Abstract
Objectives
We sought to evaluate the ethnic variation in pelvimetry and its impact as a predictor of positive surgical margins (PSM) at radical prostatectomy (RP).
Methods
Preoperative MRI was performed in 482 Caucasian and 103 African-American (AA) men undergoing RP without prior treatment from 07/03-01/05 and 11/01-06/07, respectively. We measured bony and soft tissue dimensions on MRI to evaluate the pelvic inlet, midplane, prostate size and apical depth. Analysis of covariance was performed to determine the effect of ethnicity on the midpelvic area (MPA). We performed multivariate logistic regression analysis for prediction of overall and site-specific PSM.
Results
AA men had a significantly steeper symphysis pubis angle (median 43.1° vs 41.3°, respectively, p=0.001) and smaller MPA (median 78.5 vs 83.9 cm2, respectively, p=0.004). Ethnicity and BMI were found to have a significant effect on MPA. Apical depth of the prostate was identified as a significant independent predictor of apical PSM with a more pronounced effect in AA men. Pelvimetric measures were not a significant predictor of other sites of PSM.
Conclusions
AA men have a significantly smaller MPA and steeper symphysis angle. The adverse impact of a deep pelvis, as measured by the apical prostatic depth, on apical PSM was found to be greater in AA men. Evaluation of pelvic dimensions and prostate parameters in preoperative MRI imaging may add to our understanding of their impact on surgical outcomes.
Keywords: pelvimetry, prostate cancer, ethnicity, apical depth, MRI
Introduction
We have previously reported that AA men have a higher apical PSM rate at RP, controlling for other clinical and pathologic parameters, which we postulated was due to a narrower pelvis.1 While it has been shown that the likelihood for a PSM in surgically treated rectal cancer patients is higher in patients with a narrow pelvis, presumably caused by the technical difficulty with smaller pelvic dimensions,2 the impact of pelvimetry on oncologic outcomes in prostate cancer has not been fully elucidated. Hong et al. have reported that men with a deep and narrow pelvis on MRI had a higher PSM on univariate but not multivariate analysis;3 however, this study had limited power due to small study size. The racial differences in pelvic anatomy have been well documented in early anthropologic studies by Turner with the finding that the pelvis is narrower in blacks.4 As such, we sought to determine the ethnic differences in bony and soft tissue pelvic dimensions in patients undergoing RP and the influence of pelvimetric measures and ethnicity on PSM.
Material and Methods
Patient population
Our prospective database of patients undergoing RP was used to identify 482 Caucasian patients with preoperative prostate MRI imaging undergoing RP (open (RRP) or laparoscopic (LRP)) without prior treatment between July 2003 and January 2005 and 103 AA men with preoperative MRI undergoing RP between November 2001 and June 2007 by one of five dedicated prostate surgeons. The case volume prior to their first patient in this study for each of the five surgeons was 1487, 1088, 779, 164 and 0 cases after fellowship training. Median follow-up was 38.9 months (interquartile range: 24.5 – 48.6 months).
MRI imaging
MR imaging studies were performed on a 1.5-Tesla system (Signa; GE Medical Systems, Milwaukee, WI) as previously published.5 Bony measurements were made of the pelvic inlet, midplane and outlet as well as soft tissue measurements at the pelvic midplane and prostate measurements, expressed in mm. (Figure 1). Preoperative MRI has been performed routinely in our institution for staging of prostate cancer. As such, the pelvimetric measurements have been performed retrospectively and did not influence the decision to perform surgery or the approach in this cohort of patients.
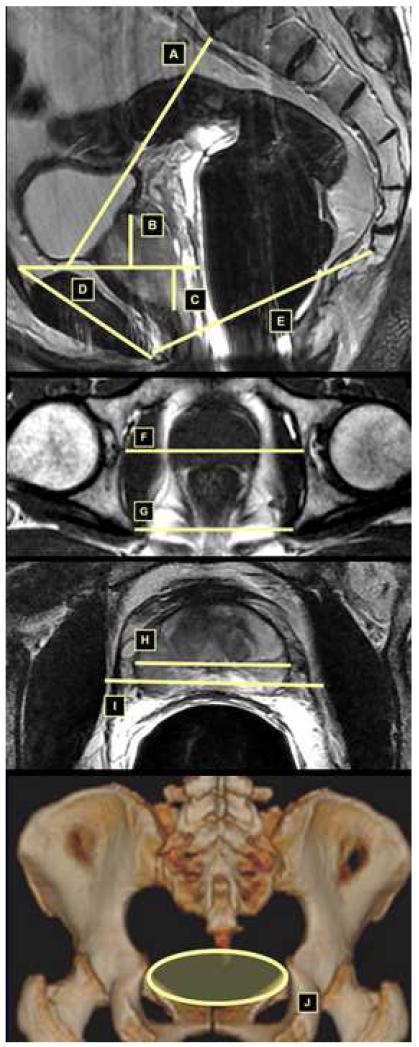
Figure 1.
A: Upper conjugate defined as distance from the innermost aspect of the top of the symphysis pubis to the sacral promontory.
B: Height of prostate defined as orthogonal distance from top of symphysis pubis to base of prostate.
C: Depth of prostate defined as orthogonal distance from top of symphysis pubis to apex of prostate.
D: Symphysis angle: defined as angle between the long axis of the symphysis pubis and the horizontal.
E: Lower conjugate defined as distance from lower inner symphysis pubis to sacrococcygeal junction.
F: Bony femoral width defined as bony width of the pelvis at the mid-femoral head level.
G: Interspinous distance defined as distance between the tips of the ischial spines.
H: Width of prostate at the same level as soft tissue width.
I: Soft tissue width defined as narrowest distance between the levator muscles.
J: Demonstration of the mid-pelvic area (MPA).
The pelvic dimension index (PDI) was defined as by Hong et al3 as interspinous distance (ISD) divided by apical depth of the prostate (AD). Given that the bony femoral width (BFW) is measured at a more anterior plane corresponding to the location of the prostate than the ISD, the BFW may be more representative of the width of the bony pelvis encountered by the urologist in performing RP. The mid-pelvic area (MPA) in cm2 was defined by the formula for an ellipse: π*anteroposterior diameter of the pelvic midplane (in mm) * BFW (in mm) /400 (Figure 1).
Statistical analysis
Statistical significance of difference in the medians of continuous variables was tested using the median test. Normality of the distribution of MPA was tested using the Lillefors modification of the Kolmogorov-Smirnov test, while equality of variances was tested using the Levene test. Analysis of covariance was performed with ethnicity as a fixed factor, surgeon and prostatectomy approach as random factors and age and body mass index (BMI) as covariates to determine the effect on the MPA. We determined the significance of the terms in the model using the F statistic and calculated the power of detecting the observed differences given the sample sizes in the study with α error = 0.05. The partial η2 statistic was determined as a measure of effect size. The pelvimetric measures of AD, ISD, BFW, and MPA were correlated with estimated blood loss (EBL) and operative time as measures of operative difficulty separately for RRP and LRP using the Kendall τb correlation coefficients. Multivariate logistic regression analysis for prediction of overall and site-specific PSM was performed with forward stepwise variable selection evaluating age, ethnicity, body mass index (BMI), RP approach (RRP vs. LRP), preoperative serum PSA, clinical stage, biopsy Gleason score, MRI volume, MPA, AD, MPA*race, AD*race, specimen weight, pathologic organ-confined status, and pathologic Gleason score as predictors. For preoperative PSA, a logarithmic transformation was used to generate odds ratios for a doubling in the predictor variable. Biopsy and pathologic Gleason score was categorized as Gleason 2-6, 7 or 8-10. Tumors were pathologic organ-confined if there was no extracapsular extension, seminal vesicle invasion or lymph node involvement. Statistical analyses were performed using the SPSS statistical package (SPSS Inc., Chicago, IL).
Results
The pelvic dimensions and prostate measurements by ethnicity are presented in Table 1. The pelvic inlet was significantly smaller in AA men as well as the bony femoral width and the soft tissue width of the pelvic midplane. We found a significantly steeper symphysis pubis angle in AA patients: median 43.1° (40.5°, 45.9°) versus 41.3° (38.1°, 44.7°), respectively (p=0.001). The MPA is significantly less in AA than Caucasian men: median 78.5 (IQR 72.4, 86.7) cm2 versus 83.9 (IQR 77.3, 90.7) cm2, respectively (p=0.004). The prostate volume was significantly smaller in AA patients whereas there was no significant difference in apical depth of the prostate. In the analysis of covariance, ethnicity (coefficient=−4.0 [95% CI:−6.7,−1.3] for AA vs Caucasian, p=0.004, partial η2=0.014, power=82%) and BMI (coefficient=0.55 [95% CI:0.33,0.77], p<0.001, partial η2=0.041, power=100%)had a statistically significant effect on MPA; but these have little clinical impact as demonstrated by the low partial η2 statistic. For both RRP and LRP patients there was no correlation between the pelvimetric measures of AD, ISD, BFW, and MPA the measures of operative difficulty of EBL and operative time, except for a weak inverse correlation between MPA and operative time in patients undergoing LRP (Kendall τb = −0.098, p = 0.023). On multivariate logistic regression analysis, PSA was a significant independent predictor of overall PSM (odds ratio [OR] 1.4, 95%CI=1.03-1.9 for doubling of PSA), apical PSM (OR 1.8, 95%CI=1.1-2.8 for doubling of PSA), and bladder neck PSM (OR 3.0, 95%CI=1.05-8.8 for doubling of PSA); pathologic non-organ confined disease was a significant independent predictor of overall PSM (OR 2.2, 95%CI=1.2-3.9) and posterior PSM (OR 3.5, 95%CI=1.5-8.3); pathologic Gleason score was a significant independent predictor of overall PSM (OR 2.1, 95%CI=1.1-4.2 for Gleason 7 vs 2-6; OR 3.1, 95%CI=1.2-7.8 for Gleason 8-10 vs 2-6) and anterior PSM (OR 14.4, 95%CI=1.5-141.7 for Gleason 8-10 vs 2-6); apical depth of the prostate was a significant independent predictor of apical PSM (OR 1.04, 95%CI=1.01-1.07 for each mm for Caucasian men and OR 1.11, 95%CI=1.02-1.21 for each mm for AA men). In particular, pelvimetric measures were not found to impact overall PSM or PSM at the bladder neck, posteriorly or anteriorly.
Table 1.
Pelvic dimensions and prostate measurements by ethnicity.
| Dimension | African-American | Caucasian | P value |
|---|---|---|---|
| BONY | |||
| Pelvic inlet | |||
| Upper conjugate | 103 (95, 108) | 110 (103, 116) | <0.001 |
| Symphysis angle | 43.1° (40.5°, 45.9°) | 41.3° (38.1°, 44.7°) | 0.001 |
| Pelvic midplane | |||
| Lower conjugate | 103 (97, 111) | 105 (98, 111) | 0.14 |
| ISD | 92 (88, 97) | 94 (88, 98) | 0.049 |
| BFW | 97 (93, 102) | 102 (98, 106) | <0.001 |
| SOFT TISSUE | |||
| Pelvic midplane | |||
| (Soft tissue width) | 51 (48, 56) | 57 (50, 60) | <0.001 |
| PROSTATE | |||
| Prostate width | 45 (41, 49) | 49 (45, 53) | <0.001 |
| Max. Prostate width | 48 (43, 53) | 52 (48, 55) | <0.001 |
| Depth of prostate | 27 (22, 31) | 29 (23, 33) | 0.064 |
| Height of prostate base |
14 (10, 19) | 14 (8.8, 21) | 0.98 |
| Prostate volume | 28.4 (22.5, 40.0) | 36.4 (27.4, 47.7) | <0.001 |
All values are median (interquartile range) expressed in mm for all measurements except symphysis angle which is expressed in degrees and prostate volume which is expressed in cc.
Comment
In surgically treated rectal cancer patients, it has been reported that the PSM rate is higher in those with a narrow pelvis.2 The impact of pelvimetry on PSMs in patients undergoing RP has not been fully elucidated. Hong et al. have suggested that pelvimetry may impact PSM with men with a deep and narrow pelvis having a higher PSM rate, but this relation was found only to be significant on univariate but not multivariate analysis 3, the power being limited by the small sample size of 190 patients. Neill et al found a narrow pelvis to be correlated with higher odds of capsular breach in prostate cancer patients undergoing RP.6 In their study, Neill et al speculated that the higher odds of capsular breach might be caused by a technically more difficult operation in an anatomically smaller pelvis. Early anthropological studies have shown that the pelvis was narrower in blacks compared to Caucasians.4 Our subjective impression that the pelvis in AA men at RP often appears to be narrower than in Caucasian patients, is consistent with these reports. Previously, we investigated if the rate of apical PSM at RP might be higher in AA men given the increased challenge of apical dissection in a deep and narrow pelvis and reported our findings.1 Controlling for clinical and pathological variables, ethnicity was found to be a significant independent predictor of apical PSM but not of overall PSMs or PSMs at other sites, with AA men having a higher apical PSM rate.1 We concluded that further evaluation is necessary to determine the effect of pelvic size on PSM at RP and in particular in AA men who might have a higher probability due to an anatomically narrower pelvis leading to a technically more challenging operation.
In the present study, we measured different bony and soft tissue dimensions in 482 Caucasian men and 103 AA patients undergoing RP using preoperative prostate MRI images. We could confirm, consistent with the anthropological studies by Turner,4 that AA men have an anatomically significantly narrower mid-pelvis than Caucasians and a smaller mid-pelvic area calculated using the measured mid-pelvic bony dimensions in the sagittal and axial planes. Furthermore, AA men have a significantly steeper symphysis pubis angle (43.1° vs 41.3°, p= 0.001), defined as the angle between the long axis of the symphysis pubis and the horizontal; the wider this angle is, the steeper the access to the apex of the prostate for the surgeon which might consequently lead to a more challenging dissection. The soft tissue width, defined as the narrowest distance between the levator muscles, which affects the actual breadth of the surgical field, besides the bony dimensions, was found to be significantly smaller in AA patients (51mm vs. 57mm, p=<0.001). Adjusting for clinical and pathological variables to exclude the possibility of detecting a higher PSM rate in AA men caused by a more advanced pathologic stage, apical prostate depth was found to be a significant independent predictor for apical PSM, with a more pronounced effect in AA men. Ethnicity was not found to be an independent predictor of overall PSM or of PSM at other sites besides the apex of the prostate. We have previously reported that AA men have a 1.76-fold increased risk of apical PSM but that the risk of overall PSM or of PSM at other sites was not increased compared to Caucasian men.1 Powell et al have reported ethnicity as an independent predictor of PSM;7 however clinical stage and preoperative Gleason score were included into the statistical model as continuous variables based on an ordinal scale which might have weakened the relative effect of these variables. Other studies have reported a higher PSM rate in AA men compared with Caucasian men but did not use a multivariate analysis to control for other clinical or pathological variables.8-9 Hence these studies might have shown a higher PSM frequency due to a more advanced pathologic stage in AA patients.
The apex of the prostate is the most frequent location for a PSM at RP.10-11 The impact of an isolated apical PSM on progression is controversial. Several authors have reported a higher BCR rate in men with apical PSMs12-14 while others have not identified any adverse prognostic significance for isolated apical PSMs.15-17 Pettus et al reported a higher risk of BCR for patients with a PSM with a similar risk of recurrence for patients with an isolated apical PSM as compared to a PSM at another site or multiple PSMs.13 Evaluating the impact of PSM site on prognosis, Eastham et al reported an increased risk for PSA progression after RP in patients with an isolated posterolateral or posterior PSM, but not for an isolated apical PSM while this location was the most common one in their study.17 Given this controversy, it would be prudent to achieve a negative apical surgical margin and all parameters potentially impacting occurrence of an apical PSM should be evaluated to minimize the surgical risk of BCR.
In our study, BMI and ethnicity had a statistically significant effect on the midpelvic area in the analysis of covariance but the clinical impact was limited as evidenced by the low partial η2 statistic. Masterson et al reviewed the current literature addressing the use of MRI in patients treated with localized prostate cancer and concluded that MRI may facilitate an enhanced ability to individualize treatment and tailor the approach to maximize cancer control.18 Hricak et al investigated the influence of preoperative MRI on surgical planning.5 In almost 40% of the cases, the MRI reading led to a either more aggressive or conservative surgical approach.5 Besides the reported use of MRI in advanced prostate cancer cases in particular, our finding that pelvimetric measures impact apical PSM improves our understanding of the role of technical factors in achieving negative margins. We did not identify pelvimetric measures to be correlated with other measures of surgical difficulty such as EBL and operative time, suggesting that the surgeon is able to adapt to challenging pelvic anatomy. Notwithstanding that in this cohort the pelvimetric measures were not used to guide treatment decisions, prospective evaluation and correlation with intraoperative findings and surgical difficulty is necessary to ultimately assess the role of pelvimetry in clinical decision-making.
In the present study, RP approach was not a significant independent predictor of PSM overall or at any site. In order to dissect around the prostate in all dimensions a certain amount of retraction of the prostate in both the anterior/posterior and medial/lateral planes must occur. This is especially true during dissection of the neurovascular bundles and the apex. While it would seem that anterior retraction might be easier with an open approach given that the anterior abdominal wall is open, the pneumoperitoneum usually provides ample room in this direction during laparoscopic surgery. A narrow pelvis, however, restricts movement in the lateral/medial direction during both open and laparoscopic surgery; thus surgery can be difficult by either approach and is compounded when dealing with a large prostate in a narrow pelvis. In these circumstances the smaller instrumentation used with laparoscopic or robotic surgery may be advantageous.
There are a number of limitations of this study. MRI imaging was performed preoperatively for staging purposes, while the pelvimetric measurements were done retrospectively. Not all patients treated with RP were preoperatively staged using MRI imaging but the distribution of pelvimetric dimensions is not expected to be different in patients having versus not having an MRI. All MRI imaging was performed placing an endorectal probe which influences the soft tissue measurements depending on the location of the probe in that the prostate may be displaced superiorly. While most institutions do not use MRI imaging for staging purposes, for institutions already using MRI for staging purposes, pelvimetric measures may potentially be useful in surgical planning; however, this awaits prospective evaluation.
Conclusion
Apical depth of the prostate was found to be a significant independent predictor of apical PSM with a more pronounced effect in AA men. The significantly smaller midpelvic area and a steeper symphysis pubis angle in AA men might lead to a more challenging operation, in particular a technically more difficult apical dissection. We would speculate that these anatomic differences may potentially account for the higher apical PSM in AA men. Our study suggests that the location of the prostate in relation to the pelvis may impact the technical difficulty of prostate cancer surgery as measured by the apical PSM rate.
Acknowledgments
Supported by: The Sidney Kimmel Center for Prostate and Urologic Cancers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabbani F, Herran Yunis L, Vora K, Eastham JA, Guillonneau B, Scardino PT, Touijer K. Impact of ethnicity on surgical margins at radical prostatectomy. BJU Int. 2009;104:904–908. doi: 10.1111/j.1464-410X.2009.08550.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyle KM, Petty D, Chalmers AG, Quirke P, Cairns A, Finan PJ, Sagar PM, Burke D. MRI assessment of the bony pelvis may help predict resectability of rectal cancer. Colorectal Dis. 2005;7:232–240. doi: 10.1111/j.1463-1318.2005.00819.x. [DOI] [PubMed] [Google Scholar]
- 3.Hong SK, Chang IH, Han BK, Yu JH, Han JH, Jeong SJ, Jeong H, Byun SS, Lee HJ, Lee SE. Impact of variations in bony pelvic dimensions on performing radical retropubic prostatectomy. Urology. 2007;69:907–911. doi: 10.1016/j.urology.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 4.Turner W. The Index of the Pelvic Brim as a Basis of Classification. J Anat Physiol. 1885;20:125–143. [PMC free article] [PubMed] [Google Scholar]
- 5.Hricak H, Wang L, Wei DC, Coakley FV, Akin O, Reuter VE, Gonen M, Kattan MW, Onyebuchi CN, Scardino PT. The role of preoperative endorectal magnetic resonance imaging in the decision regarding whether to preserve or resect neurovascular bundles during radical retropubic prostatectomy. Cancer. 2004;100:2655–2663. doi: 10.1002/cncr.20319. [DOI] [PubMed] [Google Scholar]
- 6.Neill MG, Lockwood GA, McCluskey SA, Fleshner NE. Preoperative evaluation of the “hostile pelvis” in radical prostatectomy with computed tomographic pelvimetry. BJU Int. 2007;99:534–538. doi: 10.1111/j.1464-410X.2006.06640.x. [DOI] [PubMed] [Google Scholar]
- 7.Powell IJ, Heilbrun LK, Sakr W, Grignon D, Montie J, Novallo M, Smith D, Pontes JE. The predictive value of race as a clinical prognostic factor among patients with clinically localized prostate cancer: a multivariate analysis of positive surgical margins. Urology. 1997;49:726–731. doi: 10.1016/S0090-4295(96)00618-8. [DOI] [PubMed] [Google Scholar]
- 8.Moul JW, Douglas TH, McCarthy WF, McLeod DG. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol. 1996;155:1667–1673. [PubMed] [Google Scholar]
- 9.Tiguert R, Gheiler EL, Tefilli MV, Banerjee M, Grignon DJ, Sakr W, Wood DP, Jr., Powell IJ. Pontes, J. E.: Racial differences and prognostic significance of tumor location in radical prostatectomy specimens. Prostate. 1998;37:230–235. doi: 10.1002/(sici)1097-0045(19981201)37:4<230::aid-pros4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JI. Incidence and significance of positive margins in radical prostatectomy specimens. Urol Clin North Am. 1996;23:651–663. doi: 10.1016/s0094-0143(05)70343-8. [DOI] [PubMed] [Google Scholar]
- 11.Guru KA, Perlmutter AE, Sheldon MJ, Butt ZM, Zhang S, Tan W, Wilding G, Kim HL, Mohler JL. Apical margins after robot-assisted radical prostatectomy: does technique matter? J Endourol. 2009;23:123–127. doi: 10.1089/end.2008.0398. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993;71:3582–3593. doi: 10.1002/1097-0142(19930601)71:11<3582::aid-cncr2820711120>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Pettus JA, Weight CJ, Thompson CJ, Middleton RG, Stephenson RA. Biochemical failure in men following radical retropubic prostatectomy: impact of surgical margin status and location. J Urol. 2004;172:129–132. doi: 10.1097/01.ju.0000132160.68779.96. [DOI] [PubMed] [Google Scholar]
- 14.Connolly SS, O’Toole GC, O’Malley KJ, Manecksha R, O’Brien A, Mulvin DW, Quinlan DM. Positive apical surgical margins after radical retropubic prostatectomy, truth or artefact? Scand J Urol Nephrol. 2004;38:26–31. doi: 10.1080/00365590310017334. [DOI] [PubMed] [Google Scholar]
- 15.Ohori M, Abbas F, Wheeler TM, Kattan MW, Scardino PT, Lerner SP. Pathological features and prognostic significance of prostate cancer in the apical section determined by whole mount histology. J Urol. 1999;161:500–504. [PubMed] [Google Scholar]
- 16.van den Ouden D, Bentvelsen FM, Boeve ER, Schroder FH. Positive margins after radical prostatectomy: correlation with local recurrence and distant progression. Br J Urol. 1993;72:489–494. doi: 10.1111/j.1464-410x.1993.tb16183.x. [DOI] [PubMed] [Google Scholar]
- 17.Eastham JA, Kuroiwa K, Ohori M, Serio AM, Gorbonos A, Maru N, Vickers AJ, Slawin KM, Wheeler TM, Reuter VE, Scardino PT. Prognostic significance of location of positive margins in radical prostatectomy specimens. Urology. 2007;70:965–969. doi: 10.1016/j.urology.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 18.Masterson TA, Touijer K. The role of endorectal coil MRI in preoperative staging and decision-making for the treatment of clinically localized prostate cancer. MAGMA. 2008;21:371–377. doi: 10.1007/s10334-008-0116-4. [DOI] [PubMed] [Google Scholar]
- 19.Yossepowitch O, Bjartell A, Eastham JA, Graefen M, Guillonneau BD, Karakiewicz PI, Montironi R, Montorsi F. Positive Surgical Margins in Radical Prostatectomy: Outlining the Problem and Its Long-Term Consequences. Eur Urol. 2009;55:87–99. doi: 10.1016/j.eururo.2008.09.051. [DOI] [PubMed] [Google Scholar]