Abstract
BACKGROUND CONTEXT
The possibility and likelihood of a postoperative medical complication after spine surgery undoubtedly play a major role in the decision making of the surgeon and patient alike. Although prior study has determined relative risk and odds ratio values to quantify risk factors, these values may be difficult to translate to the patient during counseling of surgical options. Ideally, a model that predicts absolute risk of medical complication, rather than relative risk or odds ratio values, would greatly enhance the discussion of safety of spine surgery. To date, there is no risk stratification model that specifically predicts the risk of medical complication.
PURPOSE
The purpose of this study was to create and validate a predictive model for the risk of medical complication during and after spine surgery.
STUDY DESIGN/SETTING
Statistical analysis using a prospective surgical spine registry that recorded extensive demographic, surgical, and complication data. Outcomes examined are medical complications that were specifically defined a priori. This analysis is a continuation of statistical analysis of our previously published report.
METHODS
Using a prospectively collected surgical registry of more than 1,476 patients with extensive demographic, comorbidity, surgical, and complication detail recorded for 2 years after surgery, we previously identified several risk factor for medical complications. Using the beta coefficients from those log binomial regression analyses, we created a model to predict the occurrence of medical complication after spine surgery. We split our data into two subsets for internal and cross-validation of our model. We created two predictive models: one predicting the occurrence of any medical complication and the other predicting the occurrence of a major medical complication.
RESULTS
The final predictive model for any medical complications had a receiver operator curve characteristic of 0.76, considered to be a fair measure. The final predictive model for any major medical complications had receiver operator curve characteristic of 0.81, considered to be a good measure. The final model has been uploaded for use on SpineSage.com.
CONCLUSION
We present a validated model for predicting medical complications after spine surgery. The value in this model is that it gives the user an absolute percent likelihood of complication after spine surgery based on the patient’s comorbidity profile and invasiveness of surgery. Patients are far more likely to understand an absolute percentage, rather than relative risk and confidence interval values. A model such as this is of paramount importance in counseling patients and enhancing the safety of spine surgery. In addition, a tool such as this can be of great use particularly as health care trends toward pay-for-performance, quality metrics, and risk adjustment. To facilitate the use of this model, we have created a website (SpineSage.com) where users can enter in patient data to determine likelihood of medical complications after spine surgery.
Keywords: Predictive model, Medical complications, Spine surgery, Multivariate analysis, Adverse event, Spinesage.com
Introduction
The possibility and likelihood of a postoperative medical complication after spine surgery undoubtedly play a major role in the decision making of the surgeon and patient alike. High-risk patients are frequently evaluated preoperatively by medical providers for risk stratification and health optimization before undergoing extensive spinal surgery. In the course of preoperative risk assessment, tools such as the widely utilized Revised Cardiac Index can be used to predict the likelihood of cardiac complication [1–3]. To date, there is no risk stratification tool specific to spine surgery.
Previously, we reported risk factors for complication after spine surgery utilizing the Spine End Result Registry (SERR) [4–8]. This registry is a prospectively collected registry for all surgical spine patients at University of Washington and Harborview Medical Center who underwent surgery from January 1, 2003, to December 31, 2004. Medical complications were defined explicitly a priori and extensive demographic, comorbidity, and surgical details were prospectively recorded for each surgical patient for at least 2 years after their surgery. From this registry, we performed multivariate analysis of risk factors for medical complications after spine surgery. We reported risk factor and confidence interval (CI) values for multiple significant risk factors for medical complications after spine surgery. These efforts represent the first step in the analysis of the data to derive a predictive model for medical complication after spine surgery. The purpose of this study was to derive and validate a predictive model for medical complication after spine surgery using the prospectively collected data from the SERR.
Methods
Patient population
This is a retrospective analysis of a prospective cohort of patients who participated in a SERR or a quality assurance/quality improvement database for the purpose of defining and assessing safety and outcomes for any patient undergoing spine surgery at one of two academic institutions. All patients were recruited to participate in the SERR to assess adverse events and provide outcome data, if patients declined to participate in the registry (N=745/1,476; 50.5%) they were followed in the quality assurance/quality improvement study and only their adverse events were tracked. However, some information about their risk factors, such as smoking status and alcohol use, are missing. The data for this group’s adverse events were found either by notification of hospital staff or record review of only the adverse event and no other comorbidity or previous risk factor assessment. Those patients in the “missing” group can be attributed to those patients who only participated in the quality assurance/quality improvement arm of the study.
Exclusions
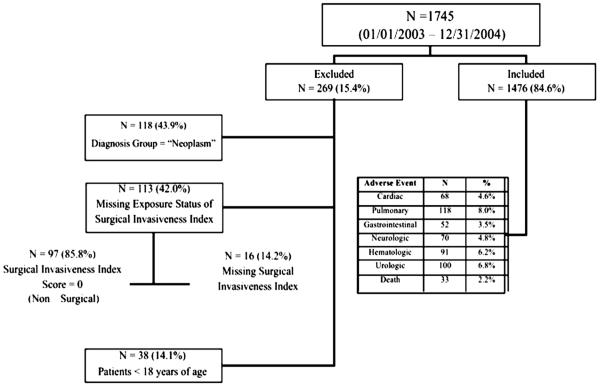
There were 1,745 patients enrolled in the study from January 1, 2003, to December 31, 2004. Of those, 269 were excluded because of a missing exposure status (N=16/113; 14%) or exposure status equal to zero (N=97/113; 86%), or because they were younger than 18 years of age (N=38/269; 25%), or because they were diagnosed with a neoplasm (N=118/269; 44%). Exposure status equal to zero included those who did not have surgical intervention, including cast and halo placement and thoracolumbarsacral orthosis (Fig. 1). Patients with a neoplastic diagnosis were excluded from this analysis because it was discovered in the validation model that those with a neoplasm had an inverse relationship with adverse events. It was found that those patients with a neoplasm (primary diagnosis or metastatic disease to the spine) were less likely to have any adverse event, which is opposite the finding in the univariate analysis. Because the intent of this prediction model is to provide predicted probabilities of any adverse event after general spine surgery, we felt it prudent to drop this complicated, neoplastic patient group from this data model. It is our impression that most general practice spine surgery centers refer patients with neoplasms to the spine or metastatic spine disease to tertiary academic institutions or cancer centers for treatment.
Fig. 1.
Participant flow diagram with inclusion and exclusion criteria.
Data collection
Classification of predictors, confounders and outcomes
The definition for each adverse occurrence can be found in the attached Appendix 1. Risk factors examined included age, gender, smoking status, alcohol use, diabetes, body mass index, insurance status, surgical approach (posterior, anterior, combined), revision surgery, surgery region (cervical, thoracic, lumbosacral), diagnosis (degenerative, trauma, neoplasm, infection, other), and surgical invasiveness. In addition, the influence of preexisting medical comorbidity (cardiac disease, congestive heart failure [CHF], chronic obstructive pulmonary disease (COPD), hypertension (HYTN), rheumatoid arthritis, renal disease, liver disease, cancer, anemia, bleeding disorder) were also be considered as predictor variables.
We utilized the Surgical Invasiveness Index (SII) as described by Mirza et al. [9]. The SII is a previously validated instrument that accounts for the number of levels decompressed, fused, or instrumented, posteriorly and anteriorly. It ranges from 0 to 48, with a higher score indicating greater invasiveness. The index is the sum of six weighted surgical components: anterior decompression (ad), anterior fusion (af), anterior instrumentation (ai), posterior decompression (pd), posterior fusion (pf), and posterior instrumentation (pi). The weights for each component represent the number of vertebral levels at which each it is performed. For example, in a C5–C6 anterior discectomy, with fusion and plating, the score is 5 (ad=1 [one disc]+af=2 [two vertebrae fused]+ai=2 [plate at both levels]). Effect modification is addressed by categorizing the SII score into six groups: 1 through 5, 6 through 10, 11 through 15, 16 through 20, 21 through 25, and greater than 25. However, for the final model we choose to model the SII as a continuous variable.
Postoperative medical adverse events (cardiac, pulmonary, gastrointestinal, hematologic, neurologic, urologic, and death) and any adverse occurrence (if a patient had any medical complication listed previously) were our dependent variables. Specific definitions for each medical adverse event have been previously published and are listed again in Appendix 1. We created two predictive models. The first predicts the likelihood of any medical complication (all complications listed Appendix 1). The second model predicts the likelihood of major medical complications (identified in Appendix 1).
Analysis of data
Continuous data are presented as means and standard deviations. Categorical data are presented as the number of cases and percentages. For categorical values, Pearson’s chi-square or Fisher’s exact tests (where cell counts were low) are used to assess the effect of various risk factors. Odds ratio (OR) and 95% CI were calculated for each of the categorical variables using univariate and multivariate log-binomial regression analysis, adjusting for confounders and other covariates. The model covariates were included if they were known risk factors based on previously published studies (obesity and smoking), to contribute to each medical complication (previous history of a myocardial infarction as a risk factor for a cardiac adverse event), were a known confounder (age) or had a univariate association of p<.10 (chronic pulmonary disease) [6–8]. Because operative approach and the number of vertebral levels operated on are a component of the invasiveness index, they are not included in the multivariate regression model.
A clinical prediction model was created to calculate the probability of any medical complication and the probability of any major medical complication after spine surgery. The beta (β) coefficients from the log-binomial regression models were used to develop a probabilistic model to predict the probability for any adverse occurrence. A random number generator was used to split the cohort into two randomly sampled groups to perform internal validation (Altman Ref). Groups A and B were then cross-validated (A vs. B and B vs. A) to evaluate the performance of the model. Models were calibrated by plotting the observed events versus the predicted events and applying the Hosmer-Lemeshow test. Discrimination was assessed by sensitivity and specificity of the predictor model using receiver operator character curve (ROC) analysis to determine how well the prediction model distinguished the appropriate probability for any adverse event based on risk factors.
Results
Descriptive data for any medical complication
Among the 1,476 participants (85%) who met our inclusion criteria and were followed for adverse events, 731 (49.5%) consented to provide detailed questionnaires of their risk factors. The mean surgical invasiveness score was 8.5 (range, 1–48.) Patients were mostly male (57%) with a mean age of 49.4 years (range, 18–98), and a body mass index of 27.7 kg/m2. Slightly more than two thirds had a degenerative condition (67%), followed by treatment for trauma (25%). Most of the operations were performed using a posterior approach (58%), followed by a combined approach (23%). The mean follow-up was 17.9 months (range, 1.02–65.5).
There were 532 adverse events recorded in 23% of participants (338/1,476;), an incidence of 18.0 per 100 persons per year undergoing spine surgery. The most common adverse event was respiratory related (N=118/532; 22%) and were 21.1 times more likely to die (95% CI, 10.2–43.7; p<.0001). The next most common adverse event was urologic (N=100/532; 19%); however, those with this event were only 3.19 (95% CI, 1.29–7.91; p=.008) times more likely to die after surgery. Those with a respiratory adverse event were 16.4 times more likely to die from surgery (95% CI, 10.2–43.7; p<.0001).
Surgical invasiveness had a strong dose response, with adverse event and was the greatest risk factor in the univariate analysis (SII>25; OR, 5.95; 95% CI, 3.43–10.3; p<.0001). The greater the SII score, the prevalence of patients who had an adverse event rose. For example, the prevalence of an adverse event among those with an invasiveness index between 1 and 5 was 14%, compared with 50% among those with a score greater than 25. The greatest medical risk factor for an adverse event in the univariate analysis was for those with a history of CHF, the odds of an adverse event in this group were 3.68 times as large than the odds for those without CHF (95% CI, 2.19–6.18; p<.0001).
Predicted probability model for any medical complication
Table 1 presents the results of our multivariate analysis demonstrating that, unlike the univariate findings, the greatest odds of developing an adverse event after spine surgery occurred in those patients diagnosed with trauma to the spine. Trauma patients were 4.12 times more likely to have an adverse event adjusting for those with other spinal diagnoses, the age of the patients, the invasiveness of the surgery, or a history of bleeding disorder or blood clot or CHF (OR, 4.12; 95% CI, 3.02–5.61; p<.0001). The predicted probability algorithm was created using the beta coefficients from the final multivariate model and yields the following formula:
Table 1.
Multivariate for any complication
| Any complication | Odds ratio |
95% CI low |
95% CI high |
p Value |
|---|---|---|---|---|
| Age | 2.401 | 1.354 | 4.260 | .003 |
| Surgical Invasiveness Score | 1.063 | 1.045 | 1.082 | <.001 |
| Bleeding disorder | 2.068 | 1.318 | 3.247 | .002 |
| Congestive heart failure | 2.401 | 1.354 | 4.26 | .003 |
| Diagnosis: Trauma | 4.116 | 3.018 | 5.613 | <.001 |
| Diagnosis: Infection | 3.005 | 1.875 | 4.815 | <.001 |
Assumptions associated with this model are:
History of CHF and bleeding or hematologic (BleedHx) disorder, trauma (Trauma), or other diagnosis (OtherDx) are binary. Thus, 1= yes, has a history or diagnosis, 0=no history or diagnosis;
The diagnosis categories are mutually exclusive; and
Age and SII are on a continuous scale with validated ages from 18 to 98 years and SII validated from 1 to 48.
Validation, calibration, and discrimination for any medical complication predictive model
The random number generator created two groups for cross-validation, group A (N=716) and group B (N=760). For the predictive modeling of any medical complication, the model under group A had a ROC of 0.78 (Fig. 2) when fit using the data from group B and the model under group B had an ROC of 0.73 when fit using the data from group A. The final predicted probability model including all the data (N=1476) was an ROC of 0.76, which is considered a fair discrimination of the ability of the final model to predict adverse events.
Fig. 2.
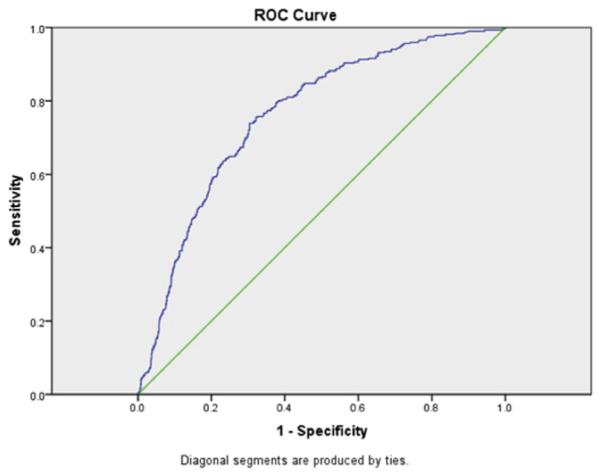
Receiver operator curve for any medical complication. Area under the curve is 0.76.
Predicted probability model for a major medical complication
Table 2 presents the multivariate for developing a major medical complication. As with any medical complication, the greatest odds of developing a major complication occurred in those patients diagnosed with trauma to the spine. In this case, however, the odds were 1.17 times higher compared with any medical complications (OR, 5.290; 95% CI, 3.236–8.648; p<.001) when adjusted for other spinal diagnoses, age and gender of the patients, invasiveness of the surgery, history of COPD, history of HYTN, and history of cardiac disease. The predicted probability algorithm was created using the beta coefficients from the final multivariate model and yields the following formula:
Table 2.
Multivariate for major complication
| Major complication | Odds ratio | 95%CI low | 95% CI high | p Value |
|---|---|---|---|---|
| Age | 1.033 | 1.018 | 1.048 | <.001 |
| Surgical Invasiveness Score |
1.064 | 1.038 | 1.089 | <.001 |
| Gender | 0.622 | 0.396 | 0.977 | .039 |
| Chronic obstructive pulmonary disease |
2.179 | 1.157 | 4.100 | .016 |
| Hypertension | 1.685 | 1.059 | 2.680 | .028 |
| Previous cardiac history |
2.440 | 1.423 | 4.182 | .001 |
| Diagnosis: Trauma | 5.290 | 3.236 | 8.648 | .001 |
| Diagnosis: Infection | 3.740 | 1.946 | 7.185 | <.001 |
Assumptions associated with this model are:
History of COPD, HYTN, and previous cardiac disease (PrevCardiac), trauma (Trauma) or other diagnosis (OtherDx) are binary. Thus, 1=yes has a history or diagnosis, 0=no history or diagnosis;
Gender (Gender) is 1=female, 0=male;
The diagnosis categories are mutually exclusive; and
Age and SII are on a continuous scale with validated ages from 18 to 98 years and SII validated from 1 to 48.
Validation, calibration, and discrimination for major medical complication predictive model
The random number generator created two groups for cross-validation, group A (N=738) and group B (N=738). For the predictive modeling for major complications, the model under group A had a ROC of 0.79 when fit using the data from group B and the model under group B had an ROC of 0.81 when fit using the data from group A. The final predicted probability model including all the data (N=1,476) was an ROC of 0.81, which is considered a good discrimination of the ability of the final model to predict adverse events (Fig. 3).
Fig. 3.
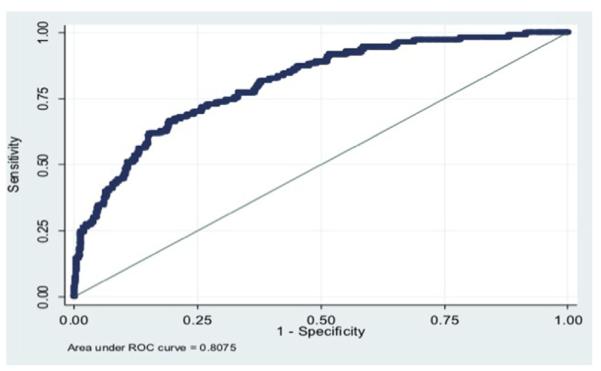
Receiver operator curve for major medical complication. Area under the curve is 0.81.
Discussion
Using the SERR, we had previously reported several significant risk factors for medical complication after spine surgery [6–8]. Although these data and calculations are valuable from a statistical and academic perspective, the translatability of these data to patients is challenging. What does a relative risk value or CI mean to a patient? If a patient is told that she has a relative risk of 3.5 for a cardiac complication, how is she to interpret that? How is the physician to interpret those values? A relative risk value is, by definition and nomenclature, relative. Without normative references, which are difficult to define, the RR value becomes somewhat abstract.
Although RR and CI values may be difficult to interpret, a gross probability of a complication is far easier to process and factor in during decision making. For example, if a patient is told that her likelihood for medical complication is 15%, then she can truly consider this risk in balance to goals and benefits of the surgery. A probability of complication value is far more user-friendly for patients and physicians, than RR and 95% CI values. The subject of this manuscript presents a validated predictive model to estimate risk of medical complication after or during spine surgery.
For predicting any medical complication, we calculated our area under the ROC to be 0.76, considered to be fair by most measures. When predicting major medical complications, we calculated our area under the ROC to be 0.81, considered to be good by most measures. For comparison, the area under the ROC for the widely utilized Revised Cardiac Risk Index was reported to be as high as 0.77 [2]. Using this tool, we estimate that, for a 65-year-old man undergoing a one-level lumbar laminectomy and posterior lateral instrumented arthrodesis, the likelihood of any medical complication to be 14.6% and the likelihood of major medical complication to be 3.06%. Although these values may seem elevated, the calculations are based on what were defined as complications a priori (Appendix 1). In the SERR, a complication was defined as “any event in the course of a patient’s treatment that has the potential for causing harm to the patient” [10]. For example, the occurrence of a postoperative ileus, or postoperative atrial fibrillation that did not result in long-term sequelae for the patient was nonetheless recorded as a complication in this registry.
The value in this predictive model is first and foremost evident when considering safety in spine surgery. When counseling a patient on whether or not to have surgery, or how much surgery to have, a predictive model such as this is of paramount importance during decision making. However, the complexity of this calculation, may prove to be unwieldy in clinical settings; thus, we have designed a website to facilitate the use of this predictive algorithm (spinesage.com).
A predictive model such as this is also of great benefit when considering of risk adjustment. Quality metrics such as reoperation, surgical site infection, deep venous thrombosis, or readmission, are becoming increasingly prevalent. A direct consequence of poor performance in these metrics is decreased reimbursement to medical centers. Thus, medical centers are further financially incentivized to perform well in quality metrics. One possible unintended consequence of the well-intended policy may be discrimination against patients with risk factors for complication and poor performance in quality metrics. A 70-year-old organ transplant recipient with diabetes undergoing a lumbar laminectomy is intuitively more likely to have complication than a healthy 50-year-old undergoing the same surgery. However, with many current quality metrics, these patients are measured by the same bar. Thus, providers and medical centers may be more likely to preferentially provide care for healthier patients to optimize their quality metric performance. As a result, patients with risk factors for complication and poor quality metric performance may unwittingly encounter discrimination in access to care. A predictive model such as this allows for risk adjustment taking into account medical comorbidity and surgical invasiveness. When considering quality metrics, a predictive model such as this may assist in “leveling the playing field.” Predictive models and risk stratification are essential for tertiary care centers to embrace the care of the sickest in our population without concern of financial penalty. If the policy of directly linking reimbursement to performance on complications-based quality metrics is to persist, then a risk adjustment process of critical to ensure that patients with risk factors for complications are not selected against.
To our knowledge, this is the first predictive model examining the occurrence of medical complication after spine surgery. Although statistically this has been validated, there are several weaknesses. This model is based on an analysis of 1,476 patients. Although this is an high number of patients compared with most studies, clearly the accuracy of such a model would be improved with greater power. Although some covariates were found to be statistically significant risk factors, it is possible that the lack of statistical significance for other risk factors may be related to lack of sufficient power. Second, this model only evaluated the risk for medical complication, not surgical complication. The occurrence of hardware failure, dural tear, iatrogenic nerve injury, and other surgical complications are not accounted for in this model.
We have presented a validated predictive model for medical complication after spine surgery (spinesage.com). A tool such as this is of substantial value in the preoperative counseling of patients, shared surgical decision making, and ultimately improving safety in spine surgery. Secondarily, as we progress into an era of quality metrics and performance assessment, a tool such as this can be beneficial in policy structuring. Future predictive models such as these are recommended for further risk stratification.
Acknowledgments
Author disclosures: MJL: Consulting: Stryker Spine-for work not related to the manuscript (C), AO Spine-Faculty (B); Endowments: Synthesis Spine (F, Paid directly to the institution); Fellowship Support: AOSpine (F, paid directly to the institution). AMC: Endowments: Synthes Spine (F, Paid directly to the institution). DH: Endowments: Synthes Spine as the Entity (F). JRC: Consulting: Synthes USA (B); Speaking and/or Teaching Arrangements: AO Spine (B), Synthes (B); Board of Directors: AOSpine North America (B per year), AOSpine Foundation (B); Endowments: Hans Joerg Wyss Foundation (Total endowment I, Paid directly to the institution); Grants: Medtronic (Fellowship support), Alseres Pharmaceuticals (Research support); Fellowship Support: AOSpine North America (Fellowship E, Paid directly to the institution).
Supported in part by the Spine End-Results Research Fund at the University of Washington Medical Center through a gift from Synthes Spine (Paoli, PA).
Appendix 1. Medical complication the model predicts for by organ system
Appendix 1.
Medical complication the model predicts for by organ system
| Complication | Definition |
|---|---|
| Cardiac complications | |
| Air embolism | Entrainment of air into the venous circulation and heart detected by any monitoring device including Doppler, TEE, or sudden decrease in end-tidal CO2, SpO2, or blood pressure or air in coronary vessels on post-mortem examination |
| Arrest* | Cardiac output insufficient to maintain a palpable central pulse, and requiring CPR, electroshock therapy and/or vasoactive drugs to maintain an adequate perfusion pressure. |
| Arrhythmia (telemetry+Tx or mc06/death) | Any cardiac rhythmthat varies frombaseline and requires either extramonitoring, drugs, consultations, or electroshock therapy, or results in hypotension or death. |
| CHF (new S3/JVD+rales/CXR+Tx) | An abnormality of cardiac function is responsible for the failure of the heart to pump blood at a rate commensurate with the requirements of the metabolizing tissues, manifested by pulmonary edema, a new S3 gallop, jugular venous distension, rales, pleural edema or effusion, and requiring treatment. |
| Hypertension | SBP >180 or DBP >100 for >5 min |
| Hypotension (SBP/MAP <50% base, >5 min) |
MAP <50% of baseline for >5 min |
| Infarction (mc09+enzymes/new Qs)* | Necrosis of heart tissue as evidenced by elevated ST segments or new Q waves or new wall motion abnormality associated with elevated cardiac enzymes (troponin, CK-MB) |
| Inappropriate or inadequate fluid therapy |
Insufficient replacement of volume with blood products, crystalloid or other colloid to maintain adequate perfusion and oxygenation of all tissues, as evidenced by inadequate urine output, low central filling pressures, elevated lactate, metabolic acidosis with pH <7.35, and/or hypotension responsive to fluids. Criteria: (1) inadequate urine output (<0.5 mL/kg/h); (2) hypotension responsive to fluid challenge; (3) elevated lactate level; (4) metabolic acidosis (pH <7.35); and/or (5) low central filling pressures |
| Ischemia (sx/1 mm ST 2 leads, ROMI/Tx)* |
Myocardial ischemia is a deficiency of the blood supply to the heart muscle, leading to symptoms, flat depression of the ST segment of >0.1 mV below the baseline (ie, the PR segment) and lasting >0.08 s, treatment, or rule-out MI monitoring |
| Thermoregulation | Temperature <35°C for >30 min |
| Other cardiac occurrence | Other circulation or cardiac-related occurrence |
| Pulmonary complications | |
| ARDS (FiO2>50/vent>48 h+ mc04/mr05)* |
Acute hypoxemic respiratory failure owing to pulmonary edema caused by increased permeability of the alveolar capillary barrier. Criteria: (1) FiO2>50%; (2) ventilator support for >48 h; (3) PaO2/FiO2≤300 mmHg; and (4) bilateral lung infiltrates on CXR |
| Empyema | Purulent fluid collection in the pleural space confirmed by imaging studies and aspiration or by surgery |
| Hemothorax | Blood in the pleural space confirmed by imaging studies and aspiration or surgery |
| Pleural effusion | Excess fluid in the pleural space |
| Postoperative hypoxia (FiO2>50× 48 h or supplemental O2 ×7 d)* |
Requirement for supplemental oxygen postoperatively, with FiO2>50% for 48 h or supplemental oxygen by nasal cannula for 7 days |
| Pneumonia (>38.0+Cx/CXR and Tx) | Infection of the lung parenchyma confirmed by fever, sputum or bronchial cultures, CXR, and requiring treatment |
| Pneumothorax | Accumulation of gas in the pleural space resulting in symptoms (tachycardia, hypotension), requiring extra surveillance (eg, repeat CXRs or pulse oximetry) or treatment (chest tube placement) |
| Pulmonary embolus (CTA/VQ/ angiography+Tx)* |
Sudden onset of shortness of breath, tachypnea, cyanosis, tachycardia, hypotension, or chest pain confirmed to be a imaging studies to be a pulmonary thrombus, and requiring treatment; or diagnosis made at autopsy |
| Respiratory arrest* | Sudden cessation of voluntary breathing, requiring CPR or mechanical ventilation |
| Other respiratory | Other respiratory problem |
| Gastrointestinal complications | |
| Ascites | Effusion and accumulation of serous fluid in the abdominal cavity leading discernable on physical examination or radiologic imaging (free peritoneal fluid > 25 mL), leading to symptoms, unplanned evaluation, or requiring treatment |
| Colitis | Inflammation of the colon manifested as diarrhea or bloody diarrhea, sepsis, abdominal pain, or toxic megacolon. Criteria: (1) Rectal discharge; (2) perineal ulceration; (3) colonoscopic and biopsy evidence of inflammation |
| GI bleeding (heme pos+drop Hct 10% or Tx) |
Blood loss through the gastrointestinal tract, including hematemesis, melena, hematochezia, occult GI bleeding may be identified in the absence of overt bleeding by special examination of the stool (eg, guaiac testing), or symptoms of blood loss or anemia such as lightheadedness, syncope, angina, or dyspnea. Criteria: (1) Bloody vomitus or stool; (2) bleeding from the rectum; (3) Hct decrease >10%; (4) lightheadedness, syncope, angina, or dyspnea |
| Ileus | Abdominal distension and no passage of stool or flatus by postoperative day 3 |
| Obstruction | Pseudo-obstruction is colonic distension in the absence of mechanical obstruction, with cecal diameter of >9 cm and air in all colonic segments on plain radiographs |
| Pancreatitis | Acute inflammation of the pancreaswith sudden onset of: (1) abdominal pain; (2) nausea; (3) vomiting; (4) high levels pancreas enzymes (serumamylase 3× normal) |
| Perforation* | Iatrogenic perforation of the stomach, small intestine, or large intestine during the procedure or perforation later caused by implants or instrumentation. Criteria: (1) Nausea, vomiting, or ileus; (2) abdominal or groin pain and referred pain; (3) air in the abdomen on plain radiograph or CT or other imaging study; (4) abdominal distension and tenderness; or surgical finding of perforation |
| Peritonitis | Inflammation or infection of the peritoneum with symptoms of (1) abdominal pain and tenderness; (2) constipation; (3) vomiting; (4) moderate fever |
| Other GI occurrence | Other GI-related occurrence |
| Neurologic complications | |
| CVA/TIA (new focal deficit or CT/MR)* |
The abrupt onset of a nonconvulsive and new focal neurologic deficit owing to a reduction of blood flow to the brain, or abnormality on imaging studies suggestive of a CNS infarct, or CNS infarction confirmed by biopsy or autopsy |
| Cerebral perfusion (ICP>20 or CPP <30 for >5 min) |
Reduction in the flow of blood to the brain during the procedure for >5 minutes, with intracranial pressure >20 or cerebral perfusion pressure <30 mmHg |
| Delirium (confusion>24 h+Tx/sitter/ restraint) |
Acute change in level of consciousness characterized by reduced ability to maintain attention to external stimuli, lethargy, or agitation, and disorganized thinking as manifested by rambling, irrelevant, or incoherent speech. Criteria: (1) Confusion>24 h; and (2) was not related to narcotics; and (3) patient required restraints or continuous supervision |
| Diabetes insipidus | Excessive urine production from reduced production or responsiveness to ADH; diagnosis can be made by relating plasma to urine osmolality, particularly in postoperative neurosurgical patients or after head trauma, where its use can permit quick differentiation of diabetes insipidus from parenteral fluid excess |
| Electrolyte change (Na <130/>150, K>5.5, other) |
The electrolyte balance of the extracellular fluid was sufficiently changed from normal to require extra monitoring, evaluation, or treatment beyond routine postoperative care. Specifically: Na <130 or>150 or K >5.5 |
| Meningitis (pos Cx/Bx or CT/MR and Tx)* |
Inflammation of the meninges (the pia-arachnoid) and the CSF of the subarachnoid space associated with symptoms of fever, headache, nausea/diarrhea/ abdominal pain, and confirmed by CSF cultures or biopsy, imaging studies, and requiring treatment |
| SAH/intracerebral hemorrhage* | Hemorrhage in the space between the arachnoid membrane and pia matter (subarachnoid) causing compression of the brain associated with sudden headache, neurologic deficit, and confirmed with imaging studies or blood in the CSF; may also occur in the spinal cord in association with sudden back pain |
| Seizure | Paroxysmal event owing to abnormal, excessive, hypersynchronous discharges from an aggregate of CNS neurons with manifestations ranging from convulsive activity to experiential phenomena not discernible by an observer, confirmed by EEG or neurology consultation |
| Withdrawal, alcohol (history+ mn03+Tx) |
A patient with history of alcohol abuse exhibits anxiety, confusion and delirium after the cessation of alcohol intake, requiring treatment |
| Withdrawal, narcotic | The patient exhibits symptoms of nausea and diarrhea, coughing, lacrimation, mydriasis, rhinorrhea, profuse sweating, twitching muscles, and piloerection, or “goose bumps”; mild elevations in body temperature, respiratory rate, and blood pressure after reduction or cessation of narcotic intake, with improvement in symptoms after opioid administration |
| Other neurologic occurrence | Other neurologic occurrence |
| Hematologic complications | |
| Coagulopathy (INR>2 or platelets <50 or Fib <100) |
Any disorder reducing the ability of the blood to clot: Severity 1, INR>1.5 and <2.0, or platelets <100k and >50k; severity 2, INR>2.0 and <3.0, or platelets <50k and >20k; severity 3, INR >3.0, or platelets <20k |
| DVT (confirmed by imaging) | The presence of thrombosis of the iliac, femoral, or popliteal or other veins confirmed by imaging studies (duplex scan, CT, or MR) with or without swelling, warmth, erythema, or tenderness |
| OR hemorrhage >3,000 mL | Blood loss >3 L during the procedure. |
| Transfusion occurrence | The patient required an unplanned transfusion during or after the procedure, or adverse reaction to blood product transfusion |
| Other hematologic occurrence | Other hematologic adverse occurrence |
| Urologic complications | |
| Foley catheter trauma | Injury to the urethra or bladder caused during normal insertion or removal of the Foley catheter, or during inadvertent removal of the catheter |
| Renal insufficiency (Cr >2 over base) | Operational definition: Failure of the kidneys characterized by rapid decline in glomerular filtration rate (hours to days), retention of nitrogenous waste products, and perturbation of extracellular fluid volume and electrolyte and acid–base homeostasis; criteria: Serum Cr >2 above baseline |
| Urinary retention | Inability to empty bladder under voluntary control |
| UTI | The presence of large amounts of bacteria (>100,000 organisms/mL) in the upper or lower urinary tract associated with symptoms or requiring treatment |
| Other urologic event | Other urologic adverse occurrence |
ADH, antidiuretic hormone; ARDS, acute respiratory distress syndrome; CHF, congestive heart failure; CK-MB, creatinine kinase myocardial band; CPP, cerebral perfusion pressure; CPR, cardiopulmonary resuscitation; Cr, creatinine; CSF, cerebrospinal fluid; CBS, central nervous system; CT, computed tomography; CTA, computed tomographic angiography; CVA, cerebrovascular accident; CXR, chest x-ray; DBP, diastolic blood pressure; DVT, deep venous thrombosis; GI, gastrointestinal; Hct, hematocrit; ICP, intracranial pressure; INR, International Normalized Ratio; MAP, mean arterial pressure; MI, myocardial infarction; OR, operating room; ROMI, rule out myocardial infarction; SAH, subarachnoid hemorrhage; SBP, systolic blood pressure; TEE, transesophageal echocardiogram; TIA, transient ischemic attack; Tx, treatment; UTI, urinary tract infection.
Major complication.
Footnotes
FDA device/drug status: Not applicable.
The disclosure key can be found on the Table of Contents and at www.TheSpineJournalOnline.com.
References
- [1].Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152:26. doi: 10.7326/0003-4819-152-1-201001050-00007. [DOI] [PubMed] [Google Scholar]
- [2].Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- [3].Thoren E, Hellgren L, Jideus L, Stahle E. Prediction of postoperative atrial fibrillation in a large coronary artery bypass grafting cohort. Interact Cardiovasc Thorac Surg. 2012;14:588–93. doi: 10.1093/icvts/ivr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baker GA, Cizik AM, Bransford RJ, et al. Risk factors for unintended durotomy during spine surgery: a multivariate analysis. Spine J. 2012;12:121. doi: 10.1016/j.spinee.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cizik AM, Lee MJ, Martin BI, et al. Using the spine surgical invasiveness index to identify risk of surgical site infection: a multivariate analysis. J Bone Joint Surg Am. 2012;94:335. doi: 10.2106/JBJS.J.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee MJ, Hacquebord J, Varshney A, et al. Risk factors for medical complication after lumbar spine surgery: a multivariate analysis of 767 patients. Spine. 2011;36:1801. doi: 10.1097/brs.0b013e318219d28d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee MJ, Konodi MA, Cizik AM, et al. Risk factors for medical complication after spine surgery: a multivariate analysis of 1,591 patients. Spine J. 2012;12:197. doi: 10.1016/j.spinee.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee MJ, Konodi MA, Cizik AM, et al. Risk factors for medical complication after cervical spine surgery: a multivariate analysis of 582 patients. Spine. 2013;38:223–8. doi: 10.1097/BRS.0b013e318268ffc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mirza SK, Deyo RA, Heagerty PJ, et al. Development of an index to characterize the “invasiveness” of spine surgery: validation by comparison to blood loss and operative time. Spine. 2008;33:2651. doi: 10.1097/BRS.0b013e31818dad07. [DOI] [PubMed] [Google Scholar]
- [10].Mirza SK, Deyo RA, Heagerty PJ, et al. Towards standardized measurement of adverse events in spine surgery: conceptual model and pilot evaluation. BMC Musculoskelet Disord. 2006;7:53. doi: 10.1186/1471-2474-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]