Abstract
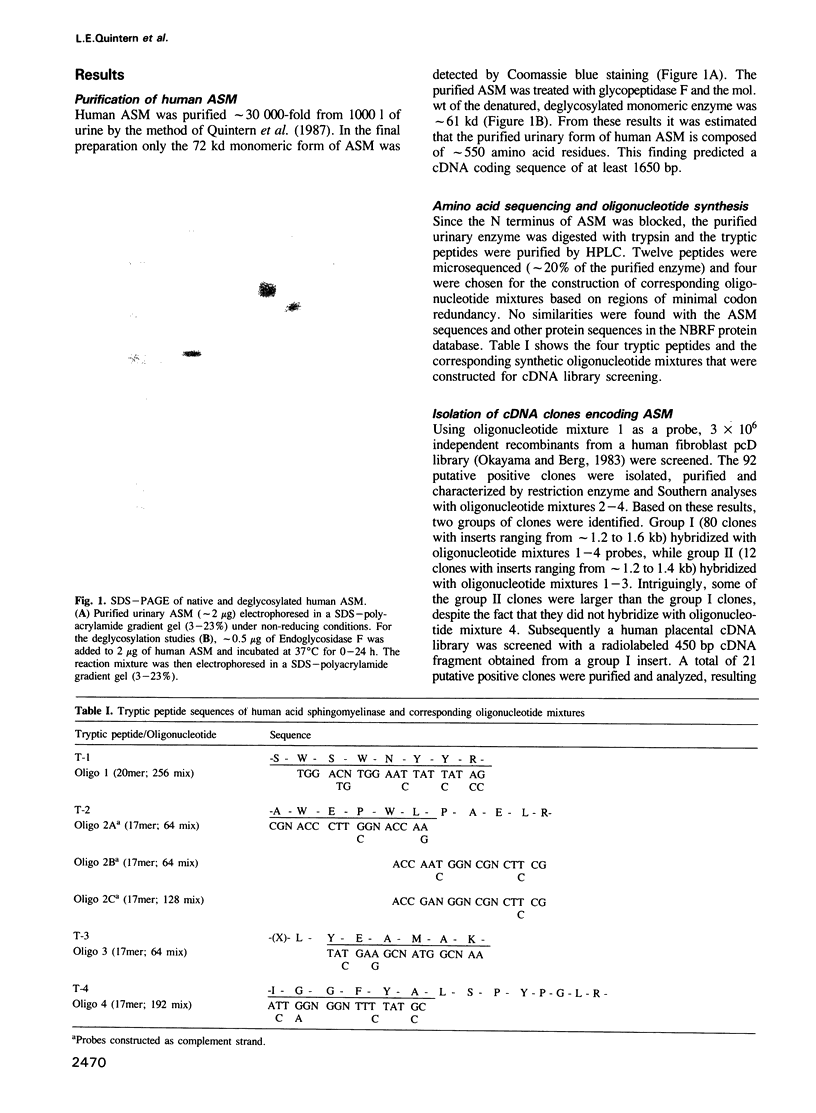
Acid sphingomyelinase (sphingomyelin phosphodiesterase, EC 3.1.4.12) was purified from human urine and 12 tryptic peptides were microsequenced (128 residues). Based on regions of minimal codon redundancy, four oligonucleotide mixtures were synthesized and oligonucleotide mixture 1 (20mer; 256 mix) was used to screen 3 X 10(6) independent recombinants from a human fibroblast cDNA library. Putative positive clones (92) were purified and analyzed by Southern hybridization with oligonucleotide mixtures 2-4. These studies revealed two groups of clones; group 1 (80 clones; inserts ranging from approximately 1.2 to 1.6 kb) hybridized with oligonucleotides mixtures 1-4, while group II (12 clones; inserts ranging from approximately 1.2 to 1.4 kb) hybridized with oligonucleotide mixtures 1-3. Several group II clones had larger inserts than those in group I, but did not hybridize with oligonucleotide mixture 4. Screening of a human placental cDNA library with a 450 bp group I fragment, also resulted in the isolation of group I and II clones. Representative clones from group I (pASM-1) and group II (pASM-2) were sequenced. pASM-1 contained a 1879 bp insert which was colinear with 96 microsequenced amino acids, while the pASM-2 1382 bp insert was colinear with 78 microsequenced residues. Notably, pASM-2 did not have an internal 172 bp sequence encoding 57 amino acid residues, but had instead an in-frame 40 bp sequence encoding 13 amino acids which was not present in pASM-1. These findings demonstrate the presence of two distinct acid sphingomyelinase transcripts in human fibroblasts and placenta and suggest the occurrence of alternative processing of the mRNA encoding this lysosomal hydrolase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besley G. T., Hoogeboom A. J., Hoogeveen A., Kleijer W. J., Galjaard H. Somatic cell hybridisation studies showing different gene mutations in Niemann-Pick variants. Hum Genet. 1980;54(3):409–412. doi: 10.1007/BF00291589. [DOI] [PubMed] [Google Scholar]
- Caruthers M. H. Gene synthesis machines: DNA chemistry and its uses. Science. 1985 Oct 18;230(4723):281–285. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]
- Hempel J., von Bahr-Lindström H., Jörnvall H. Aldehyde dehydrogenase from human liver. Primary structure of the cytoplasmic isoenzyme. Eur J Biochem. 1984 May 15;141(1):21–35. doi: 10.1111/j.1432-1033.1984.tb08150.x. [DOI] [PubMed] [Google Scholar]
- Itakura K., Rossi J. J., Wallace R. B. Synthesis and use of synthetic oligonucleotides. Annu Rev Biochem. 1984;53:323–356. doi: 10.1146/annurev.bi.53.070184.001543. [DOI] [PubMed] [Google Scholar]
- Levade T., Salvayre R., Douste-Blazy L. Sphingomyelinases and Niemann-Pick disease. J Clin Chem Clin Biochem. 1986 Apr;24(4):205–220. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A., Kyle J. W., Miller R. D., Hoffmann J. W., Powell P. P., Grubb J. H., Sly W. S., Tropak M., Guise K. S., Gravel R. A. Cloning, sequencing, and expression of cDNA for human beta-glucuronidase. Proc Natl Acad Sci U S A. 1987 Feb;84(3):685–689. doi: 10.1073/pnas.84.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintern L. E., Weitz G., Nehrkorn H., Tager J. M., Schram A. W., Sandhoff K. Acid sphingomyelinase from human urine: purification and characterization. Biochim Biophys Acta. 1987 Dec 14;922(3):323–336. doi: 10.1016/0005-2760(87)90055-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P. B., Kennedy E. P. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J Lipid Res. 1967 May;8(3):202–209. [PubMed] [Google Scholar]
- Stone K. L., Williams K. R. High-performance liquid chromatographic peptide mapping and amino acid analysis in the sub-nanomole range. J Chromatogr. 1986 May 30;359:203–212. doi: 10.1016/0021-9673(86)80074-7. [DOI] [PubMed] [Google Scholar]




