Abstract
Mitochondria form a dynamic network, in which organelles fuse or divide in response to metabolic changes or cellular stress. Inhibition of these processes leads to cell dysfunction and numerous human diseases. New work from several laboratories shows that mitochondria do not divide in isolation from other cellular structures. Rather, they carry out this process in partnership with the endoplasmic reticulum (ER) and actin filaments.
Mitochondrial division (or fission) is mediated by the dynamin-related protein Drp1 and its yeast homologue Dnm1p. Drp1/Dnm1p is a GTPase that is recruited to mitochondria by mitochondrial outer membrane proteins (Fis1p, Caf4p and Mdv1p in yeast, and Mff in metazoans), and assembles into cylindrical spirals that encircle the organelle. Upon GTP hydrolysis, Drp1/Dnm1p undergoes conformational changes that lead to contraction of the spirals and mitochondrial fission [1]. While a central role for Drp1/Dnm1p in mitochondrial fission is well established, it is clear that Drp1/Dnm1p is not the sole mediator of mitochondrial fragmentation. Specifically, structural analysis indicates that the diameter of the Drp1 ring (30–50 nm) or the Dnm1p ring (100–130 nm) is smaller than the diameter of the mitochondrion [2–4]. Thus, some other pre-constriction factor may act before Drp1/Dnm1p assembly. Here, I describe recent findings from Korobova et al. [5] that raise the very interesting possibility that ER and actin assemble into a force-generating element that works in conjunction with Drp1 to drive mitochondrial fission.
Organelles are discrete subcellular compartments in which unique environments are created for specific biochemical functions. At the same time, organelles are not autonomous: they interact physically and functionally with one another. Interaction of mitochondria with ER is critical for phospholipid biosynthesis, calcium homeostasis and anchorage of mitochondria at specific sites within cells [6–8]. Indeed, Mfn2, a protein that mediates interaction of mitochondria with ER as well as mitochondrial fusion, is a target for mutation in Charcot-Marie-Tooth Disease type IIa, a peripheral neuropathy [9].
Previous studies points to a role for mitochondria-ER interactions in mitochondrial fission [10]. Specifically, electron tomography studies revealed that ER encircles mitochondria at sites where mitochondria are constricted and are associated with fission proteins (Drp1, its yeast orthologue Dnm1p, and Mff, a mitochondrial fission factor). Importantly, constriction of mitochondria at sites of ER contact does not require Mff or Drp1. These observations support the idea that ER interacts with mitochondria at sites where mitochondria undergo early constriction events, and that Drp1/Dnm1p is recruited to those sites, where it mediates further constriction of the organelle.
Other studies support a role for actin in mitochondrial constriction. Specifically, treatment of mammalian cells with agents that inhibit mitochondrial electron transport or ATP production results in Drp1-dependent fragmentation of the organelle. Furthermore, disruption of actin inhibits recruitment of Drp1 to mitochondria and attenuates inhibitor-induced mitochondrial fission [11]. These findings support the model that Drp1 serves as a metabolic sensor that alters mitochondrial morphology in response to changes in the oxidative phosphorylation activity of the organelle. They also support a role for the actin cytoskeleton in this process, in part by recruitment of Drp1 to the organelle. However, the mechanism underlying actin function in mitochondrial fission was not well understood.
Scientists from the Higgs laboratory obtained evidence for a direct role for actin and a formin protein in mitochondrial fission [5]. Formins are conserved proteins that regulate the dynamics of actin and microtubule cytoskeletons [12]. INF2 is an “inverted” formin: its formin homology domains (FH1 and FH2) are closer to the N terminus of the protein compared to other fomrins. This inverted formin stimulates actin nucleation and elongation of F-actin, like other formins. In addition, it stimulates F-actin depolymerization at filament pointed ends. There are two INF2 isoforms in mammalian cells. One is bound to ER through its CAAX-box and regulates ER morphology [13]. The other lacks a CAAX box and is found in cytosolic actin meshworks but also stabilizes the Golgi apparatus [14].
Korobova et al. [5] find that actin localizes to sites of ER-mitochondria interaction in mammalian cell lines. Moreover, they obtained evidence that INF2 stimulates actin polymerization at sites of mitochondrial fission, and that this actin polymerization is required for recruitment of Drp1 to those sites. Specifically, they find that silencing of the ER-associated INF2 results in elongation of mitochondria, and defects in both assembly of Drp1p into punctate structures and association of Drp1 with mitochondria. Consistent with this, they show that overexpression of constitutively active ER-associated INF2 has the opposite effect: mitochondrial fragmentation that is dependent upon Drp1p and the actin polymerization activity of INF2.
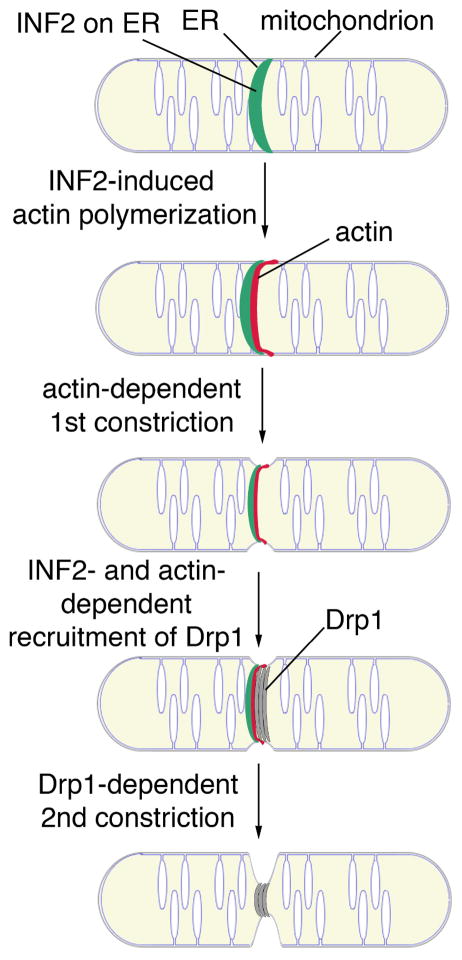
They support a model for ER, actin and formin function in mitochondrial fission (Fig. 1). According to this model, ER and its associated INF2 encircle mitochondria at sites of mitochondrial fission. INF2 then stimulates actin polymerization at that site. Actin can generate forces by different mechanisms including myosin-mediated filament sliding and polymerization-driven pushing forces. Therefore, it is likely that actin provides the force for constriction of mitochondria to a diameter that is compatible with the size of the Drp1p cylinder. This then allows for assembly of Drp1 into spirals and cylinders at that site and a second round of constriction that ultimately leads to mitochondrial fission.
Fig. 1. Model for mitochondrial fission.
Please refer to text for details.
This study revealed a novel mitochondrial-cytoskeletal interaction, an ER- and formin-dependent mechanism for establishing that interaction, and a foundation for understanding how this interaction affects mitochondrial dynamics. It also raises questions regarding the precise function of actin in mitochondrial fission. Does actin serve as a scaffold or force generator that allows ER to encircle mitochondria? Alternatively, is actin required for generating forces for constriction of mitochondria? Indeed, these models are not mutually exclusive. If actin generates forces for mitochondrial constriction, what is the mechanism underlying this process? Does actin assemble into a contractile ring, similar to the actomyosin ring that mediates cytokinesis? Alternatively, does newly polymerized actin that extends from INF2 on the ER surface exert inward pushing forces on mitochondria?
How INF2 is regulated and how it contributes to constriction of mitochondria is yet to be determined. Mutagenesis studies indicate that INF2 is regulated by autoinhibition like other formins and that actin polymerization by INF2 is required for its function in mitochondrial fission [5]. Thus, INF2 is likely activated at sites of mitochondrial fission. Moreover, INF2 is unique among formins because it stimulates actin depolymerization as well as polymerization. Is there an INF2 activator on mitochondria? What controls the length of F-actin that is polymerized at sites of mitochondrial fission? Is the actin depolymerization activity of INF2 also required for its function in mitochondrial fission?
These studies also raise questions regarding the mechanism for recruitment of Drp1 and Mff to ER-marked sites of mitochondrial fission. Does Mff bind to actin? Or does it recognize mitochondrial membrane curvature either directly or by binding to a protein that recognizes that curvature?
Finally, can budding yeast, in which actin is intimately associated with mitochondria and ER, shed light on this process in other eukaryotes? Neither of the known formins in budding yeast localizes to ER [12]. However, Bni1p is found in the cytosol, and could therefore stimulate actin polymerization at sites of ER-mitochondrial contact, like INF2 [15]. Other studies revealed a protein complex (mitochore/ERMES) that is required for association of mitochondria with the actin cytoskeleton and for mitochondrial morphology and motility [16]. Interestingly, this complex also mediates association of mitochondria with ER [17]. Thus, it is possible that mitochore/ERMES maintains actin at sites of mitochondria-ER interactions, which in turn contributes to mitochondrial fission through effects on mitochondrial constriction and recruitment of dynamin-related proteins to those sites.
Future studies that address these fundamental questions will provide a foundation for understanding interaction of mitochondria with ER and the actin cytoskeleton, mechanisms that underlie and regulate fission of the organlle, and forces that control mitochondrial plasticity. INF2 is a target for mutation in a degenerative kidney disease (focal and segmental glomerulosclerosis) and a peripheral neuropathy (Charcot-Marie-Tooth disease). Therefore, these studies will also extend our understanding of the role of mitochondrial fission in human disease.
Acknowledgments
We thank the members of the Pon laboratory and Drs. Gregg Gundersen and Bruce Goode for valued input and discussion. This work was supported by awards from the National Institutes of Health (NIH) (GM45735, GM45735S1 and GM096445) to LP. GM45735S1 was issued by the NIH under the American Recovery and Reinvestment Act of 2009.
References
- 1.Elgass K, Pakay J, Ryan MT, Palmer CS. Recent advances into the understanding of mitochondrial fission. Biochim Biophys Acta. 2013;1833:150–161. doi: 10.1016/j.bbamcr.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol. 2011;18:20–26. doi: 10.1038/nsmb.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swayne TC, Zhou C, Boldogh IR, Charalel JK, McFaline-Figueroa JR, Thoms S, Yang C, Leung G, McInnes J, Erdmann R, et al. Role for cER and Mmr1p in anchorage of mitochondria at sites of polarized surface growth in budding yeast. Curr Biol. 2011;21:1994–1999. doi: 10.1016/j.cub.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lackner LL, Ping H, Graef M, Murley A, Nunnari J. Endoplasmic reticulum-associated mitochondria-cortex tether functions in the distribution and inheritance of mitochondria. Proc Natl Acad Sci U S A. 2013;110:E458–E467. doi: 10.1073/pnas.1215232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 10.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol. 2005;15:678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 12.Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol. 2010;11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- 13.Chhabra ES, Ramabhadran V, Gerber SA, Higgs HN. INF2 is an endoplasmic reticulum-associated formin protein. J Cell Sci. 2009;122:1430–1440. doi: 10.1242/jcs.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramabhadran V, Korobova F, Rahme GJ, Higgs HN. Splice variant-specific cellular function of the formin INF2 in maintenance of Golgi architecture. Mol Biol Cell. 2011;22:4822–4833. doi: 10.1091/mbc.E11-05-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buttery SM, Yoshida S, Pellman D. Yeast formins Bni1 and Bnr1 utilize different modes of cortical interaction during the assembly of actin cables. Mol Biol Cell. 2007;18:1826–1838. doi: 10.1091/mbc.E06-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boldogh IR, Nowakowski DW, Yang HC, Chung H, Karmon S, Royes P, Pon LA. A protein complex containing Mdm10p, Mdm12p, and Mmm1p links mitochondrial membranes and DNA to the cytoskeleton-based segregation machinery. Mol Biol Cell. 2003;14:4618–4627. doi: 10.1091/mbc.E03-04-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]