Abstract
In a previous study (Sidhu et al., 1993), we demonstrated that a combination of certain cell culture media, hormone addition, and extracellular matrix (ECM) overlay coordinately modulated the expression of certain liver-selective genes in primary rat hepatocyte cultures, including the responsiveness of genes to phenobarbital. However, little is known about the interactions between the type of substratum upon which hepatocytes are adhered and the ECM overlay, as codeterminants of liver-selective gene expression. The present study was undertaken to compare specific substrata, including tissue culture-grade plastic, Primaria, and type 1 collagen-coated plastic, in combination with the presence or absence of standard ECM or a growth-factor-reduced ECM overlay. Hepatocyte cultures were assessed either as control cultures or subsequent to treatment for 24 h with phenobarbital (0.1 or 1 mM), or beta-naphthoflavone (22 μM), to monitor responses of hepatocytes to two prototypic gene-inducing agents. Analyses of maintenance and induction of cytochrome P450 and liver-selective gene expression included measures of mRNA levels using Northern blot and slot-blot hybridization and single cell immunofluorescence assays to measure levels of specific cytochrome P450 proteins. The results of these experiments demonstrated that hepatocyte-selective expression, including the absolute level of induction response (relative to those observed in the rat liver in vivo) was highly dependent on the presence of ECM overlay but independent of the substratum employed. As studied herein, the establishment of optimal conditions for primary hepatocyte culture, enabling reproduction of responses observed in vivo, is important to further prospects for in vitro toxicity testing and for investigating molecular mechanisms of phenobarbital-mediated gene regulation.
INTRODUCTION
In vivo liver function has been difficult to maintain in vitro. Although certain hepatoma cell lines are capable of expressing limited levels of liver-selective genes (e.g., albumin and transferrin) (Schulz et al., 1988; Cassio et al., 1991), usually dedifferentiation markers such as alpha-fetoprotein (Schulz et al., 1988) also are expressed. Several other highly liver-selective functions are typically poorly maintained in cell culture (Cassio et al., 1991), including the ability of hepatocytes to respond to phenobarbital (PB), a prototypic inducing agent. Recent studies have linked the loss of liver-specific gene responses, or dedifferentiation, to the loss of the expression of certain liver-specific transcription factors (DiPersio et al., 1991; Liu et al., 1991).
Primary hepatocyte cultures are promising systems to study the maintenance of liver-specific function and gene responsiveness. However, under conventional cell culture conditions, adult rat hepatocytes rapidly lose many differentiated characteristics (Bissell and Guzelian, 1980). Recent studies have focused primarily on the use of chemically defined media (Enat et al., 1984; Turner et al., 1988; Turner and Pitot, 1989; Waxman et al., 1990), complex cell culture substratum (Michalopoulos and Pitot, 1975; Sirica et al., 1979; Reid et al., 1980; Bissell et al., 1986; Fujita et al., 1987; Cima et al., 1991; Takezawa et al., 1992; Saad et al., 1993a,b), or a combination of both (Enat et al., 1984; Waxman et al., 1990; Jauregui et al., 1991). Other reports have indicated a significant role of an extracellular matrix (ECM) substratum in further enhancing liver-specific gene expression (Ben-Ze’ev et al., 1988; Bissell et al., 1990), including the maintenance of PB-mediated induction of cytochrome P450 genes (Schuetz et al., 1988; Guzelian and Schuetz, 1989).
The cytochrome P450s (CYPs) are a multigene family (Nelson et al., 1993) of hemoproteins that catalyze, predominantly in the liver, the oxidative metabolism of an extensive variety of both endogenous and exogenous compounds (Adesnik and Atchison, 1985; Okey, 1990). Certain gene families are differentially inducible in the presence of particular chemical agents, with distinct mechanisms accounting for the regulation of various constitutive and inducible CYP genes (Okey, 1990; Gonzalez, 1990). The CYP2B and CYP3A subfamilies have been well-characterized as PB-inducible genes in rodents (Waxman and Azaroff, 1992), and have been used in this investigation as markers of liver-selective expression.
To enable investigation of the molecular mechanisms involved in the PB induction response, most investigators have relied on the use of primary rat hepatocytes isolated by in situ collagenase perfusion of liver and maintained for short periods in culture (Schuetz et al., 1988; Guzelian and Schuetz, 1989; Kocarek et al., 1993). We have developed a modified technique relying on the application of an overlay of dilute concentrations of ECM directly to the culture medium, similar to that suggested by Caron (1990), subsequent to cell attachment to a conventional substratum of collagen type 1 (Sidhu et al., 1993). We demonstrated that a combination of cell culture medium, hormone addition, and concentration-dependent ECM overlay coordinately modulated PB-responsiveness and albumin gene expression in primary rat hepatocytes (Sidhu et al., 1993).
To provide for more efficient and cost-effective toxicologic end-point determinations, and also to alleviate the inhibitory effect of gel substrata on DNA transfection methodologies (Pasco and Fagan, 1989), the present study was undertaken to compare a conventional air-dried collagen type 1 substratum with standard tissue-culture plastic and Primaria substrata. The latter is a commercially available plastic in which a net positive charge has been applied to standard tissue culture dishes to mimic charge characteristics of the cell surface better (Koide et al., 1990; Yuasa et al., 1993). We also compared the standard ECM overlay to a growth factor-reduced ECM preparation. Assessment of liver-selective and dedifferentiation markers was accomplished by mRNA expression analyses and with in situ immunofluorescence. The data obtained demonstrate that liver-selective gene responsiveness does not require a complex substratum, since both tissue-culture plastic and Primaria yielded an equal if not better response than conventional collagen type 1. Our results likewise indicated that cells cultured with standard ECM preparations yielded essentially identical patterns of gene responsiveness to those cultured with growth-factor reduced ECM preparations.
MATERIALS AND METHODS
Cell Culture Materials
All cell culture media were obtained from Gibco (Grand Island, NY). Nu-Serum, insulin/transferrin/selenium/BSA-linoleic acid (ITS+), Matrigel (ECM), and growth factor-reduced Matrigel (defined ECM) were obtained from Collaborative Biomedical Products (Bedford, MA). Collagen type 1 was obtained from Sigma (St. Louis, MO). Tissue culture-treated plastic and Primaria dishes were obtained from Nunc (Naperville, IL) and Becton Dickinson (Franklin Lake, NJ), respectively. Collagenase (type 1) was obtained from Worthington Biochemicals (Freehold, NJ). Beta-naphthoflavone (βNF) was obtained from Aldrich Chemical Co. (Milwaukee, WI). All other chemicals were of the highest grade available and obtained from Sigma.
Isolation and Culture of Hepatocytes
Rat hepatocytes were isolated by a modification of the two-step collagenase perfusion in situ (Seglen, 1976) and cultured with a modification of the protocol described previously (Sidhu et al., 1993). Cells were isolated from noninduced rat liver and resuspended in Williams’ E medium as described (Sidhu et al., 1993). The dexamethasone (Dex) concentration, however, was reduced to 100 nM during the isolation and attachment period. Hepatocytes were plated at 1 × 106 cells/ml in 3-5 ml of complete Williams’ E medium on 60 mm tissue culture-treated plastic, Primaria, or air-dried collagen type 1. For cells to attach efficiently to plastic and Primaria dishes, 5% Nu-Serum was included during the 3 h attachment period. After this attachment period all further incubations were conducted with serum-free medium and the Dex concentration reduced to 25 nM. Medium changes were conducted on a daily basis.
Matrigel Overlay
A dilute concentration (233 μg/ml, final concentration) of ECM or defined ECM was added either at 4 h or delayed for 24 h after plating following initial dilution of ECM to a concentration of 5 mg/ml (Sidhu et al., 1993) with cell culture medium.
For the gene induction treatments, cells were cultured for 48 hr, as previously described (Sidhu et al., 1993), prior to addition of drugs. PB was added at 0.1 or 1 mM in 0.9% saline. Dex was added at 25 nM. βNF was added at 22 μM in DMSO (final concentration of DMSO was 0.05%). Unless otherwise stated, treatments were conducted for 24 h, at which point total RNA was isolated. All studies were repeated at least three times and representative data are shown.
Northern and Slot-Blot Analysis
Total RNA was isolated (Chomczynski and Sacchi, 1987) from cells pooled from two to three dishes for each treatment. For Northern blots, unless otherwise stated, 5 μg of each RNA sample was resolved on 1% agarose gels containing formaldehyde (Sidhu et al., 1993) and transferred to Genescreen Plus nylon membranes (NEN-DuPont, Boston, MA). Nucleic acid concentrations were estimated spectrophoto-metrically by absorbance at 260 nm. Equal RNA loading and transfer efficiencies were ascertained by ethidium bromide staining and hybridization to a radiolabeled oligonucleotide targeted to 18S ribosomal RNA (rRNA) as described (Omiecinski et al., 1990; Sidhu et al., 1993). For slot-blot evaluation, 5 μg of total RNA was applied onto a Genescreen Plus membrane under denaturing conditions and under vacuum using a Minifold II apparatus (Schleicher and Schuell, Keene, NH). The membranes were hybridized with specific 32P-radiolabeled oligonucleotides for CYP2B1, 2B2, 3A1, albumin, and 18S rRNA as described previously (Omiecinski et al., 1990; Sidhu et al., 1993). Resulting films (XAR-2, Kodak, Rochester, NY) from autoradiography were scanned densitometrically with a Howtek flatbed scanner and analyzed with Biolmage Whole Band Analysis Software (Millipore Corp, Bedford, MA) using a Sun Sparcstation II computer. Several exposures of each autoradiograph were taken to ensure that densitometry would be performed within the linear range of the x-ray film. Oligonucleotides specific for rat transferrin, C/EBPα, GST Ya1 were designed as follows:
Transferrin: 5′-TCAGGGACA GCCAGACACAG-3′
C/EBPα: 5′-AGTGTGCGATCTGGAACTGCAAGT G-3′
GST Ya1: 5′-TCGTGTTTATTATTTCAA-3′
cDNA Probes
A cDNA probe specific for CYP1A1 consisted of a 326 bp, exon 9 fragment provided by Dr. James Whitlock, Jr. (UC, Stanford, CA). cDNA probes specific for CYP1A2, CYP2E1, microsomal epoxide hydrolase (mEH), and glutathione-S-transferase Pi class (GST Pi) were derived from PCR products of rat liver cDNAs (Omiecinski et al., 1990) using the following forward (FP) and reverse (RP) primer pairs:
CYP2E1 FP, 5′-GCCTAGCGCCTCCAGCTTAG-3′
CYP2E1 RP, 5′-AGATCATAGACTCAGTCCCC-3′
CYP1A2 FP, 5′-CTGCAGAAAACAGTCCAGGA-3′
CYP1A2 RP, 5′-GGAAAAGGAACAAGGGTGGC-3′
mEH FP, 5′-CACTATGGCTTCAACTCC-3′
mEH RP, 5′-CAGGCCTCCATCCTCCAG-3′
GST Pi FP, 5′-ACGCGCATGCTGCTGGCTCA-3′
GST Pi RP, 5′-AAGTTGTCCAGGCAGCCAGG-3′
The CYP1A2, CYP2E1, mEH, and GST Pi FPs and RPs produced the expected size PCR products of 452 bp, 638 bp, 793 bp, and 470 bp, respectively. These PCR products were radiolabeled using the DECAprime DNA labeling kit (Ambion, Austin, TX) and α-32P-dCTP (>3,000 Ci/mmol, New England Nuclear/DuPont, Boston, MA). Hybridizations were performed essentially as described (Hassett et al., 1989), except that hybridizations were conducted at 60°C in the absence of formamide.
In Situ Immunofluorescence
These studies were initiated by the attachment of freshly isolated hepatocytes on tissue-culture treated plastic, Primaria, or type 1 collagen 35 mm tissue culture dishes. Cultures were maintained and exposed to inducers using the previously described protocol for 60 mm tissue culture dishes (Sidhu et al., 1993). The culture dishes were then rinsed with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde, pH 7.5 for 20 min at 4°C, and placed in methanol for 20 min at −20°C in order to permeabilize the cells. The adherent hepatocytes were subsequently rehydrated using PBS and then exposed to 1% normal goat serum in PBS to decrease nonspecific binding for at least 20 min. The anti-CYP2Bl/2B2 polyclonal primary antibody was provided by Dr. Paul Thomas (Rutgers University, NJ). The culture dishes were then exposed to the primary antibody for 60 min at room temperature, washed with PBS, exposed to the secondary antibody, fluorescein isothiocyanate-conjugated goat anti-rabbit IgG, for 60 min at room temperature, and washed again with PBS. The cell layer was protected using a glass coverslip and Aqua-mount (Lerner Laboratories, Pittsburgh, PA). The dishes were examined using scanning laser cytometry coupled with confocal microscopy.
Scanning laser Cytometry
Hepatocytes were examined for immunoreactive CYP (CYP2B1/2B2) using an ACAS 570 Ultima confocal laser cytometer (Meridian Instalments, Okemos, MI). Fields of cells were scanned in confocal mode using a 40X LWD objective (na = 0.7). The 488 nm line of an argon–ion laser was used for fluorescein excitation (200 mW; 10% scanning strength). A 530/30 nm band-pass filter was used to detect fluorescein emission, with the PMT voltage set at 45%. Image size was set for 360 × 360 pixels at a step size of 2 μm/pixel, with 32 samples/pixel. To limit the contribution of out of focus fluorescence, the pinhole aperture in front of the PMT was set to 100 μm. Images were then saved as TIFF files, and subsequently processed using NIH Image software (NCSA, Univ. of Illinois, Urbana-Champagne, IL). The resulting images were then pasted into the software package PowerPoint (Microsoft Corp, Redmond, WA) and subsequently printed using a dye sublimation printer. The pseudocolor bar represents the linear increase in fluorescence intensity.
RESULTS
Effect of ECM Overlay on Maintenance of Hepatocyte Morphology
Primary rat hepatocytes were isolated from adult rat liver and plated on collagen type 1, tissue-culture treated plastic, or Primaria. After the cells were allowed to attach for 3 h, the medium was changed and a dilute concentration of ECM or defined ECM was added and allowed to gel at 37°C. As shown in Figure 1, hepatocyte morphology was maintained over 96 h where an ECM overlay was present (panels E and F). In the absence of an ECM overlay (D), the cells became very flattened, with spreading and fibroblastlike protrusions evident at 96 h postisolation. No morphologic differences were observed between the two types of ECM examined (E,F). In addition, no morphologic differences were observed for the three different substrata tested (micrograph shown only for collagen type 1).
FIG. 1.
Morphologic comparison of hepatocytes cultured on a collagen type 1 substratum with and without an overlay of ECM. Comparison of standard Matrigel (ECM) vs growth-factor-reduced Matrigel (defined ECM). A–C 24 h postisolation: no ECM (A); ECM (B); defined ECM (C). D–F 96 h postisolation: no ECM (D); ECM (E); defined ECM (F). Representative micrographs shown for collagen substratum. Similar results were observed for plastic and Primaria (not shown).
Effect of Substratum and ECM Overlay on PB-Mediated Induction in Cultured Primary Rat Hepatocytes
The data shown in the Northern blot analysis of Figure 2 illustrate the specificity of several hybridization probes used in these analyses. Relative mRNA expression levels of three PB-inducible P450s (CYP2B1, 2B2 and 3A1), microsomal epoxide hydrolase (mEH), and albumin in hepatocyte cultures and PB- and Dex-induced rat liver are demonstrated. The present study focused on the role of tissue-culture substratum in conjunction with the type of ECM overlay as determinants of liver-selective gene expression and induction responses by prototypic xenobiotics. In the absence of ECM overlay (PB lane), expression levels of the liver-specific albumin gene, as well as three PB-inducible P450s, are poorly maintained. However, in the presence of an ECM overlay, albumin expression was clearly preserved, and induction of CYP2B1, CYP2B2, and CYP3A1 by PB was facilitated dramatically.
FIG. 2.
Northern blot analysis of the effects of ECM overlay (ECM vs. defined ECM) on PB-inducibility (1.00 mM) of CYP2B1, 2B2, 3A1 gene expression in primary rat hepatocytes. Total RNA was extracted and 5 μg resolved, as stated in Materials and Methods section. External controls (5 μg) are indicated by phenobarbital (PB)- and dexamethasone (Dex)-induced Sprague-Dawley (S.D.) liver. In addition microsomal epoxide hydrolase (mEH) and albumin mRNA expression levels are also represented. Ribosomal 18S hybridization indicates a lower than expected loading in the lane designated PB (no ECM overlay). However, the data still represent a significant difference between the expression of PB-inducible CYP genes in the presence or absence of an ECM overlay (see legend to Fig. 3).
As illustrated by slot-blot analyses in Figures 3 and 4, PB-mediated induction of CYP2B1 was maintained irrespective of the type of ECM employed. Use of defined ECM did not alter PB or constitutive expression levels relative to the standard ECM preparation. In comparing the three different substrata, when the ECM overlay was applied at 4 h postplating, no obvious differences in expression levels resulted in any of the various marker genes assessed. However, a generally higher CYP2B1 induction response was observed for plastic and Primaria substrata compared to collagen (Fig. 4). These latter observations were especially evident when ECM was added to the adherent cells after a 24 h delay (Fig. 4). Delaying ECM addition, while preserving the PB induction capability of the cells, enables a window for transient gene transfection experiments to be conducted. Even in the absence of an ECM overlay, plastic and Primaria substrata still facilitated CYP2B1 PB-induction, albeit at substantially lower levels of responsiveness than obtained with ECM overlay (Fig. 3).
FIG. 3.
Slot-blot comparison of substrata together with the absence (No ECM) or presence of standard Matrigel (ECM) vs. growth-factor-reduced matrigel (defined ECM) overlay on PB-inducibility of CYP2B1 gene expression. Hepatocytes were plated on collagen type 1, plastic or Primaria substrata and 48 h postisolation, treated with PB (0.1/1.00 mM) or BNF (22 μM) for 24 h. Total RNA was evaluated by slot-blot analysis, as stated in Materials and Methods section. The addition of ECM overlay (233 μg/ml) at 4 h postplating (4 h), and delaying the addition until 24 h postplating (24 h), also is shown.
FIG. 4.
Densitometric evaluation of slot-blot data shown in Figure 3 for CYP2B1 gene expression. The addition of ECM overlay (233 μg/ml) at 4 h postplating (4 h), and delaying the addition until 24 h postplating (24 h), also is shown. Autoradiographic data were quantified by whole-band analysis and normalized to equivalent 18S ribosomal rRNA data. Normalized signal values are represented by arbitrary O.D. units.
We also examined the role of substratum and ECM overlay on PB-mediated induction of CYP3A1. This gene is prototypically induced both by steroids (e.g. Dex) and by PB-like agonists (Gonzalez et al., 1986; Waxman and Azaroff, 1992), although optimal CYP3A1 induction by PB appears to require higher dosages than for CYP2B1 or CYP2B2 induction (Kocarek et al., 1990). We evaluated this response in the present study, and the resulting data are shown in Figures 5 and 6. A PB concentration of 1.00 mM was required for induction of CYP3A1 (vs. 0.1 mM for CYP2B1: Fig. 3). Both preparations of ECM facilitated PB-induction when ECM addition was made at 4 h postisolation (Fig. 6). However, when addition was delayed for 24 h, cells cultured on plastic and/or Primaria demonstrated dramatically higher gene responsiveness than those on collagen type 1 (Fig. 6). It is noteworthy that, similar to in vivo responsiveness, βNF did not induce CYP2B1 or CYP3A1 in these cultures, nor did PB induce CYP1A1 or CYP1A2 expression.
FIG. 5.
Slot-blot comparison of substrata and absence (no ECM) or presence of standard Matrigel (ECM) vs. growth-factor-reduced Matrigel (defined ECM) overlay on PB-inducibility of CYP3A1 gene expression. Hepatocytes were plated on collagen type 1, plastic or Primaria substrata and 48 h postisolation, treated with PB (0.1/1.00 mM) or BNF (22 μM) for 24 h. Total RNA was evaluated by slot-blot analysis, as stated in Materials and Methods section. The addition of ECM overlay (233 μg/ml) at 4 h postplating (4 h), and delaying the addition till 24 h postplating (24 h), also is shown.
FIG. 6.
Densitometric evaluation of slot-blot data shown in Figure 5 for CYP3A1 gene expression. The addition of ECM overlay (233 μg/ml) at 4 h postplating (4 h), and delaying the addition till 24 h postplating (24 h), also is shown. Autoradiographic data were quantified by whole-band analysis and normalized to equivalent 18S ribosomal rRNA data. Normalized signal values are represented by arbitrary optical density units.
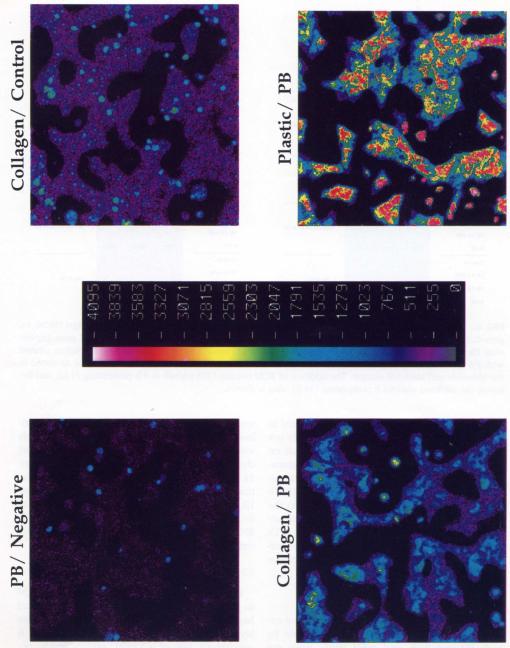
Effect of Substratum and ECM Overlay on PB-Mediated Induction of CYP2BI/2B2 Immunoreactive Protein as Detected by Immunofluorescence and Scanning Laser Cytometry
To extend the mRNA expression data discussed above we also examined PB-inducible CYP protein levels in the primary cultures. Traditionally, one would isolate cell extracts, prepare S9s and/or microsomes, and use Western analysis to detect immunoreactive protein. Although the latter technique is very useful in determining induction potential, it requires the disruption of cells from their spatial culture environment and does not, therefore, reflect the heterogeneous distribution of CYP protein normally found in adult liver (Omiecinski et al., 1990). Using confocal microscopy and scanning laser cytometry we examined the in situ distribution of PB-induced CYP2B1/2B2 protein expression in hepatocytes cultured on various substrata and in the presence of an ECM overlay. The addition of an ECM overlay was delayed for 24 h. As is evident from Figure 7, PB-induction leads to a dramatic elevation in the expression of CYP2B1/2B2 immunoreactive protein. In addition, the induction potential is higher for cells cultured on a plastic than a collagen substratum. The latter observation is consistent with the level of mRNA expression under the same conditions (Figs. 3,4). Although not shown here, similar mRNA/protein correlative expression levels were also observed using single cell fluorescence detection with antibodies specific for CYP3A and 1A proteins.
FIG. 7.
Effect of substratum on level of immunoreactive CYP2B1/2B2 protein in primary rat hepatocytes cultured with an overlay of ECM. Hepatocytes were plated either on tissue-culture treated plastic or type 1 collagen-coateci plastic, an overlay of ECM was applied at 24 h postplating and cells were subsequently induced with PB (0.1 mM). Cells were fixed and penneabilized, as stated in Materials and Methods section, then exposed to a rabbit polyclonal antibody specific for CYP2B1/2B2 followed by a Fluorescein Isothiocyanate conjugated goat antirabbit IgG. Confocal microscopy in combination with scanning laser cytometry was used to detect single cell-associated immunofluorescence due to PB-induced CYP2B1/2B2 protein. Multiple fields (360 × 360 μm) were examined and representative fluorescence micrographs are shown as follows: (PB/Negative), cells cultured on a collagen substratum, treated with PB but no primary antibody; (Collagen/Control), cells cultured on a collagen substratum, not induced with PB; (Collagen/PB), cells cultured on a collagen substratum, and induced with PB; (Plastic/PB), cells cultured on a plastic substratum, and induced with PB. The pseudocolor bar in the center of the figure represents a linear graduation of relative fluorescence intensity units.
Effect of Substratum and ECM Overlay on BNE-Mediated Induction of CYP1AI and 1A2 Genes in Cultured Primary Rat Hepatocytes
In the present investigation we also examined polycyclic aromatic hydrocarbon- (PAH) inducible responses, specifically the effect of substratum and ECM overlay on βNF-mediated (22 μM) induction of CYP1A1 and CYP1A2 mRNAs. As shown in Figure 8, CYP1A1 and CYP1A2 induction levels were comparable in both ECM formulations. Similar to the PB response, delaying additions of ECM to 24 h postisolation did not compromise the βNF inductive effects, nor did the plastic substratum have an adverse impact on the induction response relative to collagen type 1 or Primaria (normalized densitometric data not shown).
FIG. 8.
Slot-blot comparison of substrata and absence (no ECM) or presence of standard Matrigel (ECM) vs. growth-factor reduced Matrigel (defined ECM) overlay on βNF-inducibility of CYP1A1 and 1A2 gene expression. Hepatocytes were plated on collagen type 1, plastic or Primaria substrata and 48 h postisolation, treated with PB (0.1/1.00 mM) or βNF (22 μM) for 24 h. Total RNA was evaluated by slot-blot analysis, as stated in the Materials and Methods section. The addition of ECM overlay (233 μg/ml) at 4 h postplating (4 h), and delaying the addition until 24 h postplating (24 h), also is shown.
Effect of Substratum and ECM Overlay on Liver- and Tissue-Selective Gene Expression
In Figure 9, the expression of two liver-selective genes, albumin and transferrin, was assessed in cultured cells using slot blot mRNA analysis. Similar to results presented above for albumin expression using Northern blot analyses (Fig. 2), ECM overlay served to maintain the expression of both genes, with albumin expression appearing somewhat more highly dependent on ECM overlay than transferrin. In addition, the data in Figure 11 show that albumin expression was maintained, regardless of the substratum used, even at 96 h postisolation and was equivalent in level to that in freshly isolated hepatocytes. It is noteworthy that in those preparations in which reduced expression of albumin and/or mEH were observed, elevated expression levels of GST Pi also were apparent (Fig. 11). GST Pi expression was not detected in freshly isolated hepatocytes and is normally not constitutively expressed in vivo in the adult rat liver (Sato et al., 1984). Rather, GST Pi expression is considered indicative of a dedifferentiated hepatic phenotype (Sato et al., 1984). Expression of GST Pi was evident at 48 h in the absence of ECM overlay and increased dramatically between 48 and 72 h in culture (Fig. 11). However, only very low levels of GST Pi expression were apparent at 96 h in the presence of an ECM overlay.
FIG. 9.
Slot-blot comparison of substrata together with the absence (no ECM) or presence of standard Matrigel (ECM) vs. growth-factor-reduced Matrigel (defined ECM) overlay on liver-specific albumin and tissue-specific transferrin gene expression. Total RNA was evaluated by slot-blot analysis, as stated in the Materials and Methods section. The effects of adding the ECM overlay (233 μg/ml) at 4 h postplating (4 h), and upon delaying ECM additions until 24 h postplating (24 h), also are shown.
FIG. 11.
Northern blot analysis of the effects of ECM overlay and substratum on albumin, mEH, CYP2E1, C/EBPα, GST Ya1, and GST Pi expression. Hepatocytes were cultured on collagen type 1, plastic, or Primaria substrata in the presence or absence of ECM overlay for 96 h. Total RNA was isolated at the indicated time points and 10 μg resolved, as stated in the Materials and Methods section. The lanes represent the following treatments: lane 1, freshly isolated hepatocytes; lane 2, 4 h postisolation, plastic substratum; lane 3, 4 h postisolation, Primaria substratum; lane 4, 4 h postisolation, collagen type 1 substratum; lane 5, 24 h postisolation, plastic without ECM overlay; lane 6, 24 h, plastic plus ECM overlay; lane 7, 48 h postisolation, plastic without ECM overlay; lane 8, 48 h postisolation, plastic plus ECM overlay; lane 9, 72 h postisolation, plastic without ECM overlay; lane 10, 72 h postisolation, plastic plus ECM overlay; lane 11, 96 h postisolation, plastic plus ECM overlay; lane 12, 96 h postisolation, Primaria plus ECM overlay; lane 13, 96 h postisolation, collagen type 1 plus ECM overlay; lane 14, 5 μg PB-induced Sprague-Dawley rat liver RNA (S.D.). The membrane was hybridized with 32P-labeled gene-specific cDNA and oligonucleotide probes, as stated in the Materials and Methods section. The arrows on the left hand side of the figure indicate the position of the 18S rRNA marker. Note that the GST Ya1 hybridization probe cross-hybridized to some extent with 18S rRNA.
mEH Expression
Although not a liver-selective marker, the expression of mEH was induced moderately by both PB and βNF, and mEH levels were not modulated substantially by substratum selection or by the particular type of ECM preparation tested (Fig. 10). However, as was the case for albumin and transferrin, mEH expression levels were enhanced by ECM overlay. This was most clearly evident by comparing the data of Figure 11 (lane 9), corresponding to a 72 h postisolation time point in the absence of ECM, to lane 10, the same time point but in the presence of an ECM overlay. It was noteworthy that mEH RNA levels were substantially reduced by Dex treatment in vivo (Fig. 2).
FIG. 10.
Slot-blot comparison of substrata and absence (no ECM) or presence of standard Matrigel (ECM) vs. growth factor-reduced Matrigel (defined ECM) overlay on microsomal epoxide hydrolase (mEH) gene expression. Total RNA was evaluated by slot-blot analysis, as stated in the Materials and Methods section. The effects of adding the ECM overlay (233 μg/ml) at 4 h postplating (4 h), or after delaying the addition until 24 h postplating (24 h), also are shown. In addition the ribosomal 18S hybridization signal is included and was used to normalize relative gene expression levels.
CYP2E1 and GST Ya1 Expression
Despite the demonstrated capabilities of ECM in promoting liver-selective function in cultured hepatocytes, CYP2E1 and GST Ya1 expression levels were not maintained by ECM treatment. A precipitous fall of CYP2E1 mRNA levels occurred over time in culture. As apparent from the data presented in Figure 11, expression levels of CYP2E1 mRNA were maximal at 4 h postisolation but declined rapidly during the subsequent 24 h in culture. Longer autoradiographic exposures demonstrated very low expression levels at 72 and 96 h postisolation but only in the presence of an ECM overlay (data not shown). A somewhat similar profile was evident for GST Ya1 expression (Fig. 11). Between 4 and 24 h postisolation, a dramatic reduction in GST Ya1 mRNA levels was also observed.
C/EBPα Expression
We examined the expression of a liver-specific transcription factor C/EBPα in the primary hepatocyte cultures (Landschulz et al., 1988). As is evident from the Northern blot data in Figure 11, C/EBPα expression was closely linked to the presence of an ECM overlay. After 24 h in culture, C/EBPα mRNA was barely detectable (single band at 2.7 kb; Padgham et al., 1993) in the absence of an ECM overlay (lane 5), but remained relatively constant throughout the culture period of 96 h when an ECM overlay was present.
DISCUSSION
In an earlier report (Sidhu et al., 1993), we demonstrated the advantages of culturing primary rat hepatocytes with an overlay of ECM instead of plating the cells directly on an ECM substratum. This strategy not only maintained liver-specific gene expression but also facilitated PB-mediated induction of CYP genes to levels equivalent to those observed in liver in vivo. However, the previous study was conducted with cells plated on a substratum of collagen type 1. Application of this reagent to culture dishes represents a tedious undertaking and an additional expense. In addition, proteinaceous substrata have been reported to inhibit transient gene transfection efficiencies in primary rat hepatocytes (Pasco and Fagan, 1989). To overcome these deficiencies and to characterize further and optimize primary hepatocyte culture conditions, we undertook the present study and compared a variety of liver-selective and non-selective gene responses using various combinations of substrata with or without an ECM overlay; two different, commercially available preparations of ECM; the standard Matrigel and a growth factor-reduced Matrigel; and the prototypic inducing agent PB, and the PAH inducer, βNF.
The results presented demonstrated that maintenance of PB-mediated induction of rat CYP2B1, CYP2B2, and CYP3A1, as well as βNF-mediated induction of CYP1A1 and CYP1A2 genes, although critically dependent on the presence of an ECM overlay, were not dependent on the use of a complex substratum for plating of the cells. Substitution of collagen type 1 substratum with either standard tissue culture-grade plastic or Primaria maintained gene induction responsiveness equivalently or even superior to that observed with collagen.
Most studies that have attempted to maintain liver-specific gene expression have relied on the presence of complex tissue-culture substrata (Enat et al., 1984; Saad et al., 1993a,b), chemically defined culture media (Turner et al., 1988; Waxman et al., 1990), or a combination of both (Enat et al., 1984; Waxman et al., 1990). With respect to collagen-type substrata, studies with hepatocytes have traditionally employed standard air-dried (Bissell and Guzelian, 1980), covalently bonded (Macklis et al., 1985; Waxman et al., 1990), or gels derived from collagen type 1 (Michalopolous and Pitot, 1975, Sirica et al., 1979). Recent reports have further modified the gel substratum technique by culturing the cells in a “sandwich” of collagen type 1 (Dunn et al., 1989, 1992; Ezzell et al., 1993). The latter studies demonstrated that when hepatocytes were cultured between two hydrated layers of collagen, hepatocellular morphology was better maintained, as were levels of albumin and transferrin secretion. Lee et al. (1993) reported that “sandwich-mediated” maintenance of liver-specific function was further enhanced by the continued presence of l-proline in the tissue-culture medium.
Several investigations have focused on more complex ECM substrata. For example, Schuetz et al. (1988) and Guzelian et al. (1989) demonstrated that several liver-selective functions, including gene responsiveness to the prototypic inducer PB, were maintained only in hepatocytes plated on a substratum of Matrigel, a reconstituted basement membrane derived from the Engelbreth-Holm-Swarm sarcoma (Kleinman et al., 1985). Only marginal inductive effects were detected in cells cultured on a Vitrogen (commercially available collagen type 1) substratum (Schuetz et al., 1988; Kocarek et al., 1993). Lindblad et al. (1991) likewise demonstrated that the biophysical nature of the collagenous substratum was a determining factor in the maintenance of liver-specific function. The latter report concluded that the fluidity of either Matrigel or denatured type 1 collagen facilitated the maintenance of hepatocyte cell shape and corresponding gene-responsiveness to PB, while cells cultured on various rigid collagen substrata were refractive to induction by this agent. A recent investigation by Musat et al. (1993) demonstrated that when a dilute concentration (625 μg/ml) of ECM was added as an overlay to primary hepatocytes established for 24 h on a collagen type 1 substratum, cell polarity was restored. These latter authors found that in addition to the reformation of membrane domains resembling adult liver, the actin cytoskeleton reorganized into pericanalicular webs. Thus, considerable evidence is accumulating for both biophysical and molecular roles for ECM in controlling hepatocyte-specific function and gene expression.
In addition to the type of substratum used to maintain optimal hepatocyte function, we evaluated two different types of commercially available ECM preparations. Overlay of cells with a standard preparation of ECM was compared with a growth factor-reduced ECM (defined ECM). This aspect of our investigation was prompted by the need to examine a more highly defined system, due to the complex nature of soluble components found in standard ECM (Kleinman et al., 1985), and also by a recent report that was critical of the use of standard Matrigel due to the presence of proteases in the normal preparation (Mackay et al., 1993). The results presented here demonstrate that both preparations are equally efficacious in promoting induction responses and expression of liver-selective genes. Since the concentrations of various growth factors and contaminating proteases are significantly reduced in the defined ECM preparation (Taub et al., 1990; manufacturer’s specifications), our results argue against a significant role for proteases or soluble growth factor components as facilitators of liver-specific function in primary rat hepatocyte cultures. However, the use of a more defined preparation of ECM should be encouraged in situations that may involve growth factor-mediated differentiation processes or in which growth factor or protease availability is an important consideration.
In the present study we also examined the expression of certain genes that appear to be relatively labile once hepatocytes are isolated and placed in culture. For example, as reported recently, CYP2E1 mRNA (Hunt et al., 1991; Kocarek et al., 1993; Padgham et al. 1993) and immunoreactive protein (Hunt et al., 1991) undergo a rapid time-dependent decline in expression when hepatocytes are placed in culture. In the report by Padgham et al. (1993), the level of CYP2E1 expression was reduced to less than 20% of the level in freshly isolated hepatocytes after 4 h in culture. As shown in Figure 11, using our conditions no decline in CYP2E1 mRNA was detected during the initial 4 h culture period, prior to the ECM overlay, regardless of the type of substratum employed. However, a dramatic decline in CYP2E1 mRNA level was observed at subsequent time points, regardless of the presence/absence of ECM overlay, such that between 24–96 h of culture the level of the corresponding mRNA was barely detectable. These results are similar to those reported by Hunt et al. (1991) and Kocarek et al. (1993).
In contrast to Padgham et al. (1993), we did not detect any marked change in the level of C/EBPα mRNA, a liver-enriched transcription factor (Landshulz et al., 1988), over a period of 96 h unless ECM overlay was omitted (Fig. 11). Padgham et al. (1993) suggested that during the isolation procedure, hepatocytes are committed to an irreversible loss of certain CYP mRNAs and liver-specific transcription factors, but discrepancies between our respective studies appear to underscore the need for the use of more optimized isolation and culture conditions to prevent rapid dedifferentiation of hepatocyte phenotype. Despite the use of an overlay of ECM, we still noted a rise in the expression of GST Pi with time in culture (Fig. 11). The expression appeared to be greater on the collagen substratum and considerably higher when ECM overlay was omitted. In contrast, Schuetz and Schuetz (1993) observed a very high expression of GST Pi dedifferentiation marker, by 24 h in culture, in their hepatocyte system using an ECM substratum. These differences are likely attributable to a lack of Dex in the latter investigators’ culture medium, since this glucocorticoid has been shown to repress the expression of GST Pi in hepatocyte culture (Abramovitz et al., 1989; Aoki et al., 1993). GST Pi is not normally expressed in adult liver, but is dramatically elevated in preneoplastic liver cell populations (Sato et al., 1984). Therefore, our results indicate that a combination of ECM and hormonal supplements) is required to maintain the differentiation stimulus.
In conclusion, the present investigation examined a variety of culture conditions for primary rat hepatocytes. Results obtained demonstrated that standard tissue-culture grade plastic can be substituted as a preferred substratum to that of collagen type 1. This alteration is more cost-effective, simpler to effect, and should allow for better reproducibility than previous conditions relying on the coating of tissue-culture dishes with collagen, ECM, or ECM-derived components. In addition, the use of tissue-culture substrata of a nonproteinaceous nature in combination with delayed addition of ECM should facilitate gene regulation investigations requiring transient gene transfection analyses. We are currently using these improved culture conditions to examine xenobiotic biotransformation pathways in hepatocytes and in the assessment of molecular mechanisms underlying the regulation of PB-inducible CYP gene expression.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the excellent technical assistance of Richard Ramsden in the design and generation of oligonucleotides used in this study. This study was supported by USPHS grant GM32281 (CJO).
REFERENCES
- ABRAMOVITZ M, ISHIGAKI S, LISTOWSKY I. Differential regulation of glutathione-S-transferases in cultured hepatocytes. Hepatology. 1989;9:233–239. doi: 10.1002/hep.1840090212. [DOI] [PubMed] [Google Scholar]
- ADESNIK M, ATCHISON M. Genes for cytochrome P-450 and their regulation. Crit. Rev. Biochem. 1985;19:247–305. doi: 10.3109/10409238609084657. [DOI] [PubMed] [Google Scholar]
- AOKI Y, MATSUMOTO M, SUZUKI KT. Expression of glutathione-S-transferase P-form in primary cultured rat liver parenchymal cells by coplanar polychlorinated biphenyl congeners is suppressed by protein kinase inhibitors and dexamethasone. FEBS Letts. 1993;333:114–118. doi: 10.1016/0014-5793(93)80386-9. [DOI] [PubMed] [Google Scholar]
- BEN-ZE’EV A, ROBINSON GS, BUCHER NLR, FARMER ST. Cell–cell and cell–matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc. Natl. Acad. Sci. USA. 1988;85:2161–2165. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISSELL DM, GUZELIAN PS. Phenotypic stability of adult rat hepatocytes in primary monolayer culture. Ann. NY Acad. Sci. 1980;349:85–98. doi: 10.1111/j.1749-6632.1980.tb29518.x. [DOI] [PubMed] [Google Scholar]
- BISSELL DM, STAMATOGLOU SC, NERMUT MV, HUGHES RC. Interactions of rat hepatocytes with type IV collagen, fibronectin and laminin matrices. Distinct matrix-controlled modes of attachment and spreading. Eur. J. Cell Biol. 1986;40:72–82. [PubMed] [Google Scholar]
- BISSELL DM, CARON JM, BABISS LE, FRIEDMAN JM. Transcriptional regulation of the albumin gene in cultured rat hepatocytes. Role of basement-membrane matrix. Mol. Biol. Med. 1990;7:187–197. [PubMed] [Google Scholar]
- CARON J. Induction of albumin gene transcription in hepatocytes by extracellular matrix proteins. Mol. Cell Biol. 1990;10:1239–1243. doi: 10.1128/mcb.10.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASSIO D, HAMON-BENAIS C, GUERIN M, LECOQ O. Hybrid cell lines constitute a potential reservoir of polarized cells: isolation and study of highly differentiated hepatoma-derived hybrid cells able to form functional bile canaliculi in vitro. J. Cell Biol. 1991;115:1397–1408. doi: 10.1083/jcb.115.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOMCZYNSKI P, SACCHI N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CIMA LG, INGBER DE, VACANTI JP, LANGER R. Hepatocyte culture on biodegradable polymeric substrates. Biotech. Bioeng. 1991;38:145–158. doi: 10.1002/bit.260380207. [DOI] [PubMed] [Google Scholar]
- DIPERSIO CM, JACKSON DA, ZARET KS. The extracellular matrix coordinately modulates liver transcription factors and hepatocyte morphology. Mol. Cell Biol. 1991;11:4405–4414. doi: 10.1128/mcb.11.9.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN JC, YARMUSH ML, KOEBE HG, TOMPKINS RG. Hepatocyte function and extracellular matrix geometry: Long-term culture in a sandwich configuration. FASEB J. 1989;3:174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- DUNN JC, TOMPKINS RG, YARMUSH ML. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J. Cell. Biol. 1992;116:1043–1053. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENAT R, JEFFERSON DM, RUIZ-OPAZA N, GAT-MAITAN Z, LEINWAND LA, REID LM. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc. Natl. Acad. USA. 1984;81:1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EZZELL RM, TONER M, HENDRICKS K, DUNN JCY, TOMPKINS RG, YARMUSH ML. Effect of collagen gel configuration on the cytoskeleton in cultured rat hepatocytes. Exp. Cell Res. 1993;208:442–452. doi: 10.1006/excr.1993.1266. [DOI] [PubMed] [Google Scholar]
- FUJITA M, SPRAY DC, CHOI H, SAEZ HC, ROSENBERG LC, REID LM. Glycosaminoglycans and proteoglycans induce gap junction expression and restore transcription of tissue-specific mRNAs in primary liver cultures. Hepatology. 1987;7:1S–9S. doi: 10.1002/hep.1840070702. [DOI] [PubMed] [Google Scholar]
- GONZALEZ FJ, SONG BJ, HARDWICK JP. Pregnenolone l6a-carbonitrile-inducible P-450 gene family: gene conversion and differential regulation. Mol. Cell Biol. 1986;6:2969–2976. doi: 10.1128/mcb.6.8.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ FJ. Molecular genetics of the P-450 superfamily. Pharmacol. Ther. 1990;45:1–38. doi: 10.1016/0163-7258(90)90006-n. [DOI] [PubMed] [Google Scholar]
- GUZELIAN PS, LI D, SCHUETZ EG. Induction of cytochromes P-450 b/e by phenobarbital in primary culture of adult rat hepatocytes: test of differentiated liver gene expression. Drug Metab. Rev. 1989;20:793–809. doi: 10.3109/03602538909103579. [DOI] [PubMed] [Google Scholar]
- HASSETT C, TURNBLOM SM, DEANGELES A, OMIECINSKI CJ. Rabbit microsomal epoxide hydrolase: isolation and characterization of the xenobiotic metabolizing enzyme cDNA. Arch. Biochem. Biophys. 1989;271:380–389. doi: 10.1016/0003-9861(89)90287-7. [DOI] [PubMed] [Google Scholar]
- HUNT CM, GUZELIAN PS, MOLOWA DT, WRIGHT SA. Regulation of rat hepatic cytochrome P450IIE1 in primary monolayer hepatocyte culture. Xenobiotica. 1991;21:1621–1631. doi: 10.3109/00498259109044410. [DOI] [PubMed] [Google Scholar]
- JAUREGUI HO, NG S-F, GANN KL, WAX-MAN DJ. Xenobiotic induction of P-450 PB-4 (IIB1) and P-450c (1A1) and associated monooxygenase activities in primary cultures of adult rat hepatocytes. Xenobiotica. 1991;21:1091–1106. doi: 10.3109/00498259109039549. [DOI] [PubMed] [Google Scholar]
- KLEINMAN HK, MCGARVEY ML, HASSELL JR, STAR V, CANNON FB, LAURIE GW, MARTIN GR. Basement membrane complexes with biological activity. Biochemistry. 1985;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- KOCAREK T, SHUETZ E, GUZELIAN PS. Differentiated induction of cytochromes P450b/e and P450p mRNAs by dose of phenobarbital in primary cultures of adult rat hepatocytes. Mol. Pharmacol. 1990;38:440–444. [PubMed] [Google Scholar]
- KOCAREK T, SHUETZ E, GUZELIAN PS. Expression of multiple forms of cytochrome P450 mRNAs in primary cultures of rat hepatocytes maintained on matrigel. Mol. Pharmacol. 1993;43:328–334. [PubMed] [Google Scholar]
- KOIDE N, SAKAGUCHI K, KOIDE Y, ASANO K, KAWAGUCHI M, MATSUSHIMA H, TAKENAMI T, SHINJI T, MORI M, TSUJI T. Formation of multicellular spheroids composed of adult rat hepatocytes in dishes with positively charged surfaces and under other nonadherent environments. Exp. Cell Res. 1990;186:227–235. doi: 10.1016/0014-4827(90)90300-y. [DOI] [PubMed] [Google Scholar]
- LANDSHULZ WH, JOHNSON PF, ADASHI EY, GRAVES BI, MCKNIGHT SL. Isolation of a recombinant copy of the gene coding C/EBP. Genes Dev. 1988;2:7929–7933. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- LEE J, MORGAN JR, TOMPKINS RG, YARMUSH ML. Proline-mediated enhancement of hepatocyte function in a collagen gel sandwich culture configuration. FASEB J. 1993;7:586–591. doi: 10.1096/fasebj.7.6.8472895. [DOI] [PubMed] [Google Scholar]
- LINDBLAD WJ, SCHUETZ EG, REDFORD KS, GUZELIAN PS. Hepatocellular phe-notype in vitro is influenced by biophysical features of the collagenous substratum. Hepatology. 1991;13:282–288. [PubMed] [Google Scholar]
- LIU J-K, DIPERSIO CM, ZARET KS. Extracellular signals that regulate liver transcription factors during hepatic differentiation in vitro. Mol. Cell Biol. 1991;11:773–784. doi: 10.1128/mcb.11.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKLIS JD, SIDMAN RL, SHINE HD. Cross-linked collagen surface for cell culture that is stable, uniform, and optically superior to conventional surfaces. In Vitro Cell. Dev. Biol. 1985;21:189–194. doi: 10.1007/BF02621357. [DOI] [PubMed] [Google Scholar]
- MAKAY AR, GOMEZ DE, COTTAM DW, REES RC, NASON AM, THORGEIRSSON UP. Identification of the 72 kDa (MMP-2) and 92-kDa gelatinase/type IV collagenase in preparations of laminin and matrigel. Biotechniques. 1993;15:1048–1051. [PubMed] [Google Scholar]
- MICHALOPOLOUS G, PITOT HC. Primary culture of parenchymal cells on collagen membranes. Exp. Cell Res. 1975;94:70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- MUSAT AI, SATTLER CA, SATTLER GL, PITOT HC. Reestablishment of cell polarity of rat hepatocytes in primary culture. Hepatology. 1993;18:198–205. [PubMed] [Google Scholar]
- NELSON DR, KAMATAKI T, WAXMAN DJ, GUENGERICH FP, ESTABROOK RW, FEY-EREISEN R, GONZALEZ FJ, COON MJ, GUN-SALUS IC, GOTOH O, OKUDA K, NEBERT DW. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993;12:1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- OMIECINSKI CJ, HASSETT C, COSTA P. Developmental expression and in situ localization of the phenobarbital-inducible rat hepatic mRNAs for cytochromes CYP2B1, CYP2B2, CYP2C6, and CYP3A1. Mol. Pharmacol. 1990;38:462–470. [PubMed] [Google Scholar]
- OKEY AB. Enzyme induction in the cytochrome P-450 system. Pharamcol. Ther. 1990;45:241–298. doi: 10.1016/0163-7258(90)90030-6. [DOI] [PubMed] [Google Scholar]
- PADGHAM CRW, BOYLE CC, WANG XJ, RALEIGH SM, WRIGHT MC, PAINE AJ. Alteration of transcription factor mRNAs during the isolation and culture of rat hepatocytes suggests the activation of a proliferative mode underlies their de-differentiation. Biochem. Biophys. Res. Commun. 1993;197:599–605. doi: 10.1006/bbrc.1993.2521. [DOI] [PubMed] [Google Scholar]
- PASCO DS, FAGAN JB. Efficient DNA-mediated gene transfer into primary cultures of adult rat hepatocytes. DNA. 1989;8:535–541. doi: 10.1089/dna.1.1989.8.535. [DOI] [PubMed] [Google Scholar]
- REID LM, GAITMAITAN Z, ARIAS I, PONCE P, ROJKIND M. Long-term cultures of normal rat hepatocytes on liver biomatrix. Ann. N.Y. Acad. Sci. 1980;349:70–76. doi: 10.1111/j.1749-6632.1980.tb29516.x. [DOI] [PubMed] [Google Scholar]
- SAAD B, SCHAWALDER H, MAIER P. Crude liver membrane fractions as a substrate preserve liver-specific functions in long-term, serum-free rat hepatocyte cultures. In Vitro Cell Dev. Biol. 1993a;29A:32–40. doi: 10.1007/BF02634369. [DOI] [PubMed] [Google Scholar]
- SAAD B, SCHOLL FA, THOMAS H, SCHAWALDER H, STREIT V, WAECHTER F, MAIER P. Crude liver membrane fractions and extracellular matrix components as substrata regulate differentially the preservation and inducibility of cytochrome P-450 isozymes in cultured rat hepatocytes. Eur. J. Biochem. 1993b;213:805–814. doi: 10.1111/j.1432-1033.1993.tb17823.x. [DOI] [PubMed] [Google Scholar]
- SATO K, KITAHARA A, SATOH Y, ISHIKAWA T, TATEMATSU M, ITO N. The placental form of glutathione-S-transferase as a new marker protein for preneoplasia in rat chemical hepatocarcinogenesis. Jpn. J. Cancer Res. 1984;75:199–202. [PubMed] [Google Scholar]
- SCHUETZ EG, LI D, OMIECINSKI CJ, MULLER-EBERHARD U, KLEINMAN HK, ELSWICK B, GUZELIAN PS. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J. Cell Physiol. 1988;134:309–323. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- SCHUETZ J, SCHUETZ E. Extracellular matrix regulation of multidrug resistance in primary monolayer cultures of adult rat hepatocytes. Cell Growth Differ. 1993;4:31–40. [PubMed] [Google Scholar]
- SCHULZ WA, CRAWFORD N, LOCKER J. Albumin and α-fetoprotein gene expression and DNA methylation in rat hepatoma cell lines. Exp. Cell Res. 1988;174:433–447. doi: 10.1016/0014-4827(88)90313-8. [DOI] [PubMed] [Google Scholar]
- SEGLEN PO. Preparation of isolated liver cells. Methods Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- SIDHU JS, FARIN FM, OMIECINSKI CJ. Influence of extracellular matrix overlay on phenobarbital-mediated induction of CYP2B1, 2B2, and 3A1 genes in primary rat hepatocyte culture. Arch. Biochem. Biophys. 1993;301:103–113. doi: 10.1006/abbi.1993.1121. [DOI] [PubMed] [Google Scholar]
- SIRICA AE, RICHARDS W, TSUKADA Y, SATTLER CA, PITOT HC. Fetal pheno-typic expression by adult rat hepatocytes on collagen gel/nylon meshes. Proc. Natl. Acad. Sci. USA. 1979;73:283–287. doi: 10.1073/pnas.76.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEZAWA T, YAMAZAKI M, MORI Y, YON-AHA T, YOSHIZATO K. Morphological and immuno-cytochemical characterization of a hetero-spheroid composed of fibroblasts and hepatocytes. J. Cell Sci. 1992;101:495–501. doi: 10.1242/jcs.101.3.495. [DOI] [PubMed] [Google Scholar]
- TAUB M, WANG Y, SZCZESNY TM, KLEINMAN HK. Epidermal growth factor or transforming growth factor a is required for kidney tubulogenesis in matrigel cultures in serum-free medium. Proc. Natl. Acad. Sci. USA. 1990;87:4002–006. doi: 10.1073/pnas.87.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER NA, WILSON NM, JEFCOATE CR, PITOT HC. The expression and metabolic activity of cytochrome P-450 isozymes in control and phenobarbital-induced primary cultures of rat hepatocytes. Arch. Biochem. Biophys. 1988;263:204–215. doi: 10.1016/0003-9861(88)90629-7. [DOI] [PubMed] [Google Scholar]
- TURNER NA, PITOT HC. Dependence of the induction of cytochrome P450 by pheno-barbital in primary cultures of adult rat hepatocytes on the composition of the culture medium. Biochem. Pharmacol. 1989;38:2247–2251. doi: 10.1016/0006-2952(89)90461-9. [DOI] [PubMed] [Google Scholar]
- WAXMAN DJ, MORRISSEY JJ, NAIK S, JAU-REGUI HO. Phenobarbital induction of cytochrome P-450. Biochem. J. 1990;271:113–119. doi: 10.1042/bj2710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAXMAN DJ, AZAROFF L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem. J. 1992;281:577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUASA C, TOMITA Y, SHONO M, ISHIMURA K, ICHIHARA A. Importance of cell aggregation for expression of liver functions and regeneration demonstrated with primary cultured hepatocytes. J. Cell. Physiol. 1993;156:522–530. doi: 10.1002/jcp.1041560311. [DOI] [PubMed] [Google Scholar]