Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia, occurring in 1-2% of overall population, involving more than 6 millions of European people. It is associated to a reduced quality of life and an increased morbidity and mortality. The Framingham study showed the link between angina and AF. The same risk factors, such as hypertension, diabetes and obesity promote both AF and coronary artery disease (CAD). About 1/4 of AF patients develop a CAD and, in this setting, about 1/5 undergoes a percutaneous coronary intervention (PCI). In patients with both AF and CAD, the optimal medical strategy is challenging and it is still debated in cardiological community, since patients treated by dual (two antiplatelets drugs ore one antiplatelets drug and an oral anticoagulant drug) or triple therapy (two antiplatelets drugs and an oral anticoagulant drug) are exposed to divergent risk of bleeding or thromboembolic and ischemic complications.
Aim of this paper is to focus the attention on the different problems arising from the presence of AF in patients undergoing PCI, such as the risk of stroke, bleeding and stent thrombosis.
Keywords: Atrial fibrillation, Acute coronary syndromes, Dual antiplatelet therapy, Triple therapy
I. INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia, occurring in 1-2% of overall population, involving more than 6 millions of European people [1]. AF is associated to a reduced quality of life and an increased morbidity and mortality, due to its not uncommon complications, such as arterial embolism [2, 3]. Furthermore, AF development after an acute coronary syndrome is related with a worse prognosis [4]. The Framingham study showed the link between angina and AF, especially in males [5-7]. Both AF and coronary artery disease (CAD) are occurring in presence of similar risk factors, such as hypertension, diabetes and obesity. In AF patients the average CAD incidence is 34%, according to the different study populations, reaching more than 40% in patients older than 70 years [8]. Among all this patients about 1/5 undergoes a percutaneous coronary intervention (PCI), opening a controversy about the optimal antiplatelet medical strategy [8]. In patients with concomitant coronary artery disease and AF, the optimal medical strategy is challenging, since patients treated by dual (two antiplatelets drugs or one antiplatelets drug and an oral anticoagulant drug) or triple therapy (two antiplatelets drugs and an oral anticoagulant drug) are exposed to divergent risk of bleeding or thromboembolic and ischemic complications.
Aim of this paper is to focus the attention on the different problems arising from the presence of AF in patients undergoing PCI, such as the risk of stroke, bleeding and stent thrombosis.
II. RISK STRATIFICATION
According to the current guidelines of the European Society of Cardiology (ESC) for AF oral anticoagulation should be started after risk stratification [1]. The most commonly used stroke risk score in clinical practice is the CHA2DS2-Vasc-Score; it consists of eight different clinical and anamnestic parameters with the attribution of one point per each, with exception of age ≥ 75 years and previous stroke or thrombo-embolism (attribution of 2 points). Oral anticoagulation is indicated when the CHA2DS2-Vasc-Score is ≥ 2. The superiority of oral anticoagulation compared to antiplatelet therapy in prevention of thromboembolism in patients with atrial fibrillation has been already demonstrated [9]. Therefore, not all AF patients need to be treated by oral anticoagulation, but only those with an elevated embolic risk. The patients at low embolic risk should be treated by using aspirin alone; unfortunately the rate of this low risk patients is less than 10% [1]. On the other hand a more aggressive antiplatelet strategy correlates with an increased bleeding risk, that should be evaluated by using an haemorrhagic risk score, such as the HAS-BLED-Score. However some clinical variables are common in both embolic and haemorrhagic risk score, leading to a very challenging appropriate medical therapy.
III. ANTIPLATELET THERAPY AFTER STENT IMPLANTATION
According to ESC guidelines on myocardial revascularization, the dual antiplatelet therapy (DAPT) should be performed 1 month after bare metal stent (BMS) implantation in stable angina, 6-12 months after drug eluting stent (DES) implantation in all patients, and 12 months in all patients after acute coronary syndrome irrespectively of revascularization strategy [10]. By using risk score stratification a triple therapy consisting of a vitamin-K-antagonist, aspirin, and clopidogrel is recommended in all patients with an higher embolic risk. Depending on the clinical setting (acute coronary syndrome or stable angina), hemorrhagic and stroke risk, and the type of stent implanted, triple therapy should be prescribed for the shortest time possible, continuing with a vitamin-K-antagonist alone administration as lifelong therapy.
Others oral antiplatelets drugs, such as Prasugrel and Ticagrelor, are now commercially available to prevent reinfarction and stent thrombosis. The comparison in terms of efficacy and adverse events between Clopidogrel vs Prasugrel and Clopidogrel vs Ticaglelor has been performed in TRITON-TIMI 38 and PLATO studies, respectively. By using Prasugrel as well as Ticagrelor the platelets activity inhibition is faster and more effective. Unfortunately the higher efficacy of Prasugrel in platelets inhibition activity correlates to an higher rate of life threatening bleedings (1.4% vs. 0.9%; p = 0.01). Conversely, in PLATO trial no statistically significant increase of major bleeding has been reported (11.6% with Ticagrelor vs 11.2% with Clopidogrel; p = n.s.) [11, 12].
Despite the superior efficacy of these new antiplatelets drugs, we do not have data on their association with vitamin-K-antagonist, available in AF patients who underwent PCI. The major risk of bleeding carried out by these new drugs makes them potentially harmful in association with vitamin-K-antagonist. Thus dedicated randomized trials and or registries are needed in order to demonstrate their efficacy and safety in this particular clinical setting.
IV. PROBLEMS IN TRIPLE ANTIPLATELET THERAPY
Stroke, bleeding and stent thrombosis are different aspects of the same phenomenon. An aggressive antiplatelet strategy (oral anticoagulation, OAC, + DAPT) leads to an increased bleeding risk, conversely a conservative antiplatelet strategy (OAC + single antiplatelet therapy, SAPT) leads to an increased embolic risk and an increased stent thrombosis rate [13]. In 239 patients treated by SAPT, comparing efficacy and safety of OAC + Aspirin vs OAC + Clopidogrel at 12 months, the first group showed a lower incidence of major bleedings and an higher incidence of stent thrombosis (6,1 vs 11,1% and 15,2 vs 0%, respectively) [14]. According to a consensus document of the European Society of Cardiology AF patients, with moderate to high stroke risk, undergoing PCI should be treated by triple therapy (TT), consisting in oral anticoagulation, aspirin and clopidogrel after stent implantation, preferably a BMS [15]. Nevertheless the major bleedings rate increase during the first 12 months, irrespective of the type of stent implanted [16].
Despite guidelines recommendations, in clinical practice the duration of DAPT after PCI depends from the type of stent used, 1 month for a BMS and 12 months for a DES, respectively [17].
V. DES AND BMS, WHICH STENT FOR WHICH PATIENT
Thus, what is the best management in AF patient undergoing PCI?
Appropriate bleeding and embolic risk stratification should be performed.
Radial approach should be preferred, due to its lower incidence of bleeding complications [18].
INR therapeutic range should be lower, between 2.0-2.5 [19].
Gastric protection with either protonic pump inhibitors, H2-receptor antagonists or antiacid drugs is recommended [15].
TT should be performed as less as possible.
According to a consensus document of the European Society of Cardiology, in AF patients requiring a stent implantation BMS should be preferred, restricting DES implantation in few clinical and/or anatomical situations, such as age < 75 years, long lesions, small vessels, diabetes, etc, in which DES have shown a better performance than BMS [15]. In case of DES implantation, a second generation DES should be preferred, such as a tacrolimus eluting stent, a Carbostent polymer-free stent, which requires DAPT only for two months, as reported from the MATRIX study, in 572 patients [20]. Conversely, if an everolimus and zotarolimus eluting stent has been implanted, DAPT could be discontinued after 3 months without an increasing rate of stent thrombosis, as observed in more than 6800 patients and more than 2200 patients, respectively [21, 22]. In case of BMS implantation a last generation of BMS should be used. The preferred stent used should be Genous stent, requiring only 15 days of DAPT, due to its anti-hCD34 coating, which allows an accelerated re-endothelization by capturing circulating CD34+ endothelial progenitor cells [23, 24]. This peculiar aspect has been reported in 384 patients enrolled in the ARGENTO study, validating the safety of this very short time of DAPT [24]. Another possibility is represented by the Avantgarde stent, which requires less than one month DAPT, due to its peculiar projected design, favoring a better endothelization. This stent was evaluated in 42 patients requiring coronary revascularization before an undeferrable major non-cardiac surgery, performed 27 ± 9 days after PCI. Only one major cardiac adverse event was observed at one month follow-up [25]. Finally, in STEMI setting with angiographic evidence of thrombus a bare metal MGuard stent should be implanted. MGuard stent has been evaluated in 150 patients undergoing primary or rescue PCI; its ability to dramatically reduce distal thrombus embolization led to a TIMI flow grade 2.85 ± 0.40 and a myocardial blush grade 3 of 90%. Furthermore, within 30 minutes after the procedure a very high rate of complete (> or = 70%) ST-segment resolution (90%) was observed [26, 27].
Independently of the implanted stent, as suggested by the current guidelines, the radial approach should be preferred in order to its lower incidence of bleeding complications, as already demonstrated by several meta-analyses [28, 29]. Romagnoli et al. in a recently published randomized controlled trial, comparing radial versus femoral approach in ST-elevated myocardial infarction patients who underwent primary PCI, showed a significant reduction of major adverse cardiac events in the radial arm of the study [30]. However, the great enthusiasm coming from clinical trials data seems to be questioned by recent data reanalysis [31, 32]. Thus this increased enthusiasm about radial approach might be owed more to the patients and interventional cardiologist preferences, than to a true mortality rate reduction.
VI. NEW ORAL ANTICOAGULANT AGENTS
New anticoagulant drugs are safe and effective compared to warfarin in non-valvular AF patient. The use of these new anticoagulant drugs is still a matter of debate due to the controversial results observed in the different clinical trials in this particular setting. Compared with placebo, the apixaban addiction to DAPT, in treatment of non-valvular AF after acute coronary syndrome occurrence, leads to an increased rate of bleedings, with no better thromboembolic outcomes [33].
Conversely a very-low dosage of rivaroxaban, used in acute coronary patients setting, showed the reduction of thromboembolic complications with an increasing rate of nonfatal bleedings [34]. A substudy of the RE-LY trial, showed an increased bleeding risk in dabigatran addiction to DAPT compared to SAPT [35]. On this ground, further studies are strongly needed in order to evaluate the efficacy and the safety of these new drugs in association with either old and new antiplatelets drugs.
VII. CONCLUSIONS
AF and CAD are strictly related. An individualized approach with a tailored medical and interventional strategy is required in patients with concomitant AF and CAD, in order to obtain a balance between the risk of cerebrovascular events, bleeding complications and reinfarction rate. In short time TT benefits are superior to its side effects, nevertheless it should be prolonged as less as possible.
Figure 1.
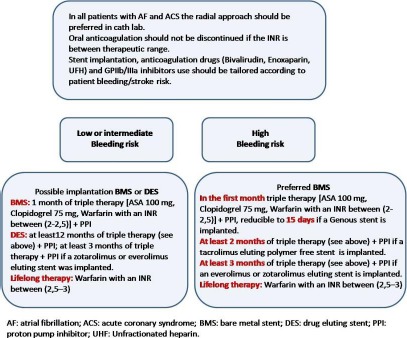
Flowchart management of atrial fibrillation patients undergoing percutaneous coronary intervention.
REFERENCES
- 1.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31(19):2369-429 [DOI] [PubMed] [Google Scholar]
- 2.Paquette M, Roy D, Talajic M, Newman D, Couturier A, Yang C, et al. Role of gender and personality on quality-of-life impairment in intermittent atrial fibrillation. Am J Cardiol 2000;86(7):764–8 [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982;306(17):1018–22 [DOI] [PubMed] [Google Scholar]
- 4.Pizzetti F, Turazza FM, Franzosi MG, Barlera S, Ledda A, Maggioni AP, et al. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI-3 data. Heart 2001; 86(5): 527–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gersh BJ, Tsang TSM, Barnes ME, Seward JB. The changing epidemiology of non-valvular atrial fibrillation: The role of novel risk factors. Eur Heart J Supplements 2005; 7(Suppl C): C5–C11 [Google Scholar]
- 6.Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250(5):382–9 [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Abbott RD, Savage DD, McNamara PM. Coronary heart disease and atrial fibrillation: The Framingham study. Am Heart J 1983;106(2):389–96 [DOI] [PubMed] [Google Scholar]
- 8.Kralev S, Schneider K, Lang S, Süselbeck T, Borggrefe M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS One. 2011; 6(9):e24964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Annals of Internal Medicine 1999;131(7): 492–501 [DOI] [PubMed] [Google Scholar]
- 10.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, et al. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Guidelines on myocardial revascularization. Eur Heart J 2010; 31(20):2501-5520802248 [Google Scholar]
- 11.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-15 [DOI] [PubMed] [Google Scholar]
- 12.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-57 [DOI] [PubMed] [Google Scholar]
- 13.Lamberts M, Olesen JB, Ruwald MH, Hansen CM, Karasoy D, Kristensen SL, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation 2012;126(10):1185-93 [DOI] [PubMed] [Google Scholar]
- 14.Karjalainen PP, Porela P, Ylitalo A, Vikman S, Nyman K, Vaittinen MA, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28(6):726-32 [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Huber K, Andreotti F, Arnesen H, Airaksinen KJ, Cuisset T, et al. European Society of Cardiology Working Group on Thrombosis. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary intervention/ stenting. Thromb Haemost 2010; 103(1):13-28 [DOI] [PubMed] [Google Scholar]
- 16.Rubboli A. The risk of bleeding of triple therapy with vitamin K-antagonists, aspirin and clopidogrel after coronary stent implantation: Facts and questions. J Geriatr Cardiol 2011; 8(4):207-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krötz F, Sohn HY, Klauss V. Antiplatelet drugs in cardiological practice: established strategies and new developments. Vasc Health Risk Manag 2008;4(3):637-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR, et al. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J 2009;157(1):132-40 [DOI] [PubMed] [Google Scholar]
- 19.Cassese S, Byrne RA, Tada T, King LA, Kastrati A. Clinical impact of extended dual antiplatelet therapy after percutaneous coronary interventions in the drug-eluting stent era: a meta-analysis of randomized trials. Eur Heart J 2012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Cassese S, De Luca G, Villari B, Berti S, Bellone P, Alfieri A, et al. Reduced antiplatelet therapy after drug-eluting stenting: multicenter Janus Flex carbostent implantation with short dual antiplatelet treatment for 2 or 6 months-MATRIX study. Catheter Cardiovasc Interv 2012;80(3):408-16 [DOI] [PubMed] [Google Scholar]
- 21.Kedhi E, Stone GW, Kereiakes DJ, Serruys PW, Parise H, Fahy M, et al. Stent thrombosis: insights on outcomes, predictors and impact of dual antiplatelet therapy interruption from the SPIRIT II, SPIRIT III, SPIRIT IV and COMPARE trials. EuroIntervention 2012;8(5):599-606 [DOI] [PubMed] [Google Scholar]
- 22.Hahn JY, Song YB, Choi JH, Choi SH, Lee SY, Park HS, et al. Three-month dual antiplatelet therapy after implantation of zotarolimus-eluting stents: the DATE (Duration of Dual Antiplatelet Therapy AfterImplantation of Endeavor Stent) registry. Circ J 2010;74(11):2314-21 [DOI] [PubMed] [Google Scholar]
- 23.Piscione F, Cassese S, Galasso G, Cirillo P, Esposito G, Rapacciuolo A, et al. A new approach to percutaneous coronary revascularization in patients requiring undeferrable non-cardiac surgery. Int J Cardiol 2011;146(3):399-403 [DOI] [PubMed] [Google Scholar]
- 24.Cassese S, Galasso G, Sciahbasi A, Scacciatella P, Muçaj A, Piccolo R, et al. Antiplatelet theRapy after Genous EPC-capturing coroNary stenT implantatiOn: The ARGENTO Study: a prospective, multicenter registry. Int J Cardiol 2013;167(3):757-61 [DOI] [PubMed] [Google Scholar]
- 25.Briguori C, Visconti G, De Micco F, Focaccio A. The avantgarde carbostent in patients scheduled for undelayable noncardiac surgery. Thrombosis. 2012;2012:372371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone GW, Abizaid A, Silber S, Dizon JM, Merkely B, Costa RA, et al. Prospective, Randomized, Multicenter Evaluation of a Polyethylene Terephthalate Micronet Mesh-Covered Stent (MGuard) in ST-Segment Elevation Myocardial Infarction: The MASTER Trial. J Am Coll Cardiol 2012; 1558-3597 [DOI] [PubMed] [Google Scholar]
- 27.Piscione F, Danzi GB, Cassese S, Esposito G, Cirillo P, Galasso G, et al. Multicentre experience with MGuard net protective stent in ST-elevation myocardial infarction: safety, feasibility, and impact on myocardial reperfusion. Catheter Cardiovasc Interv 2010;75(5):715-21 [DOI] [PubMed] [Google Scholar]
- 28.Joyal D, Bertrand OF, Rinfret S, Shimony A, Eisenberg MJ. Meta-analysis of ten trials on the effectiveness of the radial versus the femoral approach in primary percutaneous coronary intervention. Am J Cardiol 2012;109(6):813–8 [DOI] [PubMed] [Google Scholar]
- 29.Vorobcsuk A, Kónyi A, Aradi D, Horváth IG, Ungi I, Louvard Y, et al. Transradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction Systematic overview and meta-analysis. Am Heart J 2009;158(5):814–21 [DOI] [PubMed] [Google Scholar]
- 30.Romagnoli E, Biondi-Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendenza G, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012;60(24):2481-9 [DOI] [PubMed] [Google Scholar]
- 31.Mahmud E, Patel M. Radial Access for ST-Segment Elevation Myocardial Infarction Interventions: Does It Really Lower Mortality? JACC Cardiovasc Interv 2013;6(8):824-6 [DOI] [PubMed] [Google Scholar]
- 32.Di Gioia G, Piccolo R, Niglio T, D'Anna C, De Rosa R, Strisciuglio T, et al. Mortality reduction with transradial approach in patients with ST-segment elevation myocardial infarction: Is the randomized evidence conclusive? Int J Cardiol. 2013;168(2):1578-9 [DOI] [PubMed] [Google Scholar]
- 33.Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med 2011;365(8):699-708 [DOI] [PubMed] [Google Scholar]
- 34.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366(1):9-19 [DOI] [PubMed] [Google Scholar]
- 35.Van Spall HG, Wallentin L, Yusuf S, Eikelboom JW, Nieuwlaat R, Yang S, et al. Concomitant Use of Antiplatelet Therapy with Dabigatran or Warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY(R)) Trial. Circulation 2012;126(19):2309-16 [DOI] [PubMed] [Google Scholar]


