am Geriatr Soc. 2012;60:256–264. doi: 10.1111/j.1532-5415.2011.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, et al. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf) 2009;71:666–672. doi: 10.1111/j.1365-2265.2009.03548.x. [DOI] [PubMed] [Google Scholar]
- Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, et al. Vitamin D, parathyroid hormone, and sudden cardiac death: results from the Cardiovascular Health Study. Hypertension. 2011;58:1021–1028. doi: 10.1161/HYPERTENSIONAHA.111.179135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65:508–512. doi: 10.1001/archpsyc.65.5.508. [DOI] [PubMed] [Google Scholar]
- Hoang MT, Defina LF, Willis BL, Leonard DS, Weiner MF, Brown ES. Association between low serum 25-hydroxyvitamin D and depression in a large sample of healthy adults: the Cooper Center longitudinal study. Mayo Clin Proc. 2011;86:1050–1055. doi: 10.4065/mcp.2011.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Hirani V. Relationship between vitamin D levels and depressive symptoms in older residents from a national survey population. Psychosom Med. 2010;72:608–612. doi: 10.1097/PSY.0b013e3181e9bf15. [DOI] [PubMed] [Google Scholar]
- Verhoeven V, Vanpuyenbroeck K, Lopez-Hartmann M, Wens J, Remmen R. Walk on the sunny side of life–epidemiology of hypovitaminosis D and mental health in elderly nursing home residents. J Nutr Health Aging. 2012;16:417–420. doi: 10.1007/s12603-011-0361-5. [DOI] [PubMed] [Google Scholar]
- Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson ER. Vitamin D and the accurrence of depression: causal association or circumstantial evidence. Nutr Rev. 2009;67:481–492. doi: 10.1111/j.1753-4887.2009.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms LR, Burne TH, Eyles DW, McGrath JJ. Vitamin D and the brain. Best Pract Res Clin Endocrinol Metab. 2011;25:657–669. doi: 10.1016/j.beem.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Arabi A, El Rassi R, El-Hajj Fuleihan G. Hypovitaminosis D in developing countries-prevalence, risk factors and outcomes. Nat Rev Endocrinol. 2010;6:550–561. doi: 10.1038/nrendo.2010.146. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22:V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- Ju AY, Lee YJ, Jeong SN. Serium 25-hydroxyvitamin D levels and the risk of depression: a systematic review and meta-analysis. J Nutr Health Aging. 2013;17:447–455. doi: 10.1007/s12603-012-0418-0. [DOI] [PubMed] [Google Scholar]
- Bertone -Johnson ER, Powers SI, Spangler L, Brunner RL, Michael YL, Larson JC, et al. Vitamin D intake from foods and supplements and depressive symptoms in a diverse population of older women. Am J Clin Nutr. 2011;91:1104–1112. doi: 10.3945/ajcn.111.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapid MI, Cha SS, Takahashi PY. Vitamin D and depression in geriatric primary care patients. Clin Interv Aging. 2013;8:509–514. doi: 10.2147/CIA.S42838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer-Brolsma EM, Feskens AJM, Steegenga WT, de Groot LSPGM. Associations of 25-hydroxyvitamin D with fasting glucose, fasting insulin, dementia and depression in European elderly; the SENECA study. Eur J Nutr. 2013;52:917–925. doi: 10.1007/s00394-012-0399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven V, Vanpuyenbroeck K, Lopez-Hartmann M, Wens J, Remmen R. Walk on the sunny side of life–epidemiology of hypovitaminosis D and mental health in elderly nursing home residents. J Nutr Health Agingin. 2010;16:417–420. doi: 10.1007/s12603-011-0361-5. [DOI] [PubMed] [Google Scholar]
- Comijs HC, van Marwijk HW, van der Mast RC, Naarding P, Oude Voshaar RC, Beekman AT, et al. The Netherlands study of depression in older persons (NESDO); a prospective cohort study. BMC Res Notes. 2011;4:524. doi: 10.1186/1756-0500-4-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press: Washington, DC, USA; 2000. [Google Scholar]
- Penninx BW, Beekman AT, Smit JH, Zitman FG, Nolen WA, Spinhoven P, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17:121–140. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Hegeman JM, Wardenaar KJ, Comijs HC, de Waal MWM, Kok RM, van de Mast RC. The subscale structure of the Inventory of Depressive Symptomatology Self Report (IDS-SR) in older persons. J Psychiatr Res. 2012;46:1383–1388. doi: 10.1016/j.jpsychires.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Domains and facets: hierarchical personality assessment using the revised NEO personality inventory. J Pers Assess. 1995;64:21–50. doi: 10.1207/s15327752jpa6401_2. [DOI] [PubMed] [Google Scholar]
- World Health Organization Collaborating Centre for Drug Statistics Methodology . Anatomical Therapeutic Chemical (ATC) Classification http://www.whocc.no/atcddd 2010
- Doorenbos CR, de Cuba MM, Vogt L, Kema IP, van den Born J, Gans RO, et al. Antiproteinuric treatment reduces urinary loss of vitamin D-binding protein but does not affect vitamin D status in patients with chronic kidney disease. J Steroid Biochem Mol Biol. 2012;128:56–61. doi: 10.1016/j.jsbmb.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Baia LC, Humalda JK, Vervloet MG, Navis G, Bakker SJL, De Borst MH. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin J Am Soc Nephrol. 2013;8:1968–1978. doi: 10.2215/CJN.01880213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- Vieth R. What is the optimal vitamin D status for health. Prog Biophys Mol Biol. 2006;92:26–32. doi: 10.1016/j.pbiomolbio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Kriegsman DM, Penninx BW, van Eijk JT, Boeke AJ, Deeg DJ. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients' self-reports and on determinants of inaccuracy. J Clin Epidemiol. 1996;49:1407–1417. doi: 10.1016/s0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. ‘Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates: Mahwah, NJ, USA; 1988. [Google Scholar]
- Schneider B, Weber B, Frensch A, Stein J, Fritz J. Vitamin D in schizophrenia, major depression and alcoholism. J Neural Transm. 2000;107:839–842. doi: 10.1007/s007020070063. [DOI] [PubMed] [Google Scholar]
- Jaddou HY, Batieha AM, Khader YS, Kanaan SH, El-Khateeb MS, Ajlouni KM. Depression is associated with low levels of 25-hydroxy vitamin D among Jordanian adults: results from a national population survey. Eur Arch Psychiatry Clin Neurosci. 2012;262:321–327. doi: 10.1007/s00406-011-0265-8. [DOI] [PubMed] [Google Scholar]
- Marijnissen RM, Derks WJ, Gaasbeek AB, Stalpers-Konijnenburg SC, Oude Voshaar RC. Alarming vitamin D deficiency in older psychiatric inpatients. J Aging Res Clin Pract. 2013;2:137–141. [Google Scholar]
- Lee DM, Tajar A, O'Neill TW, O'Connor DB, Bartfai G, Boonen S, et al. Lower vitamin D levels are associated with depression among community-dwelling European men. J Psychopharmacol. 2011;25:1320–1328. doi: 10.1177/0269881110379287. [DOI] [PubMed] [Google Scholar]
- Brown EM. Four-parameter model of the sigmoidal relationship between parathyroid hormone release and extracellular calcium concentration in normal and abnormal parathyroid tissue. J Clin Endocrinol Metab. 1983;56:572. doi: 10.1210/jcem-56-3-572. [DOI] [PubMed] [Google Scholar]
- Diaz R, El-Hajj Fuleihan G, Brouwn EM. Handbook of Physiology, Section 7: The Endocrine System, Fray GGS (Ed) Oxford University Press: New York, NY, USA; 1999. Regulation of parathyroid function. [Google Scholar]
- May HT, Bair TL, Lappe DL, Anderson JL, Horne BD, Carlquist JF, et al. Association of vitamin D levels with incident depression among a general cardiovascular population. Am Heart J. 2010;159:1037–1043. doi: 10.1016/j.ahj.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Shardell M, Corsi AM, Vazzana R, Bandinelli S, Guralnik JM, et al. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. J Clin Endocrinol Metab. 2010;95:3225–3233. doi: 10.1210/jc.2010-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Hoogendijk W, Lips P, Heijboer AC, Schoevers R, van Hemert AM, et al. The association between low vitamin D and depressive disorders. Mol Psychiatry. advance online publication, 9 April 2013; doi:10.1038/mp.2013.36 (e-pub ahead of print). [DOI] [PubMed]
- Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264:599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- Khoraminya N, Tehrani-Doost M, Jazayeri S, Hosseini A, Djazayery A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust N Z J Psychiatry. 2013;47:271–275. doi: 10.1177/0004867412465022. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Jacka FN, Dodd S, Nicholson G, et al. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial. Br J Psychiatry. 2011;198:357–364. doi: 10.1192/bjp.bp.110.087544. [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Powers SI, Spangler L, Larson J, Michael YL, Millen AE, et al. Vitamin D supplementation and depression in the women's health initiative calcium and vitamin D trial. Am J Epidemiol. 2012;176:1–13. doi: 10.1093/aje/kwr482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaergaard M, Waterloo K, Wang CE, Almas B, Figenschau Y, Hutchinson MS, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201:360–368. doi: 10.1192/bjp.bp.111.104349. [DOI] [PubMed] [Google Scholar]
- Yalamanchili V, Gallagher JC. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause. 2012;19:697–703. doi: 10.1097/gme.0b013e31823bcec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IM, Nutt DJ, Deakin JFW. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2000;14:3–20. doi: 10.1177/026988110001400101. [DOI] [PubMed] [Google Scholar]
- Ruhe HG, Huyser J, Swinkels JA, Schene AH. Switching antidepressants after a first selective reuptake inhibitor in major depressive disorder. A systematic review. J Clin Psychiatry. 2006;67:1836–1855. doi: 10.4088/jcp.v67n1203. [DOI] [PubMed] [Google Scholar]
- Llewellyn DJ, Lang IA, Langa KM, Melzer D. Vitamin D and cognitive impairment in the elderly U.S. population. J Gerontol A Biol Sci Med Sci. 2011;66:59–65. doi: 10.1093/gerona/glq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens AP, Lang IA, Langa KM, Kos K, Llewellyn DJ. Vitamin D, cognitive dysfunction and dementia in older adults. CNS Drugs. 2011;25:629–639. doi: 10.2165/11593080-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]