Abstract
Positive affect (PA) has an important role in resilience against depression and has been shown to increase with mindfulness-based cognitive therapy (MBCT). To elucidate the underlying mechanisms of change in PA as well as develop insights that may benefit personalized medicine, the current study examined the contribution of genetic variation to individual differences in change in PA in response to MBCT. Individuals (n=126) with residual depressive symptoms were randomized to either an MBCT group or treatment as usual. PA was assessed using experience sampling methodology (ESM). Single-nucleotide polymorphisms (SNPs) in genes known to be involved in reward functioning were selected. SNPs in the genes for brain-derived neurotrophic factor (BDNF), the muscarinic acetylcholine receptor M2 (CHRM2), the dopamine receptor D4 (DRD4) and the μ1 opioid receptor (OPRM1) significantly moderated the impact of treatment condition over time on PA. Genetic variation in the genes for CHRM2 and OPRM1 specifically had an impact on the level of PA following MBCT. The current study shows that variation in response to MBCT may be contingent on genetic factors associated with the regulation of PA. These findings contribute to our understanding of the processes moderating response to treatment and prediction of treatment outcome.
Introduction
A stronger capacity to experience positive affect (PA) is associated with resilience against major depressive disorder (MDD)1, 2, 3, 4 as well as against general negative emotional experiences5, 6, 7 and other forms of psychopathology.8, 9, 10 Therefore, recent studies have examined to what degree non-pharmacological interventions are able to modify the experience of PA. Several therapies that aim at enhancing PA have been developed. Indeed, randomized controlled trials of mindfulness-based cognitive therapy (MBCT) and Loving Kindness Meditation show increases of positive emotional experience in a variety of samples.11, 12, 13, 14 However, the underlying mechanisms of how psychotherapy elevates PA remain unknown.
Therapygenetic approaches aim to investigate the impact of specific genetic variants on differences in the level of success of psychological therapies.15,16 Studies investigating PA have yielded heritability estimates between 30 and 50%,17,18 suggesting that genetic factors underlie, at least in part, individual differences in the ability to experience PA. It can therefore be hypothesized that heterogeneity in treatment outcome in terms of PA can be traced to genetic individual differences in biological systems regulating positive emotional experience.19,20 Investigating the role of genetic variation in treatment outcome may elucidate the underlying mechanism of change in PA and enhance a personalized medicine approach in treatment.21
Two brain reward pathways are considered to be essential for the experience of PA. First, the mesolimbic dopaminergic pathway appears to be at the heart of the brain reward system.22, 23, 24, 25 Second, the opioid pathway is also believed to be associated with reward.26, 27, 28 The opioid system strongly influences dopaminergic reward circuitry as the latter is heavily innervated by endogenous opioid peptide (endorphin and enkephalin) circuits.29,30 As the brain reward system depends on dopamine and opioid neurotransmission in mesolimbic and frontal areas, we hypothesize that genetic variations of genes coding for dopamine and opioid regulation may contribute to individual differences in the impact of MBCT on positive affective experiences.
Psychotherapy–genetic studies, as opposed to pharmacogenetic studies, constitute a relatively novel area of research that needs further exploration.31 Furthermore, recommendations for gene–environment interaction studies (G × E) suggest that improvements in the chosen outcome measurement—using intermediate phenotypes instead of clinical outcome measures—may reduce noise and thereby inconsistencies in results.32 This is because affective disorders are heterogeneous disease classifications and specific representations are likely associated with interactions between polygenetic clusters and the environment.33 The current study is the first to relate genotype to change in an underlying intermediate phenotype—the experiential expression of PA—which, in addition to reducing noise, may furthermore shed light on the mechanisms by which genes relate to treatment outcome.33,34
To elucidate these mechanisms, the current study aimed to examine which genetic variants have an impact on MBCT outcome—that is, which genotype is associated with a larger increase in PA following MBCT (boosting effect) compared with other genotypes within several single nucleotide polymorphisms (SNPs). To investigate which SNPs had an impact on MBCT outcome, we performed a randomized controlled trial in which individuals with residual depressive symptoms were randomized to either an 8-week intensive MBCT training group or a control group, and assessed genetic profiles in relation to modification of real-world PA experience. PA was assessed prospectively, repetitively and in-the-moment using experience sampling methodology (ESM),35 thus avoiding recall bias in the assessment of affect.
Materials and methods
Participants
Participants with residual depressive symptoms and at least one prior episode of MDD were recruited through outpatient mental health-care facilities in Maastricht (The Netherlands) as well as via posters in public places. Residual symptoms were defined as a score⩾7 on the 17-item Hamilton Depression Rating Scale for Depression (HDRS36). Exclusion criteria included the following: fulfilling criteria for a current depressive episode, schizophrenia or psychotic episodes in the past year, and recent (past 4 weeks) or upcoming changes in on-going psychological or pharmacological treatment. Currently depressed individuals were excluded since, at trial preparation, no evidence existed that currently depressed individuals were able to participate in, or benefit from, MBCT. All study procedures were approved by the Medical Ethics Committee of Maastricht University Medical Centre, and all participants signed an informed consent form. The trial was registered at the Dutch Trial Register (number NTR1084).
Procedure
Potential participants were screened by phone to check for availability during the study period along with the likelihood of meeting inclusion and exclusion criteria. During a second screening, the Structured Clinical Interview for DSM IV-Axis I37 and HDRS36 were administered by trained psychologists. All eligible participants were invited for a one-on-one explanation of the ESM procedure followed by saliva collection for DNA extraction, after which they participated in baseline assessment. The baseline assessment consisted of 6 days of ESM in the individual's own environment (see ESM below) and administration of the HDRS interview. Participants were then randomized to either the experimental (MBCT) or control (wait list) arm of the trial (for specific randomization and stratification procedures see Geschwind et al.13).
After 8 weeks of MBCT (see Intervention section), or equivalent waiting time, participants once more participated in a 6-day ESM assessment phase and administration of the HDRS. Assessment periods of control participants were matched to those of MBCT participants. All participants received compensation in the form of gift vouchers worth 50 euros. Participants in the control condition were given the opportunity to participate in an MBCT training after post-intervention assessment.
Intervention
The content of the MBCT training sessions followed the protocol of Segal et al.38 The training consisted of eight weekly sessions of 2.5 h each, involving groups of 10–15 participants. Sessions included guided meditation, experiential exercises and discussion. In addition to the weekly group sessions, participants received CDs with guided exercises and were assigned homework exercises (of 30–60 min daily). Sessions were taught by experienced trainers in a centre specialized in mindfulness trainings. Trainers were supervised by an experienced health-care professional who trained with Teasdale and Williams (co-developers of MBCT).39
ESM
ESM is a momentary assessment method to assess participants in their daily living environments, thus providing repeated in-the-moment assessments of affect in a prospective and ecologically valid manner with several advantages over retrospective questionnaires.40 In the current study, participants received both a digital wristwatch and a set of ESM self-assessment forms, the latter organized in six booklets—one for each day. The wristwatch was programmed to emit a signal (‘beep') at unpredictable moments, but certainly once every 90 min between 0730 and 2230 hours, on six consecutive days, resulting in 60 beeps per assessment period. After each beep, participants were asked to fill out the ESM self-assessment forms. The forms included reports on current mood and context, all given on a 7-point Likert scale. All reports that were not filled out within 15 min after the beep, and all participants who were with less than 20 valid reports at baseline, were excluded from the analysis because these have been shown to be less reliable.35
Measures
PA
At each beep, several ESM mood adjectives were assessed on 7-point Likert scales ranging from 1 (not at all) to 7 (very). Adjectives were selected based on previous experience of our research group within this field41,42 in combination with the following considerations: they should reflect state (rather than trait) measures and should show intra-individual variation. Items should, furthermore, preferably load on the same latent factor (PA). Consistent with previous work,3,13 principal component analysis with oblique rotation was used to generate a factor representing PA. The mood adjectives ‘happy', ‘satisfied', ‘strong', ‘enthusiastic', ‘curious', ‘cheerful', and ‘inspired' loaded on the PA factor. The mean levels of PA were then computed by averaging the above items per participant and beep moment, yielding a total of 11 513 PA observations in this sample.
17-Item Hamilton Depression Rating Scale
The HDRS36 was administered by two trained research assistants with master degrees in psychology. The HDRS is a semistructured interview designed to assess depressive symptoms over the past week. Internal, interrater and retest reliability estimates for the overall HDRS are good.43
Genotyping
Genomic DNA was obtained from saliva samples. Saliva was collected in Oragene-DNA Self Collection Kits (DNA Genotek, Ottawa, Ontario, Canada), and DNA was isolated using the AutoGenFlex DNA isolation system (Autgen, Hilliston, MA, USA) according to the manufacturer's instructions. SNPs were determined with Sequenom (Hamburg, Germany) using the Sequenom MassARRAY iPLEX platform at the facilities of the manufacturer. SNPs with a call rate of lower than 90% or that show violation of Hardy–Weinberg equilibrium (GENHWI command in STATA 12.1,44 P<0.01) were excluded from analyses.
A selection of genetic variations (see Table 1) under study was made based on (i) their involvement in dopamine and/or opioid regulation and/or (ii) previous literature showing associations between the selected variations and mental disorders associated with a deficient reward system, such as MDD.45, 46, 47, 48, 49, 50, 51, 52, 53 Of the selected 32 SNPs,
Three SNPs could not be included in any of the multiplex assays due to neighbouring SNPs or overlapping sequences (rs11030103 and rs568201086 in the brain-derived neurotrophic factor (BDNF) gene and rs1799732 in the dopamine receptor D2 (DRD2) gene);
One SNP was excluded because according information from the Database of Single Nucleotide Polymorphisms (dbSNP; http://www.ncbi.nlm.nih.gov/SNP/) its variation was suspected to be false positive due to artifacts of the presence of paralogous sequence in the genome or because evidence suggested sequencing error or computation artifacts (rs28722151 in BDNF);
One SNP was excluded because it was a multivariate SNP which was unknown at the time of genotyping (rs1799836 in the monoamine oxidase B (MAO-B) gene);
Three SNPs were excluded because they showed no, or minimal, variation: rs12273539 and rs57083135 in BDNF; rs6267 in the catechol-O-methyltransferase (COMT) gene);
One SNP was excluded because of genotyping failure (that is, call rate <0.90; rs747302 in the dopamine receptor D4 (DRD4) gene).
Table 1. Overview of selected SNPs.
| SNP | Genotypes (n) or reason of exclusion | Gene | Location | Function | Alleles | Alternative name(s) | Inclusion reference | Callrate | HW χ2 |
|---|---|---|---|---|---|---|---|---|---|
| rs6265 | G/G (78); A/G (43); A/A (5) | BDNF | 11p13 | Missense | G/A | Val66Met | Licinio et al.44 | 1.00 | 0.262 |
| rs11030101 | A/A (36); T/A (63); T/T (27) | BDNF | 11p13 | UTR-5 | A/T | Licinio et al.44 | 1.00 | 0.109 | |
| rs11030102 | C/C (76); G/C (43); G/G (7) | BDNF | 11p13 | NearGene-5 | C/G | Licinio et al.44 | 1.00 | 0.004 | |
| rs11030103 | Design failure | BDNF | 11p13 | NearGene-5 | G/A | Licinio et al.44 | / | / | |
| rs12273539 | No variation | BDNF | 11p13 | Intronic | T/C | Licinio et al.44 | 1.00 | / | |
| rs28722151 | *Suspected* | BDNF | 11p13 | UTR-5 | C/G | Licinio et al.44 | / | / | |
| rs56820186 | Design failure | BDNF | 11p13 | UTR-3 | G/T | Licinio et al.44 | / | / | |
| rs57083135 | Too little variation | BDNF | 11p13 | NearGene-5 | C/T | Licinio et al.44 | 1.00 | 0.031 | |
| rs4633 | C/C (31); C/T (63); T/T (32) | COMT | 22q11.21 | Synonymous | C/T | Diatschenko et al.51 | 1.00 | 0.009 | |
| rs4680 | G/G (31); G/A (63); A/A (32) | COMT | 22q11.21 | Missense | G/A | Val158Met | Diatschenko et al.51 | 1.00 | 0.009 |
| rs4818 | C/C (41); C/G (64); G/G (21) | COMT | 22q11.21 | Synonymous | C/G | Diatschenko et al.51 | 1.00 | 0.362 | |
| rs6267 | No variation | COMT | 22q11.21 | Missense | G/T | Ala72Ser | Diatschenko et al.51 | 1.00 | / |
| rs6269 | A/A (39); A/G (65); G/G (22) | COMT | 22q11.21 | NearGene-5 | A/G | Diatschenko et al.51 | 1.00 | 0.479 | |
| rs324650 | A/A (36); T/A (68); T/T (22) | CHRM2 | 7q31-q35 | Intronic | A/T | Wang et al.45 | 1.00 | 0.762 | |
| rs1824024 | T/T (56); G/T (50); G/G (20) | CHRM2 | 7q31-q35 | Intronic | G/T | Wang et al.45 | 1.00 | 1.649 | |
| rs2061174 | T/T (58); T/C (51); C/C (17) | CHRM2 | 7q31-q35 | Intronic | C/T | Wang et al.45 | 1.00 | 0.717 | |
| rs6276 | A/A (63); G/A (54); G/G (9) | DRD2 | 11q23 | UTR-3 | A/G | A1385G | Kraschewski et al.46 | 1.00 | 0.188 |
| rs6277 | T/T (38); C/T (63); C/C (25) | DRD2 | 11q23 | Synonymous | C/T | C957T | Kraschewski et al.46 | 1.00 | 0.002 |
| rs1799732 | Design failure | DRD2 | 11q23 | NearGene-5 | −/C | −141C del | Kraschewski et al.46 | / | / |
| rs747302 | Genotyping failure | DRD4 | 11p15.5 | NearGene-5 | C/G | C616G | Ben Zion et al.43 | 0.06 | / |
| rs936461 | G/G (58); G/A (53); A/A (15) | DRD4 | 11p15.5 | NearGene-5 | A/G | A809G | Ben Zion et al.43 | 1.00 | 0.048 |
| rs1800955 | Too little variation | DRD4 | 11p15.5 | NearGene-5 | C/T | C521T | Ben Zion et al.43 | 0.93 | 5.165 |
| rs1799836 | Multivariable SNP | MAO-B | Xp11.23 | Intronic | A/G | B-SNP 13 | Balciuniene et al.47 | / | / |
| rs495491 | T/T (65); T/C (48); C/C (13) | OPRM1 | 6q24-q25 | Intronic | C/T | Zhang et al.48 | 1.00 | 0.938 | |
| rs609148 | C/C (75); C/T (44); T/T (7) | OPRM1 | 6q24-q25 | Intronic | C/T | Zhang et al.48 | 1.00 | 0.068 | |
| rs648893 | T/T (75); C/T (44); C/C (7) | OPRM1 | 6q24-q25 | Intronic | C/T | Zhang et al.48 | 1.00 | 0.068 | |
| rs1799971 | Too little variation | OPRM1 | 6q24-q25 | Missense | A/G | Asn40Asp 118 A/G | Zhang et al.48 | 1.00 | 0.004 |
| rs3823010 | A/A (83); A/G (37); G/G (6) | OPRM1 | 6q24-q25 | Intronic | A/G | Zhang et al.48 | 1.00 | 0.484 | |
| rs563649 | Too little variation | OPRM1 | 6q24-q25 | UTR-5 | A/G | Shabalina et al.49 | 1.00 | 0.038 | |
| rs6347 | A/A (68); A/G (49); G/G (9) | SLC6A3 | 5p15.3 | Synonymous | A/G | Ex2+159 C>T | Azzato et al.50 | 1.00 | 0.006 |
| rs6350 | Too little variation | SLC6A3 | 5p15.3 | Synonymous | C/T | −3714G>T | Azzato et al.50 | 1.00 | 0.607 |
| rs6413429 | Too little variation | SLC6A3 | 5p15.3 | NearGene-5 | G/T | Ex9–55A>G | Azzato et al.50 | 1.00 | 0.853 |
Abbreviations: BDNF, brain-derived neurotrophic factor; CHRM2, cholinergic receptor muscarinic 2; COMT, catechol-O-methyltransferase; DRD2, dopamine receptor D2; DRD4, dopamine receptor D4; ESM, experience sampling methodology; OPRM1, μ1 opioid receptor; SLC6A3, dopamine transporter; SNP, single nucleotide length polymorphism; UTR, untranslated repeat. Note: call rates and X2 values of Hardy–Weinberg (HWχ2) equilibrium (both calculated on n=131; including five participants without ESM data) are indicated. SNPs with a callrate <0.90 (genotyping failure); no or too little variation (two or more genotypes with less than 30 subjects); or a X2 value above 10.83 (marked with *, corresponding with a P-value <0.001) were excluded from analyses.
We furthermore decided not to run genetic analyses on genotypes present in just a small proportion of the sample. We used the cutoff of n=30 as the minimum number of individuals for a genotype to be included in the analyses. Owing to this, five further SNPs were excluded (rs 1800955 in DRD4, rs1799971 and rs563649 in the μ1 opioid receptor (OPRM1) gene, and rs 6350 and rs6413429 in the dopamine transporter (SLC6A3) gene), resulting in a final set of 18 SNPs. An overview is provided in Table 1. Table 2 shows the correlations between SNPs, indicating that the effects of SNPs are mostly independent. Of the SNPs that did correlate highly (that is, r⩾0.70) only one SNP was analysed to avoid finding several significant effects that actually come down to one single effect as well as to avoid overcorrecting for multiple testing problems. This decision influenced the analyses of the following SNPs: rs4633, rs4680, rs4818, rs6269 in COMT (all rs⩾0.85); rs1824024 and rs206174 in CHRM2 (r=0.86); rs6276 and rs6277 in DRD2 (r=0.71); and rs495491 and rs3823010 in OPRM1 (r=0.79). For the COMT gene, only the most well-known functional SNP (rs4680) was analysed. From the other highly correlated SNPs only the one with the lowest rs number was analysed. All in all 14 SNPs were analysed. SNPs were coded in 0,1,2 format with 0 being the most frequent homozygous genotype unless it involved a functional SNP, of which the non-risk allele (according to dbSNP) was coded 0.
Table 2. Correlation coefficients between SNPs.
| rs6265 | rs11030101 | rs11030102 | rs4633 | rs4680 | rs4818 | rs6269 | rs324650 | rs1824024 | rs2061174 | rs6276 | rs6277 | rs936461 | rs495491 | rs609148 | rs648893 | rs3823010 | rs6347 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs6265 | 1.00 | |||||||||||||||||
| rs11030101 | −0.44 | 1.00 | ||||||||||||||||
| rs11030102 | −0.29 | −0.54 | 1.00 | |||||||||||||||
| rs4633 | −0.10 | 0.10 | −0.08 | 1.00 | ||||||||||||||
| rs4680 | −0.10 | 0.10 | −0.08 | 1.00 | 1.00 | |||||||||||||
| rs4818 | 0.07 | −0.11 | 0.08 | −0.85 | −0.85 | 1.00 | ||||||||||||
| rs6269 | 0.08 | −0.12 | 0.09 | −0.89 | −0.89 | 0.98 | 1.00 | |||||||||||
| rs324650 | −0.01 | 0.07 | −0.11 | 0.13 | 0.13 | −0.08 | −0.11 | 1.00 | ||||||||||
| rs1824024 | 0.02 | −0.03 | −0.09 | −0.02 | −0.02 | 0.06 | 0.06 | 0.39 | 1.00 | |||||||||
| rs2061174 | 0.06 | −0.02 | −0.14 | −0.03 | −0.03 | 0.05 | 0.05 | 0.41 | 0.86 | 1.00 | ||||||||
| rs6276 | −0.14 | −0.02 | 0.19 | 0.14 | 0.14 | −0.11 | −0.14 | −0.03 | −0.10 | −0.12 | 1.00 | |||||||
| rs6277 | −0.04 | −0.05 | 0.15 | 0.14 | 0.14 | −0.07 | −0.08 | −0.05 | −0.07 | −0.10 | 0.71 | 1.00 | ||||||
| rs936461 | −0.16 | 0.09 | −0.04 | 0.03 | 0.03 | 0.03 | 0.03 | 0.16 | 0.08 | 0.07 | 0.19 | 0.17 | 1.00 | |||||
| rs495491 | 0.04 | 0.01 | −0.05 | −0.03 | −0.03 | −0.02 | 0.00 | −0.06 | 0.02 | −0.03 | −0.06 | −0.05 | −0.13 | 1.00 | ||||
| rs609148 | −0.06 | 0.03 | 0.01 | −0.01 | −0.01 | 0.00 | 0.01 | −0.04 | −0.15 | −0.17 | −0.01 | −0.12 | −0.09 | −0.31 | 1.00 | |||
| rs648893 | −0.06 | 0.03 | 0.01 | −0.01 | −0.01 | 0.00 | 0.01 | −0.04 | −0.15 | −0.17 | −0.01 | −0.12 | −0.09 | −0.31 | 1.00 | 1.00 | ||
| rs3823010 | 0.09 | 0.09 | −0.06 | −0.12 | −0.12 | −0.01 | 0.02 | −0.06 | 0.04 | 0.02 | −0.06 | −0.07 | −0.16 | 0.79 | −0.25 | −0.25 | 1.00 | |
| rs6347 | 0.00 | −0.19 | 0.20 | −0.04 | −0.04 | 0.06 | 0.05 | 0.01 | −0.01 | −0.02 | −0.01 | −0.01 | −0.13 | −0.13 | 0.16 | 0.16 | −0.15 | 1.00 |
Note: The size of the Pearson correlation coefficients (that is, small, medium or strong correlation) are indicated as follows: r =0.1–0.3 (small, shown in italics), r =0.3–0.5 (medium, shown as underlined) and r =0.5–1.0 (strong, shown in bold).
Statistical methods
ESM data have a hierarchical structure. Thus, multiple observations (Level 1) are clustered within participants (Level 2). Multilevel analyses take the variability associated with each level of nesting into account.54 The XTMIXED command in STATA 12.144 was used to perform multilevel linear regression analyses. The large amount of observations of the outcome measure per participant increases the effective sample size.55,56 Owing to the multilevel structure of the data, the effective sample size depends on the size of the ICC. For the current data, the ICC is around 0.07, implying that the effective sample size is about 11 513/(1+0.07 × 99)=1451.
To test whether the effect of MBCT on PA depends on genotype, fourteen three-way interactions between SNP, time (baseline vs post assessment) and group (control vs MBCT) on PA were performed, yielding a total of 16 analyses (three analyses for the SNPs rs4633/rs4680, as all three genotypes were included for analysis, and one analysis per SNP for the other SNPs). The MARGINS command44 was used to calculate estimated marginal means to plot PA levels at baseline and post assessment per combination of genotype and group. For all the SNPs that returned a significant interaction effect, the TEST command, which uses a Wald test,57 was used to analyse whether (and if so, in which direction) the change in PA within a group differed per genotype.
All analyses were controlled for age and gender. Furthermore, to avoid between-person differences in average set-point of the subjective ratings, analyses were corrected for individual baseline average of PA. In addition, all multilevel models included a random intercept, covariance was set to unstructured. To correct for multiple testing problems, Holm's procedure58,59 was used. Finally, in order to graphically display the clinical effect associated with the impact of genotype on PA experience, differences in change in HDRS scores as a function of genotype and treatment arm were shown for each significant finding.
Results
Participants
At baseline, there were no significant differences between treatment groups with respect to sociodemographic and clinical characteristics: age, gender, full-/part-time work, illness/unemployment benefits, living with partner/own family, HDRS score, comorbid past/present anxiety disorder, current use of antidepressants or benzodiazepines (all P-values >0.05). Furthermore, MBCT compared with CONTROL was associated with significant increases in PA (for details of sociodemographic and clinical characteristics as well as PA levels per group and Participants flow see Geschwind et al.13). Of the 130 subjects included, one subject had to be excluded from analysis for not meeting the prespecified number of experience sampling entries for baseline assessment, and three subjects were excluded due to low call rate (that is, call rate <0.90), resulting in a final sample of 126 participants.
Gene x intervention interactions
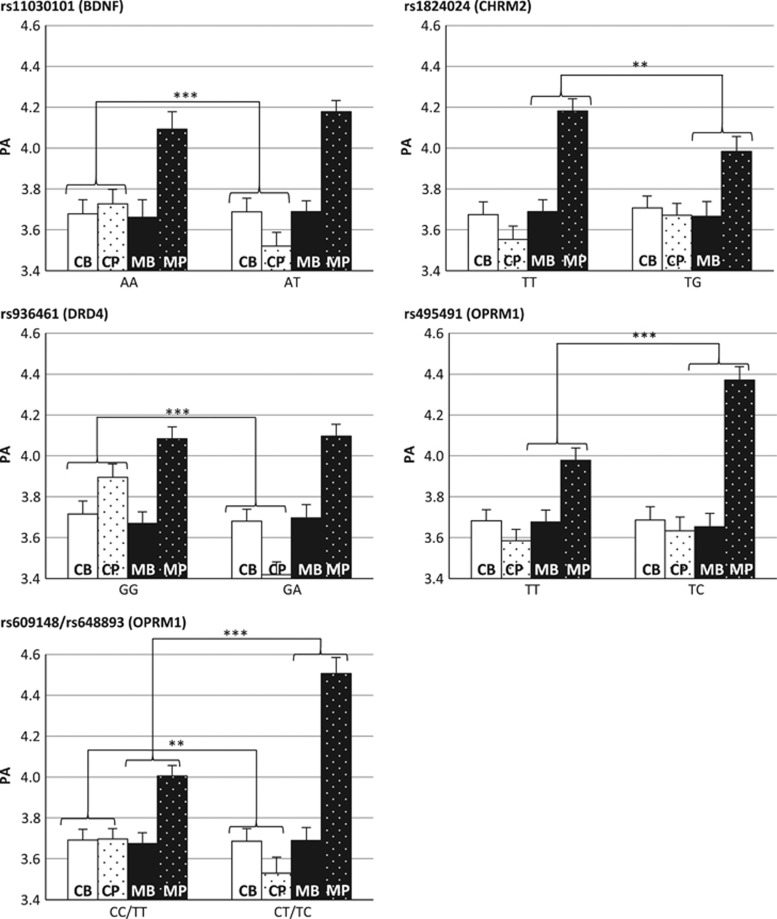
PA levels did not differ per genotype at baseline (main effect of SNP on PA; all P-values >0.05). When correcting for multiple testing with Holm's method,58,59 5 of the 16 SNP × time(baseline−post) × group(MBCT−control) analyses were significant (see Table 3). These were SNPs in BDNF, the muscarinic acetylcholine receptor M2 (CHRM2) gene, DRD4 and OPRM1. For each of these significant results, two follow-up analyses (to further examine how change in PA within a group differed per genotype) were performed (see Figure 1).
Table 3. Unstandardized effect sizes (s.e.) of the group × time × SNP interaction and their P-values.
| SNP | Gene | Effect size (s.e.) | P-value |
|---|---|---|---|
| rs6265 | BDNF | −0.067 (0.082) | 0.415 |
| rs11030101 | BDNF | 0.274 (0.089) | 0.002a |
| rs11030102 | BDNF | 0.128 (0.081) | 0.112 |
| rs4680b | COMT | (a) 0.204 (0.097) (b) −0.049 (0.110) (c) −0.254 (0.092) | 0.0360.6560.006 |
| rs4818 | COMT | −0.063 (0.085) | 0.461 |
| rs6269 | COMT | −0.104 (0.086) | 0.229 |
| rs324650 | CHRM2 | −0.206 (0.087) | 0.018 |
| rs1824024c | CHRM2 | −0.259 (0.083) | 0.002a |
| rs6276 | DRD2 | −0.206 (0.079) | 0.010 |
| rs6277 | DRD2 | −0.156 (0.090) | 0.082 |
| rs936461 | DRD4 | 0.426 (0.082) | 0.000a |
| rs495491d | OPRM1 | 0.372 (0.081) | 0.000a |
| rs609148e | OPRM1 | 0.647 (0.083) | 0.000a |
| rs6347 | SLC6A3 | −0.209 (0.081) | 0.009 |
Abbreviations: BDNF, brain-derived neurotrophic factor; CHRM2, cholinergic receptor muscarinic 2; COMT, catechol-O-methyltransferase; DRD2, dopamine receptor D2; DRD4, dopamine receptor D4; OPRM1, μ1 opioid receptor; SLC6A3, dopamine transporter; SNP, single nucleotide length polymorphism.
Note: the most common homozygotic genotype is the reference category (except for rs4680, of which the homozygote of the non-risk allele is the reference category); (a) tests group 1 to 0 (heterozygotic genotype to homozygote of non-risk allele), (b) tests group 2 to 0 (homozygote of risk-allele to homozygote of non-risk allele), (c) tests group 2 to 1 (homozygote of risk-allele to heterozygotic genotype).
Indicates a significant result, that is when P-values are ordered from smallest P1 to largest Pn, Pi ⩽α/(n−i+1), following Holm's method for correcting for multiple testing.54,55
rs4680 is perfectly correlated with rs4633 and highly correlated with rs4818 and rs6269.
rs1824024 is highly correlated with rs2061174.
rs495491 is highly correlated with rs3823010.
rs609148 is perfectly correlated with rs648893.
Figure 1.
Standardized predicted vales of PA (y axis) per combination of group (control/MBCT), assessment time (baseline/post) and SNP genotype for significant interaction effects only; controlled for the mean baseline PA, gender and age. Follow-up analyses were performed to test whether the change in PA from baseline to post intervention within a group (control/MBCT) differed per genotype: *P⩽0.05; **P⩽0.01; ***P⩽0.001. CB, baseline measurement in control group; CP, post assessment in the control group; MB, baseline measurement in the MBCT group; MP, post assessment in the MBCT group; PA, positive effect.
Prominent effects on change in PA in response to MBCT were found for SNPs in OPRM1 (rs495491, rs609148/rs648893). Compared with the reference genotype, the heterozygotic variant of these SNPs was consistently associated with larger boosts in PA from baseline to post assessment in the MBCT group. For the latter two SNPs (rs609148/rs648893), the same heterozygotic variant, compared with the reference genotype, was furthermore associated with a decrease in PA in the control group. For one SNP (rs1824024 in CHRM2), the boosting effect in PA from baseline to post assessment appeared to be greater, compared with the reference genotype, in the MBCT group with the homozygotic variant. Two more SNPs were in significant interaction with group and time: rs11030101 in BDNF and rs936461 in DRD4. However, the effect in these SNPs was driven by differences in the control group rather than the MBCT group. In the control group the heterozygotic variants of these SNPs were associated with a decrease in PA from baseline to post assessment, compared with stable or increasing PA levels from baseline to post assessment in participants with the homozygotic variant. The increase in PA in the MBCT group for these two SNPs was similar for heterozygotic and homozygotic genotypes. This indicates that people with a certain genotype would show a decrease in PA (that is, deteriorate) if left untreated, and that this deteriorating effect could be prevented with MBCT.
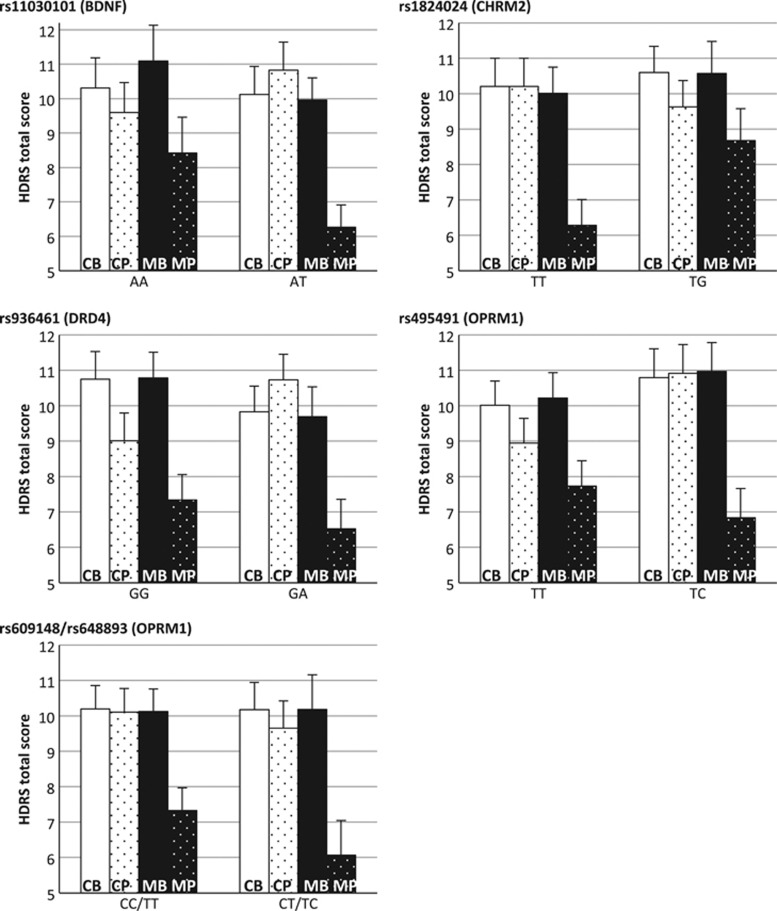
In order to visually relate these findings to clinical changes in depressive symptoms, Figure 2 shows, for each of these SNPs, the HDRS total score per combination of SNP genotype and group over time. Findings show that the difference between genotypes in change in HDRS scores is on average about 2 points. Change in HDRS scores, furthermore, generally mirrors the change in PA—that is, whenever the average PA increases in the MBCT group for a certain genotype, the average HDRS total score decreases as well.
Figure 2.
Standardized predicted vales of 17-item Hamilton Depression Rating Scale (HDRS) score (y axis) per combination of group (control/MBCT), assessment time (baseline/post) and SNP genotype; controlled for gender and age. CB, baseline measurement in control group; CP, post assessment in the control group; MB, baseline measurement in the MBCT group; MP, post assessment in the MBCT group.
A post hoc analysis was conducted to investigate whether the differential effect of the significant SNPs on PA might be mediated by differences in behavioural engagement during the MBCT training. The analysis revealed that, after correction for multiple testing, none of the SNPs significantly predicted the total amount of minutes that participants practiced. Therefore, it does not seem likely that the effect of SNPs is transferred through differences in behavioural engagement during the MBCT training.
Discussion
To the best of our knowledge, this is the first study combining assessment of DNA and ESM in a randomized controlled trial with MBCT. The study aimed at investigating the impact of SNPs on MBCT outcome in terms of PA. It was hypothesized that genetic variations in a selection of genes related to dopamine and opioid regulation and/or that have associations with mental disorders that have been linked with a deficient reward system may contribute to individual differences in the impact of MBCT on positive affective experiences. The results suggest that SNPs in CHRM2 and OPRM1 moderate the positive impact of MBCT in the sense that change in PA was on average more pronounced in participants with certain variants of these SNPs (boosting effect). An additional effect that was not hypothesized, but revealed to be associated with SNPs within BDNF and DRD4, can best be described as a deteriorating effect in the control group. The average increase in PA in participants in the MBCT group did not vary with variations within these SNPs. The MBCT intervention therefore can be conceived as counteracting a natural liability to worsen.
The impact of genetic variation in the context of previous literature
One SNP in OPRM1 that showed a boosting effect in the MBCT group additionally showed the deterioration effect in the control group, thus showing that this SNP moderates susceptibility to environmental influences in a for-better-and-for-worse manner.60 Indeed, it has been found previously that another SNP in the OPRM1 gene was associated with differential susceptibility to the environment, with one genotype making individuals simultaneously more vulnerable to the negative consequences of lower levels of maternal care (as reflected by highest levels of fearful attachment) and more responsive to the benefits of higher levels of maternal care (as reflected by lowest levels of fearful attachment).61
It is noteworthy that the interaction effect between MBCT and both analysed SNPs in the OPRM1 gene on PA turned out to be very significant, despite the fact that these SNPs were not highly correlated. Hence, the opioid system may be strongly involved in reward expression in the flow of daily life. Initially, ‘reward' was conceptualized as if it were a single psychological process or a unitary feature of a reinforcing stimulus, and dopamine in the nucleus accumbens was nominated as the ‘pleasure neurotransmitter'.62,63 However, with the separation of component processes of reward (‘liking' and ‘wanting'),64 a more complex model was debated.63,65,66 Our results coincide with recent literature showing that the perception of hedonic properties of rewards (‘liking') may be mediated more by opioids rather than dopamine.64,67
Previous literature showed that BDNF has an important role in the mesolimbic system and specific areas that are centrally involved in the regulation of reward, such as the Ventral Tegmental Area and the Nucleus Accumbens.68, 69, 70 In line with this research, the current study found a significant effect of variation in BDNF. This effect was not characterized by a boosting effect in the context of MBCT but was brought about by differential ability to maintain stable levels of PA in the waiting list control group.
The significant interaction of rs1824024 in CHRM2 in the current study is in line with a study replicating the finding of our inclusion article that this SNP was one of the most important susceptibility SNPs for alcohol dependence and affective disorders.71 Cohen-Woods et al.72 could not replicate this association in a sample of participants with recurrent unipolar depression not comorbid with substance misuse. Nevertheless, the SNP was found to be significantly associated with the severity of alcohol dependence in a Korean population,73 and more generally muscarinic receptor binding in the frontal cortex was found to be decreased in MDD subjects.74 Even though rs1824024 and CHRM2 have not been investigated extensively, their reported association with reward-related disorders is in accordance with our finding.
Significant associations have also been found between several functional polymorphisms in DRD4 genotypes and reward-associated outcomes, such as heroin addiction and schizophrenia,75 and smoking, alcohol use and food craving, as reviewed in Stice et al.76 These findings coincide with our significant result regarding variation in DRD4. However, the specific SNP in DRD4 that we found to significantly moderate the effect of MBCT on PA (rs936461) has not specifically been investigated with regard to reward.
Therapygenetics
Therapygenetics is a relatively new continuation of the more established research area of pharmacogenetics.77 Although the field is still in its infancy, the initiative of previous studies (see Lester and Eley31 for an overview of therapygenetic research in affective disorders) to extend the examination of the moderating role that genetic variations have in therapeutic outcome to non-pharmacological therapies is promising. Some of the previous work concurs in providing evidence that interactions between genetic variation and directly manipulated (positive) environmental experiences (G × E) can influence remission of mental disorder outcomes.15,78, 79, 80
Overall, however, previous therapygenetic results have been somewhat mixed.31,81 As discussed in the introduction, the specific approach in the current study aimed to continue and extend methodological developments within this field. In using PA as the main outcome measure, instead of clinical outcome measures, we directly tapped into the expression of reward, an important underlying intermediate phenotype for depression. This approach helped to reduce noise and increase power to detect G × E effects.33,34 Several significant interactions were found. Furthermore, to examine how clinically relevant the current results were, HDRS total scores were rendered visually. The finding that changes in PA indeed coincided with changes in HDRS validates and supports the current approach. Future research may adopt similar approaches to examine whether the current results will be more consistent than previous findings.
Even though it has been suggested that treatment research identifying genetic variants that predict therapeutic outcome may advance personalized treatment in psychiatry,33,82 to date no genetic predictor examined has sufficient predictive power to warrant their use as a clinical biomarker.31 This may change once there is more knowledge about specific networks of genetic variants that correlate with treatment outcome and more complicated statistical analytical methods, such as gene-network analysis83 are applied. In addition, machine-learning algorithms in which (epi-)genetic predictors are combined with clinical, demographic, neuroimaging and other predictors may be valuable to aid clinical judgement in the future. However, until all the relevant data for these options are available, it is important to systematically specify the mechanisms of recovery. The experience of PA during the flow of daily life has been shown to have a central role in the recovery of depressive symptoms.84, 85, 86 Our results add to these findings in that the effect of experiential PA in recovery seems to be associated with biological mechanisms associated with reward-processing. These biological mechanisms need further examination, as they will provide us with a deeper understanding of biological processes underlying resilience and recovery, which eventually may aid in the improvement of therapeutic interventions.
Methodological issues
One possible limitation of the current study is the relatively small number of participants for testing genetic variants. However, given that we used ESM to assess our outcome variable (PA), we could collect many observations per participant, yielding an effective sample size55 of about 1451 rather than 126, which is considerable. Nevertheless, replication of these findings in further studies is important.
Furthermore, the current study did not differentiate between high and low motivational intensity of PA items. Harmon-Jones et al.87 show that only affective states low in motivational intensity (that is, the urge to move toward/away from a stimulus) broaden cognitive scope. This suggests that only affective states low in motivational intensity could attribute to a positive upward spiral as described in the broaden-and-build theory.88 However, in the data of the current study the positive emotional states all loaded on one factor. Empirically, therefore, we have no argument to separate the two constructs in our analyses. Future research may look into what the inclusion of additional PA items such as ‘relaxed' or ‘calm' would do to the factor structure of PA as well as the effect of a potential ‘low-motivational intensity PA factor' on the results as described in the current paper.
In addition, it is important to appreciate that the homogeneity of the sample in this study imposes limitations on the generalizability of the findings. Replication in other—for example, more severely depressed—samples is required in order to determine whether the effects of SNPs is in the same direction as well as large enough to be picked up in these samples as well.
Furthermore, the current study is limited by the fact that included SNPs were selected in 2009 based on available information at that time. Research in and knowledge about genetics, however, is evolving rapidly. Therefore, we were not able to examine newly discovered SNPs with relevance to reward functioning in this paper (for example, PER2 or GABRA289,90). In addition, as the majority of our selection of SNPs is either non-coding or non-functional, biological plausibility at the level of molecular mechanisms cannot be offered. Future research can elucidate the role of genetic variations in the genes that were currently under investigation.
Lastly, the importance of gene–gene interaction (epistasis) in predisposing toward complex syndromes, such as MDD, has been emphasized previously91 and may very well account for some impact on reward-processing and changes herein following treatment. Unfortunately, this area of research is still in progress and up to now no clear a priori hypotheses for specific gene–gene interactions in this respect could be formulated. Future research might be able to explicate these mechanisms.
In conclusion, the current results indicate that changes in daily life-positive affective experiences through a non-pharmacological intervention may be moderated by individual differences in biological mechanisms regulating reward. These findings contribute to our understanding of the processes underlying response to treatment.
Acknowledgments
This study was supported by the Dutch organisation for scientific research (NWO: VENI grant nr 916.76.147 to M Wichers), the Brain Foundation of The Netherlands (grant nr 2012(1)-03 to M Wichers) and by the Weijerhorst Foundation.
The authors declare no conflict of interest.
References
- Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression. Dev Psychopathol. 2005;2005:3. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Peeters F, Jacobs N, Delespaul P, Derom C, Thiery E, et al. Meeting risk with resilience: high daily life reward experience preserves mental health. Acta Psychiatr Scand. 2010;122:129–138. doi: 10.1111/j.1600-0447.2009.01525.x. [DOI] [PubMed] [Google Scholar]
- Wichers M, Peeters F, Geschwind N, Jacobs N, Simons CJP, Derom C, et al. Unveiling patterns of affective responses in daily life may improve outcome prediction in depression: a momentary assessment study. J Affect Disord. 2010;124:191–195. doi: 10.1016/j.jad.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Myin-Germeys I, Jacobs N, Peeters F, Kenis G, Derom C, et al. Evidence that moment-to-moment variation in positive emotions buffer genetic risk for depression: a momentary assessment twin study. Acta Psychiatr Scand. 2007;115:451–457. doi: 10.1111/j.1600-0447.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequalae of negative emotions. Cogn Emot. 1998;12:191–220. doi: 10.1080/026999398379718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The undoing effect of positive emotions. Motiv Emot. 2000;24:237–258. doi: 10.1023/a:1010796329158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. J Pers Soc Psychol. 2004;86:320–333. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N, van Os J, Derom C, Thiery E, Delespaul P, Wichers M. Neuroticism explained? From a non-informative vulnerability marker to informative person-context interactions in the realm of daily life. Br J Clin Psychol. 2011;50:19–32. doi: 10.1348/014466510X491397. [DOI] [PubMed] [Google Scholar]
- Ong AD, Zautra AJ, Carrington Reid M. Psychological resilience predicts decreases in pai catastrophizing through positive emotions. Psychol Aging. 2010;25:516–523. doi: 10.1037/a0019384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Fasman R, Reich JW, Harakas P, Johnson LM, Olmsted ME, et al. Fibromyalgia: evidence for deficits in positive affect regulation. Psychosom Med. 2005;67:147–155. doi: 10.1097/01.psy.0000146328.52009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, Finkel S. Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. J Pers Soc Psychol. 2008;95:1045–1062. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayner B, Esplen MJ, DeRoche P, Wong J, Bishop S, Kavanagh L, et al. A randomized controlled trial of mindfulness-based stress reduction to manage affective symptoms and improve quality of life in gay men living with HIV. J Behav Med. 2012;35:272–285. doi: 10.1007/s10865-011-9350-8. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Peeters F, Drukker M, Van Os J, Wichers M. Mindfulness training increases momentary positive emotions and reward experience in adults vulnerable to depression: a randomized controlled trial. J Consult Clin Psychol. 2011;79:618–628. doi: 10.1037/a0024595. [DOI] [PubMed] [Google Scholar]
- Spek AA, van Ham NC, Nyklíček I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randmized controlled trial. Res Dev Disabil. 2013;34:246–253. doi: 10.1016/j.ridd.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Eley TC, Hudson JL, Creswell C, Tropeano M, Lester KJ, Cooper P, et al. Therapygenetics: the 5HTTLPR and response to psychological therapy. Mol Psychiatr. 2012;17:236–237. doi: 10.1038/mp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, McGeary JE. Therapygenetics: moving towards personalized psychotherapy treatment. Trends Cogn Sci. 2012;16:11–12. doi: 10.1016/j.tics.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Blalock CL, Button TM. Sex differences in the heritability of resilience. Twin Res Hum Genet. 2008;11:12–27. doi: 10.1375/twin.11.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid M, Riemann R, Angleitner A, Borkenau P. Sociability and positive emotionality: genetic and environmental contributions to the covariation between different facets of extraversion. J Pers. 2003;71:319–346. doi: 10.1111/1467-6494.7103003. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influence responsivity of the human reward system. Proc Natl Acad Sci USA. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M, Aguilera M, Kenis G, Krabbendam L, Myin-Germeys I, Jacobs N, et al. The catechol-O-methyl transferase Val158Met polymorphism and experience of reward in the flow of daily life. Neuropsychopharmacology. 2008;33:3030–3036. doi: 10.1038/sj.npp.1301520. [DOI] [PubMed] [Google Scholar]
- Costa e Silva JA. Personalized medicine in psychiatry: new technologies and approaches. Metabolism. 2013;62:25. doi: 10.1016/j.metabol.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Arias-Carrión O, Stamelou M, Murillo-Rodríguez E, Menéndez-González M, Pöppel E. Dopaminergic reward system: a short integrative review. Int Arch Med. 2010;3:24–29. doi: 10.1186/1755-7682-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, North RA. Neurobiology of opiate abuse. Trends Pharmacol Sci. 1992;13:185–193. doi: 10.1016/0165-6147(92)90062-b. [DOI] [PubMed] [Google Scholar]
- Van Ree JM, Gerrits MAFM, Vanderschuren LJMJ. Opioids, reward and addicition: an encounter of biology, psychology and medicine. Pharmacol Rev. 1999;5:341–396. [PubMed] [Google Scholar]
- van Ree JM, Niesink RJM, Van Wolfswinkel LV, Ramsey NF, Kornet MLMW, van Furth WR, et al. Endogenous opioids and reward. Eur J Pharacol. 2000;405:89–101. doi: 10.1016/s0014-2999(00)00544-6. [DOI] [PubMed] [Google Scholar]
- Dilts RP, Kalivas PW. Autoradiographic localization of mu-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res. 1989;488:311–327. doi: 10.1016/0006-8993(89)90723-3. [DOI] [PubMed] [Google Scholar]
- Dilts RP, Kalivas PW. Autodiographic localization of delta opioid receptors within the mesocorticolimbic dopamine system using radioioidinated [2-D-penicillamine, 5-D-penicillamine] enkephalin (125I-DPDPE) Synapse. 1990;6:121–132. doi: 10.1002/syn.890060203. [DOI] [PubMed] [Google Scholar]
- Lester KJ, Eley TC. Therapygenetics: using genetic markers to predict response to psychological treatment for mood and anxiety disorders. Biol Mood Anxiety Disord. 2013;3:2045–5380. doi: 10.1186/2045-5380-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffit TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology: concepts, research strategies, and implications for research, intervention, and public understanding of genetics. Persp Psychol Sci. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Nikolova YS, Pizzagalli DA. Neurogenetics of depression: a focus on reward processing and stress sensitivity. Neurobiol Disord. 2013;52:12–23. doi: 10.1016/j.nbd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Delespaul PAEG . Assessing Schizophrenia in Daily Life: the Experience Sampling Method. Maastricht University Press: Maastricht, The Netherlands; 2005. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Willims JBW.Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, Research Version Biometrics Research, New York State Psychiatric Institute: New York, NY, USA2002 [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD.Mindfulness-based Cognitive Therapy for Depression: a new Approach to Preventing Relapse Guilford Press: New York, NY, USA,2002 [Google Scholar]
- Teasdale JD, Williams DR. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help. Behav Res Ther. 1995;33:25–39. doi: 10.1016/0005-7967(94)e0011-7. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R. Validity and reliability of the experience sapling method. J Nerv Ment Dis. 1987;175:526–536. doi: 10.1097/00005053-198709000-00004. [DOI] [PubMed] [Google Scholar]
- Wichers M, Peeters F, Rutten BP, Jacobs N, Derom C, Thiery E, et al. A time-lagged momentary assessment study on daily life physical activity and affect. Health Psychol. 2012;31:135–144. doi: 10.1037/a0025688. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. EMotional reactivity to daily life stress in psychosis. Arch Gen Psychiatr. 2001;58:1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become lead weight. Am J Psychiatr. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. StataCorp LP: College Station, TX, 2011.
- Ben Zion IZ, Tessler R, Cohen L, Lerer E, Raz Y, Bachner-Melman R, et al. Polymorphisms in the dopamine D4 receptor gene (DRD4) contribute to individual differeneces in human sexual behavior: desire, arousal and sexual function. Mol Psychiatry. 2006;11:782–786. doi: 10.1038/sj.mp.4001832. [DOI] [PubMed] [Google Scholar]
- Licinio J, Dong C, Wong M-L. Novel sequenc variations in the Brain-Derived Neurotrophic Factor gene and association with major depression and antidepresant treatment response. Arch Gen Psychiatry. 2009;66:488–497. doi: 10.1001/archgenpsychiatry.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- Kraschewski A, Reese J, Anghelescu I, Winterer G, Schmidt LG, Gallinat J, et al. Association of the dopamine D2 receptor gene with alcohol dependence: haplotypes and subgroups of alcoholics as key factors for understanding receptor function. Pharmacogenet Genomics. 2009;19:513–527. doi: 10.1097/fpc.0b013e32832d7fd3. [DOI] [PubMed] [Google Scholar]
- Balciuniene J, Emilsson L, Oreland L, Pettersson U, Jazin EE. Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet. 2002;110:1–7. doi: 10.1007/s00439-001-0652-8. [DOI] [PubMed] [Google Scholar]
- Zhang H, Luo X, Kranzler HR, Lappalainen J, Yang B-Z, Krupitsky E, et al. Association between two μ-opioid receptor gene (OPRM1) haplotype blocks and drug or alcohol dependence. Human Mol Genet. 2006;15:807–819. doi: 10.1093/hmg/ddl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina S, Zaykin DV, Gris P, Ogurtsov AY, Gauthier J, Shibata K, et al. Expansion of the human μ-opioid receptor gene architecture: Novel functional variants. Hum Mol Genet. 2008;18:1037–1051. doi: 10.1093/hmg/ddn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzato EM, Morton LM, Bergen AW, Wang SS, Chatterjee N, Kvale P, et al. SLC6A3 and body mass index in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. BMC Med Genet. 2009;10:9–17. doi: 10.1186/1471-2350-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diatschenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel Analysis: an Introduction to Basis and Advanced Multilevel Modeling. Sage: London, UK; 1999. [Google Scholar]
- Faes C, Molenberghs G, Aerts M, Verbeke G. The effective sample size and an alternative small-sample degrees-of-freedom method. Am Stat. 2009;63:389–399. [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel Analysis: an Introduction to Basic and Advanced Multilevel Modeling. Sage Publishers: London; 1999. Design effects in two-stage samples; pp. 22–24. [Google Scholar]
- Clayton D, Hill M.Wald tests Clayton D, Hill M.(eds).Statistical Models in Epidemiology Oxford Science: Oxford, England; 1993101–102. [Google Scholar]
- Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86:726–728. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: differential susceptibility to environmental influences. Curr Direct Psychol Sci. 2007;16:300–304. [Google Scholar]
- Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, Siracusano A, et al. Variation in the μ-opioid receptor gene (OPRM1) moderates the influence of early maternal care on fearful attachment. Soc Cogn Affect Neurosci. 2012;7:542–547. doi: 10.1093/scan/nsr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. The dopamine synapse and the notion of 'pleasure centers' in the brain. Trends Neurosci. 1980;3:91–95. [Google Scholar]
- Berridge K. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience. Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dual roles of dopamine in food and drug seeking: the drive-reward paradox. Biol Psychiatry. 2013;73:819–826. doi: 10.1016/j.biopsych.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S. Opioid reward ‘liking' and ‘wanting' in the nucleus accumbens. Physiol Behav. 2008;94:675–680. doi: 10.1016/j.physbeh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30:2533–2541. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;31:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case-control structured association study. Hum Mol Genet. 2005;14:2421–2434. doi: 10.1093/hmg/ddi244. [DOI] [PubMed] [Google Scholar]
- Cohen-Woods S, Gaysin D, Craddock N, Farmer A, Gray J, Gunasinghe C, et al. Depression Case Control (DeCC) Study fails to support involvement of the muscarinic acetylcholine receptor M2 (CHRM2) gene in recurrent major depressive disorder. Hum Mol Genet. 2009;18:1504–1509. doi: 10.1093/hmg/ddp051. [DOI] [PubMed] [Google Scholar]
- Jung MH, Park BL, Lee B-C, Ro Y, Park R, Shin HD, et al. Association of CHRM2 polymorphisms with severity of alcohol dependence. Genes Brain Behav. 2011;10:256–256. doi: 10.1111/j.1601-183X.2010.00663.x. [DOI] [PubMed] [Google Scholar]
- Gibbons AS, Scarr E, McLean C, Sundram S, Dean B. Decreased muscarinic receptor binding in the frontal cortex of bipolar disorder and major depressive disorder subjects. J Affect Disord. 2009;116:184–191. doi: 10.1016/j.jad.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JH, Zhu YS, Huo ZH, Sun RF, Yu B, Wang YP, et al. Association study of polymorphisms in the promoter region of DRD4 with schizophrenia, depression, and heroin addiction. Brain Res. 2010;1359:227–232. doi: 10.1016/j.brainres.2010.08.064. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Zald D, Dagher A. Dopamine-based reward circuitry responsivity, genetics, and overeating. Curr Top Behav Neurosci. 2011;6:81–93. doi: 10.1007/7854_2010_89. [DOI] [PubMed] [Google Scholar]
- Roses AD. Pharmacogenetics and the practice of medicine. Nature. 2000;405:857–865. doi: 10.1038/35015728. [DOI] [PubMed] [Google Scholar]
- Lonsdorf T, Ruck C, Bergstrom J, Andersson G, Ohman A, Lindefors N, et al. The COMTval158met polymorphism is associated with symptom relief during exposure-based cognitive-behavioral treatment in panic disorder. BMC Psychiatry. 2010;10:99. doi: 10.1186/1471-244X-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Richter J, Straube B, Hofler M, Lueken U, Gloster AT, et al. MAOA and mechanisms of panic disorder revisited: from bench to molecular psychotherapy. Mol Psychiatry. 2013;19:122–128. doi: 10.1038/mp.2012.172. [DOI] [PubMed] [Google Scholar]
- Fullana MA, Alonso P, Gratacos M, Jaurrieta N, Jimenez-Murcia S, Segalas C, et al. Variation in the BDNF Val66Met polymorphism and response to cognitive-behavior therapy in obsessive-compulsive disorder. Eur Psychiatry. 2012;27:386–390. doi: 10.1016/j.eurpsy.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Bockting CL, Mocking RJ, Lok A, Koeter MW, Schene AH. Therapygenetics: the 5HTTLPR as a biomarker for response to psychological therapy. Mol Psychiatry. 2012;3:92. doi: 10.1038/mp.2012.92. [DOI] [PubMed] [Google Scholar]
- Owen DR, Rupprecht R, Nutt DJ. Stratified medicine in psychiatry: a worrying example or new opportunity in the treatment of anxiety. J Psychopharmacol. 2013;27:119–122. doi: 10.1177/0269881112443746. [DOI] [PubMed] [Google Scholar]
- Franke L, van Bakel H, Fokkens L, de Jong ED, Egmont-Petersen M, Wijmenga C. Reconstruction of a functional human gene network, with an application for prioritizing positional candidate genes. Am J Hum Genet. 2006;78:1011–1025. doi: 10.1086/504300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Nicolson NA, Peeters F, van Os J, Barge-Schaapveld D, Wichers M. Early improvement in positive rather than negative emotion predicts remission from depression after pharmacotherapy. Eur Neuropsychopharmacol. 2011;21:241–247. doi: 10.1016/j.euroneuro.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Batink T, Peeters F, Geschwind N, van Os J, Wichers M. How does MBCT for depression work? Studying cognitive and affective mediation pathways. PLoS One. 2013;8:e72778. doi: 10.1371/journal.pone.0072778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhn P, Menne-Lothmann C, Peeters F, Nicolson NA, Jacobs J, Derom C, et al. Moment-to-moment transfer of positive emotions in daily life predicts future course of depression in both general population and patient samples. PLoS One. 2013;8:e75655. doi: 10.1371/journal.pone.0075655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable P, Price TF. The influence of affective states varying in motivational intensity on cognitive scope. Front Integr Neurosci. 2012;10:73. doi: 10.3389/fnint.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol. 2001;56:218–226. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, Almeida JR, Ferrell RE, Nimgaonkar VL, Mansour H, et al. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biol Psychiatry. 2012;7:451–457. doi: 10.1016/j.biopsych.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Heitzeg MM, Foley S, Yau WY, Majczenko K, Zubieta JK, et al. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Mol Psychiatry. 2012;17:511–519. doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorenko EL.Epistasis and the genetics of complex traits. In: Plomin R, DeFries J, Craig IW, McGuffin P (eds).Behavioural Genetics in the Postgenomic Era American Psychological Association: Washington, DC, USA; 2003247–266. [Google Scholar]