Abstract
Background:
Piper betle Linn. (Piperaceae) is used as a remedy for gastric ulcers in traditional medicinal systems in Sri Lanka. However, the gastroprotective activity has never been proven scientifically using betel leaves grown in Sri Lanka.
Objective:
To evaluate the gastroprotective activity of hot aqueous extract (HAE) and cold ethanolic extract (CEE) of P. betle in rats as the experimental model.
Materials and Methods:
Three doses (200, 300, and 500 mg/kg/bw) of both extracts were evaluated for the gastroprotective activity against ethanol induced gastric ulcers in rats. The parameters evaluated were (a) effects of HAE on mucus content adhering to the wall of the gastric mucosa, (b) acidity (total and free), (c) volume and (d) pH of the gastric juice.
Results:
Oral administration of HAE and CEE provided marked dose dependent (HAE: r2 = 0.97; CEE: r2 = 0.96) and significant (P ≤ 0.05) protection against gastric damage caused by absolute ethanol. The gastroprotective effect of CEE was comparable with that of HAE. Further, gastroprotective activity of the highest dose of both extracts were significantly greater (P ≤ 0.05) than that of misoprostol, the reference drug. The HAE significantly (P ≤ 0.05) increased the mucus content adhering to the wall of the gastric mucosa and inhibited the volume of gastric acid. However, acidity (total and free) and pH of the gastric juice remained unaltered.
Conclusion:
It is concluded that both HAE and CEE of P. betle leaves have a strong gastroprotective activity.
Keywords: Gastric acid, misoprostol, mucus content, Piper betle
INTRODUCTION
Piper betle Linn. (Piperaceae) is a perennial dioecious, semi-woody climber. Stems are strongly swollen at the nodes, papillose when young, entirely glabrous. Leaves are alternate, large, 15-20 cm long, some broadly ovate, cordate and symmetrical at base known as female leaves and others narrower and oblique called male leaves. P. betle is cultivated in Sri Lanka, India, Malaysia, Indonesia, Philippine Islands, and East Africa.[1,2] Betel leaves have a strong pungent flavor and are widely used as a masticatory. The betel leaves contain essential oils and the content of oil varies from 0.7% to 2.6% depending upon the varieties of leaves.[3] More than 12 P. betle cultivars are reported in Sri Lanka[4] and except the cultivar called Malabulath, which is not used for chewing, other cultivars constitute “commercial betel” of Sri Lanka. According to Kumaratunga,[5] chemical constituents and their relative proportions in essential oil of “commercial betel” of Sri Lanka are different from that of other countries. The essential oil of Sri Lankan betel leaves was characterized by high content of safrole. In addition, eugenol, allyl diacetoxy benzene and chavibitol acetate were identified as major constituents of the Sri Lankan betel leaves. P. betle leaves are credited with many medicinal properties such as digestive, stimulative, carminative and aphrodisiac.[3] However, Sri Lankan P. betle inhibits male sexual behavior in rats and possesses anti-aphrodisiac activity[6] indicating the differences in biological activities of Sri Lankan betel. Further, very few investigations on the activities of P. betle grown in Sri Lanka are reported except the experiments on antifertility effects of male rats,[7] antimotility effects on washed human spermatozoa,[8] antimicrobial,[5] antidiabetic,[9] antinociceptive[10] and antioxidant[11] activities. Betel is used as a remedy for gastric ulcers in traditional medicinal systems in Sri Lanka. However, gastroprotective activity was not scientifically investigated using Sri Lankan grown betel leaves. Thus, it is of worth to scientifically investigate the gastroprotective activity of P. betle grown in Sri Lanka due to the biological and chemical differences of the plant. Therefore, this study was undertaken to investigate gastroprotective activity of betel leaves using hot aqueous extract (HAE) and cold ethanolic extract (CEE).
MATERIALS AND METHODS
Plant material
Fresh P. betle leaves were collected from the main vegetable markets of Colombo, Gampaha and Kalutara districts in the Western province of Sri Lanka in the period of March-April. The leaves were identified and authenticated by the curator of National Herbarium, Royal Botanical Gardens, Peradeniya, Sri Lanka. A voucher specimen (PS 01) was deposited in the Industrial Technology Institute, Colombo 7, Sri Lanka.
Animals
Healthy adult crossbred male albino rats (weighing 200-250 g) were used throughout the experiment. Rats were housed under standardized animal house conditions (temperature: At 28-31°C, photoperiod: Approximately, 12 h natural light and relative humidity 55-60%) with free access to pelleted food (Vet House Ltd., Colombo, Sri Lanka) and tap water. All animal experiments were conducted in accordance with the internationally accepted laboratory animal use and care guide lines and rules of the ethical committee, University of Colombo, Sri Lanka for experiment. Prior to the experiments, the rats were deprived of food for 36 h, water for 12 h and kept in raised mesh bottomed cages to prevent coprophagy.
Preparation of HAE
P. betle leaves were air dried for 3-5 days in the shade and cut into small pieces. Five Hundred grams were boiled with 2.5 L of distilled water (DW) for 4 h. HAE was concentrated under vacuum, freeze dried and stored in a refrigerator at 4°C until use (yield 26.2% w/w on the basis of dry weight).
Preparation of CEE
P. betle leaves were air dried for 3-5 days in the shade and cut into small pieces. Five Hundred grams were macerated with ethanol (80% v/v) and kept for 48 h at room temperature (28-30°C). The extract was filtered and the filtrate was evaporated to dryness under reduced pressure and stored in a refrigerator at 4°C until use (yield 15.6% w/w on the basis of dry weight).
Phytochemical screening of HAE and CEE
Phytochemical screening of the HAE and CEE for alkaloids, flavonoids, steroids, saponins and tannins was carried out.[12]
Dose administration
Dose of 200, 300, and 500 mg/kg/bw of HAE and CEE were prepared in 1 mL of DW and given orally to each groups (n = 9/group) of rats once. Doses were selected on the basis of previously reported experiment.[9]
Evaluation of gastroprotective activity
The food and water has been withdrawn before 36 h and 12 h respectively for each animal group. These rats were randomly divided into 8 groups (n = 9/group) and treated orally as per the following schedule:
Rats in group 1 received 1 ml of DW once
Rats in group 2 received 200 mg/kg/bw of HAE once
Rats in group 3 received 300 mg/kg/bw of HAE once
Rats in group 4 received 500 mg/kg/bw of HAE once
Rats in group 5 received 200 mg/kg/bw of CEE once
Rats in group 6 received 300 mg/kg/bw of CEE once
Rats in group 7 received 500 mg/kg/bw of CEE once
Rats in group 8 received 133 µg/kg/bw of misoprostol (the reference drug) once.
After 1 h of oral treatment, each rat was given 1 mL of absolute ethanol orally and kept for another 1 h. Then, the rats were sacrificed with an over dose of ether, stomachs were removed and inflated with 1% formalin solution and immersed in the same solution to fix the outer layer of the stomach. Each stomach was opened along the greater curvature, rinsed with tap water to remove gastric contents and blood clots. (a) The number of hemorrhagic lesions was counted and (b) their lengths were measured using a Vernier Caliper.[13]
Evaluation of the mode of gastroprotective activity
Mode of action by which P. betle mediates its gastroprotective effects was assessed by determining its effects on (a) mucus content of the stomach and (b) acidity and volume of gastric juice using 500 mg/kg/bw of HAE.
Determination of mucus content of stomach
Alcian blue binding to gastric wall mucus was determined by a modified method of Corne.[14] In this experiment, 12 male rats were starved for 36 h and water was withdrawn for 12 h as described previously. They were randomly divided into 2 equal groups. Rats in the two groups were orally treated with either 500 mg/kg/bw of HAE or 1 mL of DW per rat. After 1 h, these rats were laparotomised under ether anesthesia and at the pyloric end of the stomach were ligated with a cotton thread. The stomach was then carefully placed back in the abdominal cavities and the rats were sutured and allowed to regain consciousness. After 4 h, the rats were sacrificed with over dose of ether; each stomach was opened along the greater curvature, rinsed with 0.25 M sucrose solution. These stomachs were incubated in 10 mL aliquots of 0.1% alcian blue solution for 2 h at room temperature (30°C). After 2 h stomachs were removed, washed with 0.25 M sucrose solution and separately incubated in 10 mL aliquots of 0.5 M magnesium chloride solution for 2 h at room temperature while shaking at 30 min. intervals to elute the alcian blue bound to the mucosa of the stomachs. After 2 h, stomachs were removed and 5 mL of each aliquot of magnesium chloride solution containing the alcian blue eluted from each stomach was shaken with 5 mL of diethyl ether. The aqueous phase was separated out, centrifuged at 3200 rpm for 5 min. and the absorbance of the supernatant was measured at l 605 nm. The amount of alcian blue bound per stomach in µg was determined using a standard calibration curve.
Evaluation of the effects on acidity and volume of the gastric juice
A total of 12 male rats were starved for 36 h and water was withdrawn for 12 h as described previously. They were randomly divided into two equal groups. Rats in the two groups were orally treated with either 500 mg/kg/bw of HAE or 1 mL of DW per rat. After 1 h, these rats were laparotomised under ether anesthesia and at the pyloric end of the stomach were ligated with a cotton thread. The stomachs were then carefully placed back in the abdominal cavities and the rats were sutured and allowed to regain consciousness. After 4 h, the rats were sacrificed with over dose of ether, stomach was excised, gastric juice collected and centrifuged at 3500 rpm for 15 min. The volume of gastric juice from each rat was measured and acidity (total and free) was determined by titration with 0.01 M NaOH according to the method described by Reitman.[15]
Statistical analysis
Data were expressed as mean ± standard error of mean statistical comparisons were made by using one-way analysis of variance followed-by Duncan's multiple range tests. A P ≤ 0.05 was considered to be significant.
RESULTS
Phytochemical screening of HAE and CEE
Phytochemical screening revealed the presence of alkaloids, flavonoids, steroids, saponins and tannins in both HAE and CEE.
Experimentally induced gastric lesions
Both HAE and CEE of P. betle leaves caused a significant (P ≤ 0.05) inhibition of the length and the number of gastric lesions [Figures 1 and 2] induced by absolute ethanol in a dose dependent (HAE: r2 = 0.97; CEE: r2 = 0.96) manner. Among the tested doses, highest dose (500 mg/kg/bw) had shown the maximum inhibition of the length (by HAE: 91%; CEE: 95%) and number of gastric lesions (by HAE: 89%; CEE: 95%) followed-by 300 mg/kg/bw dose (length: By HAE: 67%; CEE: 73% number: By HAE: 59%; CEE: 63%) and 200 mg/kg/bw dose (length: By HAE: 25%; CEE: 28%; number: By HAE: 32%; CEE: 34%). The gastroprotective activity of 500 mg/kg/bw dose of both extracts were significantly (P ≤ 0.05) higher than that of misoprostol, the reference drug which only inhibited the length and number of gastric lesions by 78% and 85% respectively.
Figure 1.
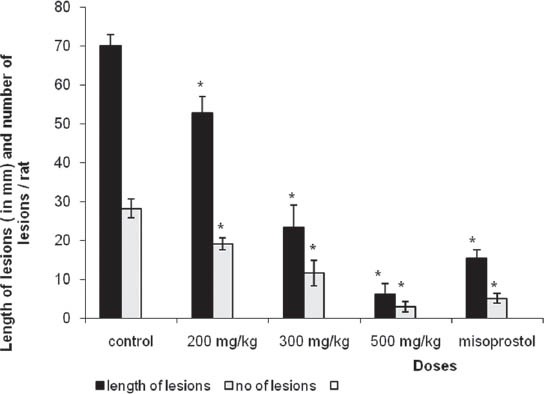
Effects of hot aqueous extract of Piper betle leaves on the length of gastric lesions (in mm) and number of lesions/rat induced by absolute ethanol (mean ± standard error of mean, n = 9). *Significant at P<0.05 as compared with control
Figure 2.
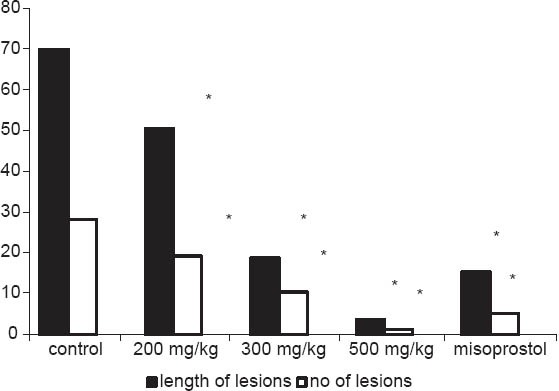
Effects of cold ethanolic extract of Piper betle leaves on the length of gastric lesions (in mm) and number of lesions/rat induced by absolute ethanol. *Significant at P<0.05 as compared with control
Gastric mucus studies
The highest dose of HAE significantly (P ≤ 0.05) increased (by 49%) the amounts of mucus content adhered to the gastric mucosa in 4 h pylorus ligated rats (control vs. treatment: 151.03 ± 9.12 vs. 224.65 ± 11.08 µg/stomach).
Acid secretion studies
In 4 h pylorus, ligated rats, the highest dose of HAE caused a significant (P ≤ 0.05) reduction (41%) in volume of gastric juice (control vs. treatment 4.52 ± 0.31 vs. 2.71 ± 0.36 ml/stomach). However, there was no significant (P ≥ 0.05) change in free acidity (control vs. treatment 0.043 ± 0.0047 vs. 0.047 ± 0.0045 mol/L/stomach) total acidity (control vs. treatment 0.076 ± 3.9 × 10−3 vs. 0.081 ± 4.3 × 10−3 mol/L/stomach) or in pH (control vs. treatment 3.5 ± 0.2 vs.3.8 ± 0.3).
DISCUSSION
Ethanol is one of the ulcerogenic agents that induce intense damage in gastric mucosa by promoting disturbances of mucosal microcirculation, ischemia and appearance of free radicals, endothelin release, degranulation of mast cells, inhibition of prostaglandins and decrease of gastric mucus production.[16] The leaf extracts of P. betle possesses marked gastroprotective properties as evidenced by its significant (P ≤ 0.05) inhibition in the formation of gastric lesions (in terms of length and number) induced by absolute ethanol. Gastroprotective activity of CEE was comparable with that of HAE. Further, the gastroprotective effects of the highest dose (500 mg/kg/bw) of both betel extracts were superior than that of reference drug, misoprostol. The gastroprotective effect was dose-dependent and dose response curve was linear.
The gastroprotective activity mediated by P. betle extracts may be mediated through any one of the listed mechanisms, which may cause enhancement of the gastric mucosal defense either through (a) increase in mucus and/or bicarbonate production or (b) reduction in the volume of gastric acid secretion or (c) by reduction of the gastric acidity.[17] In the present investigation, it has been demonstrated that the HAE can significantly enhance gastric mucus secretion while reducing the volume of the gastric juice in rats. Gastric mucus is an important protective factor for the gastric mucosa and consists of a viscous, elastic, adherent and transparent gel formed by 95% water and 5% glycoproteins that covers the entire gastrointestinal mucosa. Moreover, mucus is capable of acting as an antioxidant and thus can reduce mucosal damage mediated by free radicals. The protective property of the mucus barrier not only depends on the gel structure, but also on the amount or thickness of the layer covering the mucosal surface.[18,19]
Administration of HAE has significantly (P ≤ 0.05) increased the amount of mucus produced by the rat gastro mucosa compared with the control. Therefore, the enhanced mucus secretion after administration of HAE may help to protect against the absolute ethanol-induced damage by preventing the action of acid and free radicals on the stomach mucous epithelium. Similar mode of action has been reported with several other plants also.[20,21,22,23,24] It is also well-known that prostaglandins synthesized in large quantities by the gastrointestinal mucosa can prevent experimentally induced ulcers by ulcerogens. Thus, when the ulcer lesions are induced by absolute ethanol, the cytoprotective effect of the antiulcer agent can mediated through endogeneous prostaglandins.[22] Therefore, it can be thought that HAE may stimulate the secretion of prostaglandin or possess prostaglandin like substances.
Phytochemical screening of P. betle revealed the presence of alkaloids, flavonoids, steroids, saponins and tannins. Polyphenols, especially tannins, are phytochemicals with antioxidant properties. These compounds have also shown antiulcerogenic properties due to their protein precipitating and vasoconstricting effects.[23] Their astringent action can help precipitating microproteins on the ulcer site, thereby forming an impervious layer over the lining that hinders gut secretions and protects the underlying mucosa from toxic and other irritants.[24] The antiulcerogenic effect of many plants is related to their flavonoid content as these antioxidants inhibit lipid peroxidation and other free radical mediated processes that lead to gastric damage.[25] Moreover, saponins[26] and alkaloids[27] have been shown to have potent gastroprotective activities. Therefore, secondary metabolites such as alkaloids, saponins, tannins, flavonoids and other phenolic compounds present in P. betle may also contribute to its gastroprotective effect.
It is generally, believed that enhanced acid secretion is the most important factor for the induction of gastric lesions.[28] In this study, the highest dose of HAE did not cause significant inhibition in acidity (both total and free) or pH of gastric fluid. Therefore, the gastroprotective effect of P. betle was not mediated through inhibition of acidity in the gastric juice. Both extracts were devoid of unacceptable side-effects even following chronic administration at a dose of 1500 mg/kg/bw which was 3-fold higher than the dose that showed maximum gastroprotective activity. There were no overt signs of toxicity, hepatotoxicity (in terms of serum aspartate aminotransferase, alanine aminotransferase levels) or renotoxicity (as judged by serum urea and creatinine levels).[29]
In conclusion, our results demonstrate the gastroprotective activity and the mode of action of Sri Lankan P. betle leaves for the first time and indicate its therapeutic potential to be used as a cheap, efficacious and a safe herbal gastroprotective agent.
ACKNOWLEDGMENT
The authors express their gratitude to National Science Foundation, Sri Lanka for the Research grant (SIDA (1L) 2000/BT/03).
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Jayaweera DM. Vol. 5. Colombo: National Science Council of Sri Lanka; 1982. Medicinal Plants Used in Ceylon; p. 201. [Google Scholar]
- 2.Dassanayake MD, Fosberg FR. Vol. 6. New Delhi: Amreind; 1987. A Revised Hand Book to the Flora of Ceylon; pp. 287–8. [Google Scholar]
- 3.Anonymous. Raw Material. Revised ed. Vol. 5. New Delhi: Publication and Information Directorate, CSIR; 1992. The Wealth of India. The Dictionary of Indian Raw Materials and Industrial Products; pp. 84–94. [Google Scholar]
- 4.Ratnasoma HA, Senevirathna JM. Sri Lanka: Published by Department of Agriculture; 1995. Betel cultivation; pp. 3–5. [Google Scholar]
- 5.Kumaratunga KG. M. Phil Thesis. Sri Lanka: University of Kelaniya; 2003. Gas chromatographical and antimicrobial studies on Alpinia calcarata and Piper betle from Sri Lanka; pp. 57–91. [Google Scholar]
- 6.Ratnasooriya WD, Premakumara GA. Piper betle leaves impairs masculine sexual behavior of rats. Med Sci Res. 1996;24:303–6. [Google Scholar]
- 7.Ratnasooriya WD, Premakumara GA. Piper betle leaves reversibly inhibits fertility of male rats. Vidyodaya J Sci. 1997;7:15–21. [Google Scholar]
- 8.Ratnasooriya WD, Jayawardena KG, Premakumara GA. Antimotility effects of Piper betle (L) leaf extract on washed human spermatozoa. J Natl Sci Counc Sri Lanka. 1990;18:53–60. [Google Scholar]
- 9.Arambewela LS, Arawwawala LD, Ratnasooriya WD. Antidiabetic activities of aqueous and ethanolic extracts of Piper betle leaves in rats. J Ethnopharmacol. 2005;102:239–45. doi: 10.1016/j.jep.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Arambewela LS, Arawwawala LD, Ratnasooriya WD. Antinociceptive activity of aqueous and ethanolic extracts of Piper betle leaves. Pharm Biol. 2005;43:766–72. doi: 10.1016/j.jep.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Arambewela LS, Arawwawala LD, Rajapaksa R. Piper betle: A potential natural antioxidant. Int J Food Sci Technol. 2006;41:10–4. [Google Scholar]
- 12.Farnsworth NR. Biological and phytochemical screening of plants. J Pharm Sci. 1966;55:225–76. doi: 10.1002/jps.2600550302. [DOI] [PubMed] [Google Scholar]
- 13.Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761–7. [PubMed] [Google Scholar]
- 14.Corne SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol. 1974;242:116P–7. [PubMed] [Google Scholar]
- 15.Reitman S. Gastric secretion. In: Frankel S, Reitman S, Sonnenwirth AC, editors. Gradwohl's Clinical Laboratory Methods and Diagnosis. London: Mosby; 1970. pp. 1949–58. [Google Scholar]
- 16.Park S, Hahm KB, Oh TY, Jin JH, Choue R. Preventive effect of the flavonoid, wogonin, against ethanol-induced gastric mucosal damage in rats. Dig Dis Sci. 2004;49:384–94. doi: 10.1023/b:ddas.0000020490.34220.6d. [DOI] [PubMed] [Google Scholar]
- 17.Antonio JM, Gracioso JS, Toma W, Lopez LC, Oliveira F, Brito AR. Antiulcerogenic activity of ethanol extract of Solanum variabile (false “jurubeba”) J Ethnopharmacol. 2004;93:83–8. doi: 10.1016/j.jep.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Penissi AB, Piezzi RS. Effect of dehydroleucodine on mucus production: A quantitative study. Dig Dis Sci. 1999;44:708–12. doi: 10.1023/a:1026601506677. [DOI] [PubMed] [Google Scholar]
- 19.Repetto MG, Llesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res. 2002;35:523–34. doi: 10.1590/s0100-879x2002000500003. [DOI] [PubMed] [Google Scholar]
- 20.Moraes Tde M, Rodrigues CM, Kushima H, Bauab TM, Villegas W, Pellizzon CH, et al. Hancornia speciosa: Indications of gastroprotective, healing and anti-Helicobacter pylori actions. J Ethnopharmacol. 2008;120:161–8. doi: 10.1016/j.jep.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Hiruma-Lima CA, Rodrigues CM, Kushima H, Moraes TM, Lolis Sde F, Feitosa SB, et al. The anti-ulcerogenic effects of Curatella americana L. J Ethnopharmacol. 2009;121:425–32. doi: 10.1016/j.jep.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto K, Kakegawa H, Ueda H, Matsumoto H, Sudo T, Miki T, et al. Gastric cytoprotective anti-ulcerogenic actions of hydroxychalcones in rats. Planta Med. 1992;58:389–93. doi: 10.1055/s-2006-961498. [DOI] [PubMed] [Google Scholar]
- 23.Berenguer B, Sánchez LM, Quílez A, López-Barreiro M, de Haro O, Gálvez J, et al. Protective and antioxidant effects of Rhizophora mangle L. against NSAID-induced gastric ulcers. J Ethnopharmacol. 2006;103:194–200. doi: 10.1016/j.jep.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 24.Nwafor PA, Okwuasaba FK, Binda LG. Antidiarrhoeal and antiulcerogenic effects of methanolic extract of Asparagus pubescens root in rats. J Ethnopharmacol. 2000;72:421–7. doi: 10.1016/s0378-8741(00)00261-0. [DOI] [PubMed] [Google Scholar]
- 25.Gurbuz I, Yesilada E, Ito S. An anti-ulcerogenic flavonol diglucoside from Equisetum palustre L. J Ethnopharmacol. 2009;121:360–5. doi: 10.1016/j.jep.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa T, Li N, Nagatomo A, Matsuda H, Li X, Yoshikawa M. Triterpene saponins with gastroprotective effects from tea seed (the seeds of Camellia sinensis) J Nat Prod. 2006;69:185–90. doi: 10.1021/np058097w. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Shang JC, Zhou QX. Study of total alkaloids from rhizome Coptis chinensis on experimental gastric ulcers. Chin J Integr Med. 2005;11:217–21. doi: 10.1007/BF02836508. [DOI] [PubMed] [Google Scholar]
- 28.Konturek SJ, Konturek PC, Brzozowski T, Konturek JW, Pawlik WW. From nerves and hormones to bacteria in the stomach; Nobel prize for achievements in gastrology during last century. J Physiol Pharmacol. 2005;56:507–30. [PubMed] [Google Scholar]
- 29.Arambewela LS, Arawwawala LD, Ratnasooriya WD. Safety evaluation of Sri Lankan Piper betle leaf extracts in rats. J Trop Med Plants. 2003;4:195–8. [Google Scholar]


