Abstract
The development of hair cells in the auditory system can be separated into steps; first, the establishment of progenitors for the sensory epithelium, and second, the differentiation of hair cells. Although the differentiation of hair cells is known to require the expression of basic helix-loop-helix transcription factor, Atoh1, the control of cell proliferation in the region of the developing cochlea that will ultimately become the sensory epithelium and the cues that initiate Atoh1 expression remain obscure. We assessed the role of Wnt/β-catenin in both steps in gain- and loss-of-function models in mice. The canonical Wnt pathway mediator, β-catenin, controls the expression of Atoh1. Knock-out of β-catenin inhibited hair-cell, as well as pillar-cell, differentiation from sensory progenitors but was not required to maintain a hair-cell fate once specified. Constitutive activation of β-catenin expanded sensory progenitors by inducing additional cell division and resulted in the differentiation of extra hair cells. Our data demonstrate that β-catenin plays a role in cell division and differentiation in the cochlear sensory epithelium.
Introduction
Hair cells are mechanosensory cells that detect sound and convert it into electrical signals. Hair cells and adjacent supporting cells constitute the hearing organ, the organ of Corti. Early in development, the otic placode develops from an ectodermal thickening. Invagination of the otic placode results in the formation of the otocyst from which the auditory and vestibular systems are formed. Canonical Wnt signaling is critical for otocyst induction from Pax2-expressing preotic placodal cells (Ohyama et al., 2006), and a balance of dorsal Wnt and ventral sonic hedgehog directs the patterning of dorsal vestibular and ventral auditory systems (Riccomagno et al., 2005); Wnt signaling then instructs vestibular organ formation in both sensory and nonsensory regions (Rakowiecki and Epstein, 2013).
After separation from the vestibular system, auditory hair cells and supporting cells develop from common sensory progenitors at mid-embryogenesis. Auditory sensory progenitors exit the cell cycle at embryonic day (E)13.5–E14.5 in a gradient from apex to base, opposite their differentiation gradient from base to apex. Hair cells first appear at the midbasal region at E14.5, expressing Atoh1, a gene whose expression is required for hair cell development, and their development progresses along the longitudinal cochlear axis toward the apex and base, and laterally from the inner to outer hair cells. The initial signal that specifies hair cells from these common progenitors is unknown; however, Wnt signaling was suggested to play a role, because Atoh1 is a direct target of the Wnt pathway (Shi et al., 2010). Lgr5, a member of the Wnt-potentiating positive-feedback regulatory loop, was found to mark inner ear stem cells, and Wnt signals had dual roles in the control of their proliferation and differentiation (Chai et al., 2012; Shi et al., 2012). In cochlear explants treated with Wnt inhibitors and activators (Jacques et al., 2012), Wnt was shown to play dual roles in both proliferation and hair-cell differentiation in the developing organ of Corti. In the present study, we use gain- and loss-of-function models to study the role of β-catenin in hair-cell differentiation in vivo. We find that β-catenin is required for hair-cell and pillar-cell differentiation from sensory progenitors and that overexpression of β-catenin expands postmitotic sensory progenitors by reactivating the cell cycle but blocks longitudinal extension in the cochlea.
Materials and Methods
Animals.
β-Cateninflox(exon3) mice (Harada et al., 1999) were generously provided by Mark Taketo (Kyoto University, Kyoto, Japan), β-cateninflox(exon2–6) mice (Brault et al., 2001) by Rolf Kemler (Max-Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany), Sox2-CreER mice (Arnold et al., 2011) by Konrad Hochedlinger (Harvard Medical School, Boston, MA), Atoh1-nGFP mice (Lumpkin et al., 2003) by Jane Johnson (University of Texas Southwestern Medical Center, Dallas, TX), and Gfi1-Cre mice (Yang et al., 2010) by Lin Gan (University of Rochester, Rochester, NY). CMV-CreER mice were obtained from The Jackson Laboratory (stock no. 004453). The Cre lines were maintained as hemizygotes. Cochlear cultures were harvested from embryonic CD-1 mice of both sexes. All mouse experiments were approved by IACUCs at Massachusetts Eye and Ear Infirmary, University of California San Diego, or Sunnybrook Research Institute.
Knock-out or constitutive expression of β-catenin in vivo.
CMV-CreER, Sox2-CreER, and Gfi1-Cre mice were mated with β-cateninflox(exon2–6) or β-cateninflox(exon3);Atoh1-nGFP mice. Female β-cateninflox/flox(exon2–6);Atoh1-nGFP mice were mated with male β-cateninflox/flox(exon2–6) mice that were hemizygous for one of the Cre alleles to generate knock-outs. Female β-cateninflox/flox(exon3);Atoh1-nGFP mice were mated with Sox2-CreER mice to generate Sox2-CreER;β-cateninflox(exon3)/+;Atoh1-nGFP mice. Littermates without Cre were used as controls. Tamoxifen was given to the pregnant mice, and they were killed at the indicated time points. One-hundred microliters EdU (10 mg/ml) was given to mice twice a day for 3 d, and tamoxifen (250 mg/kg body weight, Sigma-Aldrich) and estradiol (0.5 mg/kg body weight, Sigma-Aldrich) were given once a day for two consecutive days by intraperitoneal injection. Cochleae from embryos were dissected and processed as whole mount or section preparations. Embryos and pups were genotyped after sacrifice.
Genotyping of sensory epithelium.
Cochlear tissue was harvested by removal of the cochlear capsule, lateral wall, and spiral ganglion. Genomic DNA in 100 μl was isolated from the cochlear tissue of one mouse using the Qiagen DNeasy Blood and Tissue Kit, and 10 μl DNA was then used in PCR to detect the recombination of β-catenin exons following induction of Cre activity. The primers for β-cateninflox(exon2–6) mutants were as follows: AAG GTA GAG TGA TGA AAG TTG TT (RM41); CAC CAT GTC CTC TGT CTA TCC (RM42); TAC ACT ATT GAA TCA CAG GGA CTT (RM43) to detect β-cateninflox(exon2–6) at 324 bp, β-cateninΔexon2–6 at 500 bp, and β-catenin at 221 bp. The primers for β-cateninflox(exon3) mutants were GGT AGT GGT CCC TGC CCT TGA CAC (F1); CTA AGC TTG GCT GGA CGT AAA CTC (P85) to detect β-cateninflox(exon3) at 1200 bp, and GGT AGG TGA AGC TCA GCG CAG AGC (GF2) and ACG TGT GGC AAG TTC CGC GTC ATC C (AS5) to detect β-cateninΔexon3 at 700 bp and β-catenin at 900 bp.
Histology and immunostaining.
Antibodies used in this study were myosin VIIa (1:800, Proteus), Sox2 (1:500; Santa Cruz Biotechnology), Prox1 (1:200; Millipore Bioscience Research Reagents), E-Cad (1:500; Abcam), p75 (1:100, Millipore), jagged-1 (1:100, Santa Cruz Biotechnology), β-catenin (1:200, Sigma-Aldrich), Ki67 (1:200; Thermo Scientific), and GFP (1:1000; Invitrogen). Species-specific AlexaFluor-conjugated secondary antibodies were used for detection (1:500; Invitrogen). The immunostaining was analyzed by confocal microscopy.
Cochlear explant culture.
Cochlear explants were collected at E13.5, dissected and cultured as previously described (Dabdoub et al., 2008). For the Rspo1 experiments, three independent experiments were performed for each condition. Recombinant Rspo1 (R&D systems) was added at 5 μg/ml in 2% FBS-DMEM and replenished after 24 h. Explants were cultured for 6 d then fixed in 4% PFA for 30 min. Cell counts were taken across a 100 μm region at 25, 50, and 75% points from the base along the length of the duct. For the E-cadherin experiments, explants were grown in media containing 10% FBS along with 10 mm LiCl, as a Wnt activator. Control media contained 10 mm NaCl. Some explants were cultured in BrdU (3.5 μg/ml; BD Biosciences). Experiments consisted of at least six cochleae/condition from a minimum of three independent litters.
Quantification.
The length and width of auditory and vestibular sensory epithelium were measured using ImageJ software with the overall length determined from the hook to the apex in each sample and the number of Atoh1 or myosin VIIa-positive cells were manually counted. The expression of β-catenin and E-cadherin were determined in the immunohistochemical images, taken with a Leica SP5 confocal microscopy, using fixed intensity for control and treated or mutant samples and analyzed with ImageJ software. The average fluorescence intensity of sensory epithelium in 3000 μm2 was determined by pixel counts using ImageJ software, and the data were expressed as the mean values ± SD. All cochlear explant experiments were performed on at least six ears, and p values were calculated using the two-tailed Student's t test.
Results
β-Catenin is required for cochlear hair-cell development
Previously we found that Atoh1, a gene required for hair cell development was a downstream target of canonical Wnt/β-catenin signaling (Shi et al., 2010). β-catenin gain- and loss-of-function experiments were performed using β-cateninflox(exon2–6) and β-cateninflox(exon3) mutant mice after crossing to CMV-CreER or Sox2-CreER drivers to create double-mutants. Cre-induced recombination at the LoxP sites flanking exons 2–6 of β-catenin, which contain the transcriptional start codon, results in a β-catenin-null allele (Brault et al., 2001). β-Catenin:exon 3 encodes the GSK3 phosphorylation sites for degradation, and exon 3 is not functionally required for β-catenin transcriptional activity. Deletion of β-catenin:exon3 therefore results in increased levels of β-catenin. Cre activity was induced by administration of tamoxifen on two consecutive days. In CMV-CreER;β-cateninflox(exon2–6) mutants first administered tamoxifen at E15.5, β-catenin:exon2–6 were deleted in the auditory sensory epithelium 24 h after the first dose and reached near complete deletion at 48 h (Fig. 1A). Immunohistochemistry for β-catenin revealed a concomitant decrease in protein expression at 48 h (Fig. 1B). When Sox2-CreER was used as the driver for β-cateninflox(exon2–6) mutants, deletion of β-catenin:exon2–6 in sensory epithelium was observed 24 h after the first dose of tamoxifen at E15.5 (Fig. 1C), and expression of the protein was concomitantly decreased, with near complete loss in Sox2-expressing cells at 48 h (Fig. 1D). Similarly, β-catenin:exon3 was deleted in Sox2-CreER;β-cateninflox(exon3) sensory epithelium 24 h after the first dose of tamoxifen (Fig. 1E), and protein expression was further increased at 48 h (Fig. 1F).
Figure 1.
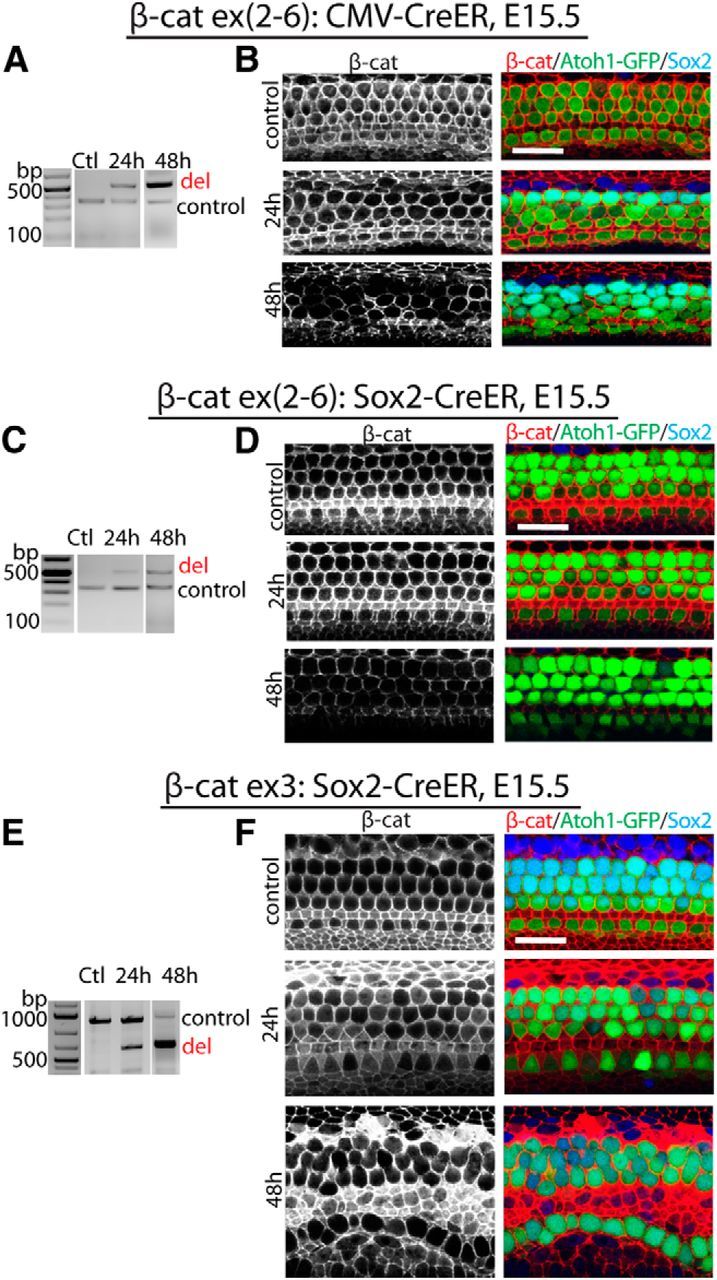
Manipulation of β-catenin expression in developing sensory epithelium. A, In CMV-CreER;β-cateninflox(exon2–6 mutants, exons 2–6 of β-catenin were deleted in sensory epithelium 24 and 48 h after the first dose of tamoxifen at E15.5. B, The decrease of β-catenin expression in sensory epithelium was at its highest point 48 h after the initial dose of tamoxifen. Hair cells were positive for GFP (compound mutants also expressed GFP under the control of Atoh1); β-catenin (red) was expressed in the entire sensory epithelium; Sox2 (blue) was expressed in supporting cells. C, In Sox2-CreER;β-cateninflox(exon2–6) mutants, exons 2–6 of β-catenin were deleted in sensory epithelium 24 and 48 h after the first dose of tamoxifen. D, β-catenin expression in sensory epithelium decreased 48 h after the first dose of tamoxifen. E, Following the initial tamoxifen injection at E15.5 in Sox2-CreER;β-cateninflox(exon3) mutants, deletion of exon 3 of β-catenin was seen at 24 and 48 h in sensory epithelium. F, A greater increase in β-catenin was apparent in sensory epithelium 48 h after the first dose of tamoxifen. The controls are littermates without Cre expression at 48 h after tamoxifen. Scale bar, 20 μm.
Hair cells first develop from postmitotic progenitors in the mid-basal region of the cochlea and express Atoh1 at E14.5, before expression of myosin VIIa. Because Cre activity was most evident 24–48 h after administration of tamoxifen in the CMV-CreER;β-cateninflox(exon2–6) embryos, we examined hair cells at E14.5 in embryos given a first dose of tamoxifen at E11.5. When examined at E14.5, cochleae with β-catenin deletion initiated at E11.5 did not contain Atoh1-expressing hair cells, whereas hair cells of the control cochlea had strong expression of Atoh1 at this time point (Fig. 2A,B). There was no apparent change in Sox2 expression in the β-catenin knock-out (Fig. 2B). Hair cells in the saccule develop 24 h before the organ of Corti and initiate expression of myosin VIIa, which is seen after Atoh1, at E14.5. Atoh1-positive hair cells could thus be seen in the saccule, but a smaller number of hair cells developed in the β-catenin deletion mutants than in Cre-negative embryos (Fig. 2C,D).
Figure 2.
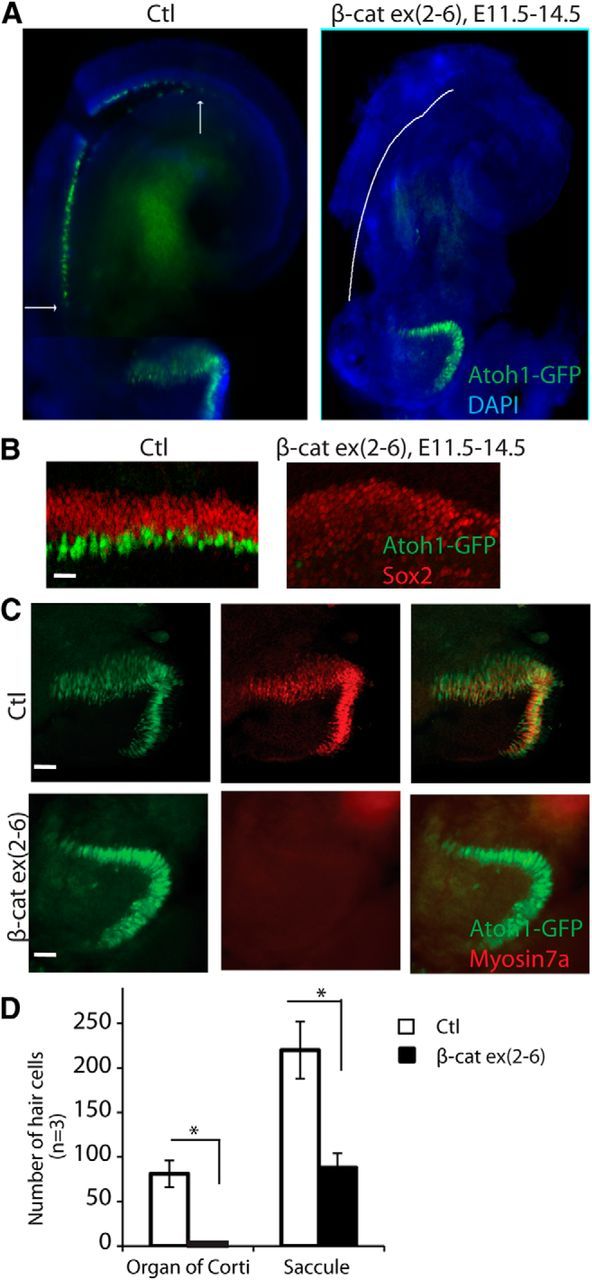
Disruption of hair cell development in β-catenin knock-out mice. A, No hair cells were evident in a cochlea analyzed at E14.5 after deletion of β-catenin at E11.5 (before hair-cell differentiation) by administration of tamoxifen to a CMV-CreER;β-cateninflox(exon2–6) embryo that also expressed nGFP under control of Atoh1. B, Hair cells were detected at E14.5 in the Cre-negative β-cateninflox(exon2–6) organ of Corti (Ctl) at the midbasal region. Cells in the prosensory domain of β-catenin knock-out ears were positive for Sox2 but did not contain Atoh1-positive cells. C, Hair cells in the saccule were Atoh1-nGFP-positive. Some were also myosin VIIa-positive in the control. The β-catenin knock-out ears had fewer Atoh1-positive cells and no myosin VIIa-positive cells. D, The number of hair cells was significantly decreased in the β-catenin mutants (p < 0.01). Scale bar, 20 μm.
β-Catenin functions in hair-cell generation and patterning during development
To examine whether inhibition of hair cell development was a direct effect of β-catenin depletion in sensory progenitors, we conditionally interrupted β-catenin function in sensory progenitors using Sox2-CreER;β-cateninflox(exon2–6) mice. Sox2 is expressed in sensory progenitors, and continues to be expressed in supporting cells but is downregulated in hair cells. Tamoxifen was initiated at E12.5 to activate Cre, and the organ of Corti was examined at E15.5. Outer hair cells differentiated lateral to inner hair cells at E15.5. When β-catenin expression was interrupted, fewer inner hair cells and no outer hair cells developed (Fig. 3A–C). Sox2 expression appeared to be normal compared with the Cre-negative littermates when β-catenin was knocked out (but the cochlea was less developed; Fig. 3B). This suggested that β-catenin was not required for maintaining progenitors in the cochlea once they were specified.
Figure 3.
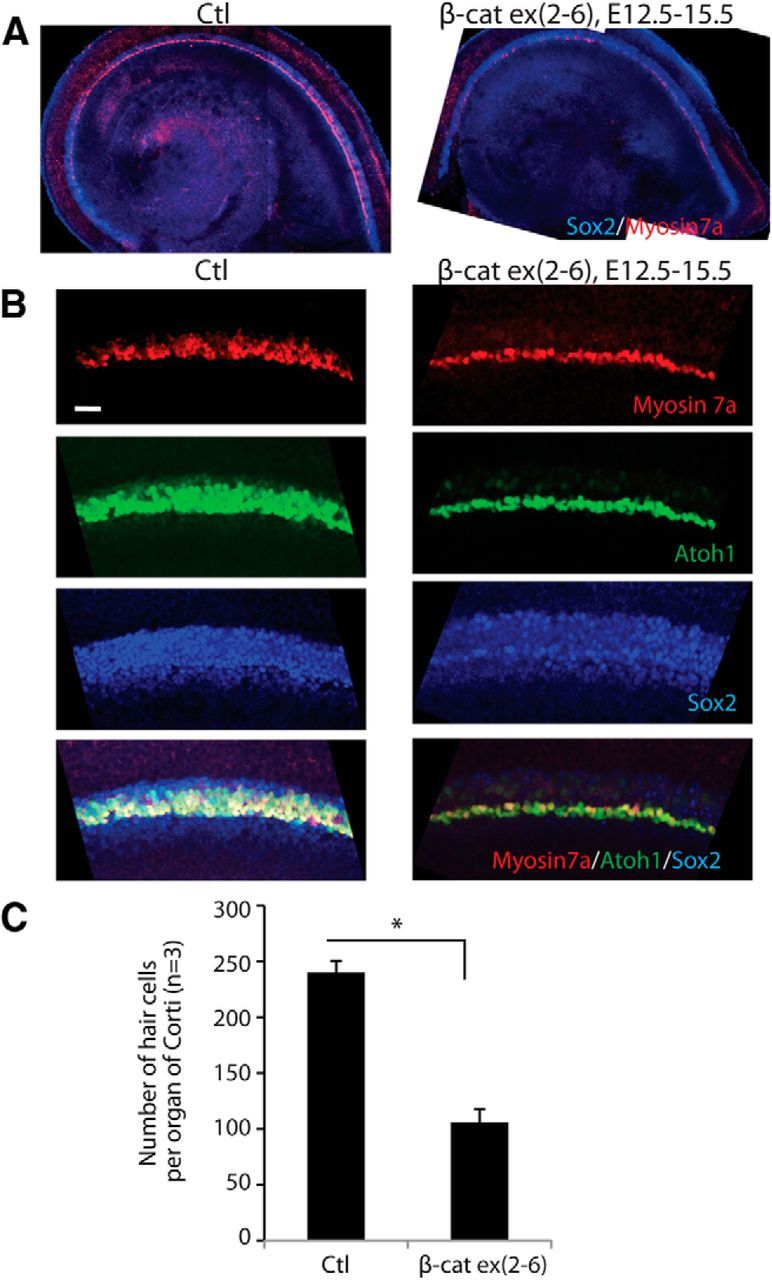
Knock-out of β-catenin in sensory progenitors inhibits hair cell development. A, The number of hair cells was decreased in the sensory region after deletion of β-catenin was induced at E12.5 in sensory progenitors (Sox2-positive) of Sox2-CreER;β-cateninflox(exon2–6) mice. Low-magnification views are shown at E15.5. B, Two to three rows of Atoh1-positive outer hair cells were observed lateral to the myosin VIIa-positive inner hair cells in the midbasal region; some outer hair cells expressed myosin VIIa in a littermate lacking Cre. A single shortened row of hair cells had developed in β-cateninΔΔexon2–6 mutants. No change in Sox2 expression was seen. C, The difference in hair cell number was significant (p < 0.01).
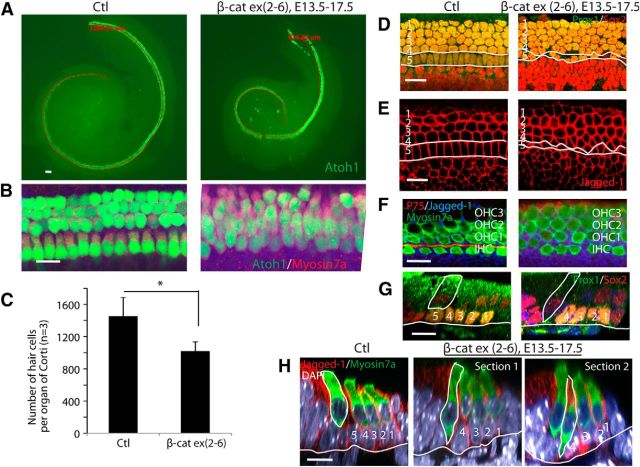
To investigate the effect of β-catenin knock-out on hair cell patterning, tamoxifen was given at E13.5 and E14.5, or at E14.5 and E15.5, and the ear examined at E17.5 or E18.5, respectively, when hair-cell differentiation is nearly complete. Loss of β-catenin expression resulted in a shortening of the organ of Corti and decreased inner and outer hair-cell differentiation (Fig. 4A). Hair cells appeared less organized, and separation of inner and outer hair cells was not observed (Fig. 4B). The organ of Corti was 29% shorter with 33% fewer hair cells in the β-cateninΔΔexon2–6 mutants (Fig. 4C). The row of elongated inner pillar cell nuclei was absent and negative for Prox1, a marker of Dieters' and pillar cell nuclei (Fig. 4D). Immunostaining for jagged-1 revealed an irregular pattern of pillar cells (Fig. 4E), and p75, a pillar cell marker, was not expressed (Fig. 4F). Due to an apparent lack of underlying pillar cells in the β-cateninΔΔexon2–6 mutants as shown by Prox1 staining (Fig. 4G), inner hair cells, and the first row outer hair cells contacted the basilar membrane (Fig. 4H).
Figure 4.
β-Catenin knock-out delays hair cell development and patterning. A, Fewer hair cells differentiated and the organ of Corti was shorter in Sox2-CreER; β-cateninflox(exon2–6); mice at E17.5 when β-catenin deletion was induced at E13.5. B, The hair cells were less organized and inner and outer hair cells had not separated in β-catenin knock-out mice. C, The organ of Corti in the mutants was 29% shorter and had 33% less hair cells (p < 0.05). D, The number of pillar cells decreased and a continuous row of inner pillar cells was not apparent (white lines) when supporting cells were labeled with Sox2 and Prox1. E, An antibody to jagged-1 revealed irregular patterning in the inner pillar cell area (white lines). F, No staining (P75) was observed in the pillar cell area. G, Staining for Prox1, a pillar cell marker, was absent and the inner hair cells contacted the basilar membrane (white line). H, Inner hair cells (outlined; Section 1) or the first outer hair cells (outlined; Section 2) contacted the basilar membrane (white line) as shown by myosin VIIa staining. Scale bar, 20 μm; H1, H2, H3 are Dieters' cells and H4 and H5 are pillar cells. IHC, Inner hair cells; OHC, outer hair cells.
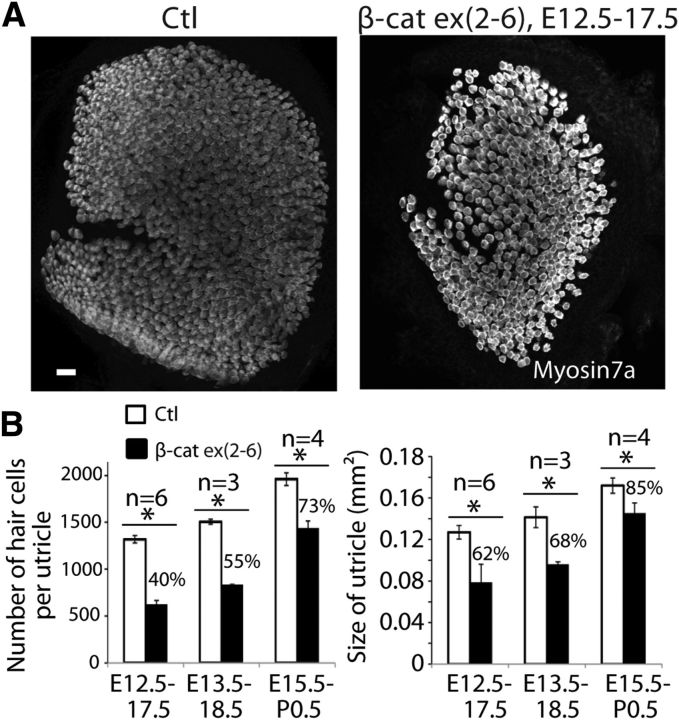
In the utricle, which continues to add new hair cells until P7, β-catenin knock-out mutants had a reduced number of hair cells (47% of normal) and were smaller at E17.5 when tamoxifen administration was initiated at E12.5 (Fig. 5A), unlike the organ of Corti, which completes hair-cell differentiation by E18.5. The effect on the size and number of hair cells in the utricle persisted when β-catenin was knocked out at a later time point (tamoxifen at E12.5, E13.5, or E15.5; Fig. 5B).
Figure 5.
Knock-out of β-catenin in sensory progenitors inhibits utricular hair cell development. A, Examination of the ear at E17.5 after deletion of β-catenin at E12.5 in Sox2-CreER;β-cateninflox(exon2–6) mice revealed a smaller utricle with less hair cells. B, The decrease in utricular hair cell number and utricular size were significant after β-catenin knock-out from E12.5 to E17.5, E13.5 to E18.5, and E15.5 to P0.5 (p < 0.01). Scale bar, 20 μm.
β-Catenin is not necessary for hair-cell maintenance
Gfi1 is expressed in the cochlea starting from E15.5 specifically in hair cells (Yang et al., 2010). Conditional deletion of β-catenin in cochlear hair cells in Gfi1-Cre;β-cateninΔΔexon2–6 mutant mice had no apparent effect on the organ of Corti (Fig. 6A). No difference was observed in hair cells or supporting cells (myosin VIIa and Sox2 expression; Fig. 6B,B′) even though β-catenin expression was significantly reduced or undetectable in hair cells of the β-catenin knock-out ears (Fig. 6C). The normal appearance of the cochlea was maintained as long as P42, and normal ABR and DPOAE thresholds at that time point confirmed the functional integrity of the hair cells and showed that the cochlea remained intact (Fig. 6D). Therefore β-catenin was required for hair-cell differentiation from sensory progenitors during development; however, it was not required for hair-cell maintenance.
Figure 6.
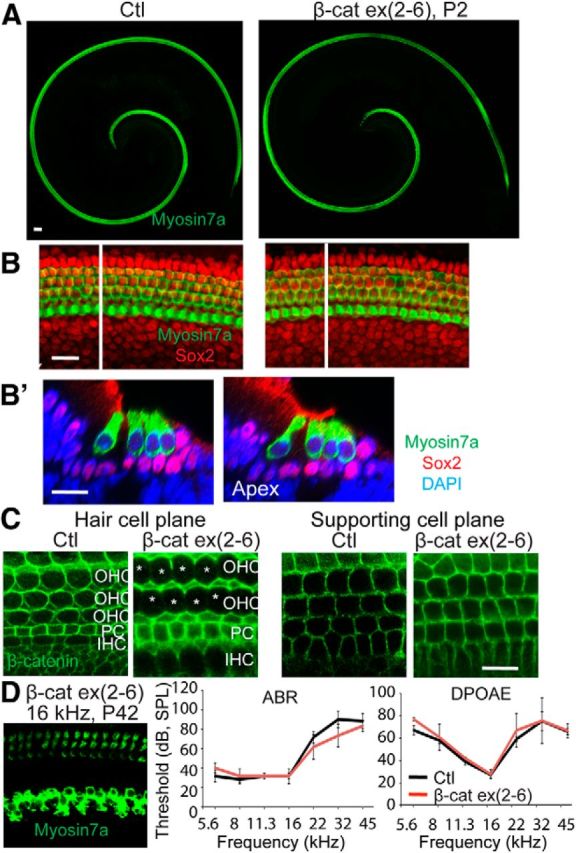
Morphology is normal after β-catenin knock-out in hair cells. A, No gross change of hair-cell morphology was seen at P2 in a Gfi-Cre;β-cateninflox(exon2–6) ear (knock-out of β-catenin in hair cells). B, Hair cells and supporting cells were stained, respectively, with myosin VIIa and Sox2. B′, An XZ scan of B at the white line shows a cross-section of the organ of Corti. C, β-catenin was expressed in both hair cells and supporting cells of the Cre-negative animal. β-catenin was not detected in hair cells (hair cell plane) but was retained in supporting cells in the hair cell-specific β-catenin knock-out. D, At P42, the cochlea in the Gfi-Cre;β-cateninflox(exon2–6) mouse had regular hair cell rows, and ABR and DPOAE thresholds did not differ from control mice.
β-Catenin overexpression at the time of hair-cell differentiation increases the number of immature hair cells
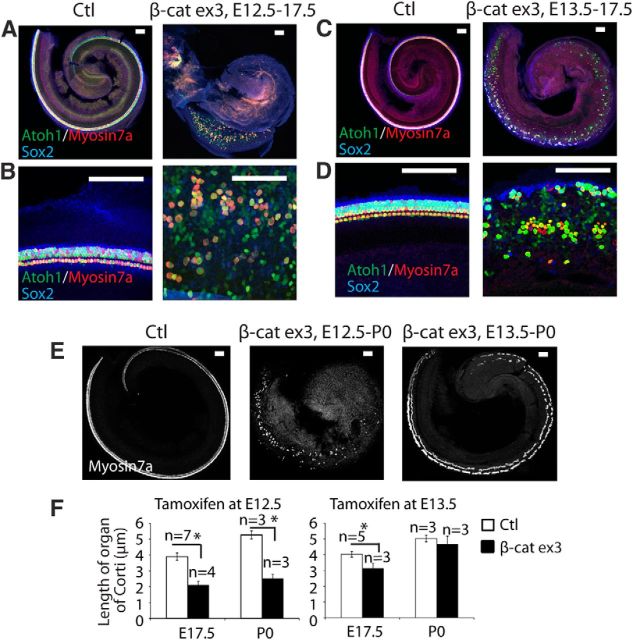
We examined the effect of β-catenin overexpression at different stages of developing sensory epithelia to assess the known effect of β-catenin signaling on cell proliferation. Sensory progenitors in the cochlea exit the cell cycle before their differentiation and remain postmitotic throughout life. By E17.5, sensory epithelial development is nearly complete and hair-cell differentiation reaches the apex of the cochlea (Chen and Segil, 1999). Overexpression of β-catenin in sensory progenitors by tamoxifen administration to Sox2-CreER;β-cateninflox(exon3) mice beginning at E12.5 resulted in a wider sensory epithelium in the midbasal region with a lack of elongation along the cochlear axis (Fig. 7A,F). Atoh1 was upregulated, and myosin VIIa was found in one third of the new cells in the expanded sensory epithelium (Fig. 7B). When tamoxifen was initiated one day later, the sensory epithelium also expanded but continued to extend toward the apex (Fig. 7C,D,F) at E17.5. Elongation of the sensory epithelium at postnatal day (P)0 was nearly normal when overexpression was initiated at E13.5 but not E12.5 (Fig. 7E,F).
Figure 7.
Overexpression of β-catenin expands and inhibits elongation of the sensory epithelium. A, When β-catenin was overexpressed in sensory progenitors at E12.5 in Sox2-CreER;β-cateninflox(exon3) mice, the sensory epithelium expanded with extra hair cells in the midbasal region, but failed to extend to the apex by E17.5, when hair cells had nearly completely developed in a Cre-negative littermate. B, At high-magnification, in the midbasal region, all Atoh1-positive cells expressed myosin VIIa. In β-catenin expression mutants, the sensory epithelium was wider, but only some of the Atoh1-positive cells expressed myosin VIIa. C, When β-catenin overexpression was initiated at E13.5, the sensory epithelium also expanded and extended toward the apex by E17.5. D, Higher-magnification of B (overexpression of β-catenin) showed an expanded area between inner and outer hair cells. E, At P0 the sensory epithelium extended nearly to the apex when β-catenin overexpression was initiated at E13.5 but not at E12.5. F, When overexpression of β-catenin was initiated at E12.5, the difference in the length of the sensory epithelium was significant at E17.5 and P0 (p < 0.01), whereas it was shorter at E17.5 but similar to the Cre-negative littermate by P0 when initiated at E13.5 (p < 0.05 at E17.5). Scale bar, 100 μm.
β-Catenin overexpression reduces membranous E-cadherin and expands the organ of Corti
The observed disorganization and expansion of the organ of Corti, in addition to the proliferation following ectopic β-catenin activation, suggested changes in the adhesion complexes within the epithelium. In addition to roles in proliferation and differentiation, β-catenin plays a role in cell adhesion by binding to the cytoplasmic tail of cadherins at the plasma membrane (Ozawa et al., 1989). Similar to β-catenin, E-cadherin is expressed within the cell membranes of the cochlear sensory epithelium (Whitlon, 1993; Leonova and Raphael, 1997; Simonneau et al., 2003). When we overexpressed β-catenin in the cochlea after initiation of tamoxifen at E13.5 in the Sox2-CreER;β-cateninflox(exon3) mouse, elongation of the sensory epithelium proceeded into the apical region (Fig. 8A) as expected, and staining for EdU as well as Ki67, a cell proliferation marker, were observed (Fig. 8B), but E-cadherin expression within the cell membranes was decreased (Fig. 8C,E). The cochlea contained multiple rows of inner hair cells expressing myosin VIIa (Fig. 8D). The number of cells in the pillar cell region was dramatically expanded from 2 to as many as 20; many of the cells expressed Sox2 (Fig. 8D). The width of the organ of Corti was increased by the extra rows of cells (Fig. 8E). Our findings showing that constitutive activation of β-catenin leads to the formation of ectopic hair cells are in agreement with activation of β-catenin signaling by secreted molecules. Application of the potent canonical Wnt activator R-spondin1 (Rspo1) to E13.5 cochlear explant cultures resulted in a statistically significant increase in the number of inner and outer hair cells (Fig. 8F). Rspo1 added at 5 μg/ml to culture medium resulted in a significant increase in the number of cells that developed as inner hair cells throughout the cochlear explant compared with untreated controls. There was also a significant increase in the number of outer hair cells as a result of the Rspo1 treatment (Control n = 12, Rspo1 treated n = 9, generated across three independent experiments). Expansion of the hair cell domain is specific to Rspo1; we have previously shown that Rspo2 (an R-spondin endogenously expressed in the cochlea) inhibits formation of outer hair cells (Mulvaney et al., 2013). These data agree with the in vivo data presented above and previous in vitro data demonstrating an increase in the number of cells that develop as hair cells after activating Wnt/β-catenin.
Figure 8.
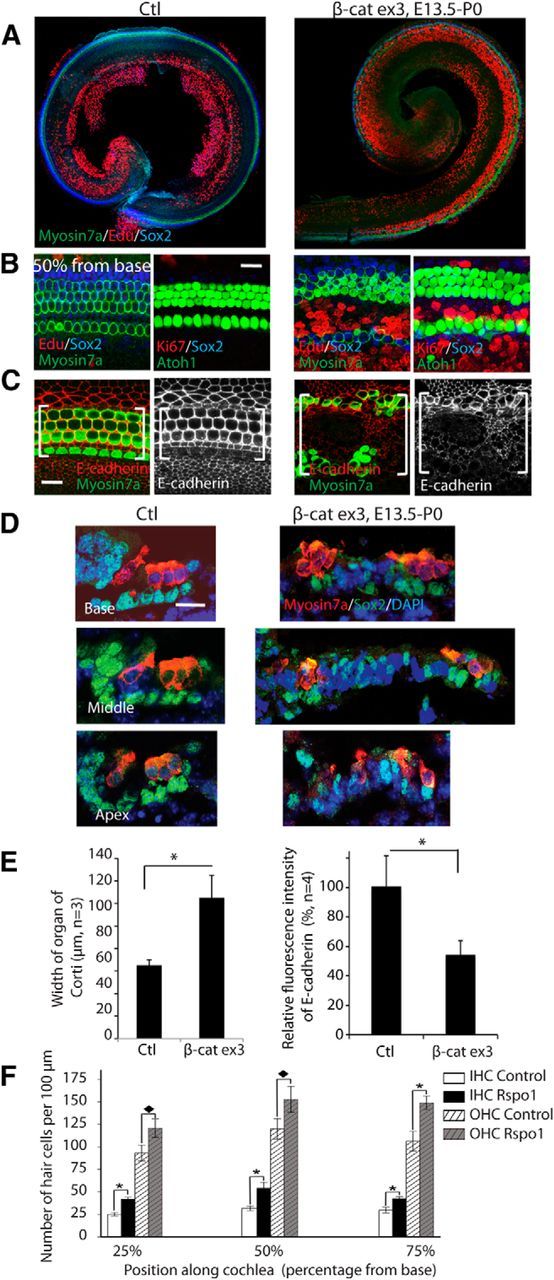
Overexpression of β-catenin expands sensory epithelium and generates more hair cells. A, The organ of Corti in β-catenin overexpression mutants was expanded when β-catenin was overexpressed from E13.5 to P0 in Sox2-CreER;β-cateninflox(exon3) mice. B, EdU was incorporated, and Ki67-positive cells were seen in the inner pillar cell region in β-catenin overexpression mutants. C, β-catenin overexpression reduced E-cadherin expression in the expanded organ of Corti (bracket). D, The cells in the pillar cell region were expanded dramatically in the β-catenin overexpressing ear. The expanded cells had decreased or abolished Sox2 expression. E, β-catenin overexpression significantly expanded the organ of Corti, and decreased the expression of E-cadherin, with p < 0.01. F, The number of hair cells in organ of Corti explant culture was increased by Rspo1 (p < 0.01).
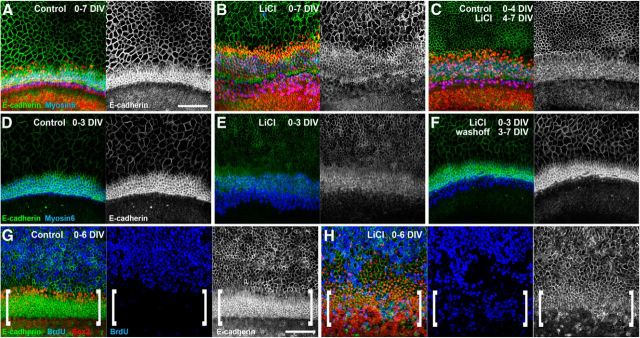
To further examine whether Wnt activation had an effect on E-cadherin in the cochlear duct and the effect on proliferation, E13 cochleae were isolated and established in culture, then maintained in control media or 10 mm LiCl (to activate canonical Wnt signaling) for up to 7 d in vitro (DIV). Immunostaining for E-cadherin identified a significant reduction in membranous localization of E-cadherin within the hair cell domain of LiCl-treated cultures compared with controls (Fig. 9A,B; n > 6). This reduction was observed throughout the cochlear duct where similar results were observed in the basal, midbasal, and apical regions (data not shown). Moreover, if LiCl treatment was delayed until the fourth DIV (equivalent to E17) and explants were maintained for an additional 3 DIV, a similar reduction in membranous E-cadherin expression was observed (Fig. 9C). This demonstrates that even at advanced stages of development, activation of canonical Wnt signaling can affect cell adhesion within the organ of Corti. Furthermore, washout experiments demonstrated that membranous E-cadherin expression was restored to control levels when explants were allowed to recover for 3–4 DIV after an initial 3 d treatment with LiCl (Fig. 9D–F), suggesting a dynamic role for β-catenin in cochlear cell adhesion.
Figure 9.
In vitro activation of Wnt/β-catenin signaling reduces the level of membranous E-cadherin expression in the developing organ of Corti. A–C, Organ of Corti explants established at E13 and maintained for 7 DIV were immunostained for E-cadherin (green), Sox2 (red), and myosin 6 (blue); merged colors are shown at left and E-cadherin is shown alone at right. Compared with explants maintained for 7 DIV in control media (A), explants maintained in 10 mm LiCl for 7 DIV (B) showed a reduction in membranous E-cadherin expression. Similarly, delayed addition of LiCl until the last 3 DIV (C) also resulted in reduced membranous E-cadherin expression within the organ of Corti domain. D–F, High levels of membranous E-cadherin could be detected after 3 DIV (D), but were significantly reduced if explants were maintained in LiCl for the first 3 DIV (E). If the LiCl was washed from the media and explants were maintained under control conditions for an additional 4 DIV, membranous E-cadherin expression was restored (F). G, H, In E13 explants maintained for 6 DIV, BrdU incorporation (blue) was only detected in cells outside of the organ of Corti domain which has the highest levels of membranous E-cadherin expression (G, brackets), whereas numerous BrdU-positive cells were found within the organ of Corti domain in LiCl-treated explants (H, brackets) where the levels of membranous E-cadherin were significantly reduced. Scale bars, 100 μm.
To determine whether the observed reduction in membranous E-cadherin expression corresponded with regions of induced proliferation, E13 cochlear explants were exposed to BrdU for 6 DIV in either control or LiCl-treated media. In control explants, BrdU incorporation was restricted to cells outside of the organ of Corti, to the region in which E-cadherin expression was lowest (Fig. 9G). In contrast, numerous BrdU-positive cells were found throughout the organ of Corti domain in LiCl-treated explants (Fig. 9H) where the levels of membranous E-cadherin were significantly reduced compared with controls.
Discussion
In this study, we temporally and spatially manipulated the expression of β-catenin, the key mediator of canonical Wnt signaling. Knock-out of β-catenin in the course of sensory epithelium development inhibited the differentiation of hair cells from sensory progenitors. In contrast, overexpression of β-catenin expanded the sensory progenitors and increased the number of hair cells.
Canonical Wnt activity in the developing cochlea
Wnt signaling has been demonstrated to control planar cell polarity during stereociliary bundle formation in developing hair cells and in convergent extension during cochlear development via a β-catenin independent noncanonical pathway. Several reporters have been used to study canonical Wnt activity during the course of hair-cell development and generated inconsistent data. BAT-gal reporter mice, which express LacZ under the control of a β-catenin/TCF responsive element, containing seven TCF/LEF-binding sites upstream of a minimal-promoter TATA box (Maretto et al., 2003) did not show activity in the cochlea from E10.5–E18.5 (Qian et al., 2007). Using similar reporters based on multimerized TCF sites upstream of other promoters, TOP-gal and TCF/LEF:H2B-GFP, we and others have found that Wnt activity parallels the course of hair-cell development (DasGupta and Fuchs, 1999; Jacques et al., 2012; Shi et al., 2013). Lgr5-GFP, a downstream Wnt target gene which successfully identified Wnt-responsive stem cells in intestine (Barker et al., 2007), reveals a similar pattern of Wnt activity in the developing cochlea (Chai et al., 2012; Shi et al., 2012).
β-Catenin is involved in cell-cycle regulation of progenitor cells
The Wnt/β-catenin pathway plays important roles in development (van Amerongen and Nusse, 2009) by controlling key stages of proliferation, specification, and cell differentiation. Wnts, short distance secretory glycopeptides, bind to frizzled receptors, and activate intracellular cascades to control cell growth, cell fate, and proliferation. The canonical Wnt pathway is mediated by β-catenin, which controls expression of downstream target genes. β-Catenin is also a structural protein and can act as a component of cadherin-based adhesion junctions and in centrosome complexes during mitosis (Valenta et al., 2012).
One of the effects of Wnt/β-catenin signaling is to stimulate cell proliferation. β-catenin overexpression increases the size of otic vesicles between E8.25 and E10, without apparent effects on cell proliferation, but at the expense of the surrounding Pax2-expressing epidermal tissue (Ohyama et al., 2006). β-Catenin stimulation with LiCl in cultured cochlear explants increases proliferation and differentiation of cochlear progenitors to result in extra hair-cell formation (Jacques et al., 2012). The effects of β-catenin activation could be cell-stage or Wnt-level dependent. At later stages of development (E12.5), when sensory progenitors exit the cell cycle and initiate terminal differentiation, we found that constitutive activation of β-catenin promoted proliferation but inhibited elongation of the cochlea along the longitudinal axis from the mid-basal region to the apex, suggesting continued longitudinal extension after cell division had ceased. Elongation continued, however, when upregulation was initiated later in development (E13.5), suggesting that elongation could proceed during cell proliferation.
The planar cell polarity pathway, which is one of the noncanonical Wnt pathways and is distinct from canonical Wnt signaling through β-catenin, reportedly drives elongation of the cochlear duct via a process of convergent extension (Wang et al., 2005), although the cochlea elongated normally after conditional knock-out of Vangl2 (Copley et al., 2013), one of the core planar cell polarity components. Our observation of disruption of cochlear elongation after the forced expression of β-catenin suggest that the canonical pathway may also play a role, and interactions between the two pathways have been reported (Hayes et al., 2013), but the effect of β-catenin is most likely to be due to the increased number of progenitors that result from canonical Wnt signaling.
β-Catenin is required for hair-cell specification but not for maintaining hair- cell fate
Atoh1 is a key transcription factor in hair cell development, with roles in the initial differentiation as well as the maturation and stability of hair cells (Bermingham et al., 1999; Cai et al., 2013; Chonko et al., 2013). Overexpressing Atoh1 in developing and young organ of Corti induces generation of hair cells (Zheng and Gao, 2000; Gubbels et al., 2008). We have identified Atoh1 as a direct downstream target of Wnt/β-catenin signaling (Shi et al., 2010). The current study supports our hypothesis that β-catenin is crucial for Atoh1 expression during hair-cell development; interrupting β-catenin expression inhibits Atoh1 expression and prevents hair cell generation. Furthermore, Wnt signaling inhibitors significantly reduced hair cell generation in an in vitro model (Jacques et al., 2012); a similar reduction in hair cell generation was observed in vivo after interruption of β-catenin. Constitutive activation of β-catenin generated more Atoh1-expressing cells in the middle and basal regions, whereas proliferation of progenitors dominated over differentiation into hair cells in the apex of the cochlea. The specification of supporting cells in the developing cochlea is secondary to hair-cell differentiation and lateral inhibition of the neighboring cells (Lanford et al., 1999). Without hair cells, we were not able to assess the role of β-catenin in supporting cell differentiation. We find that β-catenin is not required to maintain Atoh1 expression, once hair-cell fate is established using a hair cell-specific Cre, Gfi-1, to interrupt the function of β-catenin, as no disorganization of hair cells was seen after β-catenin knock-out in hair cells. β-Catenin also acts as an adhesion molecule at cell–cell junctions, suggesting that compensatory mechanisms exist to maintain the junctions (Rudloff and Kemler, 2012).
β-Catenin is required for cell patterning in developing organ of Corti
In the absence of β-catenin in sensory progenitors, the gap between inner and outer hair cells did not form, suggesting an inhibitory effect on pillar cell development. This phenotype is similar to the effect of interruption of FGF8–Fgfr3 interaction (Mueller et al., 2002; Hayashi et al., 2007; Jacques et al., 2007; Puligilla et al., 2007). Altered Wnt signaling reduces the number of cells developing as inner hair cells and could thus decrease the differentiation of inner pillar cells through a deficit of FGF8, because inner hair cells are the source of FGF8 required for the differentiation of inner pillar cells. Alternatively, the lack of inner pillar cell development could be a direct result of the absence of Wnt signaling since inner pillar cells are part of the subset of supporting cells where Lgr5 continues to be expressed during development, and cells expressing Lgr5 respond to Wnt overexpression long after they exit the cell cycle (Shi et al., 2013). Furthermore, Lgr5 acts as a receptor for R-spondins. At postnatal stages, addition of Rspo1 to dissociated Lgr5-expressing cochlear cells induces expression of hair cell markers (Chai et al., 2012; Shi et al., 2012), and we show that addition of Rspo1 to embryonic cochleae leads to an increase in hair cells. This implies that the Lgr5-positive cells respond to stimulation with the Wnt potentiator, Rspo1, during development. No expansion of pillar cell-like cells was observed, likely due to application of Rspo1 after Wnt-induced cell proliferation had ceased and Wnt-induced expression of Atoh1 had been initiated.
β-Catenin overexpression inhibits E-cadherin before induction of proliferation
At later developmental stages, and throughout adulthood, E-cadherin is highly expressed in the lateral regions of the organ of Corti beginning in the pillar cells and extending laterally toward the outer hair cells and Hensen's cells (Simonneau et al., 2003). Canonical Wnt/β-catenin activity has been shown to negatively regulate E-cadherin expression during epithelial bud development (Jamora et al., 2003), and in the inner ear, reduced levels of E-cadherin were observed in vestibular sensory epithelia following Wnt activation (Lu and Corwin, 2008).
E-cadherin regulates the proliferative state of cancer cells under the control of Wnt/β-catenin signaling (Jeanes et al., 2008). It has been suggested that the balance between cell adhesion and cell proliferation is mediated by competition for limited amounts of β-catenin within a cell (Nelson and Nusse, 2004). In a previous study, we showed that activation of Wnt/β-catenin signaling induced proliferation in typically quiescent cells of the developing organ of Corti (Jacques et al., 2012), and here we show that Wnt/β-catenin both altered the localization of E-cadherin from membranous to cytoplasmic and increased proliferation. Given that E-cadherin/β-catenin complexes are stabilized by the presence of GSK3β, we hypothesize that the loss of membranous E-cadherin in the cochlea following treatment with the GSK3β inhibitor LiCl, frees up β-catenin from the membrane and shifts the balance between cell adhesion and proliferation. Reduction in E-cadherin expression at the cell membrane in mouse utricle caused by canonical Wnt activation and translocation of β-catenin mechanically frees cells from their tight epithelial junctions and induces cell division (Meyers and Corwin, 2007; Lu and Corwin, 2008).
We conclude that β-catenin signaling is a necessary upstream pathway for the development of the sensory epithelium and plays a key role in the division of progenitors, elongation of the cochlea and initiation of differentiation of inner pillar cells as well as hair cells. Canonical Wnt signaling decreases β-catenin at the membrane and results in increased proliferation to produce the cells of the maturing cochlea. Signaling via this pathway continues to be important for development of hair cells, and once these cells are established in the developing sensory epithelium; their functional properties are maintained without further contribution by the Wnt/β-catenin pathway.
Footnotes
This work was supported by grants from the National Institute on Deafness and other Communicative Disorders (RO1 DC007174, P30 DC05209, and RO3 DC010270); by a grant from the National Natural Science Foundation of China (No. 81300821); and by David H. Koch, the Shulsky Foundation, and Robert Boucai.
The authors declare no competing financial interests.
References
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Cai T, Seymour ML, Zhang H, Pereira FA, Groves AK. Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J Neurosci. 2013;33:10110–10122. doi: 10.1523/JNEUROSCI.5606-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, Liu Z, Taketo MM, Oghalai JS, Nusse R, Zuo J, Cheng AG. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012;109:8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chonko KT, Jahan I, Stone J, Wright MC, Fujiyama T, Hoshino M, Fritzsch B, Maricich SM. Atoh1 directs hair cell differentiation and survival in the late embryonic mouse inner ear. Dev Biol. 2013;381:401–410. doi: 10.1016/j.ydbio.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley CO, Duncan JS, Liu C, Cheng H, Deans MR. Postnatal refinement of auditory hair cell planar polarity deficits occurs in the absence of Vangl2. J Neurosci. 2013;33:14001–14016. doi: 10.1523/JNEUROSCI.1307-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236:525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- Hayes M, Naito M, Daulat A, Angers S, Ciruna B. Ptk7 promotes noncanonical Wnt/PCP-mediated morphogenesis and inhibits Wnt/beta-catenin-dependent cell fate decisions during vertebrate development. Development. 2013;140:1807–1818. doi: 10.1242/dev.090183. [DOI] [PubMed] [Google Scholar]
- Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development. 2007;134:3021–3029. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- Jacques BE, Puligilla C, Weichert RM, Ferrer-Vaquer A, Hadjantonakis AK, Kelley MW, Dabdoub A. A dual function for canonical Wnt/beta-catenin signaling in the developing mammalian cochlea. Development. 2012;139:4395–4404. doi: 10.1242/dev.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Leonova EV, Raphael Y. Organization of cell junctions and cytoskeleton in the reticular lamina in normal and ototoxically damaged organ of Corti. Hear Res. 1997;113:14–28. doi: 10.1016/S0378-5955(97)00130-5. [DOI] [PubMed] [Google Scholar]
- Lu Z, Corwin JT. The influence of glycogen synthase kinase 3 in limiting cell addition in the mammalian ear. Dev Neurobiol. 2008;68:1059–1075. doi: 10.1002/dneu.20635. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–395. doi: 10.1016/S1567-133X(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JR, Corwin JT. Shape change controls supporting cell proliferation in lesioned mammalian balance epithelium. J Neurosci. 2007;27:4313–4325. doi: 10.1523/JNEUROSCI.5023-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, Jacques BE, Kelley MW. Fibroblast growth factor signaling regulates pillar cell development in the organ of corti. J Neurosci. 2002;22:9368–9377. doi: 10.1523/JNEUROSCI.22-21-09368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvaney JF, Yatteau A, Sun WW, Jacques B, Takubo K, Suda T, Yamada W, Dabdoub A. Secreted factor R-Spondin 2 is involved in refinement of patterning of the mammalian cochlea. Dev Dyn. 2013;242:179–188. doi: 10.1002/dvdy.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236:1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowiecki S, Epstein DJ. Divergent roles for Wnt/beta-catenin signaling in epithelial maintenance and breakdown during semicircular canal formation. Development. 2013;140:1730–1739. doi: 10.1242/dev.092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudloff S, Kemler R. Differential requirements for beta-catenin during mouse development. Development. 2012;139:3711–3721. doi: 10.1242/dev.085597. [DOI] [PubMed] [Google Scholar]
- Shi F, Cheng YF, Wang XL, Edge AS. Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3′ enhancer. J Biol Chem. 2010;285:392–400. doi: 10.1074/jbc.M109.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Kempfle JS, Edge AS. Wnt-responsive lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32:9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Hu L, Edge AS. Generation of hair cells in neonatal mice by beta-catenin overexpression in Lgr5-positive cochlear progenitors. Proc Natl Acad Sci U S A. 2013;110:13851–13856. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneau L, Gallego M, Pujol R. Comparative expression patterns of T-, N-, E-cadherins, beta-catenin, and polysialic acid neural cell adhesion molecule in rat cochlea during development: implications for the nature of Kolliker's organ. J Comp Neurol. 2003;459:113–126. doi: 10.1002/cne.10604. [DOI] [PubMed] [Google Scholar]
- Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlon DS. E-cadherin in the mature and developing organ of Corti of the mouse. J Neurocytol. 1993;22:1030–1038. doi: 10.1007/BF01235747. [DOI] [PubMed] [Google Scholar]
- Yang H, Gan J, Xie X, Deng M, Feng L, Chen X, Gao Z, Gan L. Gfi1-Cre knock-in mouse line: a tool for inner ear hair cell-specific gene deletion. Genesis. 2010;48:400–406. doi: 10.1002/dvg.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]