Abstract
Different doses of an adenosine A2A receptor antagonist MSX-3 [3,7-dihydro-8-[(1E)-2-(3-ethoxyphenyl)ethenyl]-7 methyl-3-[3-(phosphooxy)propyl-1-(2 propynil)-1H-purine-2,6-dione] were found previously to either decrease or increase self-administration of cannabinoids delta-9-tetrahydrocannabinol (THC) or anandamide in squirrel monkeys. It was hypothesized that the decrease observed with a relatively low dose of MSX-3 was related to blockade of striatal presynaptic A2A receptors that modulate glutamatergic neurotransmission, whereas the increase observed with a higher dose was related to blockade of postsynaptic A2A receptors localized in striatopallidal neurons. This hypothesis was confirmed in the present study by testing the effects of the preferential presynaptic and postsynaptic A2A receptor antagonists SCH-442416 [2-(2-furanyl)-7-[3-(4-methoxyphenyl)propyl]-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine] and KW-6002 [(E)-1, 3-diethyl-8-(3,4-dimethoxystyryl)-7-methyl-3,7-dihydro-1H-purine-2,6-dione], respectively, in squirrel monkeys trained to intravenously self-administer THC. SCH-442416 produced a significant shift to the right of the THC self-administration dose–response curves, consistent with antagonism of the reinforcing effects of THC. Conversely, KW-6002 produced a significant shift to the left, consistent with potentiation of the reinforcing effects of THC. These results show that selectively blocking presynaptic A2A receptors could provide a new pharmacological approach to the treatment of marijuana dependence and underscore corticostriatal glutamatergic neurotransmission as a possible main mechanism involved in the rewarding effects of THC.
Keywords: adenosine A2A receptor, cannabinoids, drug abuse, monkey, self-administration, THC
Introduction
Classical psychostimulants, such as cocaine and amphetamine, produce reinforcing effects by directly increasing extracellular levels of dopamine in the striatum, through actions on dopamine transporters localized in striatal dopaminergic terminals (Schmitt and Reith, 2010). In contrast, opioids produce reinforcing effects mostly by acting on mesencephalic dopaminergic nuclei [particularly the ventral tegmental area (VTA)], in which they release dopaminergic cells from inhibitory tone provided by GABAergic inhibitory neurons (Shippenberg and Elmer, 1998). Contrary to psychostimulants and opioids, the mechanism behind the reinforcing effects of cannabinoids is still a matter of debate, and both striatal and mesencephalic mechanisms have been invoked (Tanda et al., 1997; Gardner, 2005). Our studies suggest another mechanism: an increase in corticostriatal neurotransmission, probably mediated by a cannabinoid CB1 receptor-mediated decrease in cortical GABAergic neurotransmission. The concomitant striatal glutamate release would then locally produce a glutamate-dependent striatal dopamine release. These assumptions are based on the ability of drugs that decrease corticostriatal glutamatergic neurotransmission to decrease reinforcing effects of cannabinoids, but not cocaine, in experimental animals. Those include acetylcholine nicotinic α7 receptor and adenosine A2A receptor antagonists (Solinas et al., 2007; Justinová et al., 2011, 2013). Thus, activation of nicotinic α7 or A2A receptors, localized in striatal glutamatergic terminals, potently stimulates, and their blockade significantly inhibits, striatal glutamate release and, secondarily, dopamine release (Kaiser and Wonnacott, 2000; Rassoulpour et al., 2005; Quiroz et al., 2009).
However, nicotinic α7 receptors are also localized presynaptically and postsynaptically in excitatory synapses in the cortex (Yang et al., 2013) and in glutamatergic terminals in the VTA, and their blockade could contribute to the decrease in the reinforcing effects of cannabinoids produced by nicotinic α7 receptor antagonists. Furthermore, the reported decrease in reinforcing effects of cannabinoids produced by putative striatal presynaptic A2A receptor blockade was observed with only one dose of the A2A receptor antagonist MSX-3 [3,7-dihydro-8-[(1E)-2-(3-ethoxyphenyl)ethenyl]-7 methyl-3-[3-(phosphooxy)propyl-1-(2 propynil)-1H-purine-2,6-dione]. MSX-3 significantly decreased self-administration of Δ9-tetrahydrocannabinol (THC) and anandamide in squirrel monkeys at a relatively low dose (1 mg/kg) but produced the opposite effect at a higher dose (3 mg/kg) (Justinová et al., 2011). The results were interpreted as a preferential presynaptic effect of MSX-3 at the lower dose and the appearance of a postsynaptic effect at higher doses. Thus, striatal A2A receptors are mainly localized postsynaptically, and their blockade produces locomotor activation (Orru et al., 2011). In previous studies, MSX-3 was found to have a predominant presynaptic A2A receptor profile in rats, with a low dose producing a significant decrease in corticostriatal transmission without eliciting locomotor activation. However, in agreement with the results obtained in monkeys, MSX-3 provided only a small window of selective presynaptic effects (Quiroz et al., 2010; Orru et al., 2011). By taking advantage of the recently established presynaptic and postsynaptic profiles of two A2A receptor antagonists, SCH-442416 [2-(2-furanyl)-7-[3-(4-methoxyphenyl)propyl]-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine] and KW-6002 [(E)-1, 3-diethyl-8-(3,4-dimethoxystyryl)-7-methyl-3,7-dihydro-1H-purine-2,6-dione] (Orru et al., 2011), we now confirm that presynaptic and postsynaptic A2A receptor blockade counteracts and potentiates THC self-administration in monkeys, respectively. These results have important therapeutic implications for the treatment of marijuana dependence.
Materials and Methods
Animals.
Eight adult male squirrel monkeys (Saimiri sciureus) weighing 0.8–1.2 kg were housed in individual cages in a temperature- and humidity-controlled room with access to water ad libitum. Monkeys were fed (approximately 2 h after the session) a daily ration of five biscuits of high-protein monkey diet (Lab Diet 5045; PMI Nutrition International) and two pieces of Banana Softies (Bio-Serv) that maintained body weights constant throughout the study. Nutritional and environmental enrichment were provided daily. Animals were maintained in facilities fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and experiments were conducted in accordance with guidelines of the Institutional Animal Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services. Two groups of experienced monkeys were used: (1) a group that had previous experience with THC self-administration (subjects 453, 434, 66B2, 37B, and 25B); and (2) a group used to study the effects of A2A antagonists on responding reinforced by food (subjects 27B, 30A, and 1549).
Apparatus and self-administration procedure.
Experimental chambers and other apparatus, as well as the general self-administration procedure, were as described previously (Justinová et al., 2003). Monkeys were surgically prepared with chronic indwelling venous catheters (polyvinyl chloride; Goldberg 1973). At the start of the session, a white house light was turned off, green stimulus lights were turned on, and monkeys were required to make 10 responses on a lever [10-response, fixed-ratio schedule of reinforcement (FR10)], which turned off the green lights and produced an intravenous injection of 4 μg/kg THC (0.2 s, 0.2 ml) paired with a 2 s amber light. Each injection was followed by a 60 s timeout period, during which the lever presses had no programmed consequences. The same schedule and conditions were used in a group of monkeys that learned to respond for delivery of food (190 mg of banana-flavored food pellets; F0035; Bio-Serv).
We first tested different doses of the A2A receptor antagonists SCH-442416 (0.3–3 mg/kg, i.m., 10 min before the session) and KW-6002 (0.1–1 mg/kg, i.m., 30 min before the session) in monkeys responding for food. Each dose was tested for three consecutive sessions, preceded and followed by 3 d of vehicle pretreatment. Testing stopped when we found a dose of each compound that significantly disrupted responding for food, which was 3 mg/kg for SCH-442416 and 1 mg/kg for KW-6002. Based on these results, we selected the highest doses of SCH-442416 (1 mg/kg) and KW-6002 (0.3 mg/kg) that did not disrupt food-maintained responding for tests on THC self-administration.
In monkeys trained previously to respond for THC, we started testing different doses of the A2A receptor antagonists when responding for the training dose of THC, 4 μg/kg per injection (maintains maximal rates of responding), was stable for at least five consecutive sessions (<15% variability). We tested each pretreatment dose of SCH-442416 (0.03–1 mg/kg, i.m., 10 min before the session) and KW-6002 (0.3 and 1 mg/kg, i.m., 30 min before the session) for five consecutive sessions, preceded and followed by at least 3 d of vehicle pretreatment. After testing these different doses of both drugs, monkeys were allowed to self-administer the training dose of THC for four to five sessions, followed by vehicle substitution (extinction; four to five sessions).
After reaching a stable extinction baseline, pretreatment with vehicle or SCH-442416 (1 mg/kg) was tested for three sessions. After completing this step, monkeys were returned to self-administration of the training dose of THC, followed by vehicle extinction. Then, the dose of THC was varied to construct the THC dose–response curves, and 3 d pretreatment with SCH-442416 preceded by 3 d treatment with vehicle was tested with each THC dose. The order of THC doses during testing was 0.5, 8, 16, and 32 μg/kg per injection. Testing with each THC dose was followed by vehicle extinction (three to five sessions).
KW-6002 was tested similarly to SCH-442416. First, we tested KW-6002 (0.3 mg/kg) during extinction and then with different doses of THC. The order of THC doses during testing was 1, 0.5, 8, and 0.1 μg/kg per injection, and testing with each THC dose was followed by vehicle extinction (three to five sessions).
Data analysis.
Cumulative-response records were obtained during all sessions to assess within-session patterns of responding. Reinforcements per session represent total number of injections or pellets delivered per session. Rates of responding are expressed as responses per second averaged over the 1 h session, with responding during timeouts not included in calculations. Effects of A2A receptor antagonists on THC self-administration are expressed as mean ± SEM of total numbers of reinforcements per session and rates of responding over consecutive sessions. Data for dose–effect curves and effects of A2A receptor antagonists on food-maintained behavior are expressed as mean ± SEM response rates and numbers of reinforcements per session over the last three sessions.
Statistical analysis (SigmaPlot 12.5; Systat Software) of food self-administration data (Fig. 1) and THC self-administration dose–effect curves (see Fig. 3) was done using one-way or two-way repeated-measures ANOVA. Significant main effects were analyzed further by subsequent paired comparisons with control values using Bonferroni's test or pairwise multiple comparisons using Tukey's test, respectively. For statistical evaluation of effects of A2A receptor antagonists over five consecutive sessions, we used Proc Mixed (SAS Institute) restricted maximum likelihood analysis, followed by planned comparisons with Holm correction, with session and dose as factors. Session factor included sessions 4–8 (Fig. 2) compared with “baseline,” which was the average of the three sessions with vehicle pretreatment (sessions 1–3; Fig. 2). Differences were considered statistically significant when p < 0.05. Because of the small number of subjects in our study, statistical power of each test was carefully examined to correctly interpret statistical difference or lack thereof.
Figure 1.
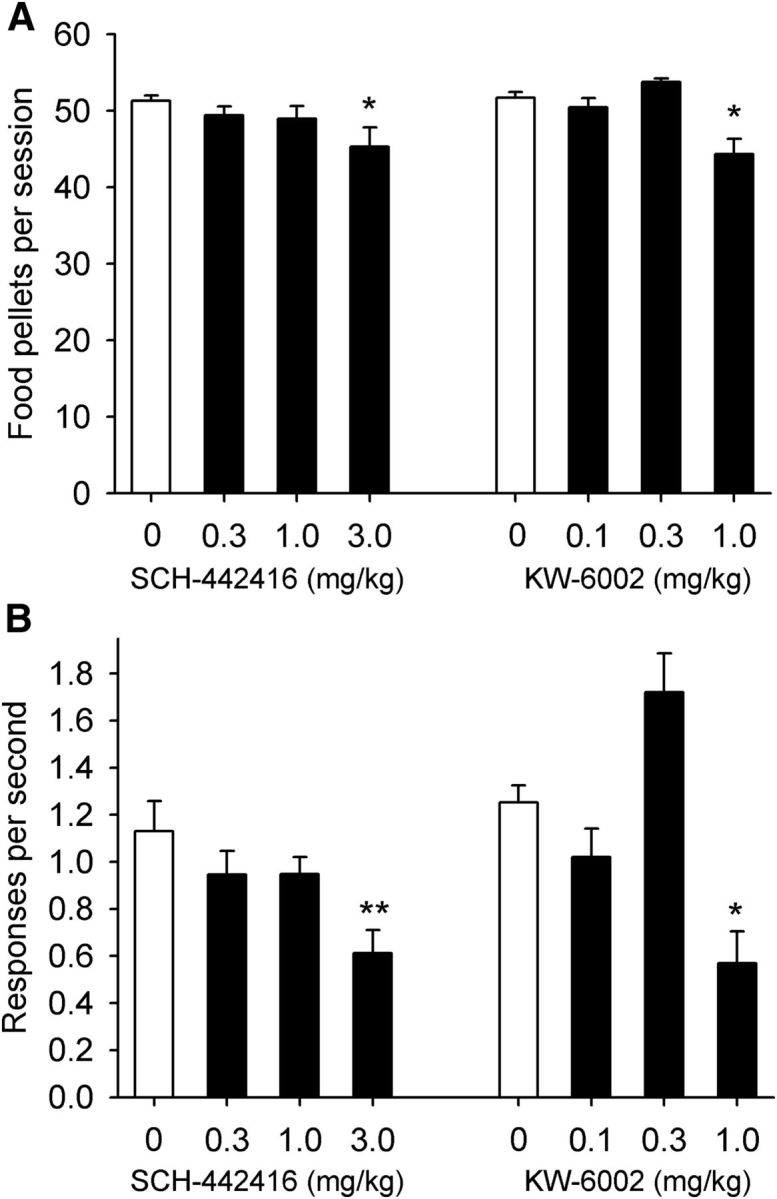
Effects of pretreatment with A2A receptor antagonists on responding maintained by food under an FR10 schedule in squirrel monkeys. A, B, Effects of pretreatment with SCH-442416 (0.3–3 mg/kg, i.m.), KW-6002 (0.1–1 mg/kg, i.m.), or vehicles on food-maintained responding are shown for the number of food pellets self-administered over a session (A) and overall rates of responding (B). Each bar represents the mean ± SEM from three monkeys over three sessions under each condition. *p < 0.05, **p < 0.01, post hoc comparisons versus vehicle pretreatment (0 mg/kg), Bonferroni's test.
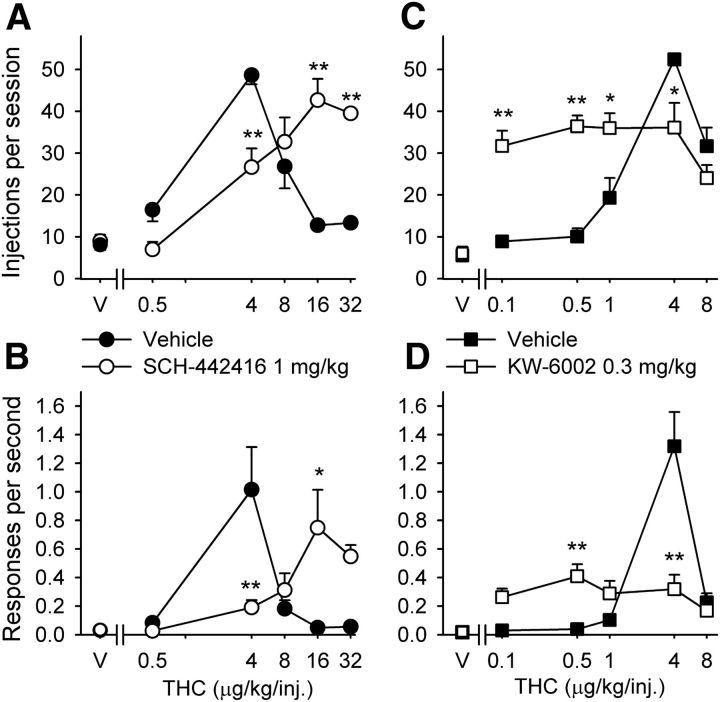
Figure 3.
Effects of A2A receptor antagonists on self-administration of different doses of THC under an FR10 schedule in squirrel monkeys. A, B, Dose–response curves for THC self-administration after intramuscular pretreatment with SCH-442416 (1 mg/kg) or vehicle. C, D, Dose–response curves for THC self-administration after intramuscular pretreatment with KW-6002 (0.3 mg/kg) or vehicle. The number of THC injections per session (A, C) and overall response rates in the presence of the green light signaling THC availability (B, D) are shown as a function of THC dose. Each data point represents the mean ± SEM of the last three sessions under each THC condition and under vehicle conditions (n = 4). *p < 0.05, **p < 0.01, post hoc comparisons of the effects of pretreatment with vehicle versus SCH-442416 or KW-6002 within each THC dose, Tukey's test. V, Vehicle.
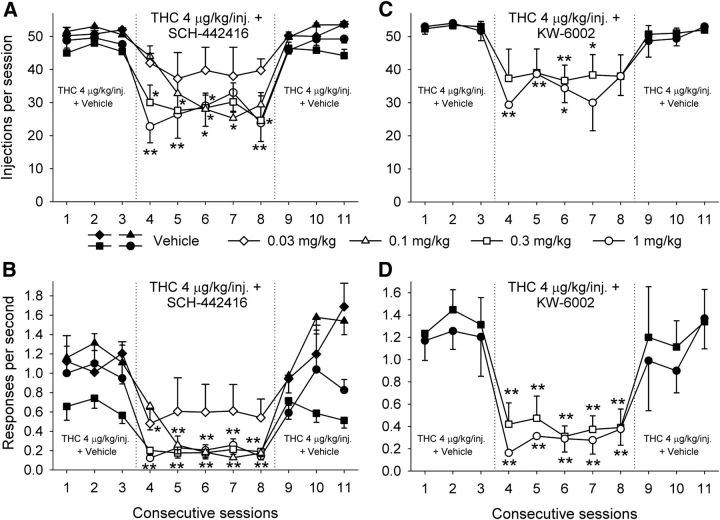
Figure 2.
Effects of A2A receptor antagonists on self-administration of a THC dose (4 μg/kg per injection) that maintains maximum rates of responding under an FR10 schedule in squirrel monkeys. A, B, SCH-442416 (0.03–1 mg/kg, i.m.) significantly decreased the number of THC injections self-administered during 1 h sessions (A) and decreased overall response rates (B) (n = 5). C, D, KW-6002 (0.3 and 1 mg/kg, i.m.) significantly decreased the number of THC injections self-administered during the sessions (C) and decreased overall response rates (D) (n = 4). The number of THC injections per session (A, C) and overall response rates in the presence of the green light signaling THC availability (B, D) are shown over consecutive sessions. Each data point represents the mean ± SEM. *p < 0.05, **p < 0.01, planned comparisons with Holm correction versus the mean of the three sessions with vehicle pretreatment (sessions 1–3).
Drugs.
THC (NIDA Drug Supply Program) was dissolved in a vehicle containing 1% ethanol and 1% Tween 80 and saline to obtain stock solution of concentration 0.4 mg/ml, which was further diluted with saline as needed. SCH-442416 was dissolved in 5% DMSO and 5% Tween 80 and saline. KW-6002 was dissolved in 8% DMSO and 8% Tween 80 and saline. All chemicals (except for THC) were purchased from Sigma-Aldrich. SCH-442416 and KW-6002 were injected intramuscularly to monkeys in a volume of 0.33 ml/kg.
Results
First, different doses of the A2A receptor antagonists SCH-442416 and KW-6002 were tested in monkeys self-administering food to establish dose ranges for THC self-administration experiments. In the present study, monkeys self-administered on average 51.54 ± 0.56 food pellets per session at a rate of 1.26 ± 0.11 responses/s. SCH-442416 had no effect on food self-administration at doses of 0.3 and 1 mg/kg, but at a dose of 3 mg/kg, it slightly, but significantly, decreased the number of self-administered pellets (∼12% decrease when compared with baseline levels after vehicle pretreatment; Fig. 1A; F(3,6) = 5.63, p = 0.03, one-way repeated-measures ANOVA, followed by Bonferroni's post hoc test, p = 0.021 for the dose of 3 mg/kg vs vehicle). More importantly, this dose of SCH-442416 significantly decreased rates of responding by ∼45% compared with baseline rates (Fig. 1B; F(3,6) = 10.69, p = 0.008, one-way repeated-measures ANOVA, followed by Bonferroni's post hoc test, p = 0.004 for the dose of 3 mg/kg vs vehicle). KW-6002 had no effect on food self-administration at a dose of 0.1 mg/kg. At a dose of 0.3 mg/kg, KW-6002 produced a nonsignificant increase, and at a dose of 1 mg/kg, it significantly (∼15%) decreased the number of self-administered pellets (Fig. 1A; F(3,6) = 11.15, p = 0.007, one-way repeated-measures ANOVA, followed, by Bonferroni's post hoc test, p = 0.015 for the dose of 1 mg/kg vs vehicle) and significantly decreased rates of responding by ∼55% compared with baseline rates after vehicle pretreatment (Fig. 1B; F(3,6) = 10.94, p = 0.008, one-way repeated-measures ANOVA, followed by Bonferroni's post hoc test, p = 0.047 for the dose of 1 mg/kg vs vehicle). Thus, we established that a SCH-442416 dose of 3 mg/kg and a KW-6002 dose of 1 mg/kg significantly affected operant responding for food in monkeys.
Next, we studied the effects of different doses of SCH-442416 and KW-6002 on self-administration of THC at an injection dose that maintained maximal responding in the present study, which, similarly to our previous studies under the same FR10 conditions (Tanda et al., 2000; Justinová et al., 2003, 2011, 2013), was 4 μg/kg. Monkeys self-administered on average 48.62 ± 1.10 injections of 4 μg/kg THC per session at a rate of 0.94 ± 0.13 responses/s. Pretreatment with SCH-442416 at 0.03 mg/kg did not significantly alter responding for THC, but at doses of 0.1, 0.3, and 1.0 mg/kg, SCH-442416 caused a significant decrease in the number of self-administered THC injections (Fig. 2A; effect of session, F(5,20) = 11.30, p < 0.0001), as well as decreases in rates of responding (Fig. 2B; effect of session, F(5,20) = 14.95, p < 0.0001). Proc Mixed analysis did not reveal significant differences between the effects of different doses of SCH-442416 on the number of self-administered THC injections (Fig. 2A; effect of dose, F(3,10) = 2.03, p < 0.17), but differences were revealed in the effects on rates of responding (Fig. 2B; effect of dose, F(3,10) = 5.6, p < 0.016). Planned comparisons showed that the effect of the 0.03 mg/kg dose was different from 0.3 and 1 mg/kg (p < 0.014 and p < 0.035, respectively). Pretreatment with KW-6002 at 0.3 and 1.0 mg/kg caused a significant dose-dependent decrease in the number of self-administered THC injections (Fig. 2C; effect of session, F(5,10) = 11.95, p < 0.0006), as well as in rates of responding (Fig. 2D; effect of session, F(5,10) = 32.21, p < 0.001). However, at the dose of 1 mg/kg, KW-6002 also significantly altered responding for food (Fig. 1); thus, a decrease in motor output may have contributed to the effects of KW-6002 at this dose. Proc Mixed analysis did not reveal significant differences between the effects of different doses of KW-6002 (Fig. 2C, effect of dose, F(1,2) = 0.53, p < 0.54; Fig. 2D, effect of dose, F(1,2) = 0.89, p < 0.44).
We then studied effects of the two A2A receptor antagonists on THC self-administration dose–response curves to ascertain possible differential shifts (Fig. 3A–D). Based on results from monkeys self-administering food (Fig. 1), we selected the highest dose of each compound that did not affect food-maintained responding: 1 mg/kg SCH-442416 and 0.3 mg/kg KW-6002. Pretreatment with 1 mg/kg SCH-442416 significantly shifted the THC dose–response curve for injections per session to the right (Fig. 3A; interaction of THC and SCH-442416, F(4,11) = 32.73, p < 0.001, two-way repeated-measures ANOVA), consistent with antagonism of the reinforcing effects of THC. After pretreatment with 1 mg/kg SCH-442416, previously ineffective doses of 16 and 32 μg/kg THC were self-administered. This SCH-442416 dose also produced a significant rightward shift for response rates (Fig. 3B; interaction of THC and SCH-442416, F(4,11) = 8.65, p = 0.002, two-way repeated-measures ANOVA). Post hoc pairwise comparisons revealed significant differences in the effects of THC at the doses of 4, 16, and 32 μg/kg per injection after SCH-442416 versus vehicle pretreatment on number of self-administered injections per session (all p < 0.001) and at the THC doses of 4 and 16 μg/kg per injection also on response rates (4 μg/kg per injection, p = 0.002; 16 μg/kg per injection, p = 0.011). Conversely, pretreatment with KW-6002 (0.3 mg/kg) significantly shifted the THC dose–response curve for injections per session to the left (Fig. 3C; interaction of THC dose and KW-6002, F(4,9) = 10.89, p = 0.002, two-way repeated-measures ANOVA), consistent with a potentiation of the reinforcing effects of THC. This KW-6002 dose also produced a significant leftward shift for response rates (Fig. 3D; interaction of THC dose and KW-6002, F(4,9) = 19.26, p < 0.001, two-way repeated-measures ANOVA). After pretreatment with 0.3 mg/kg KW-6002, previously ineffective THC doses of 0.1 and 0.5 μg/kg per injection were self-administered. Post hoc pairwise comparisons revealed significant differences in the effects of THC at the doses of 0.1, 0.5, 1, and 4 μg/kg per injection after KW-6002 versus vehicle pretreatment on the number of self-administered injections per session (0.1 and 0.5 μg/kg per injection, p < 0.001; 1 and 4 μg/kg per injection, p < 0.05) and, at the doses of 0.5 and 4 μg/kg per injection, also on response rates (in both cases, p < 0.01).
Discussion
This study shows that systemic administration of a preferentially presynaptic A2A receptor antagonist SCH-442416 reduces reinforcing effects of THC in squirrel monkeys, as demonstrated by a rightward shift of THC dose–response curves. In contrast, treatment with a preferentially postsynaptic A2A receptor antagonist KW-6002 shifts dose–response curves for THC to the left, consistent with potentiation of the reinforcing effects of THC.
From these results, we can draw two conclusions. First, as shown previously in rodents, different selective A2A receptor antagonists can produce very different qualitative behavioral results, which depend on their ability to act differentially as presynaptic or postsynaptic ligands (Orru et al., 2011). We showed previously that presynaptic and postsynaptic effects of A2A receptor antagonists depend on the differential affinity of ligands for different A2A receptor heteromers, which are differentially localized in different striatal neuronal elements. Thus, postsynaptic A2A receptors are mostly localized in striatal medium spiny neurons (MSNs) that project to the external segment of the globus pallidus, in which they form heteromers with dopamine D2 receptors, which modulate neuronal excitability (Azdad et al., 2009). Blockade of postsynaptic A2A receptors mediates the locomotor activating effects of A2A receptor antagonists and is also involved in the locomotor activating effects of the nonselective adenosine receptor antagonist caffeine (Ferré et al., 2008; Orru et al., 2011). Presynaptic A2A receptors are localized in terminals of corticostriatal neurons that make synaptic contact with MSNs that project to the substantia nigra and internal segment of the globus pallidus (Quiroz et al., 2009), in which they form heteromers with A1 receptors, which modulate glutamate release (Ciruela et al., 2006). Blockade of presynaptic A2A receptors counteracts motor output and glutamate release induced by cortical stimulation (Quiroz et al., 2009, 2010; Orru et al., 2011). By determining the potency for blocking motor output and striatal glutamate release induced by cortical electrical stimulation and the potency for inducing locomotor activation in rats as respective in vivo measures of presynaptic and postsynaptic activities of several A2A receptor antagonists, SCH-442416 and KW-6002 showed a preferential presynaptic and postsynaptic profile, respectively (Orru et al., 2011).
The present results confirm the interpretation of previous experiments obtained with the A2A receptor antagonist MSX-3 (Justinová et al., 2011), which decreased THC and anandamide self-administration in squirrel monkeys at a relatively low dose but produced the opposite effect with a threefold higher dose. Based on results obtained in rats (see Introduction), it was hypothesized that the completely different dose-dependent effects of MSX-3 could be related to a slightly selective presynaptic effect at lower doses with an overriding postsynaptic effect at larger doses.
The second conclusion that can be drawn from our results is that they strongly support our hypothesis about corticostriatal transmission being involved in the reinforcing effects of cannabinoids in experimental animals, which was based on our previous studies with nicotinic α7 receptor antagonists (Solinas et al., 2007; Justinová et al., 2013). However, as mentioned in Introduction, nicotinic α7 receptors are not only localized in glutamatergic terminals in the striatum but also in the VTA (Schilström et al., 1998; Kaiser and Wonnacott, 2000; Jones and Wonnacott, 2004), which could have a role in the counteracting effects of α7 receptor antagonists on the reinforcing and dopamine-releasing effects of THC (Solinas et al., 2007; Justinová et al., 2013). The present results agree with the corticostriatal hypothesis, because functional A2A receptors are found in the striatum but not in the VTA and the presynaptic A2A receptor antagonist SCH-442416 is a potent and selective modulator of corticostriatal transmission (Orru et al., 2011).
In summary, the present study suggests that striatal presynaptic A2A receptors can provide a new target for treatment of cannabis abuse. Furthermore, the results strongly support our hypothesis that an increase in corticostriatal neurotransmission is a main mechanism mediating the reinforcing effects of cannabinoids.
Footnotes
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), National Institutes of Health, Department of Health and Human Services. THC was provided by the NIDA Drug Supply Program. We thank Dr. Leigh V. Panlilio for his excellent assistance with statistical analysis.
The authors declare no competing financial interests.
References
- Azdad K, Gall D, Woods AS, Ledent C, Ferré S, Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology. 2009;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des. 2008;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Goldberg SR. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. J Pharmacol Exp Ther. 1973;186:18–30. [PubMed] [Google Scholar]
- Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinová Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology. 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Justinová Z, Ferré S, Redhi GH, Mascia P, Stroik J, Quarta D, Yasar S, Müller CE, Franco R, Goldberg SR. Reinforcing and neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist. Addict Biol. 2011;16:405–415. doi: 10.1111/j.1369-1600.2010.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinová Z, Mascia P, Wu HQ, Secci ME, Redhi GH, Panlilio LV, Scherma M, Barnes C, Parashos A, Zara T, Fratta W, Solinas M, Pistis M, Bergman J, Kangas BD, Ferré S, Tanda G, Schwarcz R, Goldberg SR. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat Neurosci. 2013;16:1652–1661. doi: 10.1038/nn.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, Wonnacott S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- Orru M, Bakešová J, Brugarolas M, Quiroz C, Beaumont V, Goldberg SR, Lluís C, Cortés A, Franco R, Casadó V, Canela EI, Ferré S. Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists. PLoS One. 2011;6:e16088. doi: 10.1371/journal.pone.0016088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz C, Luján R, Uchigashima M, Simoes AP, Lerner TN, Borycz J, Kachroo A, Canas PM, Orru M, Schwarzschild MA, Rosin DL, Kreitzer AC, Cunha RA, Watanabe M, Ferré S. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. ScientificWorldJournal. 2009;9:1321–1344. doi: 10.1100/tsw.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz C, Pearson V, Gulyani S, Allen R, Earley C, Ferré S. Up-regulation of striatal adenosine A(2A) receptors with iron deficiency in rats: effects on locomotion and cortico-striatal neurotransmission. Exp Neurol. 2010;224:292–298. doi: 10.1016/j.expneurol.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulpour A, Wu HQ, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- Schilström B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–1009. doi: 10.1016/S0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Elmer GI. The neurobiology of opiate reinforcement. Crit Rev Neurobiol. 1998;12:267–303. doi: 10.1615/CritRevNeurobiol.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, Fratta W, Goldberg SR. Nicotinic α7 receptors as a new target for treatment of cannabis abuse. J Neurosci. 2007;27:5615–5620. doi: 10.1523/JNEUROSCI.0027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Yang Y, Paspalas CD, Jin LE, Picciotto MR, Arnsten AF, Wang M. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc Natl Acad Sci U S A. 2013;110:12078–12083. doi: 10.1073/pnas.1307849110. [DOI] [PMC free article] [PubMed] [Google Scholar]