SUMMARY
Chromosomal inversion between 3q21 and 3q26 results in high-risk acute myeloid leukemia (AML). Here, we identified a mechanism whereby a GATA2 distal hematopoietic enhancer (G2DHE or −77-kb enhancer) is brought into close proximity to the EVI1 gene in inv(3)(q21;q26) inversions, leading to leukemogenesis. We examined the contribution of G2DHE to leukemogenesis by creating a bacterial artificial chromosome (BAC) transgenic model that recapitulates the inv(3)(q21;q26) allele. Transgenic mice harboring a linked BAC developed leukemia accompanied by EVI1 overexpression, neoplasia that was not detected in mice bearing the same transgene but missing the GATA2 enhancer. These results establish the mechanistic basis underlying the pathogenesis of a severe form of leukemia through aberrant expression of the EVI1 proto-oncogene.
INTRODUCTION
Leukemias are often induced by the rearrangement of chromosomes through translocations or inversions (Mitelman et al., 2007; Rowley, 2001). These chromosomal rearrangements can result in two types of abnormalities. The majority results in coding sequence fusions whereby a new protein is created. For example, in chronic myeloid leukemia, the t(9;22)(q34;q11) translocation creates a BCR-ABL fusion protein that functions as a constitutively active form of ABL tyrosine kinase (Rowley, 1973). A minor form of proto-oncogene activation is induced by linking the coding sequences of one gene to the regulatory sequences of a second gene, but approaches for exploring the underlying mechanisms by which regulatory sequence changes cause leukemias have proven to be technically challenging. One example of the latter mechanism is when MYC (located on 8q24) is placed under the regulatory control of the immunoglobulin heavy chain (IGH) locus on 14q32, thus creating the t(8;14)(q24;q32) translocation allele (ar-Rushdi et al., 1983).
In this regard, EVI1 is known to be a proto-oncogene that can be activated by chromosomal translocation and inversion (Morishita et al., 1992), although the underlying mechanism(s) that lead to leukemia are obscure. EVI1 is a transcription factor that is required for maintenance of hematopoietic stem cells (Goyama et al., 2008), and overexpression of EVI1 has been observed in high-risk myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) (Lugthart et al., 2008). Indeed, EVI1 harbors several hallmark functions that are normally associated with leukemogenesis, with the following four possibly the most intriguing. First, EVI1 regulates the transcription of the transcription factor genes GATA2 and PBX1, both of which play critical roles in the maintenance of hematopoietic stem cells, as well as the tumor suppressor gene Pten (Sato et al., 2008; Shimabe et al., 2009; Yoshimi et al., 2011; Yuasa et al., 2005). Second, EVI1 binds to the transcription factors RUNX1, PU.1 and GATA1 thereby inhibiting their activity and blocking the differentiation of hematopoietic progenitors (Laricchia-Robbio et al., 2006; Laricchia-Robbio et al., 2009; Senyuk et al., 2007). Third, EVI1 interacts with multiple epigenetic modification enzymes, each of which can alter specific target gene expression profiles (Chi et al., 2003; Goyama et al., 2010; Izutsu et al., 2001; Lugthart et al., 2011; Shimahara et al., 2010; Spensberger et al., 2008; Yoshimi et al., 2011). Finally, forced EVI1 overexpression disrupts normal centrosome duplication, creating increased genomic instability (Stein et al., 2010).
It has been reported that both t(3;3)(q21;q26) and inv(3)(q21;q26), chromosomal translocation and inversion between 3q21 and 3q26, respectively, cause inappropriate EVI1 gene expression (Morishita et al., 1992; Suzukawa et al., 1994). In both the translocated and inverted alleles, 3q21 has been shown to always include sequences both 5’ and 3’ to the EVI1 gene on 3q26. Since EVI1 is overexpressed in both kinds of mutant alleles, we speculated that the 3q21 region that is brought into close proximity of EVI1 as a result of the translocation or inversion might harbor an enhancer that then ectopically activates EVI1 gene expression. In this regard, as all breakpoints on 3q21 are located downstream of the Ribophorin I (RPN1) gene (that encodes a transmembrane glycoprotein that is localized in the rough endoplasmic reticulum; (Kreibich et al., 1978), one attractive possibility was that RPN1 enhancers might provide the ectopic activation required for malignant EVI1 transcription.
The GATA2 gene is located ~160-kb 3’ to the RPN1 gene on 3q21. GATA2 function is required in hematopoietic stem cells, but its expression diminishes during hematopoietic differentiation (Akashi et al., 2000; Lim et al., 2012; Minegishi et al., 2003; Suzuki et al., 2006; Tsai and Orkin, 1997; Yamamoto et al., 1990). In hematopoietic stem and progenitor cells (HSPC), GATA2 positively regulates the expression of GATA2. Multiple phylogenetically conserved regions containing GATA factor binding sites reside in the mouse Gata2 locus (at −3.9, −2.8, −1.8 and +9.5 kb from the Gata2 hematopoietic and neuronal specific promoter IS), all of which bear some degree of Gata2 enhancer activity (Grass et al., 2003; Grass et al., 2006; Johnson et al., 2012; Kobayashi-Osaki et al., 2005; Lim et al., 2012; Snow et al., 2011). In addition to these promoter and internal regulatory regions, another putative regulatory element has been identified by Bresnick and colleagues that is located 77-kb 5’ to the Gata2 gene. This element binds to GATA2 in the endogenous locus and acts as an enhancer in transfection assays (Grass et al., 2006). This element is located between RPN1 and the breakpoints that occur in both the 3q21 and 3q26 translocations and inversions, thus localizing this −77-kb 5’ GATA2 site in close proximity to the rearranged EVI1 gene in the resulting recombinant alleles. We therefore hypothesized that the −77-kb GATA2 binding site(s) were in an enhancer that misdirects EVI1 transcription in the translocated or inverted alleles between 3q21 and 3q26, and that this ectopic expression underlies the mechanism leading to leukemogenesis.
In this study, therefore, we asked whether the GATA2-bound sequences located at −77 kb from the GATA2 transcription start site indeed function as an enhancer in mouse HSPC in vivo. We also linked two bacterial artificial chromosomes (BACs) to generate a single recombinant human BAC clone that recapitulates juxtaposition of the sequences just as they are found in the inverted human allele, and then generated transgenic mice harboring this recombinant BAC to create a mouse model for leukemias that are associated with chromosomal rearrangements.
RESULTS
Identification of a Gata2 gene distal hematopoietic enhancer
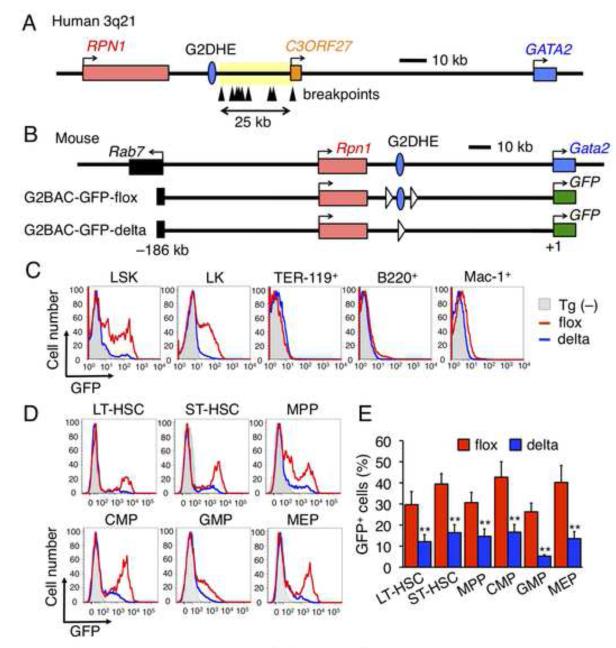
The breakpoints in 3q21 AMLs all clustered within an approximately 25-kb region (Figure 1A, arrowheads in yellow region) (Suzukawa et al., 1994; Suzukawa et al., 1997). We found that a region orthologous to the mouse Gata2 gene −77-kb GATA sites is located upstream (i.e., closer to RPN1) of the breakpoint cluster (Figure 1A, blue circle). We first tested whether this region exhibited enhancer activity for Gata2 in HSPC in vivo. To monitor Gata2 gene expression in hematopoietic cells, we generated green fluorescent protein (GFP) reporter transgenic (Tg) mouse lines using a BAC clone bearing 186 kb of 5’ sequence flanking the Gata2 gene (G2BAC-GFP, Figure 1B). LoxP sites were then inserted on both sides of the −77-kb GATA sites to test its contribution to GFP expression as a surrogate for Gata2. We injected the floxed BAC (G2BAC-GFP-flox) DNA into fertilized ova and generated G2BAC-GFP-flox Tg mice (Figure 1B). The G2BAC-GFP-flox Tg mice were then crossed to Ayu1-Cre mice that express Cre recombinase in their germ line (Niwa et al., 1993) to generate lines bearing the GATA binding site-deleted BACs (G2BAC-GFP-delta). Deletion of the element was confirmed by PCR (Figures S1A and S1B available online).
Figure 1. Identification of the Gata2 gene distal hematopoietic enhancer (G2DHE).
(A) Schematic structure of human 3q21 region. Arrowheads indicate documented breakpoints of translocation and inversion between 3q21 and 3q26. The breakpoints clustered in an approximately 25-kb region (yellow region). A blue circle indicates Gata2 distal hematopoietic enhancer (G2DHE). (B) Schematic structure of the G2BAC-GFP transgenes. The mouse Gata2 locus is depicted at the top. G2BAC-GFP transgene (G2BAC-GFP-flox or -delta) are shown beneath the endogenous Gata2 locus. White triangles represent loxP sequences. (C) Representative GFP histogram of bone marrow LSK, LK, TER-119+, B220+ and Mac-1+ cells from G2BAC-GFP-flox (red lines) and -delta (blue lines) mice. The gray shaded histograms represent GFP fluorescence in wild-type mice without a transgene (Tg (−)). (D) Representative GFP histogram in LT-HSC, ST-HSC, MPP, CMP, GMP and MEP cells from G2BAC-GFP-flox (red lines) and -delta (blue lines) mice. The gray shaded histograms represent GFP fluorescence in Tg (−) mice. (E) Percentages of GFP-positive cells in the indicated fractions of G2BAC-GFP-flox (red bars) and -delta (blue bars) mice (n=4). Data are represented as mean +/− SD. **, p<0.01 compared with G2BAC-GFP-flox mice.
See also Figure S1.
In G2BAC-GFP-flox Tg mice, GFP fluorescence was intense in a subset of Lin−Sca-1+c-kit+ (LSK) and Lin−Sca-1−c-kit+ (LK) progenitor cells, whereas little fluorescence was detected in differentiated erythroid (TER-119+), B (B220+) or myeloid lineage (Mac-1+) cells (Figure 1C, red lines). Thus the BAC transgene accurately recapitulates endogenous Gata2 gene expression in hematopoietic cells (Akashi et al., 2000; Suzuki et al., 2006; Suzuki et al., 2003). Of note, in the G2BAC-GFP-delta Tg mice, the percentages of GFP+ cells in both LSK and LK fractions were significantly reduced compared to those in cells recovered from the G2BAC-GFP-flox Tg mice (i.e. prior to deletion of the GATA sequences; Figure 1C, blue lines).
We further examined GFP fluorescence in long-term hematopoietic stem cells (LT-HSC; CD34−Flt3−), short-term hematopoietic stem cells (ST-HSC; CD34+Flt3−) and multipotent progenitors (MPP; CD34+Flt3+) in the LSK fraction (Yang et al., 2005), as well as in common myeloid progenitors (CMP; CD34+FcγRII/III3low), granulocyte/macrophage progenitors (GMP; CD34+FcγRII/III3high) and megakaryocyte/erythrocyte progenitors (MEP; CD34−FcγRII/III3low) in the LK fraction (Akashi et al., 2000). The fraction of GFP-positive cells was significantly reduced at all stages in hematopoietic progenitors in the G2BAC-GFP-delta Tg mice (Figures 1D and 1E). Therefore, we refer to this sequence as the Gata2 distal hematopoietic enhancer (G2DHE).
Gata2 is also expressed in non-hematopoietic cells; neuronal, endothelial and urogenital cells. As the G2BAC-GFP Tg contains a neuronal enhancer, but not endothelial or urogenital enhancers (Khandekar et al., 2007; Khandekar et al., 2004), the G2BAC-GFP Tg recapitulates Gata2 expression only in neural tissues, especially in midbrain (Nozawa et al., 2009). Therefore, we examined the contribution of G2DHE to Gata2 expression in the midbrain and found that GFP fluorescence and mRNA levels in the midbrain of the G2BAC-GFP-delta Tg mice were comparable to the G2BAC-GFP-flox Tg mice (Figures S1C-E). These results indicate that G2DHE is dispensable for Gata2 gene expression in the midbrain.
Generation of 3q21q26 Tg mice by linking two BACs
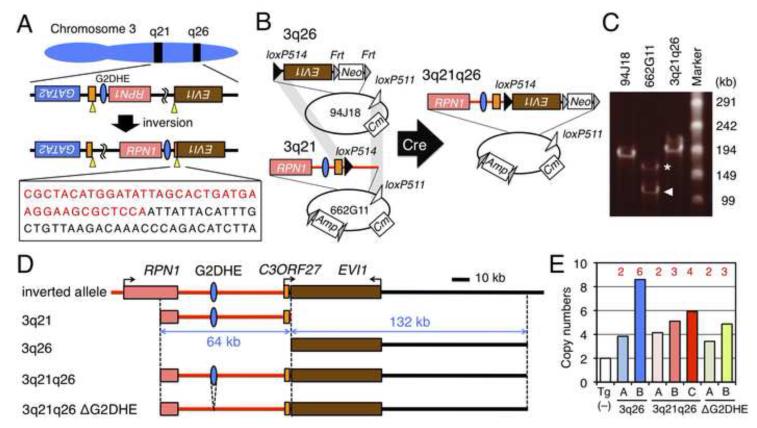
To determine whether G2DHE activates EVI1 transcription in the rearranged allele, we generated a Tg model mouse line that would precisely recapitulate the breakpoints in inv(3)(q21;q26). To do so, we duplicated the specific inversion breakpoint in the MOLM-1 cell line, which was originally established from a patient with megakaryoblastic leukemia bearing the inv(3)(q21;q26) inversion (Matsuo et al., 1991; Suzukawa et al., 1997). In MOLM-1, inversion occurred between the 3rd exon of the C3ORF27 gene in 3q21 and the 15th intron of EVI1 in 3q26 (Suzukawa et al., 1997). As the position of the precise breakpoint has not been reported, we determined the sequence of the MOLM-1 inversion breakpoint (Figure 2A).
Figure 2. Generation of 3q21q26 mice by linking two BACs.
(A) Chromosomal inversion of the region between breakpoints (yellow arrowheads) in EVI1 (brown box) and C3ORF27 (orange box) genes in MOLM-1 cells. The boxed sequence shows the breakpoint between 3q21 (red) and 3q26 (black). (B) Strategy for linking two BAC clones. Human BACs RP11-94J18 (94J18) and RP11-662G11 (662G11) contain 3q26 and 3q21, respectively. We used site-specific Cre-mediated recombination to link the two BAC clones by simultaneous intermolecular homologous recombination between the loxP514 (black triangles) and loxP511 (white triangles) sites. Neo, Cm and Amp indicate the neomycin-, chloramphenicol- and ampicillin-resistant genes, respectively. (C) Pulse-field gel electrophoresis of BAC clones. We found two types of 662G11 clones; full-length (asterisk) and partially deleted (arrowhead) clones. (D) Schematic representations of the inverted allele, 3q21, 3q26, 3q21q26 and 3q21q26ΔG2DHE BAC transgenes. Red and black lines represent contributions from 3q21 and 3q26, respectively. (E) Copy numbers of integrated transgenes. Copy numbers were determined by q-PCR using Tg (−), 3q26 (lines A and B), 3q21q26 (lines A, B and C) and 3q21q26ΔG2DHE (lines A and B) mice (n=3-6). Red numbers depicted above the bar graphs represent copy numbers of transgenes.
See also Figure S2, Table S1 and Supplemental Experimental Procedures.
We next generated a BAC clone harboring recombinant human DNA that precisely recapitulated the inv(3)(q21;q26) genomic region in MOLM-1 cells by linking two BAC clones (RP11-662G11 and RP11-94J18) containing the 3q21 and 3q26 sequences, respectively (Figure 2B) (Brandt et al., 2008). During the process of BAC modification, we found both full length (indicated by an asterisk) as well as a partially deleted RP11-662G11 clone (Figure 2C, arrowhead; hereafter RP11-662G11d). Closer analysis revealed that the recombinant insert of the deleted clone lacked approximately 50-kb of flanking sequences 5’ to the RPN1 gene (data not shown). We therefore chose to use this deleted clone in subsequent studies, thus allowing us to exclude any possible contribution of RPN1 expression to EVI1 transcription. We targeted loxP514 sequences into the breakpoint positions of the RP11-662G11d and RP11-94J18 BACs (Figure 2B; Figures S2A and S2B) and subsequently linked these two BAC clones by simultaneous Cre-mediated recombination between the loxP514 sites inserted at the breakpoints and between the loxP511 sites in the BAC vector backbones; the linked BAC clone was named 3q21q26. We verified that the 3q21q26 clone contains the 64-kb region of 3q21 and the expected 132-kb region of 3q26 by pulse-field gel electrophoresis, Southern blotting and fingerprinting (Figure 2C; Figures S2C-G).
Utilizing the 3q21q26 clone, we generated three lines of Tg mice (3q21q26 mice, Figure 2D). As one negative control, we also generated two lines of Tg mice harboring the unlinked 3q26 BAC clone (3q26 mice). To directly test whether the G2DHE could activate EVI1 in the inverted allele, we also deleted the G2DHE from 3q21q26 (referred to as 3q21q26ΔG2DHE) and generated two additional lines of Tg mice (Figure 2D; Figures S2H-J). All of the 3q21q26, 3q26 and 3q21q26ΔG2DHE Tg mice were born in the expected Mendelian ratios, grew normally, and were fertile (Table S1). The copy numbers for the integrated transgenes was determined by quantitative PCR (q-PCR) and revealed that the mice retained from 2 to 6 copies of the transgenes (Figure 2E).
Expression of the human EVI1 transgene in HSPC of 3q21q26 mice
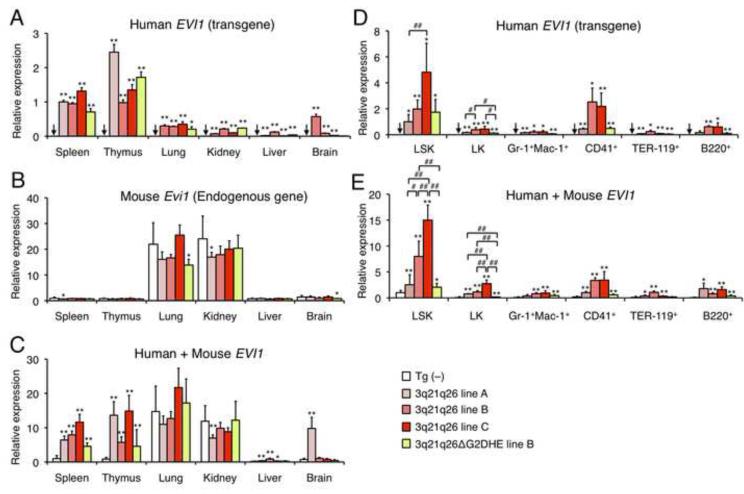
Whereas only hematopoietic lineage cells harbor the rearranged allele in human patients, every cell type should share the rearranged allele in the 3q21q26 mice. Therefore, it was important to determine the expression profile of the human EVI1 transgene in the 3q21q26 mice. We quantified EVI1 expression levels using three primer pairs that either detected exclusively the human transgene EVI1, the endogenous murine Evi1 gene, or both simultaneously. Interestingly, the human EVI1 transgene was specifically expressed in hematopoietic tissues (spleen and thymus) in the 3q21q26 mice (Figure 3A), whereas the endogenous Evi1 gene was additionally highly expressed in the lung and kidney (Figure 3B). In these mice, the abundance of human EVI1 mRNA in hematopoietic tissues is comparable to that of the mouse Evi1 mRNA in lung and kidney (Figure 3C). These results are consistent with the notion that the human 3q21q26 transgene bears enhancer activity that can activate EVI1 in hematopoietic tissues.
Figure 3. Expression profiles of the human EVI1 transgene in 3q21q26 mice.
(A-C) Expression levels of EVI1 gene in hematopoietic and non-hematopoietic tissues. Expression levels of human EVI1 mRNA (A), mouse endogenous Evi1 mRNA (B) and total (human + mouse) EVI1 mRNA (C) in hematopoietic tissues (spleen and thymus) and non-hematopoietic tissues (lung, kidney, liver and brain) of Tg (−) (n=10), 3q21q26 mouse lines (line A, n=3; line B, n=3; line C, n=3) and 3q21q26ΔG2DHE line B (n=4) were determined. The level of each mRNA was normalized to rRNA abundance. In panels B and C, average values for Tg (−) spleen cells were set to 1, while in panel A spleen cells of 3q21q26 line A was set to 1. (D, E) Abundance of EVI1 gene expression in hematopoietic cells. Expression levels of human EVI1 mRNA (D) and total EVI1 mRNA (E) in hematopoietic stem and progenitor cells and differentiated cells in Tg (−) (n=19), 3q21q26 (line A, n=3-6; line B, n=3-7; line C, n=4) and 3q21q26ΔG2DHE line B (n=4) bone marrows were determined. The abundance of each mRNA was normalized to the rRNA abundance. Average values for 3q21q26 line A LSK cells (D) and Tg (−) LSK cells (E) were set to 1.
Bar graphs are represented as mean +/− SD. Arrows indicate undetectable expression levels. *, p<0.05; **, p<0.01 compared with Tg (−). #, p<0.05; ##, p<0.01.
To more precisely examine the expression profiles of the EVI1 transgene in hematopoietic lineages, bone marrow cells were fractionated into stem cells, progenitors or various lineage-committed cells. The EVI1 transgene was highly expressed in the LSK fraction, which contains HSPC, but its expression diminished upon differentiation (Figures 3D and 3E). This expression pattern of the rearranged EVI1 transgene recapitulates that of the endogenous Gata2 gene (Minegishi et al., 2003; Suzuki et al., 2006; Tsai and Orkin, 1997) and is similar to that of the endogenous (murine) Evi1 gene in hematopoietic cells (Kataoka et al., 2011). The abundance of EVI1 mRNA was dependent on the copy number of the integrated transgenes in LSK and LK fractions of the 3q21q26 mice (Figure 3E).
In the 3q21q26ΔG2DHE mice, expression of EVI1 transgene was also detected in hematopoietic tissues (Figures 3A-C). Of note, we found that, when mice harbor the same copy number of transgenes, the total EVI1 mRNA level is clearly diminished in LSK and LK fractions of the 3q21q26ΔG2DHE mice when compared to the 3q21q26 mice (Figure 3E). These results indicate that G2DHE contributes to elevated EVI1 gene expression in hematopoietic cells.
3q21q26 mice develop G2DHE-dependent leukemia
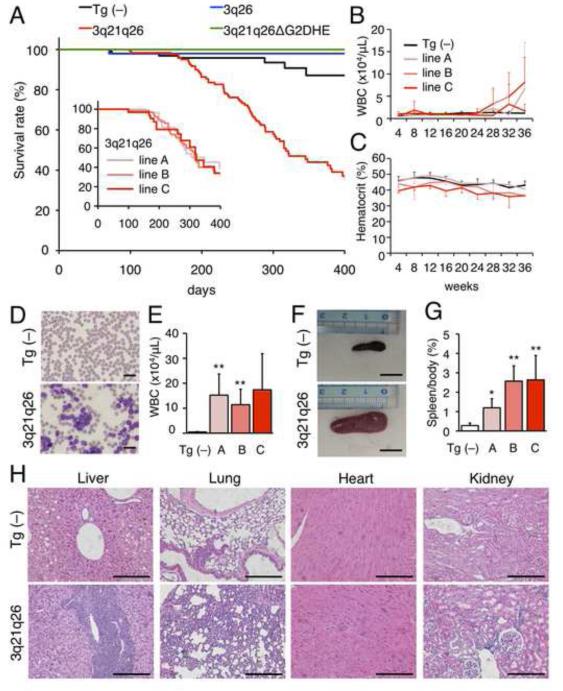
To determine whether the 3q21q26 mice develop leukemia, we examined a cohort of these animals, and determined from Kaplan-Meier analyses that the 3q21q26 mice survived far shorter than control (Tg (−)) or 3q26 mice (Figure 4A). All three lines (line A, B and C) of 3q21q26 mice exhibited similar survival and began to die around 200 days after birth (Figure 4A, insert graph). Hematological indices of the 3q21q26 mice revealed that white blood cell numbers in the peripheral blood increased markedly after 24 weeks (Figure 4B) with constitutive mild anemia (Figure 4C). 3q21q26 mice that had increased (> 5×104/μL) white blood cell counts were sacrificed (Figures 4D and 4E) and determined to display marked splenomegaly (Figures 4F and 4G). Leukocytes had infiltrated into the liver, lung, heart and kidney in the 3q21q26 mice (Figure 4H), suggesting that the 3q21q26 mice developed leukemia.
Figure 4. 3q21q26 mice develop G2DHE-dependent leukemia.
(A) Kaplan-Meier survival curve of Tg (−) (n=96), 3q26 (n=55), 3q21q26 (n=121) and 3q21q26ΔG2DHE (n=26) mice. Survival rates are calculated by compiling data from multiple Tg mouse lines. The inserted graph shows the overall survival of each line of 3q21q26 mice (lines A, B and C). (B, C) White blood cell counts (B) and hematocrits (C) in the peripheral blood of Tg (−) (n=11-46) and 3q21q26 line A (n=6-19), line B (n=5-15) and line C (n=3-9) mice. (D) Representative smears of peripheral blood taken from Tg (−) and leukemic 3q21q26 mice. Scale bar, 20 μm. (E) The numbers of white blood cells in leukemic 3q21q26 line A (n=6), line B (n=7) and line C (n=3) mice and Tg (−) (n=5) littermates. (F) Representative spleens from Tg (−) and leukemic 3q21q26 mice. Scale bar, 1 cm. (G) Average spleen weights from Tg (−) (n=5) and leukemic 3q21q26 line A (n=6), line B (n=7) and line C (n=3) mice. (H) Hematoxylin-eosin staining of tissues of Tg (−) (top panels) and leukemic 3q21q26 line B (bottom panels) mice. Scale bar, 100 μm.
Bar graphs are represented as mean +/− SD. *, p<0.05; **, p<0.01 compared to Tg (−).
When we carried out cohort studies of the 3q21q26ΔG2DHE mice to determine whether G2DHE contributed to the leukemogenesis, we determined that 3q21q26ΔG2DHE survived similarly to control (Tg (−)) mice and did not develop leukemia over the 400-day time course of the experiment (Figure 4A). These results firmly support the contention that G2DHE is essential for EVI1-dependent leukemogenesis in the 3q21q26 mice.
3q21q26 mice develop different types of leukemia
In human chromosomal rearrangements between 3q21 and 3q26, EVI1 ectopic expression results primarily in myeloid rather than lymphoid leukemia (Lugthart et al., 2008). To determine whether leukemia in the 3q21q26 mice recapitulates the human leukemia most often associated with EVI1 misexpression, we analyzed the peripheral blood and bone marrow of the 3q21q26 mice. In all three lines of the 3q21q26 mice, CD34+ and c-kit+ blast cells were observed in the peripheral blood (Figure 5A), whereas blast cells were only rarely observed in Tg (−) mice. CD34+ and c-kit+ cells were also more frequent in the bone marrow of 3q21q26 mice (Figure S3A). Additionally, B220+ antigen was detected in the 3q21q26 line A mice, while Gr-1+ and Mac-1+ antigens were detected in the 3q21q26 line B and C mice (Figure 5A; Figure S3A).
Figure 5. 3q21q26 mice suffer from three types of leukemia.
(A) Expression profiles of CD34, c-kit, B220, CD3a, Gr-1, Mac-1 and TER-119 in peripheral blood (PB) leukemic cells of 3q21q26 line A (n=5), line B (n=5-6) and line C (n=3) mice as well as mononuclear cells of Tg (−) mice (n=4-5). *, p<0.05; **, p<0.01. (B) Flow cytometric analyses of leukemic cells in 3q21q26 mice. Representative flow cytometric patterns of B-type (left panel), B+GM-type (middle panel) and GM-type (right panel) leukemias are shown. (C) Wright-Giemsa staining of leukemic cells in the peripheral blood of 3q21q26 mice. Scale bar, 10 μm. As a control, mononuclear cells from Tg (−) mice are shown. (D) Frequencies of B-type (white), B+GM-type (gray) and GM-type (black) leukemia developed in the three 3q21q26 mouse lines.
See also Figure S3.
When different combinations of surface markers were analyzed, three distinct types of leukemia could be discerned in the 3q21q26 mice. The first is a B-cell type in which B220+ cells had preferentially expanded (Figure 5B, left panel). The second is a GM type in which Gr-1+Mac-1+ cells had abundantly expanded (right panel). Interestingly, the third type is one in which both B220+ and Gr-1+Mac-1+ had increased (center panel). Morphological analyses of the peripheral blood showed that lymphoid cells, neutrophils, or both were expanded in the B, GM and B+GM types of leukemia, respectively (Figure 5C). In the B- and B+GM-type leukemia, B220+ cells expressed c-kit and Flt3, but not CD19 (Figures S3B and S3C), indicating that these cells correspond to pre-pro B cells (Nutt and Kee, 2007).
We note that the incidence of each type of leukemia correlates with increased copy numbers of the integrated transgenes. 3q21q26 line A mice bear two copies of the transgene and developed B cell leukemia, whereas GM type leukemias were observed in 3q21q26 mice lines B and C, bearing three or four copies of the transgene, respectively (Figure 5D). These results taken together suggest that the 3q21q26 mice, especially mice of lines B and C that express more abundant human EVI1, most accurately recapitulate the phenotype and pathophysiology of human leukemia that is accompanied by EVI1 ectopic expression.
3q21q26 leukemic cells expand autonomously
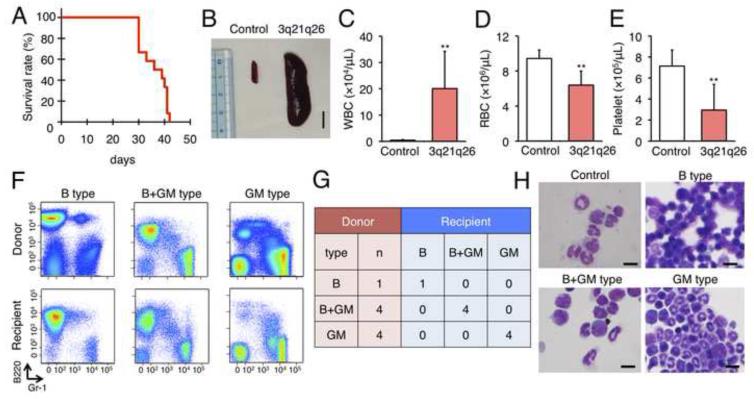
To determine whether the leukemic cells developed in the 3q21q26 mice progress autonomously if supported by wild-type cells in the leukemic niche, we isolated bone marrow cells from the 3q21q26 mice and transplanted them into nude mice. All recipient nude mice died with latencies of approximately 30-40 days following transplant (Figure 6A). As in the donor mice, the recipient nude mice exhibited marked splenomegaly (Figure 6B). In the peripheral blood of the recipients, the number of white blood cells dramatically increased over time, whereas red blood cells and platelets both diminished, indicating that the recipient mice developed transplant-dependent leukemia (Figures 6C-E).
Figure 6. Leukemic cells of 3q21q26 mice expand autonomously.
(A) Kaplan-Meier survival curve of recipient nude mice (n=12) transplanted with 107 mononuclear bone marrow cells from leukemic 3q21q26 mice. (B) Representative spleens from control or recipient nude mice 40 days after transplant. Scale bar, 1 cm. (C-E) Numbers of white blood cells (C), red blood cells (D) and platelets (E) in peripheral blood in control (n=3) and transplanted (n=11) nude mice. **, p<0.01 compared with control nude mice. (F) Representative flow cytometric analyses of peripheral blood cells from donor mice suffering from B-, B+GM-, and GM-type leukemia and comparison to their respective recipient nude mice. (G) Leukemia types of donor and recipient mice. Numbers in the table represent those of mice suffering from each type of leukemia. (H) Wright-Giemsa staining of leukemic cells in the peripheral blood of transplant recipient nude mice suffering from B-, B+GM-, and GM-type leukemia. Scale bar, 10 μm.
Bar graphs are represented as mean +/− SD.
We next carried out flow cytometry and morphological analysis of leukocytes in the peripheral blood and examined whether the phenotypes of the leukemia that developed in the recipient nude mice recapitulated that of the donors. All recipient nude mice developed leukemias (Figures 6F and 6G). In perfect agreement with analyses of the various donor animals, leukemic cells in the recipient mice developed B-, GM- or B+GM-type leukemias in lines A, B and C, and exhibited identical morphologies in lymphoid and/or neutrophil populations (Figure 6H). These data demonstrate that leukemic cells from the 3q21q26 mice progress autonomously in the transplanted nude mice, indicating that the leukemic cells in primary mice do not require a mutant microenvironment for their propagation.
Expansion of LSK cells and emergence of an abnormal cell population in 3q21q26 mice
As the human EVI1 transgene was highly expressed in LSK cells of the 3q21q26 mice, we hypothesized that misexpression of EVI1 in transgenic HSPC contributed to the leukemogenesis. To address this hypothesis, we quantified the absolute number of HSPC in the 12-week-old 3q21q26, 3q26 and 3q21q26ΔG2DHE mouse bone marrows prior to the onset of frank leukemia. Bone marrow cellularity in the 3q21q26 mice was comparable to that of 3q26 mice, while the number of LSK cells increased in the 3q21q26 mice (Figures 7A and 7B). Of specific note, the number of LSK cells in 3q21q26ΔG2DHE mice was much lower than that in 3q21q26 mice.
Figure 7. G2DHE drives EVI1 overexpression in HSPC.
(A-F) Representative flow cytometric profiles of Lin− cells (A), LSK cells (C) and LK cells (E) in 12-week-old Tg (−), 3q26 line B, 3q21q26 line B and 3q21q26ΔG2DHE line B mice. Cells in red boxes were analyzed in panels B, D and F. Absolute cell numbers of LSK and LK cells (B), LT-HSC, ST-HSC, MPP and CD34highFlt3− (CD34high) cells (D), and CMP, MEP and GMP cells (F) in the Lin− cell population in 12-week-old Tg (−) (white, n=4), 3q26 line B (blue, n=4), 3q21q26 line B (pink, n=4) and 3q21q26ΔG2DHE line B (green, n=4) mice are depicted. (G-I) Expression levels of human EVI1 mRNA (G), mouse endogenous Evi1 mRNA (H), and total EVI1 mRNA (I) in LT-HSC, ST-HSC, MPP, CD34high, CMP, MEP and GMP fractions in 12-week-old Tg (−) (white, n=4-8), 3q26 line B (blue, n=4-8), 3q21q26 line B (pink, n=4-8) and 3q21q26ΔG2DHE line B (green, n=4-8) mice. The expression level of each mRNA was normalized to rRNA abundance. Average values for 3q26 LT-HSC cells (G) and Tg (−) LT-HSC cells (H, I) were set to 1.
Bar graphs are represented as mean +/− SD. Arrows indicate undetectable or slight expression levels. N.D.; not determined. *, p<0.05; **, p<0.01 compared with Tg (−). #, p<0.05; ##, p<0.01.
See also Figure S4.
We fractionated the LSK fraction of the 3q21q26 mice into LT-HSC, ST-HSC and MPP as usual, and discovered an abnormal CD34highFlt3− cell fraction. The CD34highFlt3− cells accumulated abundantly in LSK cells of the 3q21q26 mice, but not in 3q26 animals or in the LSK faction of control (Tg (−)) mice (Figures 7C and 7D). The CD34highFlt3− cells were still observed in 3q21q26 mice suffering from myeloid leukemia, suggesting that these cells are associated with myeloid leukemia (Figures S4A and S4B). We next examined the LK fractions containing CMP, MEP and GMP cells, and found that the number of CMP was fewer in 3q21q26 mice than in 3q26 or control (Tg (−)) mice, indicating that differentiation of the CMP progenitors was deficient in the 3q21q26 mice (Figures 7E and 7F). The phenotypic accumulation of CD34highFlt3− cells was partially reversed in the LSK cells of 3q21q26ΔG2DHE mice, indicating that the differentiation defects observed in the 3q21q26 progenitor cells were rescued, at least in part, by deletion of the GATA2 enhancer from the linked BAC (Figures 7C and 7D).
G2DHE drives EVI1 overexpression in HSPC
To elucidate whether the 3q21 region, and specifically G2DHE, is able to direct ectopic EVI1 gene expression in HSPC, we examined EVI1 transcription. The abundance of human EVI1 mRNA was significantly elevated in LT-HSC, ST-HSC and MPP from the 3q21q26 mice in comparison to 3q26 mice (Figure 7G). In contrast, human EVI1 abundance in the CD34highFlt3− fraction was much lower than in the LT-HSC, ST-HSC and MPP fractions. Overexpression of the human EVI1 transgene was significantly diminished in the enhancer-deleted 3q21q26ΔG2DHE mice, demonstrating that G2DHE could be directly responsible for activating the human EVI1 transgene in LT-HSC, ST-HSC and MPP cells. Unexpectedly, we found that the levels of endogenous mouse Evi1 mRNA diminished to undetectable levels in the 3q21q26 and the 3q21q26ΔG2DHE mice (Figure 7H). The total EVI1 level (human EVI1 + mouse Evi1 mRNAs) in the 3q21q26 mice was higher than in the 3q26 mice, but the level in the 3q21q26ΔG2DHE mice was comparable to that in the 3q26 mice (Figure 7I). These results indicate that the G2DHE located in the 3q21 region activates EVI1 transcription in the inverted 3q26 allele, leading to the observed leukemogenesis.
In LT-HSC of 3q21q26 mice, the levels of known EVI1 target genes Gata2 and Pbx1 mRNAs (Shimabe et al., 2009; Yuasa et al., 2005), but not Myb, Foxo3a and Meis1, were increased compared with Tg (−) mice (Figure S4C). These results indicate that EVI1 up-regulates a subset of target genes, which are important for the maintenance of HSCs, in LT-HSC of the 3q21q26 mice. Additionally, the abundance of Pten tumor suppressor (Yoshimi et al., 2011) and the cyclin-dependent kinase inhibitor p57 (Matsumoto et al., 2011) was lower in 3q21q26 LT-HSC. We envisage that EVI1 may provoke the progression of cell cycle by regulating Pten and p57 expression. In the CD34highFlt3− cells from the 3q21q26 mice, the abundance of Meis1, Gata2, Pbx1 and p57 gene transcripts, which are all highly expressed in LT-HSCs, had largely decreased, while in contrast, we detected high level of PU.1. These results collectively suggest that the CD34highFlt3 − cells retain gene expression profiles similar to myeloid progenitors, rather than stem cells.
DISCUSSION
Translocation or inversion between 3q21 and 3q26 induces aberrant EVI1 gene expression, which gives rise to human AML that have a poor prognosis. Here we describe the contribution of a far upstream hematopoietic enhancer of the GATA2 gene (G2DHE) to EVI1 gene expression in a typically rearranged human inversion. We generated 3q21q26 mice by linking two separate BAC clones containing the 3q21 and 3q26 regions in the same physical orientation as they are found in a common AML cell line, and found that the Tg mice develop leukemias only when EVI1 is ectopically activated by G2DHE when the two genetic elements are brought into close proximity as a consequence of the inversion. We propose a model for EVI1-misexpressed leukemia that is associated with chromosomal rearrangements (Figure 8). In normal hematopoietic cells, the G2DHE is (at least partially) responsible for activating GATA2 gene expression (top panel). In leukemia cells bearing inversions or translocations between 3q21 and 3q26, G2DHE becomes localized either 3’ or 5’ to the EVI1 gene, respectively (medium and bottom panels). G2DHE activates the EVI1 gene instead of GATA2 in HSPC, thereby leading to leukemogenesis. This model is further supported by the conclusions from a study employing a completely different approach examining leukemic cells from human patients bearing the rearranged allele (Gröschel et al., 2014).
Figure 8. A model for the involvement of G2DHE in normal and 3q21q26 inverted/translocated hematopoietic cells.
In normal hematopoietic progenitors, G2DHE enhances GATA2 transcription (top panel). In AML, G2DHE becomes localized downstream or upstream of the EVI1 gene in inv(3)(q21;q26) (middle panel) or t(3;3)(q21;q26) (bottom panel) alleles, respectively. In both cases, the chromosomal rearrangement-dependent juxtaposition of G2DHE induces EVI1 gene transcription instead of GATA2 transcription which subsequently leads to leukemia.
G2DHE was originally identified as a phylogenetically conserved region containing binding sites for transcription factors GATA1 and GATA2 in the mouse Gata2 locus (Grass et al., 2006). It has been shown that the Gata2 gene regulation is highly dependent on these GATA factors. The Gata2 gene is activated by GATA2 itself in HSPC, but becomes repressed by GATA1 as progenitors differentiate into erythroid cells (Grass et al., 2003; Grass et al., 2006; Kobayashi-Osaki et al., 2005; Snow et al., 2010). GATA2 and GATA1 bind to several sites in the Gata2 locus, including three sites in G2DHE. We assume that GATA2 and GATA1 differentially bind to these motifs in a differentiation-dependent manner as erythroid progenitors switch from proliferation to terminal differentiation (Bresnick et al., 2010; Kaneko et al., 2010; Suzuki et al., 2013; Suzuki et al., 2011). Similarly, we surmise that EVI1 gene activation by G2DHE occurs in a manner that is dependent on these GATA factors. Additionally, a number of transcription factors, such as GFI1, SCL, MEIS1, RUNX1, ERG, LMO2 and FLI1 bind to G2DHE in human hematopoietic cell lines (Moignard et al., 2013). Therefore, identification of the mechanisms that regulate EVI1 gene expression though G2DHE is a critical future objective for identifying potential therapeutic targets for EVI1-expression based leukemia.
Several mouse models for EVI1-expressing leukemia have been reported (Buonamici et al., 2004; Louz et al., 2000; Yoshimi et al., 2011). In particular, Yoshimi et al. established a mouse model by transplanting bone marrow cells in which EVI1 was introduced using a retrovirus (Yoshimi et al., 2011). Of these experimental leukemia models, the present approach is unique since in the 3q21q26 mice, i) the expression level of EVI1 is evenly controlled being borne in a BAC, ii) the expression profile of EVI1 is defined by human 3q21 locus and is highly reproducible, and iii) the timing of EVI1 expression recapitulates that of the human leukemia cases bearing chromosome rearrangements. However, the leukemias that develop in the 3q21q26 mouse model appear to be similar to those using retroviral infection of EVI1 in mice, in which the EVI1 gene is expressed in hematopoietic cells. Both models develop leukemia with long latency (more than 6 months) and both models develop both myeloid and B-cell leukemia. Nonetheless, these observations further support the contention that the leukemogenesis in the 3q21q26 mice is caused by dysregulated EVI1 expression in hematopoietic cells, while we need further experiments to exclude decisively the possibility that a mutant microenvironment is necessary for de novo development of the leukemia in the 3q21q26 mice.
It is still unknown how the EVI1 misexpression induces B-cell leukemia in addition to myeloid leukemia. In this regard, it should be noted that the 3q21q26 cellular analysis clearly revealed that both types of leukemia correlate with EVI1 expression levels. EVI1 overexpression has also been observed in pediatric acute lymphoblastic leukemia (ALL) (Konantz et al., 2013). In very good agreement with these observations in the 3q21q26 mouse model, the EVI1 level in pediatric ALL is lower than that in AML, and EVI1 levels in the ALL patients has been shown to correlate with disease prognosis (Konantz et al., 2013).
Whereas the EVI1 gene expression level diminishes significantly in the GATA2 enhancer-deleted BAC transgenic mice and the mice no longer develop leukemia, the EVI1 gene is still expressed to a certain extent in the enhancer-deleted mouse bone marrow cells. We do not have a salient explanation for this observation at present, but the following points may be relevant. One plausible explanation is that EVI1 may be activated in only a minor subset of cells in the LSK fraction of 3q21q26 mice, and it is these cells that play a critical role in the leukemogenesis. As EVI1 is an essential transcription factor for normal hematopoietic stem cell maintenance (Goyama et al., 2008), it may play equally important roles in the maintenance of leukemic stem cells. Monitoring EVI1 expression at the single-cell level using novel transgenic mice in which a reporter is expressed under the control of the 3q21q26 BAC would be valuable to investigate this possibility.
An alternative explanation is that there may be a strict threshold requirement for EVI1-dependent leukemogenesis, and that impaired activation of EVI1 in the 3q21q26ΔG2DHE mice may be insufficient to surmount that threshold. The threshold model has already been proposed for the onset of leukemia that depends on the gene dosage of transcription factor PU.1 (Rosenbauer et al., 2004; Steidl et al., 2006). Deletion of the upstream regulatory element of the Sfpi1 gene encoding PU.1 gives rise to an 80% reduction in the levels of PU.1 compared with its normal abundance, which specifically induced leukemia. Meanwhile, complete or even a 50% reduction in PU.1 did not induce leukemia, indicating that sensitive fine-tuning of transcription factor levels by specific enhancers may be critical for the suppression of leukemogenesis.
We believe that this transgenic mouse approach, exploiting the BAC-linking technique, is equally applicable to the analysis of other leukemias or diseases that are associated with chromosomal rearrangements. Especially, by applying BAC clones from human genomic libraries to Tg mouse studies, we can generate very precise mouse models of human rearranged alleles. The BAC-linking method enables us to generate rearranged alleles with any combination of alleles or breakpoints, and the technique of transgenic mouse generation combined with the BAC-linking method is relatively straightforward to manipulate. Therefore, this approach using linked BACs in transgenic mice should prove to be useful in providing vivid insights into the mechanistic basis for the pathogenesis of various leukemias that are caused by chromosomal rearrangements.
EXPERIMENTAL PROCEDURES
Mice
Generation of Tg mice using BAC recombination is described in Supplemental Experimental Procedures. All mice were handled according to the regulations of the Standards for Human Care and Use of Laboratory Animals of Tohoku University and Guidelines for Proper Conduct of Animal Experiments the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Hematological analysis
30 to 50 μL of peripheral blood was collected from individual mice. Hematopoietic indices were determined using a hemocytometer (Nihon Koden). Hemogram profiles were examined by Wright-Giemsa staining. Flow cytometric analysis is described in Supplemental Experimental Procedures.
Histological analysis
Each organ was fixed with 10% formalin (Mildform 10N; Wako) and embedded in paraffin using standard procedures. Sections (5 μm) were stained with hematoxylin-eosin.
Quantitative RT-PCR (q-RT-PCR)
Gene expression levels were determined by q-RT-PCR as described in Supplemental Experimental Procedures.
Statistical analysis
Statistical significance of differences between parameters was assessed using a two-tailed unpaired Student’s t test.
Supplementary Material
SIGNIFICANCE.
Chromosomal rearrangements commonly create fusion oncoproteins, but can also induce leukemia by linking foreign enhancer elements to proto-oncogenes, resulting in their misexpression. Misexpression of the human EVI1 gene caused by translocation or inversion between 3q21 and 3q26 induces an acute myelogenous leukemia that has a poor prognosis. In this study, we determined that a distant GATA2 hematopoietic enhancer on 3q21 misdirects EVI1 expression on 3q26 when inverted, and that this enhancer is critical for AML neoplasia. This finding suggests strategies for therapies that target regulatory regions that misdirect the activity of proto-oncogenes. Additionally, this model establishes a mechanism for studying leukemias using transgenic BACs as a powerful way to create and analyze various human cancers that result from chromosomal rearrangements.
HIGHLIGHTS.
A GATA2 enhancer is adjacent to breakpoints of inv(3)(q21;q26) AMLs.
Transgenic mice can recapitulate the human inv(3)(q21;q26) allele.
3q21q26 mice misexpress EVI1 in stem/progenitor cells.
EVI1 leukemogenesis in the 3q21q26 mice depends on the GATA2 enhancer.
ACKNOWLEDGEMENTS
We thank Drs Takashi Moriguchi, Maki Kobayashi-Osaki and Naoko Minegishi for critical advice. We also thank Aya Goto, Koichiro Kato, Eriko Naganuma, Yohei Sato, and Hiromi Suda for technical assistance, laboratory members for useful discussions, and Biomedical Research Core of Tohoku University Graduate School of Medicine for technical support. This work was supported in part by the NIH (AI094642 and HL114368 to JDE, and DK68634 to EHB), the JSPS (KAKENHI 22118001 and 24249015 to MY, and 22790269 and 24790957 to MS), CREST JST (to MY), the NAITO Foundation, Mitsubishi Life Science Foundation, and the Takeda Science Foundation (to MY). HY was a JSPS Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- ar-Rushdi A, Nishikura K, Erikson J, Watt R, Rovera G, Croce CM. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983;222:390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]
- Brandt W, Khandekar M, Suzuki N, Yamamoto M, Lim KC, Engel JD. Defining the functional boundaries of the Gata2 locus by rescue with a linked bacterial artificial chromosome transgene. J Biol Chem. 2008;283:8976–8983. doi: 10.1074/jbc.M709364200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. GATA switches as developmental drivers. J Biol Chem. 2010;285:31087–31093. doi: 10.1074/jbc.R110.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonamici S, Li D, Chi Y, Zhao R, Wang X, Brace L, Ni H, Saunthararajah Y, Nucifora G. EVI1 induces myelodysplastic syndrome in mice. J Clin Invest. 2004;114:713–719. doi: 10.1172/JCI21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Senyuk V, Chakraborty S, Nucifora G. EVI1 promotes cell proliferation by interacting with BRG1 and blocking the repression of BRG1 on E2F1 activity. J Biol Chem. 2003;278:49806–49811. doi: 10.1074/jbc.M309645200. [DOI] [PubMed] [Google Scholar]
- Goyama S, Nitta E, Yoshino T, Kako S, Watanabe-Okochi N, Shimabe M, Imai Y, Takahashi K, Kurokawa M. EVI-1 interacts with histone methyltransferases SUV39H1 and G9a for transcriptional repression and bone marrow immortalization. Leukemia. 2010;24:81–88. doi: 10.1038/leu.2009.202. [DOI] [PubMed] [Google Scholar]
- Goyama S, Yamamoto G, Shimabe M, Sato T, Ichikawa M, Ogawa S, Chiba S, Kurokawa M. Evi-1 is a critical regulator for hematopoietic stem cells and transformed leukemic cells. Cell Stem Cell. 2008;3:207–220. doi: 10.1016/j.stem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Grass JA, Boyer ME, Pal S, Wu J, Weiss MJ, Bresnick EH. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc Natl Acad Sci U S A. 2003;100:8811–8816. doi: 10.1073/pnas.1432147100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass JA, Jing H, Kim SI, Martowicz ML, Pal S, Blobel GA, Bresnick EH. Distinct functions of dispersed GATA factor complexes at an endogenous gene locus. Mol Cell Biol. 2006;26:7056–7067. doi: 10.1128/MCB.01033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröschel S, Sanders MA, Hoogenboezem R, de Wit E, Bouwman BAM, Erpelinck C, van der Velden VHJ, Havermans M, Avellino R, van Lom K, et al. A single oncogenic enhancer-rearrangement causes concomitant deregulation of EVI1 and GATA2 in leukemia. Cell. 2014 doi: 10.1016/j.cell.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Izutsu K, Kurokawa M, Imai Y, Maki K, Mitani K, Hirai H. The corepressor CtBP interacts with Evi-1 to repress transforming growth factor beta signaling. Blood. 2001;97:2815–2822. doi: 10.1182/blood.v97.9.2815. [DOI] [PubMed] [Google Scholar]
- Johnson KD, Hsu AP, Ryu MJ, Wang J, Gao X, Boyer ME, Liu Y, Lee Y, Calvo KR, Keles S, et al. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J Clin Invest. 2012;122:3692–3704. doi: 10.1172/JCI61623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Shimizu R, Yamamoto M. GATA factor switching during erythroid differentiation. Curr Opin Hematol. 2010;17:163–168. doi: 10.1097/MOH.0b013e32833800b8. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Sato T, Yoshimi A, Goyama S, Tsuruta T, Kobayashi H, Shimabe M, Arai S, Nakagawa M, Imai Y, et al. Evi1 is essential for hematopoietic stem cell self-renewal, and its expression marks hematopoietic cells with long-term multilineage repopulating activity. J Exp Med. 2011;208:2403–2416. doi: 10.1084/jem.20110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandekar M, Brandt W, Zhou Y, Dagenais S, Glover TW, Suzuki N, Shimizu R, Yamamoto M, Lim KC, Engel JD. A Gata2 intronic enhancer confers its pan-endothelia-specific regulation. Development. 2007;134:1703–1712. doi: 10.1242/dev.001297. [DOI] [PubMed] [Google Scholar]
- Khandekar M, Suzuki N, Lewton J, Yamamoto M, Engel JD. Multiple, distant Gata2 enhancers specify temporally and tissue-specific patterning in the developing urogenital system. Mol Cell Biol. 2004;24:10263–10276. doi: 10.1128/MCB.24.23.10263-10276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi-Osaki M, Ohneda O, Suzuki N, Minegishi N, Yokomizo T, Takahashi S, Lim KC, Engel JD, Yamamoto M. GATA motifs regulate early hematopoietic lineage-specific expression of the Gata2 gene. Mol Cell Biol. 2005;25:7005–7020. doi: 10.1128/MCB.25.16.7005-7020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konantz M, André MC, Ebinger M, Grauer M, Wang H, Grzywna S, Rothfuss OC, Lehle S, Kustikova OS, Salih HR, et al. EVI-1 modulates leukemogenic potential and apoptosis sensitivity in human acute lymphoblastic leukemia. Leukemia. 2013;27:56–65. doi: 10.1038/leu.2012.211. [DOI] [PubMed] [Google Scholar]
- Kreibich G, Czakó-Graham M, Grebenau R, Mok W, Rodriguez-Boulan E, Sabatini DD. Characterization of the ribosomal binding site in rat liver rough microsomes: ribophorins I and II, two integral membrane proteins related to ribosome binding. J Supramol Struct. 1978;8:279–302. doi: 10.1002/jss.400080307. [DOI] [PubMed] [Google Scholar]
- Laricchia-Robbio L, Fazzina R, Li D, Rinaldi CR, Sinha KK, Chakraborty S, Nucifora G. Point mutations in two EVI1 Zn fingers abolish EVI1-GATA1 interaction and allow erythroid differentiation of murine bone marrow cells. Mol Cell Biol. 2006;26:7658–7666. doi: 10.1128/MCB.00363-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laricchia-Robbio L, Premanand K, Rinaldi CR, Nucifora G. EVI1 Impairs myelopoiesis by deregulation of PU.1 function. Cancer Res. 2009;69:1633–1642. doi: 10.1158/0008-5472.CAN-08-2562. [DOI] [PubMed] [Google Scholar]
- Lim KC, Hosoya T, Brandt W, Ku CJ, Hosoya-Ohmura S, Camper SA, Yamamoto M, Engel JD. Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. J Clin Invest. 2012;122:3705–3717. doi: 10.1172/JCI61619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louz D, van den Broek M, Verbakel S, Vankan Y, van Lom K, Joosten M, Meijer D, Lowenberg B, Delwel R. Erythroid defects and increased retrovirally-induced tumor formation in Evi1 transgenic mice. Leukemia. 2000;14:1876–1884. doi: 10.1038/sj.leu.2401887. [DOI] [PubMed] [Google Scholar]
- Lugthart S, Figueroa ME, Bindels E, Skrabanek L, Valk PJ, Li Y, Meyer S, Erpelinck-Verschueren C, Greally J, Löwenberg B, et al. Aberrant DNA hypermethylation signature in acute myeloid leukemia directed by EVI1. Blood. 2011;117:234–241. doi: 10.1182/blood-2010-04-281337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugthart S, van Drunen E, van Norden Y, van Hoven A, Erpelinck CA, Valk PJ, Beverloo HB, Lowenberg B, Delwel R. High EVI1 levels predict adverse outcome in acute myeloid leukemia: prevalence of EVI1 overexpression and chromosome 3q26 abnormalities underestimated. Blood. 2008;111:4329–4337. doi: 10.1182/blood-2007-10-119230. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Takeishi S, Kanie T, Susaki E, Onoyama I, Tateishi Y, Nakayama K, Nakayama KI. p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2011;9:262–271. doi: 10.1016/j.stem.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Adachi T, Tsubota T, Imanishi J, Minowada J. Establishment and characterization of a novel megakaryoblastic cell line, MOLM-1, from a patient with chronic myelogenous leukemia. Hum Cell. 1991;4:261–264. [PubMed] [Google Scholar]
- Minegishi N, Suzuki N, Yokomizo T, Pan X, Fujimoto T, Takahashi S, Hara T, Miyajima A, Nishikawa S, Yamamoto M. Expression and domain-specific function of GATA-2 during differentiation of the hematopoietic precursor cells in midgestation mouse embryos. Blood. 2003;102:896–905. doi: 10.1182/blood-2002-12-3809. [DOI] [PubMed] [Google Scholar]
- Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer. 2007;7:233–245. doi: 10.1038/nrc2091. [DOI] [PubMed] [Google Scholar]
- Moignard V, Macaulay IC, Swiers G, Buettner F, Schütte J, Calero-Nieto FJ, Kinston S, Joshi A, Hannah R, Theis FJ, et al. Characterization of transcriptional networks in blood stem and progenitor cells using high-throughput single-cell gene expression analysis. Nat Cell Biol. 2013;15:544. doi: 10.1038/ncb2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K, Parganas E, William CL, Whittaker MH, Drabkin H, Oval J, Taetle R, Valentine MB, Ihle JN. Activation of EVI1 gene expression in human acute myelogenous leukemias by translocations spanning 300-400 kilobases on chromosome band 3q26. Proc Natl Acad Sci U S A. 1992;89:3937–3941. doi: 10.1073/pnas.89.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Araki K, Kimura S, Taniguchi S, Wakasugi S, Yamamura K. An efficient gene-trap method using poly A trap vectors and characterization of gene-trap events. J Biochem. 1993;113:343–349. doi: 10.1093/oxfordjournals.jbchem.a124049. [DOI] [PubMed] [Google Scholar]
- Nozawa D, Suzuki N, Kobayashi-Osaki M, Pan X, Engel JD, Yamamoto M. GATA2-dependent and region-specific regulation of Gata2 transcription in the mouse midbrain. Genes Cells. 2009;14:569–582. doi: 10.1111/j.1365-2443.2009.01289.x. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Rosenbauer F, Wagner K, Kutok JL, Iwasaki H, Le Beau MM, Okuno Y, Akashi K, Fiering S, Tenen DG. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat Genet. 2004;36:624–630. doi: 10.1038/ng1361. [DOI] [PubMed] [Google Scholar]
- Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Rowley JD. Chromosome translocations: dangerous liaisons revisited. Nat Rev Cancer. 2001;1:245–250. doi: 10.1038/35106108. [DOI] [PubMed] [Google Scholar]
- Sato T, Goyama S, Nitta E, Takeshita M, Yoshimi M, Nakagawa M, Kawazu M, Ichikawa M, Kurokawa M. Evi-1 promotes para-aortic splanchnopleural hematopoiesis through up-regulation of GATA-2 and repression of TGF-b signaling. Cancer Sci. 2008;99:1407–1413. doi: 10.1111/j.1349-7006.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repression of RUNX1 activity by EVI1: a new role of EVI1 in leukemogenesis. Cancer Res. 2007;67:5658–5666. doi: 10.1158/0008-5472.CAN-06-3962. [DOI] [PubMed] [Google Scholar]
- Shimabe M, Goyama S, Watanabe-Okochi N, Yoshimi A, Ichikawa M, Imai Y, Kurokawa M. Pbx1 is a downstream target of Evi-1 in hematopoietic stem/progenitors and leukemic cells. Oncogene. 2009;28:4364–4374. doi: 10.1038/onc.2009.288. [DOI] [PubMed] [Google Scholar]
- Shimahara A, Yamakawa N, Nishikata I, Morishita K. Acetylation of lysine 564 adjacent to the C-terminal binding protein-binding motif in EVI1 is crucial for transcriptional activation of GATA2. J Biol Chem. 2010;285:16967–16977. doi: 10.1074/jbc.M110.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JW, Trowbridge JJ, Fujiwara T, Emambokus NE, Grass JA, Orkin SH, Bresnick EH. A single cis element maintains repression of the key developmental regulator Gata2. PLoS Genet. 2010;6:e1001103. doi: 10.1371/journal.pgen.1001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JW, Trowbridge JJ, Johnson KD, Fujiwara T, Emambokus NE, Grass JA, Orkin SH, Bresnick EH. Context-dependent function of "GATA switch" sites in vivo. Blood. 2011;117:4769–4772. doi: 10.1182/blood-2010-10-313031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spensberger D, Vermeulen M, Le Guezennec X, Beekman R, van Hoven A, Bindels E, Stunnenberg H, Delwel R. Myeloid transforming protein Evi1 interacts with methyl-CpG binding domain protein 3 and inhibits in vitro histone deacetylation by Mbd3/Mi-2/NuRD. Biochemistry. 2008;47:6418–6426. doi: 10.1021/bi800267f. [DOI] [PubMed] [Google Scholar]
- Steidl U, Rosenbauer F, Verhaak RG, Gu X, Ebralidze A, Otu HH, Klippel S, Steidl C, Bruns I, Costa DB, et al. Essential role of Jun family transcription factors in PU. knockdown-induced leukemic stem cells. Nat Genet. 2006;38:1269–1277. doi: 10.1038/ng1898. [DOI] [PubMed] [Google Scholar]
- Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A, Schmidt M, Krämer A, Schwäble J, Glimm H, et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med. 2010;16:198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- Suzukawa K, Parganas E, Gajjar A, Abe T, Takahashi S, Tani K, Asano S, Asou H, Kamada N, Yokota J. Identification of a breakpoint cluster region 3′ of the ribophorin I gene at 3q21 associated with the transcriptional activation of the EVI1 gene in acute myelogenous leukemias with inv(3)(q21q26) Blood. 1994;84:2681–2688. [PubMed] [Google Scholar]
- Suzukawa K, Taki T, Abe T, Asoh H, Kamada N, Yokota J, Morishita K. Identification of translocational breakpoints within the intron region before the last coding exon (exon 12) of the EVI1 gene in two cases of CML-BC with inv(3)(q21q26) Genomics. 1997;42:356–360. doi: 10.1006/geno.1997.4732. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kobayashi-Osaki M, Tsutsumi S, Pan X, Ohmori S, Takai J, Moriguchi T, Ohneda O, Ohneda K, Shimizu R, et al. GATA factor switching from GATA2 to GATA1 contributes to erythroid differentiation. Genes Cells. 2013;18:921–933. doi: 10.1111/gtc.12086. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Shimizu R, Yamamoto M. Transcriptional regulation by GATA1 and GATA2 during erythropoiesis. Int J Hematol. 2011;93:150–155. doi: 10.1007/s12185-011-0770-6. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Ohneda O, Minegishi N, Nishikawa M, Ohta T, Takahashi S, Engel JD, Yamamoto M. Combinatorial Gata2 and Sca1 expression defines hematopoietic stem cells in the bone marrow niche. Proc Natl Acad Sci U S A. 2006;103:2202–2207. doi: 10.1073/pnas.0508928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Suwabe N, Ohneda O, Obara N, Imagawa S, Pan X, Motohashi H, Yamamoto M. Identification and characterization of 2 types of erythroid progenitors that express GATA-1 at distinct levels. Blood. 2003;102:3575–3583. doi: 10.1182/blood-2003-04-1154. [DOI] [PubMed] [Google Scholar]
- Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89:3636–3643. [PubMed] [Google Scholar]
- Yamamoto M, Ko LJ, Leonard MW, Beug H, Orkin SH, Engel JD. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990;4:1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen SE. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3− short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- Yoshimi A, Goyama S, Watanabe-Okochi N, Yoshiki Y, Nannya Y, Nitta E, Arai S, Sato T, Shimabe M, Nakagawa M, et al. Evi1 represses PTEN expression and activates PI3K/AKT/mTOR via interactions with polycomb proteins. Blood. 2011;117:3617–3628. doi: 10.1182/blood-2009-12-261602. [DOI] [PubMed] [Google Scholar]
- Yuasa H, Oike Y, Iwama A, Nishikata I, Sugiyama D, Perkins A, Mucenski ML, Suda T, Morishita K. Oncogenic transcription factor Evi1 regulates hematopoietic stem cell proliferation through GATA-2 expression. Embo j. 2005;24:1976–1987. doi: 10.1038/sj.emboj.7600679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.