Abstract
For brown adipose tissue (BAT) to be effective at consuming calories, its blood flow must increase enough to provide sufficient fuel to sustain energy expenditure and also transfer the heat created to avoid thermal injury. Here we used a combination of human and rodent models to assess changes in BAT blood flow and glucose utilization.
Methods
99mTc-methoxyisobutylisonitrile (MIBI) SPECT (n=7) and SPECT/CT (n=74) scans done in adult humans for parathyroid imaging were reviewed for uptake in regions consistent with human BAT. Site-directed biopsies of subcutaneous and deep neck fat were obtained for electron microscopy and gene expression profiling. In mice, tissue perfusion was measured with 99mTc-MIBI (n=16) and glucose uptake with 18F-FDG (n=16). Animals were fasted overnight, anesthetized with pentobarbital, and given intraperitoneally either the β3-adrenergic receptor agonist CL-316,243 (CL) 1mg/kg (n=8) or saline (n=8) followed by radiotracer injection 5 minutes later. After 120 minutes, mice were imaged using SPECT/CT or PET/CT. Vital signs were recorded over 30 minutes during the imaging. BAT, white adipose tissue (WAT), muscle, liver, and heart were resected, and tissue uptake of both 99mTc-MIBI and 18F-FDG were quantified by percent injected dose (%ID) per gram of tissue and normalized to per kilogram of total body weight (%ID/g*kg).
Results
In 5.4% (4/74) patients, 99mTc-MIBI SPECT/CT showed increased retention in cervical and supraclavicular fat that displayed multilocular lipid droplets, dense capillary investment, and a high concentration of ovoid mitochondria. Expression levels of the tissue-specific uncoupling protein-1 (UCP1) were 180x higher in the BAT compared to the subcutaneous WAT (P < 0.001). In mice, BAT tissue perfusion increased by 61% (P < 0.01), with no significant changes in blood flow to WAT, muscle, heart, or liver. CL increased glucose uptake in BAT even more, by 440% (P < 0.01).
Conclusion
Pharmacologic activation of BAT requires increased blood flow to deliver glucose and oxygen for thermogenesis. However, the glucose consumption far exceeds the vascular response. These findings demonstrate that activated BAT increases glucose uptake beyond what might occur by increased blood flow alone and suggest that activated BAT likely uses glucose for non-thermogenic purposes.
Keywords: brown fat, blood flow, glucose uptake
INTRODUCTION
The contemporary pandemics of obesity and diabetes are devastating in size, breadth, and rate of growth (1). Central to these pathological processes is the adipose tissue depot. White adipose tissue (WAT) stores energy and in excess results in obesity and insulin resistance, while brown adipose tissue (BAT) consumes calories for the purpose of thermogenesis via tissue-specific UCP1 (2). BAT was initially known for its role in non-shivering and diet-induced thermogenesis in infants and small mammals (3), but it has been recently shown that adult humans have detectable BAT (4–10). When activated by mild cold exposure, human BAT consumes more glucose per gram than any other tissue (11), suggesting that BAT activity may be utilized to treat metabolic dysfunction.
Although there is great therapeutic potential in the use of human BAT energy expenditure, very little is known about the details underlying its physiological response to stimulation. Currently, BAT is routinely imaged via 18F-FDG PET/CT, which has been useful in epidemiological (12) and functional (13–16) studies. This approach has its limitations since glucose retention may not reflect the actual energy expenditure of the tissue (17). PET studies using 15O and 11C have been helpful in demonstrating BAT perfusion (11) and aerobic respiration (18), respectively, but their short half-lives substantially reduce their utility. An attractive alternative is 99mTc-methoxyisobutylisonitrile (MIBI), a perfusion-mediated radiotracer that is readily accessible and can provide essential information about BAT blood flow in adult humans (19), (20).
BAT activity in both humans and rodent models is routinely achieved through cold exposure (6), (8),(21), which acts centrally to stimulate the sympathetic nervous system to release norepinephrine from postganglionic neurons and activate UCP1 uncoupling and thermogenesis in BAT (22). Observational studies support the possibility that 99mTc-MIBI could localize to human BAT (23–26). However, it is not yet known how BAT tissue perfusion responds to pharmacological stimulation. In the current study, we provide the first direct physical evidence that 99mTc-MIBI localizes to adult human BAT in vivo. We then use a rodent model to evaluate uptake following stimulation with CL-316,243 (CL) (27), an agonist for the β3-adrnergic receptor, which is highly expressed in adipose tissue. Using 99mTc-MIBI and 18F-FDG, we establish the relationship between the increase in tissue perfusion and glucose uptake after pharmacological stimulation of mouse BAT.
MATERIALS AND METHODS
Patients
This study followed institutional guidelines and was approved by the ethics committees of Beth Israel Deaconess Medical Center, Partners HealthCare, and Joslin Diabetes Center, all in Boston, MA. For the medical records review and discarded material analyses, the consent of patients was not required. The Harvard Medical School Shared Pathology Informatics Network Patients was used to identify patients at Massachusetts General Hospital who had undergone both 99mTc-MIBI SPECT to locate parathyroid adenomas and also had tissue processed for clinical indications during subsequent parathyroidectomies that were officially documented by staff pathologists as having brown adipocytes. For the 99mTc-MIBI SPECT/CT dataset at Beth Israel Deaconess Medical Center, consecutive studies over a 22-month period were assessed for the presence of activity in regions associated with brown fat. Informed consent was obtained from the patient from whom the prospective biopsy was obtained.
Human Parathyroid Imaging with 99mTc-MIBI
For planar imaging, patients were injected intravenously with 20 mCi (740 MBq) of 99mTc-MIBI. Immediately and then 1.5 hours after injection, 10-min anterior, 35° right anterior oblique, and 35 left anterior oblique planar images were acquired in a 128 × 128 matrix, with a 20% window centered around the 140-keV photopeak, using a low-energy, high-resolution parallel collimator in a Siemens e.cam nuclear gamma camera (Siemens Medical Systems).
For SPECT/CT, imaging was carried out as described (28)(Supplementary Methods). All patients were injected intravenously with 20 mCi (740 MBq) of 99mTc-MIBI, and planar dual-phase imaging was performed with standard parameters after 20 minutes and 2 hours using a Philips Medical Systems (Andover, MA) Precedence SPECT/CT. CT Images were used for attenuation correction and anatomic localization. Static views were done 20 minutes after tracer injection, then SPECT/CT imaging after the 20-minute static views, and finally 2-hour planar views.
Processing of Human Tissue
99mTc-MIBI SPECT/CT scans were done for the clinical purpose of identifying parathyroid adenomas. Patients whose scans indicated the presence of BAT were selected. The precise anatomical location of the adipose tissue with increased uptake of 99mTc-MIBI was reviewed with the surgeon who then resected the fat tissue providing site-directed biopsies of human neck subcutaneous white fat and deeper brown fat. The fat was prepared for light and electron microscopy as described (29).
Human Gene Expression
These experiments were carried out as described (30). BAT and WAT tissue was collected and stored in liquid nitrogen at −80 °C. RNA was extracted from tissue using Qiazol and RNEasy kit (QIAGEN). cDNA was made using Applied Biosystems High Capacity cDNA kit, and quantitative real-time PCR was performed using cDNA with SybrGreen Master Mix (Fisher)(Supplementary Table 1). PCR was run using ABI Prism 7900 sequence detection system (Applied Biosystems). Expression levels of genes associated with BAT (UCP1, DIO2, CideA, PGC1α) and WAT (leptin) were normalized to 36B4.
Rodent Imaging Protocol
All animal studies were approved by the Institutional Animal Care and Usage Committees at Joslin Diabetes Center and Beth Israel Deaconess Medical Center. For the full details of the imaging protocols, please see the Supplementary Methods. In brief, a total of 32 129SVE mice (Taconic), 8–12 weeks old, 20–30g, was fasted overnight, then anesthetized with sodium pentobarbital (50–75mg/kg intraperitoneally, followed by 20mg/kg intraperitoneally every 30 minutes starting 1 hr after the initial dose), then given either the β3-adrenergic receptor agonist CL-316,243 (CL) 1mg/kg (n=16) or saline (n=16) followed 5–10 minutes later by either 18F-FDG, 275 μCi (n=8 for each of the two treatments) or 99mTc-MIBI, 225 μCi (n=8 for each of the two treatments). Two hours later, mice were imaged using PET/CT or SPECT/CT, respectively. Imaging was performed on a NanoSPECT/CT (Bioscan, Washington, DC) and a NanoPET/CT (Mediso Medical Imaging Systems, Budapest, Hungary). Helical micro-SPECT was performed using a four-headed gamma camera outfitted with multi-pinhole collimators 1.0mm diameter pinholes for a single mouse scan. Reconstruction was performed using Nucline software (Mediso Medical Imaging Systems, Budapest, Hungary) in 2D mode. OSEM reconstruction algorithm was used with a SSRB 2DLOR rebinning method. Respiratory rate was measured using the Small Animal Monitoring and Gating System; Model 1025T (SA Instruments, Stonybrook, NY), and heart rate using 3M™ Red Dot™ Neonatal, Pre-Wired, Radiolucent Monitoring Electrode with clear tape (3M, St. Paul MN). Vital signs were recorded over 30 minutes during the imaging. At the end of the imaging session, the mice were sacrificed and interscapular BAT, posterior subcutaneous WAT, muscle, liver, and heart tissue were dissected and weighed. Tissue radioactivity for both 99mTc-MIBI and 18F-FDG was measured using percent injected dose (%ID) per gram of tissue and normalized to per kilogram of total body weight (%ID/g × kg).
Statistical Analyses
Data were analyzed using JMP Pro 9.0.0 software (SAS Institute, Inc., Cary, NC). All P values presented are two tailed, and P < 0.05 was considered to indicate statistical significance. Comparison of the gene expression in different anatomical depots was done using the Student’s t-test.
RESULTS
99mTc-MIBI Localizes to BAT in Adult Humans
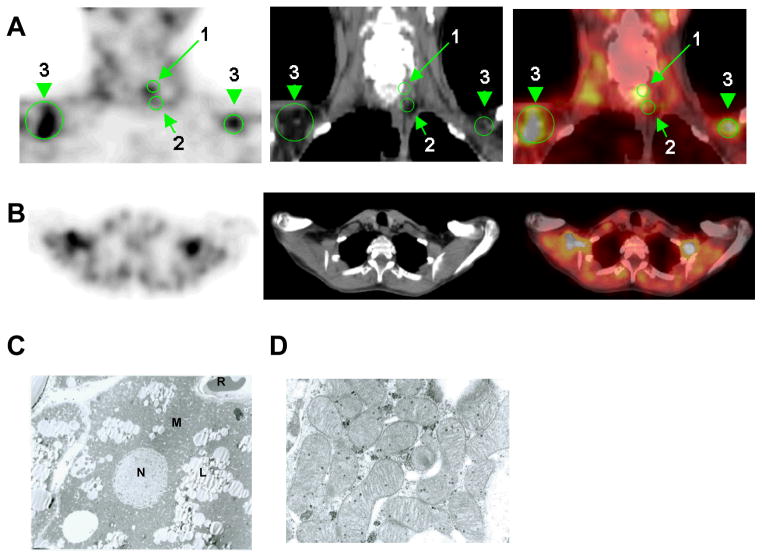
To prove that 99mTc-MIBI was localizing to BAT, we first reviewed all SPECT scans done over a three year period in which biopsy tissue was available and showed the presence of brown adipocytes. Nine patients met these criteria. The delayed images showed increased tracer retention in a mantle-like appearance in the regions of the neck and upper thorax, areas known to be enriched with human BAT typically (Fig. 1A–B). Biopsy of the tissue revealed brown adipocytes with typical multilocular lipid droplets and polygonal cells (8), (31)(Fig. 1C). We next turned to SPECT/CT to provide a definitive correlation between adipose tissue identified as MIBI-avid and histological proof of brown adipocytes. Every 99mTc-MIBI SPECT/CT scan done for the purpose of localizing parathyroid adenomas over a 22-month interval was reviewed. Of the 74 patients (19M/49F, age 58 ±15y), four (5.4%) demonstrated substantially increased uptake in the distinct fascial plane within the cervical and supraclavicular regions where BAT is located (7) (Fig. 2A–B). From a representative patient, biopsies from the subcutaneous WAT and MIBI-avid deeper neck adipose tissue were obtained. The microscopic and ultrastructural appearances were consistent with WAT and BAT, respectively (Fig. 2C–D). WAT from the subcutaneous depots was yellow, with large unilocular adipocytes and little cytoplasm. In contrast, the MIBI-localized fat was brown and multilocular with a very high density of ovoid mitochondria.
Figure 1.
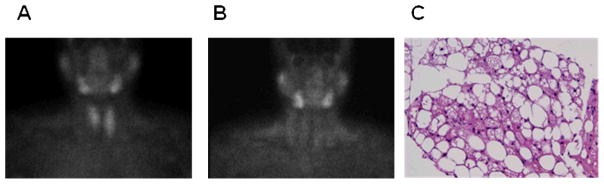
A 54 year-old woman with clinical hyperparathyroidism. Coronal images of parathyroid adenomas using 99mTc-MIBI SPECT at (A) 20 minutes and (B) 2 hours showed increased uptake in neck and upper thorax, areas associated with human BAT. (C) The tissue was resected and studied using hematoxylin and eosin staining.
Figure 2.
57 year-old woman with clinical hyperparathyroidism. (A) Coronal and (B) transaxial slices of SPECT, CT, and fused SPECT/CT imaging of 99mTc-MIBI uptake. Scans performed showed focal abnormal uptake posterior to mid pole of the left thyroid lobe, compatible with the reported parathyroid adenoma. The green arrows indicate (1) the parathyroid adenoma; (2) site of deep tissue biopsy; and (3) the main depot of BAT in the supraclavicular space. Osmium tetroxide staining of the biopsied tissue shows individual (C) individual brown adipocyte; (D) focus on ovoid mitochondria with dense cristae. Labels are the following: lipid droplets (L), mitochondria (M), nucleus (N), and red blood corpuscle (R).
Gene Expression Profile of 99mTc-MIBI-avid Adipose Tissue
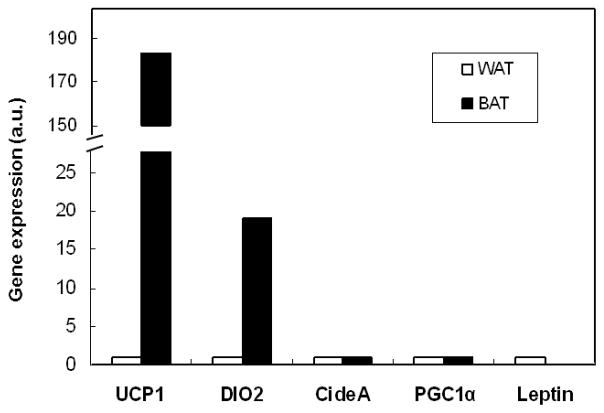
Quantitative reverse transcriptase PCR (qRT-PCR) was used to examine the gene expression profile of the two different adipose tissue depots. Compared with subcutaneous WAT, MIBI-avid fat had 180x greater tissue-specific UCP1 expression levels (P < 0.001) (Fig. 3). Additionally, genes found enriched in human BAT such as DIO2, PGC1α, CideA (8) were increased, while WAT-associated leptin (32), (33) had lower expression levels in the deeper fat.
Figure 3.
Quantitative reverse transcriptase PCR (qRT-PCR) of subcutaneous WAT and site-directed biopsy of MIBI-avid tissue from a single volunteer. Letpin is a marker of WAT and UCP1 is a marker of BAT. Expression levels are in arbitrary units normalized to TBP.
Comparing Blood Flow and Glucose Uptake in Activated Rodent BAT
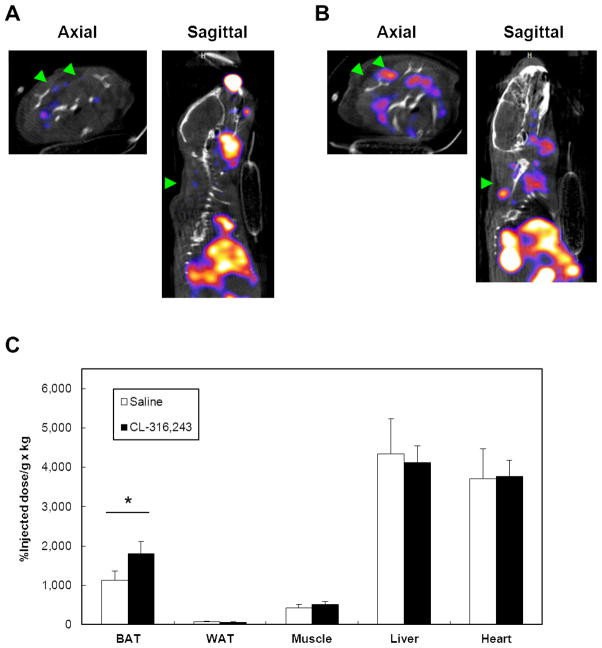
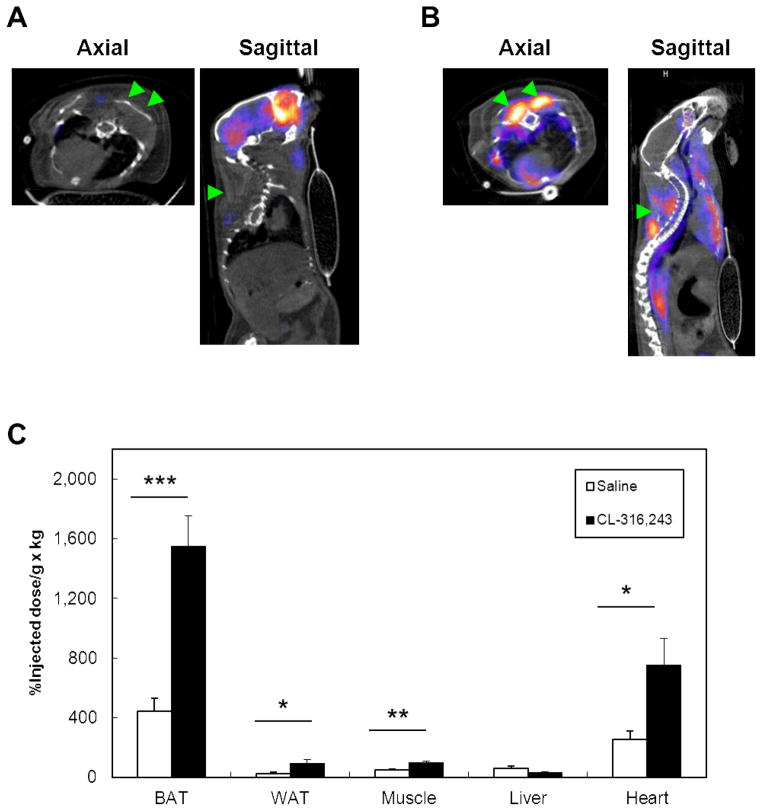
After locating -MIBI-avid adipose tissue is BAT in human tissue, we turned to the mouse model to define the physiological changes resulting from pharmacological stimulation of BAT. For each tracer, male 129SVE mice were administered either the β3-adrenergic receptor agonist CL (1mg/kg) (n=16) or saline control (n=16). 18F-FDG PET/CT (n=8 for each of the two treatments) was used to reflect glucose uptake, and 99mTc-MIBI SPECT/CT (n=8 for each of the two treatments) was given to measure tissue perfusion. CL increased heart rate by 23% and respiratory rate by 85% (P < 0.001) (Supplementary Figure 2). 18F-FDG PET/CT imaging done 2 hours post-stimulation revealed significantly increased uptake in the interscapular BAT compared to saline control (Fig. 5A–B). Uptake in the myocardium was also significantly elevated. To confirm the observed changes, we resected BAT, WAT, skeletal muscle, heart, and liver and quantified the percentage injected dose (%ID) per gram of tissue normalized by kilogram of body weight (%ID/g × kg [%ID/body weight]) (21). Even prior to stimulation, BAT retained more FDG than the other tissues and rose by 440% when stimulated by CL (P < 0.01) (Fig. 4C). Other tissues showing CL-induced increases in FDG were WAT, skeletal muscle, and heart. When the analogous study was done with 99mTc-MIBI, uptake in BAT was comparatively less than in liver and heart (Fig. 5A–B). The retained tracer in the resected tissues confirmed these findings, as %ID/body weight in BAT increased by only 61% (P < 0.01), and there were no significant changes in WAT, skeletal muscle, liver, or heart (all P > 0.05) (Fig. 5C). Thus, specific pharmacological stimulation of BAT led to a much greater increase in glucose uptake than tissue perfusion.
Figure 5.
SPECT-CT axial and sagittal images of 129SVE mice demonstrating uptake in interscapular BAT (green arrows) 120 minutes after injection of 99mTc-MIBI. Mice were given either (A) saline control or (B) CL-316,243 (1 mg/kg). After imaging, the tissues were resected, and activity was quantified (C) as the percent injected dose (%ID) per gram of tissue and normalized to per kilogram of total body weight (%ID/g × kg). *, P < 0.05.
Figure 4.
PET-CT axial and sagittal images of 129SVE mice demonstrating uptake in interscapular BAT (green arrows) 120 minutes after injection of 18F-FDG. Mice were given either (A) saline control or (B) CL-316,243 (1 mg/kg). After imaging, the tissues were resected, and activity was quantified (C) as the percent injected dose (%ID) per gram of tissue and normalized to per kilogram of total body weight (%ID/g × kg). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
From the time that it was identified as a functional tissue in adult humans (6–10), there has been great interest in utilizing BAT energy expenditure to treat obesity and metabolic dysregulation. When activated by mild cold exposure, adult human BAT has an energy expenditure of up to several hundred kcal/day (18). The broad reliance on 18F-FDG PET/CT imaging has allowed the comparison of results among investigators, but the ability to describe other features of BAT physiology becomes more limited. Progress is being made in this area, with the introduction of new tracers such as 18F-fluorobenzyl triphenyl phosphonium to measure thermogenesis (34) and the simultaneous use of combinations of 15O-H2O, 15O-O2, and 11C-acetate along with 18F-FDG to quantify blood flow, aerobic respiration, and glucose uptake (11), (17),(18). However, these and other radiotracers are either not yet approved for use in humans or are not readily available. 99mTc-MIBI meets these criteria and provides the opportunity to measure BAT mass and activity using SPECT/CT imaging. Prior studies have already indicated the possibility that 99mTc-MIBI could be used to detect BAT in adult humans (23–26). Here we have presented evidence strongly suggestive that 99mTc-MIBI localizes to human BAT and have demonstrated its utility in measuring BAT activity in response to pharmacological activation.
Both the rate of detection of unstimulated BAT (5.4%) and the anatomical location seen using 99mTc-MIBI SPECT/CT imaging match the findings reported with 18F-FDG PET/CT (7),(35), indicating that both tracers are localizing to the same tissue. This pattern of tracer avidity is consistent with their molecular structures. The glucose analog FDG localizes to tissues with high metabolic activity. MIBI is a lipophilic cation that is retained in tissues with high perfusion rates, particularly those enriched with mitochondria, which have a large negative transmembrane potential (36). The ultrastructural and gene expression findings presented here demonstrate how human brown adipocytes can be particularly avid for both FDG and MIBI. These features also have direct clinical relevance since human neck BAT lies close to the parathyroid glands. Clinicians should be careful to ensure that increased 99mTc-MIBI uptake is from parathyroid tissue and not a false-positive signal from BAT.
As an organ designed to generate heat at a rate of up to 300W/kg (32), a high rate of BAT perfusion is necessary to provide oxygen and fuel and transfer away the heat before suffering thermal injury. When studied in response to cold exposure, BAT tissue perfusion increases, but less than glucose uptake (11). These two independent measures of metabolism correlate significantly, which justifies the wide use of FDG to measure relative changes in BAT activity. However, it remains unclear what the fate of the glucose is. The fact that glucose utilization exceeds that of blood flow indicates that activated BAT likely uses glucose for several metabolic purposes. Internal lipid stores are the primary fuel for BAT and are used rapidly in response to cold exposure (32), (37). The glucose may be used for lipogenesis, glycolysis, anaplerosis, and uncoupled aerobic respiration (38). The simultaneous activity of these multiple pathways could explain the relatively higher rate of glucose utilization seen during BAT activation. Additional studies are required to determine how glucose is utilized in quiescent and activated BAT and how the measured uptake with FDG correlates with these different processes.
A limitation of this study is that we did not prospectively administer 99mTc-MIBI to humans to show changes in tracer uptake in response BAT stimulation. Rather, we relied on the site-directed biopsies to support the presence of human BAT and used the rodent studies to show the dynamic changes in BAT function. In addition, we did not simultaneously evaluate the changes in uptake of 99mTc-MIBI and 18F-FDG. In the mice, to permit detection of both tracers, this approach would have required doing the studies sequentially and not being able to accurately measure each tracer’s uptake on biopsy. However, the degree of BAT stimulation was likely to be similar with both tracers as evidenced by the non-significant difference between the heart and respiratory rates seen after treatment with CL and vehicle. Another issue is that uptake of 99mTc-MIBI is driven not only by perfusion but also the expression of p-glycoprotein (39), levels of which are unknown in BAT. Future studies should examine the expression of p-glycoprotein in rodent and human BAT. Furthermore, stimulation of BAT leads to mitochondrial uncoupling and a lowering of the transmembrane potential, which could reduce MIBI uptake. An alternative measure to quantify blood flow is contrast ultrasound, which has been used in rodent BAT (40) and could be correlated with MIBI uptake. Nevertheless, our findings are qualitatively similar to what has been reported with other markers of blood flow after cold-mediated activation of BAT in humans (11) and rodents (21).
CONCLUSION
We demonstrate that the accessible tracer 99mTc-MIBI may be used to detect BAT via SPECT/CT in humans and can provide information about changes in its blood flow. Understanding the physiological function and clinical utility of BAT energy expenditure will likely require multiple complementary tracers.
Supplementary Material
Acknowledgments
This work was supported by NIH grants DK087317, DK081604, DK046200, DK077097, and P30 DK036836 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), RR025757, the Clinical Translational Science Award UL1RR025758 to Harvard University and BIDMC from the National Center for Research Resources, Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers), the Clinical Investigator Training Program (Beth Israel Deaconess Medical Center–Harvard/MIT Health Sciences and Technology, in collaboration with Pfizer and Merck), and the Eli Lilly Foundation.
We thank Elaine Lunsford and Kyle Robichaud for their the excellent technical support at the Longwood Small Animal Imaging Facility; Michael Rourk and Graham Smythe for their expertise in maintain the animal colonies; Hillary Keenan for her biostatistical advice; Chris Cahill at the Joslin Diabetes Center Microscopy Core for his histological work; the teams of OR nurses for their assistance in tissue collection; J. Anthony Parker for his thoughts on the physiological role of MIBI; and C. Ronald Kahn for his input in preparing this manuscript. Finally, we are grateful to our volunteers for their commitment to the studies.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:143–149. doi: 10.1097/MED.0b013e328337a81f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 4.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 5.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 6.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 7.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 9.Zingaretti MC, Crosta F, Vitali A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 10.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Pfannenberg C, Werner MK, Ripkens S, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–1793. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bredella MA, Fazeli PK, Freedman LM, et al. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab. 2012;97:E584–E590. doi: 10.1210/jc.2011-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cypess AM, Chen YC, Sze C, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109:10001–10005. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vosselman MJ, van der Lans AA, Brans B, et al. Systemic beta-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes. 2012;61:3106–3113. doi: 10.2337/db12-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vrieze A, Schopman JE, Admiraal WM, et al. Fasting and postprandial activity of brown adipose tissue in healthy men. J Nucl Med. 2012;53:1407–1410. doi: 10.2967/jnumed.111.100701. [DOI] [PubMed] [Google Scholar]
- 17.Muzik O, Mangner TJ, Granneman JG. Assessment of oxidative metabolism in brown fat using PET imaging. Front Endocrinol (Lausanne) 2012;3:15. doi: 10.3389/fendo.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouellet V, Labbe SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wackers FJ, Berman DS, Maddahi J, et al. Technetium-99m hexakis 2-methoxyisobutyl isonitrile: human biodistribution, dosimetry, safety, and preliminary comparison to thallium-201 for myocardial perfusion imaging. J Nucl Med. 1989;30:301–311. [PubMed] [Google Scholar]
- 20.Marshall RC, Leidholdt EM, Jr, Zhang DY, Barnett CA. Technetium-99m hexakis 2-methoxy-2-isobutyl isonitrile and thallium-201 extraction, washout, and retention at varying coronary flow rates in rabbit heart. Circulation. 1990;82:998–1007. doi: 10.1161/01.cir.82.3.998. [DOI] [PubMed] [Google Scholar]
- 21.Baba S, Engles JM, Huso DL, Ishimori T, Wahl RL. Comparison of uptake of multiple clinical radiotracers into brown adipose tissue under cold-stimulated and nonstimulated conditions. J Nucl Med. 2007;48:1715–1723. doi: 10.2967/jnumed.107.041715. [DOI] [PubMed] [Google Scholar]
- 22.Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol. 2011;110:1137–1149. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higuchi T, Kinuya S, Taki J, et al. Brown adipose tissue: evaluation with 201Tl and 99mTc-sestamibi dual-tracer SPECT. Ann Nucl Med. 2004;18:547–549. doi: 10.1007/BF02984575. [DOI] [PubMed] [Google Scholar]
- 24.Kyparos D, Arsos G, Georga S, et al. Assessment of brown adipose tissue activity in rats by 99mTc-sestamibi uptake. Physiol Res. 2006;55:653–659. doi: 10.33549/physiolres.930890. [DOI] [PubMed] [Google Scholar]
- 25.Belhocine T, Shastry A, Driedger A, Urbain JL. Detection of 99mTc-sestamibi uptake in brown adipose tissue with SPECT-CT. Eur J Nucl Med Mol Imaging. 2007;34:149. doi: 10.1007/s00259-006-0244-x. [DOI] [PubMed] [Google Scholar]
- 26.Goetze S, Lavely WC, Ziessman HA, Wahl RL. Visualization of brown adipose tissue with 99mTc-methoxyisobutylisonitrile on SPECT/CT. J Nucl Med. 2008;49:752–756. doi: 10.2967/jnumed.107.048074. [DOI] [PubMed] [Google Scholar]
- 27.Chen YI, Cypess AM, Sass CA, et al. Anatomical and Functional Assessment of Brown Adipose Tissue by Magnetic Resonance Imaging. Obesity (Silver Spring) 2012;20:1519–1526. doi: 10.1038/oby.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akram K, Parker JA, Donohoe K, Kolodny G. Role of single photon emission computed tomography/computed tomography in localization of ectopic parathyroid adenoma: a pictorial case series and review of the current literature. Clin Nucl Med. 2009;34:500–502. doi: 10.1097/RLU.0b013e3181abb619. [DOI] [PubMed] [Google Scholar]
- 29.Cypess AM, White AP, Vernochet C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torriani M, Fitch K, Stavrou E, et al. Deiodinase 2 expression is increased in dorsocervical fat of patients with HIV-associated lipohypertrophy syndrome. J Clin Endocrinol Metab. 2012;97:E602–E607. doi: 10.1210/jc.2011-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 33.Timmons JA, Wennmalm K, Larsson O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madar I, Isoda T, Finley P, Angle J, Wahl R. 18F-fluorobenzyl triphenyl phosphonium: a noninvasive sensor of brown adipose tissue thermogenesis. J Nucl Med. 2011;52:808–814. doi: 10.2967/jnumed.110.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med. 2003;44:1267–1270. [PubMed] [Google Scholar]
- 36.Chiu ML, Kronauge JF, Piwnica-Worms D. Effect of mitochondrial and plasma membrane potentials on accumulation of hexakis (2-methoxyisobutylisonitrile) technetium(I) in cultured mouse fibroblasts. J Nucl Med. 1990;31:1646–1653. [PubMed] [Google Scholar]
- 37.Baba S, Jacene HA, Engles JM, Honda H, Wahl RL. CT hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med. 2010;51:246–250. doi: 10.2967/jnumed.109.068775. [DOI] [PubMed] [Google Scholar]
- 38.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun SS, Shiau YC, Lin CC, Kao A, Lee CC. Correlation between P-glycoprotein (P-gp) expression in parathyroid and Tc-99m MIBI parathyroid image findings. Nucl Med Biol. 2001;28:929–933. doi: 10.1016/s0969-8051(01)00259-1. [DOI] [PubMed] [Google Scholar]
- 40.Baron DM, Clerte M, Brouckaert P, et al. In vivo noninvasive characterization of brown adipose tissue blood flow by contrast ultrasound in mice. Circ Cardiovasc Imaging. 2012;5:652–659. doi: 10.1161/CIRCIMAGING.112.975607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.