Abstract
A large body of evidence shows that methamphetamine (METH) causes sustained damage to the brain in animal models and human METH users. In chronic users there are indications of cognitive and motor deficits. Striatal neuropeptides are in a position to modulate the neurochemical effects of METH and consequently striatal neural damage. Somatostatin (SST) is an intrinsic striatal neuropeptide that has been shown to inhibit glutamate transmission; glutamate is integral to METH toxicity and contributes to nitric oxide (NO) synthesis. We hypothesize that SST will protect from METH by inhibition of NO synthesis and thus reducing oxidative stress. To this end, the SST analogue octreotide (OCT) was microinjected into the striatum prior to a systemic injection of METH (30 mg/kg). We then assessed 3-nitrotyrosine (3-NT), an indirect index of NO production, tyrosine hydroxylase (TH) protein levels (dopamine terminal marker) and Fluoro-Jade C positive cells (degenerating cells). The SST agonist OCT dose dependently attenuated the METH-induced accumulation of striatal 3-NT. Moreover, pretreatment with OCT effectively mitigated cell death but failed to protect dopamine terminals. Next we co-infused OCT and NMDA and measured 3-NT and Fluoro-Jade C staining. Treatment with OCT had no effect on these parameters. The data demonstrate that SST attenuates the METH-induced production of NO protecting the striatum from the METH-induced cell loss. However, SST failed to prevent the toxicity of the dopamine terminals suggesting that pre- and post-synaptic striatal damage occur via independent mechanisms.
Keywords: methamphetamine, glutamate, somatostatin, nitric oxide, striatum
1. Introduction
The illicit psychostimulant methamphetamine (METH) is a longer lasting and more potent derivative of the stimulant amphetamine. METH exerts its effects on the brain through its influence on the monoaminergic system. Its efficacy is governed by the resemblance of its chemical structure to the neurotransmitter dopamine allowing it to enter the dopamine terminal, cause the excessive release of dopamine, and prevent its reuptake (Sulzer et al., 1995; Jones et al., 1998; Krasnova and Cadet, 2009; Logan, 2002; Sulzer, 2011). Exposure to METH results in a plethora of neurochemical dysfunctions including but not limited to the prolonged decrease in the enzyme tyrosine hydroxylase (Hotchkiss and Gibb, 1980), tissue dopamine and metabolite levels (Fumagalli et al., 1998; Villemagne et al., 1998), reduction in dopamine transporters (Baucum et al., 2004) and cell death in some regions of the brain including the striatum and the cortex (O'Dell, 1992; Deng et al., 2001; Eisch and Marshall, 1998; Pu et al., 1996; Zhu et al., 2005). The METH-induced dopamine overflow may be the triggering event but it is not the sole causative factor of METH toxicity, compelling evidence implicates glutamate transmission in METH neurotoxicity (Sonsalla et al., 1989; Stephans and Yamamoto, 1994). For example, pharmacological inhibition of the NMDA receptor diminishes the damage caused by METH (O'Dell et al, 1992; Riddle et al., 2006; Sonsalla et al., 1991). It is postulated that the generation of reactive oxygen species from NMDA-mediated excitotoxicity and oxidation of excessive cytoplasmic dopamine may serve as the mediator of damage in METH neurotoxicity through oxidative stress (Yamamoto and Zhu, 1998; Thomas et al., 2008). Several experiments have shown that free radical scavengers prior to METH attenuate METH neurotoxicity (Kawasaki et al., 2006; Yamamoto and Zhu, 1998).
Excessive activation of the NMDA receptor has been linked to activation of nitric oxide (NO) synthesis (Dawson and Dawson, 1996). During pathophysiological conditions excessive NO synthesis can result in the production of peroxynitrite, which can serve as both a reactive nitrogen and oxygen species (Beckman, 1996; Boje, 2004; Bruckdorfer, 2005). NO is synthesized by three different isoforms of the enzyme nitric oxide synthase (NOS), neuronal (nNOS), inducible (iNOS), and endothelial (eNOS) (Boje, 2004; Bruckdorfer, 2005). nNOS is considered the primary source of NO in the toxic cascade set-off by METH since several studies have shown that nNOS expression can be dynamically regulated by toxic insults including METH (Dawson et al., 1998; Deng and Cadet, 1999; Desaiah et al., 2000). Also, treatment with METH elevates the expression of nNOS (Dawson et al., 1998; Deng and Cadet, 1999; Desaiah et al., 2000), which contributes to METH toxicity since pharmacological and genetic inhibition of nNOS attenuated striatal dopamine terminal toxicity (Desaiah et al., 2000; Itzhak and Ali, 1996; Itzhak et al., 2000).
Previous work in our lab provides experimental evidence that the striatal neuropeptide substance P participates in the METH-induced striatal injury. Histological observation showed that exposure to METH resulted in a robust internalization of the neurokinin-1 receptor in the nNOS-expressing interneuron (Wang and Angulo, 2011b; Wang et al., 2008) and infusion of a substance P agonist by itself into the striatum resulted in increased 3-nitrotyrosine (3-NT) immunoreactivity, an indirect index of NO production (Wang and Angulo, 2011a; Ayata et al., 1997; Schulz et al., 1995). Moreover, suppression of substance P signaling reduced METH-induced NO synthesis and afforded protection from METH-induced striatal injury (Wang et al., 2008; Yu et al., 2004; Zhu et al., 2006). Recently, our group demonstrated utilizing selective agonists and antagonists of the neuropeptide Y Y1 and Y2 receptors that this neuropeptide modulates the METH-induced striatal production of NO (Yarosh and Angulo, 2012). In addition to substance P and neuropeptide Y, somatostatin (SST) is an intrinsic striatal neuropeptide well placed to modulate NO production in the presence of METH. In the striatum, SST is synthesized and stored in the SST/NPY/nNOS interneuron (Kawaguchi et al., 1995). SST is a neuroprotectant in pathologies attributed to glutamate-induced excitotoxicity (Cervia et al., 2008; Forloni et al., 1997). For example, following middle cerebral artery occlusion, infusion of SST or an agonist, reduced infarct volume (Rauca et al., 1999).
SST appears to exert an inhibitory influence on glutamatergic release and transmission in addition to intracellular calcium influx, which have been attributed as the means by which it protects from excitotoxic insults (Forloni et al., 1997). SST's influence on striatal signaling and growing evidence of neuroprotection in several excitotoxic models makes it a compelling candidate of further investigation in METH toxicity. It was the aim of the present study to test the hypothesis that SST is neuroprotective during METH toxicity. Moreover, we aimed to test that the influence of SST on NO synthesis and its possible neuroprotection is attributable to its inhibition on glutamate transmission, particularly via the NMDA subtype receptor.
2. Results
2.1. SST attenuates the METH-induced NO production and cell loss but not terminal degeneration
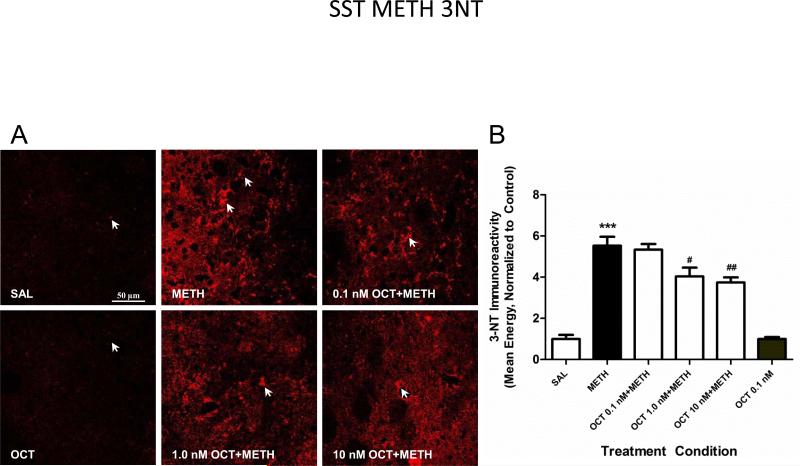
Animals received a 1 μl (rate of 0.1 μl per minute) intrastriatal infusion of the SST analogue OCT (0.1, 1.0 and 10 nM) in one hemisphere and aCSF in the other hemisphere. Fifteen minutes after the surgery both the control and experimental group were injected IP, the control group (aCSF) received saline and the experimental condition METH (30 mg/kg). We measured 3-NT immunoreactivity in striatal tissue sections by confocal microscopy. The SST agonist OCT dose dependently attenuated the METH-induced production of 3-NT (Figure 1). Based on these results the 10 nM dosage was then chosen for the subsequent series of experiments (METH and NMDA).
Figure 1.
SST analogue's modulation of METH-induced nitric oxide. (A) Pretreatment with the SST analogue octreotide (OCT) resulted in a dose dependent attenuation of METH-induced NO synthesis as measured by 3-nitrotyrosine (3-NT) immunohistochemistry (confocal images taken at 63x). Mice (n=6) received intrastriatal infusions of aCSF (right striatum) or OCT (left striatum); followed 15 minutes later by an injection of METH (30 mg/kg, i.p.) or saline. Animals were sacrificed 6 hours after METH treatment. (B) 3-NT immunoreactivity was determined utilizing confocal microscopy and Leica imaging software to measure staining intensity. (***p<0.001 as compared to the aCSF group; #p<0.05, ##p<0.01 as compared to the METH group).
Degeneration of neurons native to the striatum was measured 24 hours post-METH by staining with Fluoro-Jade C and utilizing stereological cell counts. Dopamine terminal damage was determined by quantifying striatal TH protein levels 72 hours after METH by Western blot analysis. TH is the rate-limiting enzyme necessary for the production of catecholamines such as dopamine (Fibiger and McGeer, 1971). The presence of TH in the striatum is used as an indicator of DA terminal viability. As seen in Figure 2, pretreatment with OCT showed a significant protection that almost reached control baseline levels. The agonist by itself showed no effect whereas METH as expected had a substantial and significant increase in cell loss (Figure 2). Alternatively, animals pretreated with OCT did not demonstrate protection of dopamine terminals (Figure 3), TH levels remained almost equivalent to METH levels. Treatment with METH showed the expected and significant reduction in TH levels and the OCT alone group's TH levels were comparable to baseline levels (Figure 3).
Figure 2.
Protection from METH-induced cell death by SST analogue. Cell death of striatal cells was measured using Fluoro-Jade C (A). Fluorescent images taken at 63X demonstrate that pretreatment with an SST analogue (OCT) had a significant protective effect on METH-induced striatal cell loss. Male ICR mice (n=6) received intrastriatal infusions of aCSF or the SST analogue OCT; followed 15 minutes later by an injection of METH (30 mg/kg, ip) or saline. Animals were sacrificed at 24 hours after METH treatment. (B) Striatal cell loss was measured using automated stereological cell counts of Fluoro-Jade C positive cells. The control group, which received an intrastriatal infusion of aCSF followed by an IP injection of saline, served as the baseline. Treatment with OCT protected from METH-induced cell loss. (**p<0.01 as compared to the aCSF group, ##p<0.01 as compared to the METH group).
Figure 3.
Somatostatin does not protect dopamine terminals from METH toxicity. (A) Western blot analysis was used to measure striatal tyrosine hydroxylase (TH) protein levels and thus determine dopamine terminal viability. (B) Pretreatment with the SST analogue octreotide (OCT) failed to protect from METH-induced dopamine terminal degeneration. Mice (n=6) received intrastriatal infusions of aCSF (right striatum) or OCT (left striatum); followed 15 minutes later by an injection of METH (30 mg/kg, i.p.) or saline. Animals were sacrificed at 72 hours after METH treatment. (* p<0.05, **p<0.01 as compared to the aCSF group).
2.2. SST has no influence on NMDA-induced NO synthesis or cell death
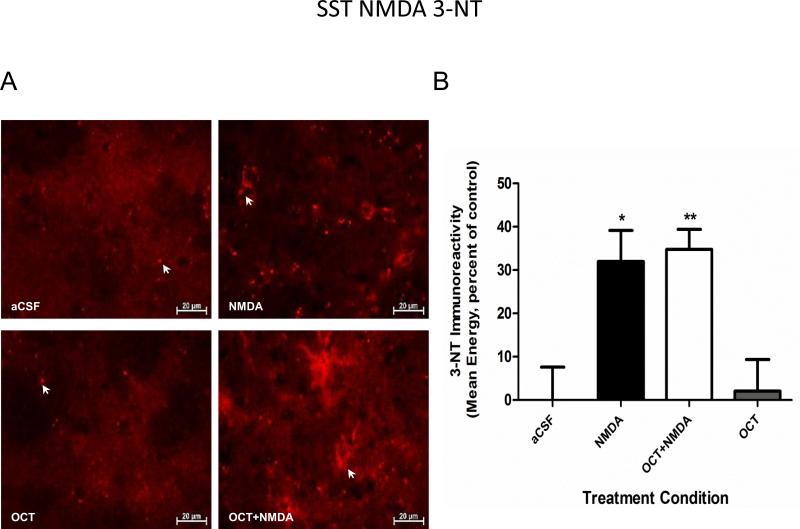
To investigate the possible connection between SST and striatal glutamate transmission, OCT was administered as described above with the exception that in the experimental condition, NMDA (20 nM) was dissolved in aCSF and infused into one hemisphere whereas OCT together with NMDA was infused into the contralateral hemisphere. The animals were sacrificed 24 hours later. In a previous study we had already determined that in glutamate-induced toxicity the peak time point of NO synthesis is 24 hours (data not shown). NO production was assessed by measuring the immunoreactivity of 3-NT in striatal tissue sections by confocal microscopy. Figure 4 shows that treatment with only the SST analogue OCT matched baseline levels in 3-NT immunoreactivity. Moreover, pretreatment with OCT did not have any effect on NMDA-induced NO production (Figure 4). Likewise when considering striatal cell loss, the agonist by itself matched baseline levels of Fluoro-Jade C positive cells (Figure 5). Additionally, treatment with OCT failed to protect from NMDA-induced apoptosis (Figure 5).
Figure 4.
Somatostatin did not influence NMDA mediated striatal NO production. (A) 3-Nitrotyrosine (3-NT) immunoreactivity was used as an indicator of striatal nitric oxide (NO) production (images taken at 63X). (B) Co-infusion of NMDA with OCT (SST analogue) had no effect on the NMDA-induced increase in 3-NT striatal levels. Mice (n=6) received an intrastriatal microinjection of a mixture of OCT and NMDA. The control group only received an infusion of aCSF. Animals were then sacrificed at 24 hours after NMDA treatment. (* p<0.05, **p<0.01 as compared to the aCSF group).
Figure 5.
Somatostatin does not confer protection from NMDA-mediated striatal injury. (A) Cell death of neurons in the striatum was assessed via the fluorescent stain Fluoro-Jade C. Fluorescent images (63X) taken show that NMDA results in a significant amount of cell loss. Co-infusion into the striatum of an SST receptor agonist (OCT) and NMDA had no effect on this cell loss. (B) Cell death was measured 24 hours after METH via unbiased stereological cell counts of Fluoro-Jade C positive cells. Treatment with OCT had no effect on the NMDA-induced striatal cell death whereas OCT on its own did not cause any significant increase in cell loss (***p<0.001 as compared to the aCSF group).
3. Discussion
Our results suggest that the striatal neuropeptide SST is neuroprotective during METH toxicity insofar as abrogating striatal cell loss. As a modulator of striatal activity and many of its transmitters primarily through inhibition, SST has the capability to play a pivotal role in the events following METH as implicated by our data. In the striatum it is synthesized and stored in the SST/NPY/nNOS interneuron, colocalizing with NPY and nitric oxide synthase (Rajput et al., 2011a). Although this particular interneuron comprises approximately 0.8-2% of the striatal neuronal population (Cicchetti et al., 2000; Tepper and Bolam, 2004), they are localized throughout the striatum (Allen et al., 2003). Additionally, they synapse with projection neurons and with the terminals of glutamate releasing neurons from the cortex (Galarraga et al., 2007; Hathway et al., 2001; Vuillet et al., 1989); both are neuronal populations central to the events following METH. We demonstrate here that OCT (SST analogue) had a mitigating influence on NO synthesis thus reducing the oxidant state caused by METH. OCT was chosen based on a number of criteria, first that it has the highest affinity for the SST receptors 2 and 5, which according to several receptor expression studies may be the predominant subtypes expressed within the rodent striatum (Allen et al., 2003; Galarraga et al., 2007; Rajput et al., 2011a, 2011b). Therefore, they would be the most plausible receptors to have an impact on METH-induced toxicity. Second, in multiple lines of excitotoxicity research in rodent models most of SST's protective effects are observed to be a function of the SST2 receptor (Cervia et al., 2008; Mastrodimou et al., 2008; Rauca et al., 1999), particularly in models testing glutamate-based neurotoxicity. In our study we infused OCT directly into the striatum prior to the systemic administration of a toxic dose of METH (30 mg/kg).
Our data demonstrate that SST attenuated the METH-induced striatal cell loss but failed to protect the striatal dopamine terminals as assessed by tyrosine hydroxylase protein levels by Western blot. Previous work in our laboratory suggested that dopamine terminal degeneration is a distinct mechanism from that underlying striatal cell loss (Zhu et al., 2006) which is further strengthened when one considers that their time course differs as well. Peak cell loss is reached 24 hours after treatment with METH whereas dopamine terminal degeneration reaches its peak at least another 24 hours later (Zhu et al., 2005) implying that either an additional set of events must occur for the initiation of dopamine terminal degeneration or cell death must precede terminal degeneration. Some studies suggest that unlike dopamine terminal toxicity, METH induces striatal apoptosis in part via cross-talk between the endoplasmic reticulum and mitochondrial destabilization involving caspase-dependent and –independent pathways (Jayanthi et al., 2001, 2004). Moreover, there have been some indicators that SST receptors can become rapidly downregulated in response to endogenous SST (Tallent and Qiu, 2008; Vasilaki et al., 2004). Additionally, they may also become desensitized to SST agonists whether through internalization or uncoupling of the cell surface receptor to the intracellular machinery necessary for second messenger signaling (Cervia et al., 2008). It is possible that SST's protective influence is better suited for the early events following METH thus rendering it ineffective in preserving dopamine terminals. None-the-less the data supports that SST can and did serve a protective role during exposure to METH.
In light of the intrinsic role glutamate plays in METH toxicity, an additional purpose of this study was to conduct an initial investigation into the mechanisms underlying SST's protective actions, namely exploring whether SST would depress NMDA-mediated NO synthesis and retain its protective effect in NMDA-mediated striatal injury. Several studies have indicated that there is a relationship between glutamate and SST within the striatum, in both in vitro and in vivo murine models application of NMDA caused the release of SST (Forloni et al., 1997; Hathway et al., 2001, Kumar, 2008). In a kindling rodent model of epilepsy, investigators found a higher baseline level of SST released in the hippocampus than in the naïve animals (Marti et al., 2000). Generally, in these models of hyperexcitability an altered biosynthesis of SST has been observed and is theorized as a compensatory mechanism attempting to establish homeostatic balance in the affected region (Tallent and Qiu, 2008). Also, SST has been shown to serve as a neuroprotectant in several paradigms of excitotoxicity such as middle cerebral artery occlusion (Rauca et al., 1999) and retinal ischaemia (Cervia et al., 2008; Kigiadaki and Thermos, 2008; Kigiadaki et al., 2010), this has been attributed to its ability to dampen neuronal hyperexcitability (Allen et al., 2003; Mastrodimou et al., 2008).
Neurochemical analysis of mice lacking the SST 1 or 5 receptor revealed that they shared many similarities to a transgenic Huntington's murine model (Rajput et al., 2011a). One such similarity was the critical loss of a large percentage of striatal projection neurons thus implicating SST signaling as playing a central role in the regulation of neurodegeneration. In fact, one neurochemical index of an Alzheimer's brain is the depletion of cortical SST (Forloni et al., 1997). The selective loss of SST-containing neurons is also a hallmark of an epileptic hippocampus in rodents as well as in humans suffering from temporal lobe epilepsy (Tallent and Qiu, 2008). All of the above mentioned neurological diseases share certain similarities, one of which is aberrant glutamatergic transmission as either a primary or secondary factor in their pathology as well as excessive NO synthesis whether by a calcium-dependent pathway or as part of a hyperactive inflammatory response (Boje, 2004; Duncan and Heales, 2005). We expected SST to attenuate the NMDA-induced striatal neural damage, however, our data show that OCT failed to protect striatal neurons from NMDA.
What alternative mechanism might be utilized by SST to explain our results? The SST/NPY/nNOS interneuron has been shown to synapse with corticostriatal neurons (Hathway et al., 2001; Vuillet et al., 1989). Inhibition of glutamate release from corticostriatal neurons would reduce activation of the ionotropic NMDA receptors located on SST/NPY/nNOS interneuron (Kawaguchi, 1997). Thus diminishing the influx of extracellular calcium, which is a necessary ingredient for the activation of nNOS and thus NO synthesis. There is experimental evidence indicating that SST2 receptors are present on the terminals of murine corticostriatal neurons (Hathway et al., 2001). Moreover, SST has the ability to depress presynaptic calcium currents (Selmer et al., 2000). Research by Boehm and Betz (1997) suggests that activation of the G-protein coupled SST receptors results in the second messenger depression of voltage gated calcium channels located on the terminal. SST's ability to influence calcium current influx suggests that it is inhibiting transmitter release. It is theorized that one manner SST protects from excitotoxicity is by inhibiting glutamate release (Tallent and Qiu, 2008). There is corroborating data that shows a decrease in glutamate release after application of OCT to the retina of mice (Cervia et al., 2008). Momiyama and Zaborszky (2006) showed that exogenous application of SST to slices of the basal forebrain of rats reduced glutamate release in a calcium dependent manner. Moreover, the inhibitory action of SST on glutamate release could serve as a means to stem the secondary or rather continual loop of glutamate release that occurs with METH thus reducing activation of the NMDA/nNOS cascade.
An additional possibility is an autocrine mechanism in which SST depresses nNOS catalytic activity through intracellular second messenger signaling. There have been several studies indicating that SST exerts direct stimulation or inhibition on NO production (Arena et al., 2005; Cordelier et al., 2006; Lopez et al., 2001; Vasilaki et al., 2004). The differences have been attributed to SST receptor subtype as well as cellular strain and or tissue differences, which would provide different intracellular substrates for second messenger signaling (Arena et al., 2005). However, two independent groups utilizing the same cell lines (CHO-K1) expressing rat SST receptors found that SST analogues were able to depress nNOS catalytic activity through the activation of second messenger pathways (Arena et al., 2005; Cordelier et al., 2006). Cordelier et al. (2006) demonstrated that an analogue for SST5 receptor depressed NO synthesis by inactivation of nNOS via phosphorylation, whereas Arena et al. (2005) showed that activation of SST2/3 receptors blocked intracellular signaling for the mobilization of calcium from intracellular stores (endoplasmic reticulum). Although the exact receptor subtype expressed by individual murine striatal neuronal populations is still uncertain, the overall consensus is that the striatum as well as individual neurons express a heterogeneous mixture (Selmer et al., 2000). In a histological analysis, Rajput et al. (2011b) showed that markers for all SST receptor subtypes colocalized with a marker for rat striatal projection neurons. In addition, Allen et al. (2003) found that in the mouse striatum the SST2 receptor was localized on cholinergic interneurons and on projection neurons that release substance P. Therefore, it is quite feasible that the SST/NPY/nNOS interneuron may possess the SST receptor necessary to activate the above discussed autocrine mechanisms and depress nNOS catalytic activity in a more direct fashion.
In conclusion, our data demonstrate that the striatal neuropeptide SST can serve as a neuroprotectant in the presence of a toxic dose of METH. However, its protective capacity is limited to striatal cell death. Although SST is known to modulate glutamate signaling, our study suggests that this is not the means by which it confers protection. Although the results do not completely eliminate the possibility that SST may instead inhibit the release of glutamate rather than its transmission via the NMDA-subtype receptor. Overall, more work is merited to further evaluate SST's function during METH toxicity and the associated mechanism. A more thorough understanding of the role it plays during METH toxicity could prove valuable to the identification of novel therapeutic targets. Especially in light of the fact that METH and neurodegenerative diseases impact similar brain areas and pathways.
4. Materials and Methods
4.1. Animal care and use
All procedures regarding animal use were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Hunter College of the City University of New York. The Hunter College animal facility is certified by the American Association for Accreditation of Laboratory Animal Care (AAALAC). Male ICR (Imprinting Critical Region) mice (Taconic, Germantown, NY) between 11 to 12 weeks of age were housed individually on a 12-h light/dark cycle with food and water available ad libitum. Mice were habituated to the housing environment and the experimenters for two weeks prior to commencement of intraperitoneal (ip) drug administration. The work described in this article was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for animal experiments.
4.2. Drug preparation and treatment
(+)-Methamphetamine hydrochloride (Sigma, St. Louis, MO) was dissolved in 0.9% physiological saline and administered as a bolus ip injection (30 mg/kg). Control animals received an equivalent volume of 0.9% physiological saline. The SST analogue OCT (Bachem, Torrance, CA) was dissolved in artificial cerebral spinal fluid (aCSF). It was microinjected directly into the striatum at a concentration of 10 nM 15 minutes prior to METH treatment. However for the NMDA-induced lesion, OCT and NMDA were infused concomitantly as a mixture. NMDA (20 nM) was also dissolved in aCSF. Intrastriatal infusion of compounds was performed as delineated in the subsequent section.
Animals used for western blot analysis of tyrosine hydroxylase levels were sacrificed 72 hours after treatment by decapitation. Their brains were then dissected, frozen on dry ice, and stored in −80°C until use. Animals used for 3-NT immunofluorescence or Fluoro-Jade C staining were sacrificed 24 hours post-treatment via intracardial perfusion. They were anesthetized by an ip injection of ketamine (100mg/kg) and acepromazine (3 mg/kg), perfused intracardially with 20 ml of phosphate buffered saline (PBS); followed by 20 ml 4% paraformaldehyde in PBS. Brains were removed and post-fixed overnight in 4% paraformaldehyde at 4°C. Brains were then cryo-protected in 30% sucrose in 0.1M PBS until they sank in the solution and then stored at −80°C until use.
4.3. Intrastriatal infusion of NMDA and OCT
Stereotaxic microinjection procedure was adapted from Ayata et al. (1997). Mice were anesthetized with inhaled isoflurane (2.5% for induction, 2.0% for maintenance). Their heads were immobilized in a stereotaxic frame (Model 5000; David Kopf Instruments, Tujunga, CA) and a burr hole was drilled into the skull at the following coordinates: +0.5 mm rostral-caudal from bregma; +/− 2.0 mm medial-lateral from bregma; −2.5 mm dorsal-ventral from dura (Franklin and Paxinos, 1997). A 2 μL microinjection needle (25 ga, Hamilton, Reno, NV) was lowered into the striatum, specifically into the caudate-putamen, and allowed to remain in position for 5 minutes. NMDA and or OCT were injected into the striatum using the quintessential stereotaxic injector (Stoelting, Wheat Lane, IL) at a rate of 0.1μl/minute and the needle remained in place for an additional 5 minutes before its removal. The wound was closed with VetBond (n-butyl cyanoacrylate, 3M) tissue adhesive and the animal was allowed to recover.
4.4. 3-NT fluorescent immunohistochemistry
Sectioning and staining was carried out by the free-floating method. Striatal 30 μm coronal sections were cut on a cryostat at −20°C. The sections were collected serially between Bregma 0.02 and 1.4 mm, with each tissue sample separated from the next sample in the series by 180 μm. Thus each sample well represents an entire striatum. They were then stored in a solution of 30% glycerin, 30% ethylene glycol, 40% PBS at −20°C until used. The sections were then rinsed in PBS and incubated 3X for 10 minutes in 10 mM citric acid at 65°C. Washed with PBS 3X for 5 minutes each; followed by incubation in the M.O.M (mouse on mouse) kit blocking reagent (Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Rinsed with PBS 3X for 5 minutes each and then incubated for 10 minutes at room temperature in M.O.M kit diluent solution. Proceeded by incubation overnight at 4°C in mouse monoclonal anti-3-NT (1:200, Santa Cruz Biotech, Santa Cruz, CA) in M.O.M diluent buffer. The next day they were washed with PBS 3X for 5 minutes each. Sections were incubated in a solution of 5% goat serum in 0.2% triton PBS for 1 hour at room temperature. Then another 1 hour at room temperature in Cy3 goat anti-mouse (1:500, Millipore, Temecula, CA) in 1% goat serum and 0.2% triton PBS. They were then washed an additional 3X with PBS for 5 minutes each. Mounted and coverslipped using Vectashield fluorescent hardset™ mounting medium with DAPI (Vector Laboratories, Burlingame, CA). The slides were imaged with a Leica TCS SP2 scanning confocal microscope (Leica Microsystems, Germany) and quantified using the Leica imaging software.
4.5. Measurement of 3-NT immunoreactivity
Quantification procedure was adapted from Gazzaley et al. (1996). From each slide at least 4 out of 6 tissue sections were selected for imaging with the Leica scanning confocal (Leica Microsystems, Germany). All tissues selected must have a visible needle tract to ensure that the effect observed is due to the injected solution. Per tissue, 4 areas were chosen within each hemisphere. The regions chosen were adjacent to each side of the needle tip but avoiding the visible needle damage. They were then scanned only once to prevent quenching, which would affect the results. Images were taken by individuals that were blind to the experimental conditions. The images were all scanned at 63X with preset parameters that give the most resolved image in the baseline condition. Said parameters were set per individual study to account for slight variations that may occur during the staining process from one individual study to another. All the tissue for a study was sectioned and processed simultaneously. Hardware and software settings were maintained the same for all images scanned thereafter. The overall settings were as follows: area scanned was 56889.33 mm2, line average of 1, zoom factor of 1.00, and a scan speed of 400 Hz. The digitized image is 512×512 pixels and an 8 bit grey resolution with a range in intensity of 0-255. The settings for the pinhole, frame average, gain, and offset were adjusted per study to accommodate for expected minor staining differences. The image includes both the cells as well as the neuropil. Analysis of 3-NT immunoreactivity was done using the Leica confocal software. Background produced by nonspecific binding was removed using the baseline correction feature (eliminate autofluorescence) in the image process option. Then using the histogram feature under the quantify tab we were able to get the mean energy of each image, which represents the staining intensity of the image. The average of the mean energy of the animal was obtained and then statistically analyzed.
4.6. TH western blot analysis
Using a brain matrix (ASI-Instruments, Warren, MI) on ice a 2 mm thick coronal section of the striatum was removed at the site of the injection. The samples were homogenized in approximately 150 μl of lysis buffer (40 mM Tris-HCL, 274 mM NaCL, 2.0 mM EGTA, 20% glycerol, 1 mM Na3VO4, 1 mM PMSF, 1 mM ß-glycerophosphate, 2.5 Na4P2O7, 50 mM NaF, 1% NP40, and protease inhibitor cocktail: 1.0 mM AEBSF, 0.8 μM aprotinin, 0.02 mM leupeptin, 0.04 mM bestatin, 0.015 mM pepstatin A, and 0.014 mM E-64) with a QSonica Sonicator 3000 cup horn at 7 cycles of 30 seconds of sonication and 60 seconds of cooling. Centrifuged at 4°C first at 3000 g for 5 minutes and then the supernatant was centrifuged at 2000 g for 5 minutes. The supernatant was removed once more and centrifuged for one final cycle of 1000 g for 10 minutes. The protein content was assayed by the Bradford method (Bio-Rad, Hercules, CA). Ten μg of protein were loaded on a 10% Tris-HCL (Invitrogen, Carlsbad, CA) SDS-PAGE and transferred to an iBlot stack membrane (Invitrogen, Carlsbad, CA). After blocking nonspecific binding using Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE) for 1 hour at room temperature, membranes were probed overnight with polyclonal rabbit anti-TH (1:5000, Millipore, Temecula, CA) antibody and monoclonal mouse anti-β-actin antibody (1:20,000, Sigma, St. Louis, MO) in Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE) with 0.2% tween 20 at 4°C. The next day the membranes were rinsed with 0.1% tween 20 PBS followed by 3 washes at 5 minutes each. They were then incubated in a mixture of Odyssey's IRDye® secondary antibodies donkey anti-rabbit 800CW (1:15,000) and donkey anti-mouse 680LT (1:30,000) within Odyssey's blocking buffer for 1 hour at room temperature. After an additional 3 washes at 5 minutes each with 0.1% tween 20 PBS as well as a final 15 minute wash with PBS alone, the proteins bands were then detected via the Odyssey infrared imager. Bands were quantified using the Odyssey Imager analysis software and normalized against β-actin.
4.7. Fluoro-Jade C stain
To stain first the tissue was rinsed with dH20 then mounted on Superfrost® Plus slides (VWR, West Chester, PA) and left to air dry. Once dried a border was drawn around the tissue using an ImmEdge™ pen (Vector Laboratories, Burlingame, CA). The tissue then went through washes in ethanol. First for 3 minutes in 100% percent ethanol followed by a 2 minute wash in 70% percent ethanol finally a 2 minute wash with dH2O. The slides were then exposed to 0.06% potassium permanganate for 10 minutes, the mixture was then removed and the slides allowed to air dry overnight at room temperature. The next day the slides were rinsed 2X with dH2O for 2 minutes per wash. The tissue was then immersed in 0.00005% Fluoro-Jade C (Millipore, Temecula, CA) in 0.1% acetic acid for 10 minutes. They were then washed 3X with dH2O for 1 minute per wash. The slides were then air dried at room temperature and the ImmEdge™ was removed using xylene. The slides were then coverslipped using Vectashield fluorescent hardset™ mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and no. 1.5 coverslips.
4.8. Fluoro-Jade C stereological cell counts
Cell counts were performed by two individuals that were blind to the experimental conditions using the AxioVision Rel. 4.8 (Imaging Associates Ltd, Carl Zeiss Group, Jena, Germany) unbiased stereology software for PC. Hardware components of the AxioVision system included a Zeiss microscope (Carl Zeiss Group, Germany) attached to a mechanical stage 75×50 mot; CAN (D) (Carl Zeiss Group, Germany), a high resolution AxioCam MRm Rev. 3 (Carl Zeiss Group, Germany), and a PC computer. Software parameters were set before the commencement of all counts and included a sampling frame area of 3873.904 μm2; the frame moved in steps automatically set at (x-step =900 μm, y-step=750 μm; 675000 μm2). A grid size was chosen so that an average of 10 or more probes per section was counted. Twenty-five micrometers was defined as the z-dimension in which cells were counted, giving a 2.5μm window for error on the top and bottom surface of the tissue. This area was excluded to account for the damage incurred by the tissue during the sectioning and staining process, which can lead to counting false positives. For all groups, AxioVision Rel. 4.8 Gundersen coefficient of error was less than or equal to 0.1. We counted four sections per animal; each section had to have a visible needle tract so that the affect seen was due to the respective treatment.
In brief, a cross section of the striatum from one hemisphere per tissue sample was outlined in 10X magnification to derive an estimate of the structure. The estimated striatal volume was automatically calculated by the software. The immediate area around the needle tract was removed from the outline ensuring that the resulting damage caused by the needle's entry is not included in the data. Actual cell counts were done at 100X magnification using the dissector probe in AxioVison Rel. 4.8 with the optical fractionator. All Fluoro-Jade C positive cells displaying the morphological features of an apoptotic cell had to be within the boundaries of the inclusion lines without touching the exclusion lines to be counted. Cells had to fulfill specific morphological criteria to be classified as striatal cells undergoing apoptosis, said criteria was established during the design stage and was strictly adhered to in the course of all counts. Criteria used were as follow: only cell bodies were counted to exclude degenerating DA terminals. In addition, the soma had to completely display a bright yellow-green color (Schmued et al., 1997); cell bodies that were only partially stained or that the stain was localized within the dendrites were excluded. Cells with a disrupted cell membrane were counted since dying neurons do not solely display a rounded shape but can have what is referred to as blebbling (interspersed bulging) of the membrane due to cytoskeletal decoupling as well as membrane disintegration in the final stages (Kroemer et al., 2009). Finally, cells with a densely stained nucleus were also counted since it indicates the degradation of the genetic material contained therein as expected in a cell undergoing apoptosis (Kroemer et al., 2009). Once counts were complete and the software automatically generated the results, the data was compiled and analyzed by a separate individual that did not participate in the cell counts.
4.9. Statistical analysis
Statistical analysis of the data was conducted using GraphPad prism (GraphPad Software, Inc., La Jolla, CA) statistical analysis software. The differences between groups were determined utilizing one way ANOVA mean±SEM. The analysis was followed by Tukey's post-hoc test. Differences between two groups were analyzed by Student's t-test. All statistical analysis were conducted with a significance criterion value set at p<0.05.
We investigated somatostatin and methamphetamine induced striatal nitric oxide
Somatostatin inhibited methamphetamine induced production of nitric oxide
Somatostatin protected from the methamphetamine induced apoptosis
Somatostatin failed to affect glutamate induced nitric oxide and apoptosis
ACKNOWLEDGMENTS
We would like to thank Dr. Maria Figueiredo-Pereira, Dr. Kai-Yvonne Shivers, Saranna Husband, and Melissa Rosso for their generous technical support and advice. This work was supported by R01 DA020142 from the National Institute on Drug Abuse to JAA. Support for infrastructure came from a grant from the National Center for Research Resources (G12 RR003037) and the National Institute on Minority Health Disparities (8 G12 MD007599) awarded to Hunter College by the NIH.
Abbreviations used
- 3-NT
3-nitrotyrosine
- ICR
Institute for Cancer Research
- ip
intraperitoneal
- METH
(+)-methamphetamine hydrochloride
- NO
nitric oxide
- NOS
nitric oxide synthase
- OCT
octreotide
- SST
somatostatin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen JP, Hathway GJ, Clarke NJ, Jowett MI, Topps ST, Kendrick K, Humphrey PPA, Wilkinson LS, Emson P. Somatostatin receptor 2 knockout/lacZ knockin mice show impaired motor coordination and reveal sites of somatostatin action within the striatum. Eur. J. Neurosci. 2003;17:1881–1895. doi: 10.1046/j.1460-9568.2003.02629.x. [DOI] [PubMed] [Google Scholar]
- Arena S, Pattarozzi A, Corsaro A, Schettini G, Florio T. Somatostatin receptor subtype-dependent regulation of nitric oxide release: involvement of different intracellular pathways. Mol. Endocrinol. 2005;19:255–267. doi: 10.1210/me.2004-0280. [DOI] [PubMed] [Google Scholar]
- Ayata C, Ayata G, Hara H, Matthews RT, Beal MF, Ferrante RJ, Endres M, Kim A, Christie RH, Waeber C, Huang PL, Hyman BT, Moskowitz MA. Mechanisms of reduced striatal NMDA excitotoxicity in type I nitric oxide synthase knock-out mice. J. Neurosci. 1997;17:6908–6917. doi: 10.1523/JNEUROSCI.17-18-06908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucum AJ, 2nd, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J. Neurosci. 2004;24:3436–43. doi: 10.1523/JNEUROSCI.0387-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- Boehm S, Betz H. Somatostatin inhibits excitatory transmission at rat hippocampal synapses via presynaptic receptors. J. Neurosci. 1997;17(11):4066–4075. doi: 10.1523/JNEUROSCI.17-11-04066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boje KMK. Nitric oxide neurotoxicity in neurodegenerative diseases. Front. Biosci. 2004;9:763–776. doi: 10.2741/1268. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer R. The basics about nitric oxide. Mol. Aspects Med. 2005;26:3–31. doi: 10.1016/j.mam.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Cervia D, Martini D, Ristori C, Timperio AM, Bagnoli P, Casini G. Modulation of the neuronal response to ischaemia by somatostatin analogues in wild-type and knock-out mouse retinas. J. Neurochem. 2008;106:2224–2235. doi: 10.1111/j.1471-4159.2008.05556.x. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, Prensa L, Wu Y, Parent A. Chemical anatomy of striatal interneurons in normal individuals and in patients with Huntington's disease. Brain. Res. Rev. 2000;34:80–101. doi: 10.1016/s0165-0173(00)00039-4. [DOI] [PubMed] [Google Scholar]
- Cordelier P, Estève J-P, Najib S, Moroder L, Vaysse N, Pradayrol L, Susini C, Buscail L. Regulation of neuronal nitric-oxide synthase activity by somatostatin analogs following sst5 somatostatin receptor activation. J. Biol. Chem. 2006;281:19156–19171. doi: 10.1074/jbc.M602024200. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Sasaki M, Gonzalez-Zulueta M, Dawson VL. Regulation of neuronal nitric oxide synthase and identification of novel nitric oxide signaling pathways. Prog. Brain Res. 1998;118:3–11. doi: 10.1016/s0079-6123(08)63196-9. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J. Chem. Neuroanatomy. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- Deng X, Cadet JL. Methamphetamine administration causes overexpression of nNOS in the mouse striatum. Brain Res. 1999;851:254–257. doi: 10.1016/s0006-8993(99)02087-9. [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res. Mol. Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Desaiah D, Reddy SLN, Imam SZ, Ali SZ. Role of neuronal nitric oxide in methamphetamine neurotoxicity and protection by nNOS inhibitor. Pure Appl. Chem. 2000;72:1001–1006. [Google Scholar]
- Duncan AJ, Heales JR. Nitric oxide and neurological disorders. Mol. Aspects Med. 2005;26:67–96. doi: 10.1016/j.mam.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Marshall JF. Methamphetamine neurotoxicity: dissociation of striatal dopamine terminal damage from parietal cortical cell body injury. Synapse. 1998;30:433–445. doi: 10.1002/(SICI)1098-2396(199812)30:4<433::AID-SYN10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, McGeer EG. Effect of acute and chronic methamphetamine treatment on tyrosine hydroxylase activity in brain and adrenal medulla. Eur. J. Pharm. 1971;16:176–180. doi: 10.1016/0014-2999(71)90008-2. [DOI] [PubMed] [Google Scholar]
- Forloni G, Lucca E, Angeretti N, Chiesa R, Vezzani A. Neuroprotective effect of somatostatin in nonapoptotic NMDA-induced neuronal death: role of cyclic GMP. J. Neurochem. 1997;68:319–327. doi: 10.1046/j.1471-4159.1997.68010319.x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG. Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J. Neurosci. 1998;18:4861–4869. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarraga E, Vilchis C, Tkatch T, Salgado H, Tecuapetla F, Perez-Rosello T, Perez-Farci R, Hernandez-Echeagaray E, Surmeier DJ, Bargas J. Somatostatinergic modulation of firing patter and calcium-activated potassium currents in medium spiny neostriatal neurons. Neurosci. 2007;146:537–554. doi: 10.1016/j.neuroscience.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathway GJ, Humphrey PPA, Kendrick KM. Somatostatin release by glutamate in vivo is primarily regulated by AMPA receptors. Brit. J. Pharmacol. 2001;134:1155–1158. doi: 10.1038/sj.bjp.0704362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J. Pharm. Exp. Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J. Neurochem. 1996;67:1770–1773. doi: 10.1046/j.1471-4159.1996.67041770.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Ali SF. nNOS inhibitors attenuate methamphetamine-induced dopaminergic neurotoxicity but not hyperthermia in mice. NeuroReport. 2000;11:2943–2946. doi: 10.1097/00001756-200009110-00022. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neoxcortex. FASEB J. 2001;15:1745–1752. doi: 10.1096/fj.01-0025com. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles P-AH, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238–251. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J. Neurosci. 1998;18:1979–86. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci. Res. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurons--chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Ishihara K, Ago Y, Nakamura S, Itoh S, Baba A, Matsuda T. Protective effect of the radical scavenger edaravone against methamphetamine-induced dopaminergic neurotoxicity in mouse striatum. Eur. J. Pharmacol. 2006;542:92–99. doi: 10.1016/j.ejphar.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Kiagiadaki F, Thermos K. Effect of intravitreal administration of somatostatin and sst2 analogs on AMPA-induced neurotoxicity in rat retina. Invest. Ophth. Vis. Sci. 2008;49:3080–3089. doi: 10.1167/iovs.07-1644. [DOI] [PubMed] [Google Scholar]
- Kiagiadaki F, Savvaki M, Thermos K. Activation of somatostatin receptor (sst5) protects the rat retina from AMPA-induced neurotoxicity. Neuropharmacol. 2010;58:297–303. doi: 10.1016/j.neuropharm.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzil L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein PP, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U. Somatostatin in medium-sized aspiny interneurons of striatum is responsible for their preservation in quinolinic acid and N-methyl-D-aspartate-induced neurotoxicity. J. Mol. Neurosci. 2008;35:345–354. doi: 10.1007/s12031-008-9093-3. [DOI] [PubMed] [Google Scholar]
- Logan BK. Methamphetamine - Effects on human performance and behavior. Forens. Sci. Rev. 2002;14:133–151. [PubMed] [Google Scholar]
- Lopez F, Ferjoux G, Cordelier P, Saint-Laurent N, Estève J-P, Vaysse N, Buscail L, Susini C. Neuronal nitric oxide synthase is a SHP-1 substrate involved in sst2 somatostatin receptor growth inhibitory signaling. FASEB J. 2001;15:2300–2302. doi: 10.1096/fj.00-0867fje. [DOI] [PubMed] [Google Scholar]
- Marti M, Bregola G, Morari M, Gemignani A, Simonato M. Somatostatin release in the hippocampus in the kindling model of epilepsy: A microdialysis study. J. Neurochem. 2000;74:2497–2503. doi: 10.1046/j.1471-4159.2000.0742497.x. [DOI] [PubMed] [Google Scholar]
- Mastrodimou N, Kiagiadaki F, Thermos K. The role of nitric oxide and cGMP in somatostatin's protection against retinal ischemia. Invest. Ophth. Vis. Sci. 2008;49:342–349. doi: 10.1167/iovs.07-0341. [DOI] [PubMed] [Google Scholar]
- Momiyama T, Zaborszky L. Somatostatin presynaptically inhibits both GABA and glutamate release onto rat basal forebrain cholinergic neurons. J. Neurophysiol. 2006;96:686–694. doi: 10.1152/jn.00507.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell SJ, Weihmuller FB, Marshall JF. MK-801 prevents methamphetamine-induced striatal dopamine damage and reduces extracellular dopamine overflow. Ann. N. Y. Acad. Sci. 1992;648:317–319. doi: 10.1111/j.1749-6632.1992.tb24567.x. [DOI] [PubMed] [Google Scholar]
- Pu C, Broening HW, Vorhees CV. Effect of methamphetamine on glutamate-positive neurons in the adult and developing rat somatosensory cortex. Synapse. 1996;23:328–334. doi: 10.1002/(SICI)1098-2396(199608)23:4<328::AID-SYN11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Rajput PS, Kharmate G, Norman M, Liu S-H, Sastry BR, Brunicardi CF, Kumar U. Somatostatin receptor 1 and 5 double knockout mice mimic neurochemical changes of Huntington's disease transgenic mice. PLoS ONE. 2011a;6:e24467. doi: 10.1371/journal.pone.0024467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput PS, Kharmate G, Kumar U. Colocalization of somatostatin receptors with DARPP-32 in cortex and striatum of rat brain. J. Mol. Neurosci. 2011b doi: 10.1007/s12031-011-9678-0. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Rauca C, Schafer K, Hollt V. Effects of somatostatin, octreotide, and cortistatin on ischaemic neuronal damage following pretreatment middle cerebral artery occlusion in the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:633–638. doi: 10.1007/s002109900136. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced neurotoxicity. A. A. P. S. J. 2006;8:E413–E418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;75:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Matthews RT, Jenkins BG, Ferrante RJ, Siwek D, Henshaw DR, Cipolloni PB, Mecocci P, Kowall NW, Rosen BR, Beal MF. Blockade of neuronal nitric oxide synthase protects against excitotoxicity in vivo. J. Neurosci. 1995;15:8419–8429. doi: 10.1523/JNEUROSCI.15-12-08419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmer I-S, Schindler M, Allen JP, Humphrey PPA, Emson PC. Advances in understanding somatostatin receptors. Regul. Peptides. 2000;90:1–18. doi: 10.1016/s0167-0115(00)00108-7. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Nicklas WJ, Heikkila RE. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989;243:398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Riordan DE, Heikkila RE. Competitive and noncompetitive antagonists at N-methyl-D-aspartate receptors protect against methamphetamine-induced dopaminergic damage in mice. J. Pharmacol. Exp. Ther. 1991;256:506–512. [PubMed] [Google Scholar]
- Sulzer D, Chen T-K, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J. Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallent MK, Qiu C. Somatostatin: An endogenous antiepileptic. Mol. Cell. Endocrinol. 2008;286:96–103. doi: 10.1016/j.mce.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J. Neurochem. 2008;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki A, Papadaki T, Notas G, Kolios G, Mastrodimou N, Hoyer D, Tsilimbaris M, Kouroumalis E, Pallikaris I, Thermos K. Effect of somatostatin on nitric oxide production in human retinal pigment epithelium cell cultures. Invest. Ophth. Vis. Sci. 2004;45:1499–1506. doi: 10.1167/iovs.03-0835. [DOI] [PubMed] [Google Scholar]
- Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J. Neurosci. 1998;18:419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillet J, Kerkeria L, Kachidian P, Bosler O, Nieoullon A. Ultrastructural correlates of functional relationships between nigral dopaminergic or cortical afferent fibers and neuropeptide Y-containing neurons in the rat striatum. Neurosci. Lett. 1989;100:99–104. doi: 10.1016/0304-3940(89)90667-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Angulo JA. Synergism between methamphetamine and the neuropeptide substance P on the production of nitric oxide in the striatum of mice. Brain Res. 2011a;1369:131–139. doi: 10.1016/j.brainres.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Angulo JA. Methamphetamine induces striatal neurokinin-1 receptor endocytosis primarily in somatostatin/NPY/NOS interneurons and the role of dopamine receptors in mice. Synapse. 2011b;65:300–308. doi: 10.1002/syn.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xu W, Ali SF, Angulo JA. Connection between the striatal neurokinin-1 receptor and nitric oxide formation during methamphetamine exposure. Ann. N. Y. Acad. Sci. 2008;1139:164–171. doi: 10.1196/annals.1432.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J. Pharmacol. Exp. Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- Yarosh HL, Angulo JA. Modulation of methamphetamine-induced nitric oxide production by neuropeptide Y in the murine striatum. Brain Res. 2012;1483:31–38. doi: 10.1016/j.brainres.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Wang J, Cadet JL, Angulo JA. Histological evidence supporting a role for the striatal neurokinin-1 receptor in methamphetamine-induced neurotoxicity in the mouse brain. Brain Res. 2004;1007:124–131. doi: 10.1016/j.brainres.2004.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Angulo JA. Disparity in the temporal appearance of methamphetamine-induced apoptosis and depletion of dopamine terminal markers in the striatum of mice. Brain Res. 2005;1049:171–181. doi: 10.1016/j.brainres.2005.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Angulo JA. Distinct mechanisms mediating methamphetamine-induced neuronal apoptosis and dopamine terminal damage share the neuropeptide substance P in the striatum of mice. Ann. N. Y. Acad. Sci. 2006;1074:135–148. doi: 10.1196/annals.1369.013. [DOI] [PMC free article] [PubMed] [Google Scholar]