Abstract
Over the past decade, the microenvironment of gastrointestinal tumors has gained increasing attention because it is required for tumor initiation, progression, and metastasis. The tumor microenvironment has many components and has been recognized as one of the major “hallmarks” of epithelial cancers. Although therapeutic strategies for gastrointestinal cancer have previously focused on the epithelial cell compartment, there is increasing interest in reagents that alter the microenvironment, based on reported interactions among gastrointestinal epithelial, stromal, and immune cells during gastrointestinal carcinogenesis. We review the different cellular components of the gastrointestinal tumor microenvironment and their functions in carcinogenesis, and discuss how improving our understanding of the complex stromal network could lead to new therapeutic strategies.
Introduction
Digestive cancers are a significant health care burden worldwide1. In the US in 2008, it was estimated that more than 270,000 patients were diagnosed with, and more than 135,000 died from, cancers of the digestive system1. Most of the research and treatment strategies for patients with gastrointestinal (GI) cancers have focused on cell-autonomous mechanisms in the epithelial compartment. However, there is accumulating in vivo evidence that epithelial cells respond to their microenvironment, comprising mesenchymal cells and immune cells, the enteric nervous system, and matrix. The luminal content—particularly the microbiome—is another important feature of this complex network; its effects on immunity and tumorigenesis are only beginning to be understood2.
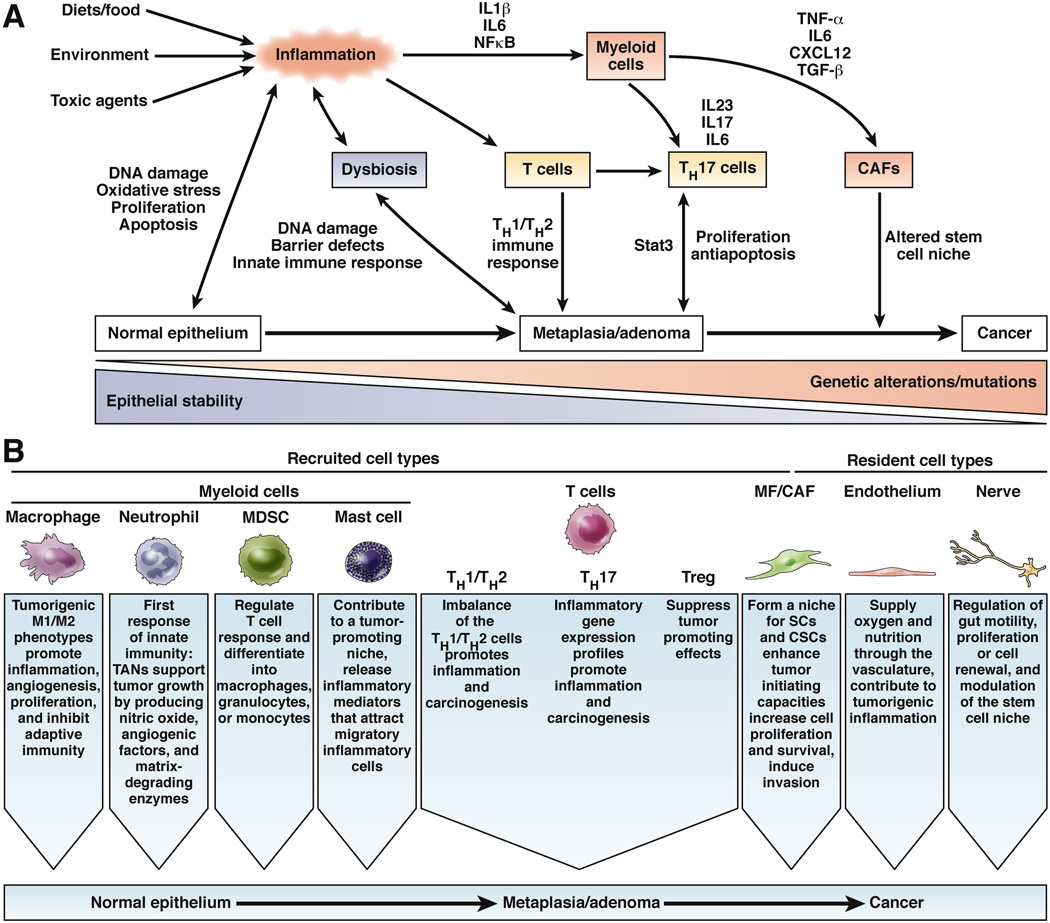
Many tumors of the digestive system arise under conditions of chronic inflammation (Fig. 1), including esophageal adenocarcinoma (from Barrett’s esophagus), gastric cancer (from Helicobacter pylori-associated gastritis), hepatocellular cancer (from viral hepatitis), colon cancer (from inflammatory bowel disease), and perhaps even pancreatic cancer (from chronic pancreatitis)3. Furthermore, eradication of infectious agents (such as H pylori) and treatment with anti-inflammatory therapies (such as prostaglandin synthase inhibitors or mesalazine) prevent cancer 4,5. Tumorigenesis under conditions of chronic inflammation is likely to be mediated by immune cells and the factors they produce, which alter the microenvironment to support tumor formation and progression6. Importantly, sporadic tumors, which do not develop as a direct consequence of chronic inflammation, are also characterized by an inflammatory microenvironment.
Figure 1.
a) Different cellular mechanisms of inflammation-induced carcinogenesis. External and host factors can create an inflammatory environment in the intestine that induces DNA damage and leads to genetic alterations over chronic injury. During carcinogenesis, different myeloid cells, T cells, and CAFs (see Figure 2) are recruited to sites of inflammation and create a microenvironment that promotes epithelial proliferation and prevents apoptosis, due to an imbalance of pro- and anti-tumorigenic factors. A network of multiple cytokines and chemokines generates a niche that enables the appearance and growth of tumor-initiating cells.
b) Types of cells that are recruited (myeloid cells, T cells, CAFs) or resident (CAFs, endothelial cells, nerves) in the tumor microenvironment, and their tumor-promoting or -inhibiting functions. The complex network of signaling among these cell types allows progression from normal to metaplastic to dysplastic gastrointestinal epithelium (carcinogenesis).
Not all tumor-associated inflammation supports tumor formation—the adaptive immune response has effective roles in immune surveillance and tumor suppression7. Nevertheless, it appears that chronic overt and/or low-level inflammation more commonly stimulates tumorigenesis rather than prevents it. Cytokines and growth factors can promote tumor cell proliferation, inhibit apoptosis, and suppress anti-tumor immunity; matrix metalloproteases (MMPs) and angiogenic factors contribute to invasion, metastasis, and tumor blood vessel formation. So it appears that an altered epithelium itself alters the surrounding stroma, and with it the mediators that ultimately create a tumorigenic microenvironment. To design better surveillance strategies and therapeutics, it is important to understand the function and contribution of diverse stromal cell types to the tumor microenvironment.
Cell Types in the Tumor Microenvironment
Although it is not clear whether inflammation precedes or is activated by tumorigenesis, the stroma of GI tumors is infiltrated by a range of bone marrow-derived cells that contribute to an expanding stroma (Fig. 2). The inflammatory environment contains cells of the innate immune system such as tumor-associated macrophages (TAMs), neutrophil granulocytes, myeloid-derived suppressor cells (MDSCs) or immature myeloid cells (iMCs), mast cells (MCs), and dendritic cells (DCs). It also contains cells of the adaptive immune system, such as T and B cells8 (Fig. 1). The complex network of immune cells in the tumor stroma affects almost every aspect of tumor biology10.
Figure 2.
The niche for tumor-initiating cells, or CSCs, is likely formed by different types of cells in the tumor microenvironment that produce tumorigenic cytokines and chemokines. These create a proliferative environment that allows tumor-initiating cells to escape apoptosis and proliferate despite acquired genetic alterations. It is likely that the epigenetic regulation of epithelial cells, and their signaling pathways, is altered by the niche cells. Moreover, the microbiota on the luminal side of the epithelium also contributes to the niche and is responsible for barrier defects and maintenance of chronic inflammation.
Innate and adaptive immune cells can promote tumorigenesis. Innate immune cells, such as TAMs, MCs, neutrophils, and MDSCs, promote tumor development, whereas subsets of adaptive immune cells, such as T-helper (Th)17 and Th2 cells, also contribute to inflammation and can promote tumorigenesis. The tumorigenic properties of these cells require their production of cytokines, growth factors, enzymes, and angiogenic mediators. Immune cells recruited to the tumors not only promote proliferation and invasion, but are also required for anti-tumor immune responses.
The density and location of T cells in colon tumors are associated with patient outcomes; they could be better prognostic factors than histology-based TNM classification 9,10. High densities of cytotoxic and memory T cells in the tumor center and at invasion fronts are associated with reduced recurrence of disease and are positive prognostic factors 9 for patients with colon cancer and even those with liver metastases. Moreover, the composition of the immune infiltrate in metastases has been associated with patients’ response to therapy 11.
These observations have led to the idea of an ‘immune score’ that quantifies the intra-tumor location and density of cytotoxic and memory T cells. Because of its powerful prognostic value, it has been proposed that the immune-score could be incorporated into the routine diagnostic and prognostic assessment of patients with tumors12. Furthermore, immune-based therapies might be developed that disrupt tumor-induced immune suppression and allow induction of anti-tumor immune responses13. So far, the precise mechanism of differential accumulation of T-cell subsets is only partially understood. However, elucidating the regulation of the adaptive T-cell response would have great therapeutic potential.
TAMs are a heterogeneous and plastic population of immune cells; large numbers of these cells in tumors indicate a poor prognosis14. Accordingly, depletion of macrophages from ApcΔ716 mice resulted reduced intestinal polyposis15. Analogous to the Th1 and Th2 classification of T cells, macrophages can be categorized as classically activated M1 or alternatively activated M2 macrophages in vitro. M1 macrophages are induced by interferon-γ and microbial products, produce high levels of inflammatory cytokines, express MHC II molecules, and have cytotoxic activity8. M2 macrophages express high levels of the immunosuppressive cytokine interleukin (IL)10, and have reduced antigen presentation and tumoricidal activities but increased tissue remodeling capacities than M1 macrophages. Macrophages accumulate in the stroma of solid tumors, are considered to have a tumorigenic M2 phenotype, and are involved in almost every aspect of tumor biology16. However, tumor infiltration by M1 macrophages has been correlated with better outcomes of patients with colorectal cancer (CRC), in a stage-dependent manner, but is also accompanied by a concomitant increase in M2 macrophages17. However, TAMS have partially overlapping cytokine profiles in GI tumors, so it is not possible to distinguish between M1 and M2 macrophages in vivo. The classification of M1s as anti-tumorigenic and M2s as tumorigenic is therefore an oversimplification of the real phenotypic diversity of the macrophage population in vivo.
MDSCs are CD11b+Gr-1+ (Ly6G–Ly6Chigh), and are a heterogeneous population of activated iMCs that is expanded in tumor-bearing mice and cancer patients18. iMCs can be detected in the bone marrow and occasionally in the spleen of cancer-free animals19, but accumulate in the spleen in animals with chronic inflammation and in response to carcinogens. In the setting of carcinogenesis, iMCs acquire suppressor properties and become MDSCs20. In particular, in models of inflammation-induced gastric and esophageal cancer, MDSCs appear to mediate carcinogenesis21,22. Within the tumor microenvironment, MDSCs suppress anti-tumor immunity by disrupting T-cell functions and promoting differentiation of the immunosuppressive regulatory T (Treg) cells23.
Beside their well-recognized role in suppressing cytotoxic T cells, MDSCs can also directly promote tumor progression and metastasis. Cis-Apc/Smad4 mice develop invasive colon tumors that are characterized by strong myeloid cell infiltration specifically at the invasion fronts. iMCs recruited to the cis-Apc/Smad4 tumors produce MMP2 and MMP9, which can degrade the extracellular matrix and collagen IV. These enzymes promote its invasion by tumor cells24. Mice with conditional knockout of p120-catenin (p120ctn) develop esophageal squamous cancer, and cytokines upregulated in the dysplastic epithelium promote mobilization and recruitment of MDSCs—a microenvironment that promotes tumor formation25.
Numbers of MCs are increased in intestinal polyps and colorectal tumors from mice and patients, and are likely to contribute to tumor growth26,27. Depletion of MCs from ApcΔ468 mice by bone marrow transplantation reduced the number of polyps that formed and the densities of their blood vessels26. So far, however, functional analyses of MCs have been limited to ablation of this lineage, without more direct evidence such as MC-specific genetic pathways.
In addition to innate immune cells, T-cell subsets can also promote development of CRC. Patients with tumors infiltrated by a high number of T cells that produce IL17 (Th17 cells) have a poor prognosis 28. These cells are derived from naïve T cells is response to transforming growth factor-β (TGFB)1, IL6 and IL23, which might all be present at high concentrations in the tumor microenvironment, and induce T-cell production of IL1729. IL17 activates expression of genes that mediate the inflammatory response, which promotes tumor growth and angiogenesis. Inhibiting the function of Th17 cells or blocking IL17 in ApcMin/+ mice (or mice with intestinal epithelial cell [IEC]-restricted deletion of Apc) reduces intestinal tumorigenesis, probably by preventing establishment of an inflammatory microenvironment30–32,39.
Th17 cells produce not only IL17 but also IL22, a member of the IL10 family that activates the transcription factor STAT333. Similar to IL17, production of IL22 is induced in response to bacterial products and has an important role in intestinal homeostasis34. Interestingly, IL22, which is also secreted by innate lymphoid cells, has a dual function in colorectal tumorigenesis. In mice with colitis-associated cancer (CAC), IL22 deficiency accelerated inflammation, probably by impairing colonic repair, but increased tumor cell proliferation after the recovery phase35. Similarly, IL4, produced mainly by Th2 cells, contributes to tumor growth by increasing proliferation of colon tumor cells. Mice that that lack the IL4Rα chain of the IL4 and IL13 receptor complexes develop fewer and smaller tumors36. Moreover IL4 and IL13 promote differentiation of tumor-promoting macrophages, which contribute to tumor formation and progression37. In contrast, tumor infiltration by cytotoxic T cells, memory T cells, and Th1 cells is associated with longer survival times of patients with CRC28.
Treg cells are required for intestinal mucosal immune homeostasis. They suppress adaptive, and to some extent innate, immunity, mainly by producing the anti-inflammatory cytokine IL10. Detection of Treg cells is associated with poor prognosis for patients with some types of cancer, because these cells suppress anti-tumor immunity and allow the tumor to evade the immune response. Surprisingly, for patients with CRC, the presence of Treg cells in tumors is a good prognostic factor28, 38. Tumor development in the colon is accompanied by the translocation of bacteria, leading to induction of a strong anti-microbial immune response that involves the activation of Th17 cells. Treg cells in the colonic mucosa can inhibit the Th17 cell responses and therefore reduce the tumorigenic effects of inflammation.35 Adoptive transfer of Treg cells suppresses sporadic and inflammation-induced intestinal tumorigenesis, probably because Treg cells can inhibit the inflammatory response39, 40. On the other hand, in the presence of specific cytokines, Treg cells can differentiate Th17 cells. Treg cells present in the polyps of ApcΔ468 mice do not produce IL10 but rather IL17 and are not able to suppress CD4+ T cells40. Moreover, IL10 activates STAT3, which upregulates several genes involved in proliferation and survival41. Given the different prognostic value of Treg cells for patients with different types of cancers, and their ability to simultaneously suppress tumor-promoting inflammation and anti-tumor immunity, it is important to develop a better understanding of the roles of Treg cells in CRC pathogenesis.
The immune response determines the progression of gastric atrophy, metaplasia, and dysplasia, because Th1 cells produce specific cytokines in response to infection with H pylori. These include IL1, tumor necrosis factor (TNF)-α, and IL10, which cause chronic inflammation and increase the risk for cancer progression42. However, production of individual cytokines could affect predisposition to gastric cancer. During infection with helicobacter, the Th1 cytokine interferon-γ can promote or inhibit inflammation-induced gastric cancer, indicating that a more specific immune response mediates cancer surveillance or promotion. Although previous studies have reported that interferon-γ might promote the development of gastric preneoplasia53, overexpression of low levels of interferon-γ in the stomach was recently shown to prevent IL1B- and Helicobacter felis-dependent carcinogenesis43. In addition, interferon-γ counteracted the development of Th17 cells. So, different composition of the same cells and cytokines in the tumor microenvironment can promote or inhibit carcinogenesis.
Apart from cancer cells and infiltrating immune cells, the stromal environment of tumors includes a mixture of mesenchymal cells, comprising mostly cancer-associated fibroblasts (CAFs). CAFs closely resemble normal myofibroblasts in the GI mucosa. However, CAFs behave differently from myofibroblasts and fibroblasts of normal tissues; based on clinical and preclinical studies, these stromal cells contribute to the development and metastasis of GI cancers44, 45. Patients with tumors surrounded by desmoplasia, which contains many CAFs, have a worse prognosis than patients with tumors that do not contain desmoplasia46. A number of studies have tried to determine the origin of CAFs, investigating resident fibroblasts49, smooth muscle cells, endothelial cells (via endothelial-mesenchymal transition [EndMT]), epithelial cells (via epithelial-mesenchymal transition [EMT]), fibrocytes, and recruited bone marrow-derived cells such as mesenchymal stem cells (MSCs)47, 48. MSCs are a minor population of multi-potent, non-hematopoietic cells in the bone marrow that are actively recruited to the sites of inflammation and injury49. The differentiation of MSCs into CAFs or myofibroblasts has been analyzed; a significant portion of CAFs were found to originate from the bone marrow-derived MSCs,44 and contribute to the normal bone marrow MSC niche and to MSC self-renewal44. These bone marrow niche cells are expanded in a TGFB-dependent manner and recruited through CXCR4 and CXCL12 signaling, together with MSC, to incipient tumors where they appear as CAFs.
CAFs contribute to tumorigenesis by promoting cancer cell proliferation and invasion. CAFs express a number of tumorigenic factors, including IL11 (which activates STAT3), hepatocyte growth factor (HGF) and fibroblast growth factor (FGF), which activate mitogen-activated protein kinase (MAPK) and phosphatidylinositide 3-kinase (PI3K) 50, 51. Under normal conditions, HGF release is suppressed by the serine-threonine kinase tumor progression locus-2 (Tpl2). Deletion of Tpl2 specifically from intestinal myofibroblasts leads to increased production of stromal HGF, resulting in phosphorylation of c-Met and activation of AKT in tumor cells. Tpl2-deficient mice given azoxymethane and dextran sodium sulphate to induce CAC develop more and larger tumors than wild-type mice 52. Increased production of HGF by the tumor microenvironment reduces the sensitivity of melanoma and colon cancer cells to Raf inhibitors by activating c-Met receptors on the tumor cells, supporting the concept that the tumor microenvironment is an important determinant of response to therapy53, 54. Tumor-associated fibroblasts contribute to colorectal tumorigenesis not only by direct signaling to cancer cells, but also indirectly, by recruiting and polarizing cells of the adaptive and innate immune systems to tumor-promoting phenotypes55.
The tumor vasculature is another important component of GI tumor microenvironment. Tumors must form new blood vessels to maintain a supply of oxygen and nutrients needed for growth. To achieve sufficient perfusion of the neoplastic tissue, tumor cells and stromal cells produce several factors that induce the formation of new blood vessels56. The tumor vasculature is involved in many different processes that promote tumor growth. Activated endothelial cells of the tumor vasculature can recruit inflammatory cells and endothelial progenitors, contributing to tumorigenic inflammation and neoangiogenesis. Furthermore, tumor endothelial cells secrete many paracrine factors that directly foster tumor cell proliferation and maintain cancer stem cells57.
The tumor vasculature is also required for metastasis. Newly formed tumor vessels are usually abnormal in structure, which enhances the intravasation of cancer cells58. Endothelial cells also produce chemokines that recruit myeloid cells to metastatic sites, which contribute to a hostile microenvironment at the metastases59. CCR2 signaling in endothelial cells in response to cancer cell-derived CCL2 alters vascular permeability, promoting extravasation of cancer cells into new tissues and metastasis60.
Nerves are an important component of normal GI physiology and could also be involved in malignancies. In addition to regulating GI motility, neural input regulates proliferation in the GI epithelium and there is evidence that the nervous system could regulate stem cell renewal61. The nervous system was shown to regulate interactions between hematopoietic stem cells and their niche66, and there is evidence that intestinal epithelial stem and progenitor cells are controlled by mucosal afferent nerves61. Preneoplastic and neoplastic tissues induce neurogenesis 62. However, the exact role and function of nerves in normal GI homeostasis and during carcinogenesis requires further investigation.
Signaling Pathways Activated by Infiltrating Cells
The types and quantities of immune cells recruited to GI sites, along with the cytokines and growth factors they produce, determine whether the tissue environment permits tumor formation (see Fig. 2, Table 1). These factors act in paracrine, autocrine, and juxtacrine manners to activate signaling pathways that converge at ‘hubs’ in cancer and stromal cells. Several of the central signaling pathways have been extensively studied and their hubs have been determined. This comprehensive knowledge led to the idea of targeting therapies to the hubs, to block multiple pathways required for GI tumor growth and progression. The same signaling pathways that are required for normal biological processes and maintenance of GI homeostasis are also involved in GI carcinogenesis. These include the Wnt, Notch, TGFB, and Hedgehog signaling pathways, which are required for the self renewal of the intestine and stem cell maintenance; the transcription factors NFκB and STAT3, which are required for tissue repair and immune homeostasis; toll-like receptor (TLR) signaling, which regulates tolerance vs an immunity against the intestinal microbiota; and growth factor signaling pathways, such those that include MAPK or Akt/PKB, which provide mitogenic and survival signals.
Table 1.
Factors that define the tumor microenvironment (related to Fig. 3)
| Chemokines | Tumorigenic | Antitumorigenic |
|---|---|---|
| Ccl9, Ccl15 | Recruitment of iMC 83,128 | Modulation of T cell responses against leukemic cells (CML) 129 |
| Cxcl1, Cxcl2, Cxcl5 | Recruitment of granulocytes 130 | |
| Ccl2 | Recruitment of macrophages 131 endothelial cell activation and increased endothelial permeability 60 | |
| Cxcl12 | Recruitment of MSCs/CAFs 132 | |
| Cx3cl1, Cxcl9, Cxcl10 | Recruitment of memory and cytotoxic T cells 133 | |
| Ccl5 | Recruitment of Th17 cells 55 | Recruitment of cytotoxic T cells 134 |
| Ccl22 | Recruitment of regulatory T cells 134 | Recruitment of regulatory T cells 134 |
| Ccl25 | Suppression of invasion and metastasis 135 | |
| Cytokines | ||
| IL1β | NFκB activation, modulation of the Wnt pathway 136 | |
| IL4 | Tumor cell proliferation 36; alternative activation of TAMs 37 | |
| IL6 | STAT3 activation, proliferation and survival of colonocytes 70; induction of Th17 differentiation | Enhancement of CD8+ T cell homing by modifying the tumor vasculature 137 |
| IL8 | MDSC recruitment, myofibroblast expansion, tumor angiogenesis 138,139 | |
| IL10 | STAT3 activation, immmunosuppression 41, 74 | Limiting Th17 responses 140 |
| IL17 | Inducing expression of inflammatory genes | Mediation of antitumor immunity 141 |
| IL18 | Downregulation of IL22BP and therefore increasing tumor cell proliferation 35 | Regulation of intestinal microbiota 142; downregulation of IL22BP and therefore reducing inflammation 35 induction of interferon-γ 88 |
| IL23 | Induction of Th17 cells, STAT3 activation 31, 32 | |
| IL22 | STAT3 activation and tumor cell proliferation 35 | Tissue repair upon epithelial injury resulting in the reduction of inflammation, protective in CAC 35 |
| TNFα | NFκB activation, production of proinflammatory cytokines 143, 144 induction of EMT 68; activation of Notch signaling 145 | |
| TGFB | Induction of EMT 84; immunosuppression 81; myofibroblast differentiation and expansion of CAFs 44; polarization of N2 TANs 146 and Th17 differentiation | Negative regulation of HGF production 82; suppression of chemokine production by epithelial cells 83 and cytokine production by T cells 79 |
| Growth factors | ||
| HGF/MET | Modulation of Wnt pathway in CSCs | Promotion of cell death induced by |
| 147; induction of an migratory phenotype 148 | TRIAL (medulloblastoma) 149 | |
| VEGF/VEGFR | Tumor angiogenesis; recruitment of mesenchymal cells that augment the proliferation of colonocytes 150; tumor cell proliferation 151 | |
| PDGF/PDGFR | Tumor angiogenesis 152; enhancement of lymphangiogenesis and lymphatic metastasis 153 | Increased pericyte coverage of the tumor vessels, decreased tumor growth 154 |
| EGF/EGFR | Control of proliferation, survival 155 and invasion 156 | Control of epithelial regeneration and inflammation, protective in CAC 157 |
| Bacterial products | ||
| Colibactin (E coli) | DNA double strand breaks, mutations, chromosomal aberrations 158 | |
| BFT (B fragilis) | Cleavage of epithelial E cadherin resulting in increased epithelial barrier permeability and wnt activation 159 | |
| Superoxid (E feacalis) | DNA double strand breaks 160 | |
| Butyrate (F prausnitzii,Roseburia spp.) | Induction of apoptosis in colon cancer cell lines 161 | |
| ECM components and ECM modifying enzymes | ||
| MMP2, MMP9 | Degradation of the basal membrane enhancing invasion 83, cleavage of ECM bound VEGF | Protective in CAC by modulating Notch activation (MMP9) 162 |
| MMP7 | Tumor initiation and growth163 | |
| MMP10 | Resolution of colonic epithelial damage 23 | |
| TNC | Tumor cell invasion148 | |
| LOX | Induction of an more proliferative and invasive phenotype through increasing ECM stiffness 164 tumor angiogenesis 165 | |
NFκB regulates genes that control many physiologic and cellular processes, such as survival, apoptosis, immunity, and inflammation, but NFκB also promotes tumorigenesis63 via distinct mechanisms. In epithelial tissues, inflammation increases survival of initiated epithelial cells by activating NFκB, which leads to transcription of genes that promote cell survival and prevent apoptosis64,65. Interestingly, constitutive activation of NFκB in epithelial cells only leads to destructive forms of inflammation when it is accompanied by MAPK activation, induced by microbial stimuli66. Furthermore, persistent activation of NFκB in IECs upregulates inducible nitric oxide synthase, which can lead to DNA damage and loss of heterozygosity to promote transformation67. NFκB contributes to inflammation-induced EMT by inducing the COP9 signalosome, which blocks the ubiquitination and subsequent degradation of the transcription factor Snail68. In the myeloid cells, NFκB controls the production of inflammatory cytokines such as IL6, IL11, and TNFα. These cytokines in turn activate proliferation and survival pathways in epithelial cells, via NFκB and STAT364, 65. Blocking the activity of NFκB in myeloid cells of mice reduces the number and size of colitis-associated tumors 64
STAT3 regulates genes involved in the process of wound healing, and like NFκB, controls survival and proliferation of epithelial cells. Inhibition of STAT3 in epithelial tissues prevents azoxymethane-induced formation of adenomas in mice, but accelerates inflammation, due to reduced expression of genes that promote cell survival and prevent cell death69, 70. Conversely, mice that express a mutant form of the gp130 chain (the signal transducing subunit of the IL6-type cytokine receptor), which increases IL11-induced activation of STAT3, spontaneously develop gastric polyps71, 72. Similarly, in mice with CAC, mutations in gp130 increase STAT3 activation by IL6 and IL11, which protects against epithelial damage and inflammation from dextran sodium sulphate but accelerates tumorigenesis69. In gastric cancer cells, STAT3 activates expression of TLR2; inhibitors of TLR2 prevent gastric tumorigenesis, but not inflammation, indicating its role in the oncogenic functions of STAT373.
STAT3 not only affects proliferation and survival of epithelial cells, but also controls expression of inflammatory cytokines and increases production of immunosuppressive factors that can inhibit DC maturation by activating STAT3 in progenitor cells74. STAT3 activation in immune cells that infiltrate tumors promotes development of the tumor. In TAMs, STAT3 activates transcription of IL23, but in DCs it inhibits NFκB-dependent expression of the anti-tumor cytokine IL1275. IL23 produced by macrophages subsequently activates STAT3 in IL23R–expressing regulatory T cells, leading to upregulation of the immunosuppressive cytokine IL10, which also activates STAT3 and can contribute to immune evasion. IL23 and STAT3 have each been implicated in the generation and expansion of the tumorigenic Th17 cells and production of IL1731, 32. The effects and regulation of STAT3 and NFκB are connected at many levels; they regulate each other in paracrine and cell-autonomous manners. Unphosphorylated STAT3, together with NFκB, promotes transcription of NFκB-regulated genes by mediating acetylation of RelA, which retains NFκB in the nucleus 76, 77. Interestingly, STAT3 activation also interferes with TGFB signaling, by inducing transcription of the TGFB signaling antagonist Smad7 71.
TGFB signaling has dual roles in tumor development—it has pro- and anti-carcinogenic activities 78. TGFB signaling in the T cells suppresses inflammation and therefore can inhibit tumor formation. Transgenic mice with Smad4 deficiency in the T cells develop spontaneous GI tumors, produce increased levels of inflammatory cytokines, and have higher concentrations of Th17 cells79, 80. On the other hand, TGFB-mediated immune suppression can lead to immune evasion and reduce anti-tumor immunity. Mice with a transgene that overexpresses Smad7 in T cells develop severe colitis, but have increased anti-tumor immunity and develop fewer tumors81.
TGFB signaling is also important in other stromal cells, such as fibroblasts. The loss of responsiveness of fibroblasts to TGFB leads to development of invasive squamous cell carcinoma of the forestomach, associated with increased production of HGF and epithelial cell proliferation. These findings indicate that under normal conditions, HGF production is negatively regulated by TGFB in fibroblasts82. Beside the important role of TGFB signaling in the immune cells, TGFB signaling by epithelial cells regulates tumor progression. Loss of epithelial TGFB signaling results in increased chemokine production and recruitment of MMP-producing iMCs to the tumor margins, where they promote invasion83. Interestingly, however, platelet-derived TGFB, which synergistically activates TGFB signaling to Smad and NFκB in cancer cells, promotes metastasis by inducing an invasive mesenchymal-like phenotype84.
Consistent with the observations from mouse models that alterations in TGFB activity affect tumor development, alterations in TGFB signaling are often found in human colorectal tumors. Many GI tumors, including colorectal tumors, have decreased expression or loss of the TGFB receptor (TGFBR). Moreover, mutations in TGFBRII or in Smad4, and a lesser extent in Smad2, are present in a considerable proportion of human colonic tumors78.
Tumor development is associated with tissue rupture and translocation of bacteria, so signaling pathways that are activated in response to microbial stimuli might be involved in GI tumorigenesis. In the tumor microenvironment, bacteria and their products are sensed via pattern recognition receptors (PRRs), such as TLRs and Nod-like receptors (TLRs and NLRs). The ability of the human body to discriminate between useful commensals and pathogenic bacteria largely depends on the function of the PRRs. TLRs are expressed in almost every cell in the intestine and signal via MAPK and NFκB, with the help of several adaptor proteins, such as MyD88. Activation of these pathways leads to the production of chemokines, inflammatory cytokines, and anti-microbial peptides85.
Preventing TLR signaling by deleting the adaptor protein MyD88 reduces tumor growth and tumor number in ApcMin+/− mice86. However, MyD88-deficient mice develop severe inflammation and have accelerated growth of colitis induced tumors 87. These seemingly contradictory data indicate that MyD88 signaling has distinct roles in inflammation-induced intestinal tumorigenesis and in sporadic or familial cancers that have no obvious link to inflammation. During inflammation, MyD88 signaling is likely to be indispensable for efficient tissue repair; the lack of Myd88 signaling results in accelerated inflammation and increased tumor development. In sporadic or familial CRC, however, MyD88 might contribute to tumor growth by inducing an inappropriate tissue repair response, leading to tumor cell proliferation and increased tumor growth. Interestingly, mice with disruptions in IL18 signaling, which is mediated by the inflammasome, are similar to Myd88-deficient mice in their susceptibility to CAC87. In addition, deficiencies in components of the inflammasome, such as Nlrp3, Nlrp6, caspase-1, and ASC, reduce levels of IL18 and accelerate inflammation-associated tumorigenesis, leading to a phenotype similar to that observed in IL18-deficient mice88. Moreover, it was recently shown that IL18 is required for downregulation of IL22 binding protein (IL22BP)35. IL22BP is a soluble, high-affinity receptor for IL22 that limits the availability of IL22 under steady-state conditions89. However, upon epithelial damage, IL22BP is downregulated35, allowing the binding of IL22 to its transmembrane receptor and induction of the tissue repair process. So lack of IL22, via reduced levels of IL18 and dysregulation of inflammasome signaling, results in more-pronounced inflammation and subsequently to accelerated tumor development.
The Tumor Microenvironment is a Niche for GI Tumor Stem Cells
The homeostasis of the untransformed stem cell niche depends on its microenvironment (Fig. 3). Normal intestinal stem cells (ISC) reside at the bottom of the crypt and depend on adhesive and soluble stroma–epithelial interactions that create a niche90. Genetic lineage-tracing studies in mice identified markers of 2 different types of ISC. Crypt base columnar cells (CBC) are fast-cycling stem cells that express Lgr5 and CD133 (Prom-1)91, 92. Slower cycling cells, which are usually found at the +4 position of the crypt, are characterized by high expression of Bmi1 and Tert93, 94. Although these 2 types of cells are functionally interconnected95, their exact hierarchical relationship has not been determined.
Figure 3.
Potential mechanisms of tumor initiation. A) the normal intestinal stem cell niche consists of epithelial stem cells that are surrounded by mesenchymal cells and matrix. A tightly regulated gradient of different signaling molecules determines a stem cell zone, a proliferative zone, and a differentiation zone. The epithelial cells are replaced regularly through renewal of stem and progenitor cells. Three different mechanisms of tumor initiation have been described in the gut: B) Tumor initiation through expansion of stem and progenitor cells at the base of the crypts in the stem cell zone. Deregulation of the signaling network induced by chronic inflammation and the recruitment of different mesenchymal and immune cells allows an expansion of the proliferative zone and provides a niche for tumor initiating cells that derive from tissue stem cells. C) Dedifferentiation of differentiated epithelial cells at the top of the crypts/villi is induced by chronic inflammation, NFκB up regulation and genetic alterations of β-catenin or KRas. Normal enterocytes are reprogrammed to tissue stem cells with the ability to proliferate and escape apoptosis despite new genetic mutations. D) In the cardia and esophagus, migration of stem cells towards the inflammatory niche increases proliferation of stem cells that acquire genetic alteration and escape apoptosis in the inflammatory niche the cells migrate to.
Regardless of their location (CBC or +4 position) or their function, ISCs depend on signals from stem cell niche cells such as pericryptal myofibroblasts and neighboring differentiated epithelia96. Important signaling pathways required for ISC maintenance and proliferation comprise the Wnt, Notch, Bone morphogenetic protein, and Hedgehog pathways90. Several markers of CRC stem cells (CSCs) have been proposed, such as CD24, CD44, CD133, and CD16697–99. However, a robust marker of CSCs has not been identified. For example, CD133 is ubiquitously expressed in differentiated murine and human colonic epithelium100 and therefore not specific for either multi-potent stem cells or progenitor cells. Interestingly, doublecortin-like kinase 1 (Dclk1) could be a valid marker of CSCs—it is detected in potential cancer stem cells but not normal stem cells101.
Although a specific niche for colon CSCs has not been defined, a microenvironment that maintains tumor-initiating cells is likely to exist. Wnt signaling is required by tumor initiating cells and recently ISC (Lgr5+)-specific negative feedback regulators have been identified102 (Fig. 3). Moreover, secretion of HGF by stromal fibroblasts increases Wnt signaling in tumor-initiating cells,103 and a higher proportion of tumor cells within myofibroblast regions (compared with other regions) have nuclear β-catenin104. Importantly, TNFα-dependent activation of NFκB can increase β-catenin signaling and transcription of the stem cell transcriptome,105 resulting in expansion of Lgr5+ stem cells106. More importantly, in post-mitotic epithelial cells, the interacting effects of NFκB and β-catenin activation induce dedifferentiation, or reprogramming of non-stem cells into tumor-initiating stem cells (Fig. 3).
The capacity to initiate tumorigenesis is therefore no longer an exclusive property of normal stem cells. Instead, an inflammatory microenvironment (such as in the intestines of patients with inflammatory bowel diseases) can lead to the conversion of normal, differentiated epithelia into CSCs. However, this concept also applies to established tumors, and to therapies that aim to eradicate CSCs by depleting, for example, Dclk1 + cells101. The inflammatory microenvironment of advanced tumors could allow the dedifferentiation of tumor cells into CSCs, to replenish eliminated stem cells.
Additional evidence for the interaction between the inflammatory microenvironment and CSC comes from a transgenic mouse model of Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC)21. Bile acid-induced IL1β- and IL6-dependent inflammation activated formation of gastric cardia stem cells, which migrated into the distal esophagus and gave rise to columnar-like metaplasia and dysplasia. These findings indicate that BE and EAC can arise from gastric progenitors21 (Fig. 3). So, in addition to upregulating reactive oxygen and nitrogen species that can lead to DNA damage and mutations in CSCs, an inflammatory microenvironment is also required for formation and maintenance of a CSC niche. It will be a challenge to further define and characterize this specific niche, but reagents designed to target it could have great therapeutic potential.
Microbiota Contribute to the Tumor Microenvironment
The composition of the human microbiome has been associated with the incidence of a number of human diseases, including cancer107, and is therefore another important compartment of the tumor microenvironment (Fig. 4). A number of individual bacterial species can initiate and promote intestinal tumorigenesis in animal models. However, it is not known whether one particular species is primarily responsible for inducing GI cancers, or whether a small community of different species contributes to transformation.
Figure 4.
Dysbiosis of the microbiota in the intestine can be induced by 1) hereditary or acquired epithelial mutations that cause barrier defects and impair epithelial cell function, 2) external factors such as dietary changes, toxic agents, environmental conditions that all influence the microbiota and select for a certain type of bacteria, and 3) chronic inflammation, which induces barrier defects and inflammatory responses that select for a specific microbiome.
H pylori infection is the best-described bacterial risk factor for gastric cancer. However, dysplasia and cancer tend to develop when H pylori colonization has either been greatly reduced or, in some cases, disappeared from the stomach altogether. Gastric cancer almost always occurs in the setting of prolonged gastric atrophy and hypochlorhydria, a condition that predisposes to enteric bacterial overgrowth. Although antibiotic therapy to eradicate H pylori delays and inhibits development of gastric cancer in mice108, antibiotics eradicate not only H pylori but also other microorganisms that colonize the atrophic, hypochlorhydric stomach. Monoassociation of otherwise germ-free INS-GAS mice with H pylori delayed development of gastric cancer, compared to H pylori-infected INS-GAS mice colonized with conventional flora109. H pylori could therefore be the initial, or the most prevalent, microbial factor responsible for gastric cancer development.
It is important to characterize the bacteria associated with CRC, which might help to identify individuals at increased risk of CRC and develop new therapeutic approaches. However, more-sensitive techniques and bigger cohorts will likely be required to precisely identify the species specifically associated with CRC. Furthermore, because the composition of the CRC-associated microbiota might change as the tumor progresses, tumor-stage specific definition of CRC-associated bacteria should be also considered. A recent analysis of frequencies of molecular features (CpG island methylator phenotype [CIMP-high], microsatellite instability [MSI-high], and BRAF mutation) that increase linearly from the rectum to the ascending colon according to a continuum model110, indicate a continuous change of the microbiota in from proximal to distal colon.
With the advent of high-throughput sequencing techniques, the possibility of comprehensively mapping dysbiosis associated with human CRC has become feasible. Studies have revealed considerable changes in the composition of the microbiota in patients with CRC, and have shown that different species of bacterial tend to colonize either the tumor tissue or the adjacent healthy mucosa. One of the most striking findings was the association of Fusobacterium spp. with CRC tissue111. Interestingly, opportunistic pathogens belonging to the family Enterobacteriaceae, such as Shigella, Salmonella, Cronobacter, and Citrobacter are more abundant in the adjacent normal tissue than in tumor tissue111. Consistent with these findings, the composition of intestinal microbiota of CRC patients is enriched in pathogenic bacteria, compared to healthy controls. Conversely, Roseburia and other butyrate-producing members of the Lachnospiraceae family are less abundant in the gut microbiome of CRC patients112.
Determining exactly how these bacteria initiate or influence tumorigenesis remains an important objective for future research (Fig. 5). One tumor-promoting property of certain colonic bacteria involves the release of bacterial toxins that induce colitis and formation of colonic tumors by a Th17-cell-mediated response in mice with multiple intestinal neoplasia113. An alternative mechanism comprises the propagation of specific genotoxic bacterial strains114. In Il10-deficient mice, inflammation creates an environment that supports carcinogenesis via its effects on the mice and their microbiota; it promotes expansion of E coli strains that contain the polyketide synthase (pks) genomic island, which encodes the bacterial genotoxin colibactin115. These E coli adhere to the colonic mucosa, resulting in increased delivery of pks products, which leads to epithelial DNA damage116.
Figure 5.
The intestinal microbiota contributes to tumorigenesis. In the normal intestine, interactions between bacteria and the epithelial cells, protected by a mucus layer and functioning tight junctions, enable necessary homeostasis. During cancer initiation, dysbiosis and/or a barrier defect contribute to a microenvironment that allows for DNA damage, genetic alterations, and a tumor-promoting signaling network. Under conditions of dysbiosis, some intestinal bacteria release toxins that affect the barrier function (reduced mucus layer, defect tight junctions) of the epithelial cells and might also induce genetic alterations. This induces inflammation and enables migration of bacteria in the stroma, leading to recruitment of more inflammatory cells that finally form a tumorigenic environment that leads to carcinogenesis. The barrier defect can also be caused by inherited or acquired genetic alterations that impair epithelial function and cause dysbiosis. The 2 pathways to an epithelial barrier defect likely account for an inflammatory condition that leads to the formation of a tumor microenvironment, and could function independently or synergistically.
Importantly, in addition to direct genotoxic effects on the intestinal epithelium, an intestinal barrier defect can lead to translocation of non-pathogenic bacteria, which affects intestinal immune system homeostasis by shifting the balance towards tumorigenic immune responses. Bacterial products are sensed via PRRs such as TLRs and NLRs. Activation of these pathways leads to the production of chemokines, inflammatory cytokines, and anti-microbial peptides85. The barrier defect is induced by Wnt activation, causing aberrant expression and mislocalization of tight junction proteins, including occludin and various claudins in epithelial cells, as well as concurrent downregulation of protective mucins. During early stages of tumorigenesis, independent of the composition of the microbiome, bacterial invasion induces a complex inflammatory reaction that involves upregulation of IL17 and IL23, which promote tumor development32. During later stages, loss of p53 function in mutagenized epithelia aggravates this barrier defect further and amplifies the generation of an inflammatory microenvironment that involves activation of NFκB and STAT3 in tumor epithelia and myeloid cells. Collective activation of these signaling pathways in epithelial and myeloid cells supports development of the EMT in p53-deficient tumor cells, which is required for invasion and metastasis117.
Therapeutic Outlook
There is accumulating evidence for the importance of the tumor microenvironment, with its complex network of cells, cytokines, and chemokines, in tumor initiation, formation, growth, and metastasis. The distinct cell types and signaling pathways have important influences on stem cell biology, malignant transformation, and the microbiome. It seems essential to gain a better understanding of the interaction between stromal cells and epithelial cells, and the specific signals of this network that promote or inhibit tumorigenesis, to develop new therapeutic options for GI cancers. The overarching goal should be to identify factors (cytokines, chemokines, bacteria) that help activate an inflammatory and tumor-promoting microenvironment; disrupting these could make cancer cells more susceptible to direct cytotoxic therapies. Although a number of existing treatments already target broadly some stromal components, major advances in stromal-specific therapies are still required.
Considering the strong association of GI cancers with inflammatory conditions, and the remarkable influence of inflammation on tumor development, it is plausible that aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) might be used in prevention and treatment4,118. Aspirin use after cancer diagnosis can affect patient survival, depending on the presence or absence of PIK3CA mutations in tumor cells119. Considering a gradual transition of intestinal biogeography, as proposed in the colorectal continuum hypothesis110, aspirin might have different effects on tumors that develop at specific sites or have different molecular features. Nevertheless, the finding that aspirin-induced alteration of the inflammatory response can determine patients’ prognoses, along with specific cancer-cell mutations, indicates the promise of reagents designed to target the tumor microenvironment.
Anti-angiogenic therapies such as bevacizumab (a monoclonal antibody against vascular endothelial growth factor [VEGF]) are well-established class of anti-stromal therapies, but a true therapeutic benefit is lacking. Antibodies against VEGF and endothelial growth factor have not been translated to the adjuvant therapy setting120, although a survival benefit was previously observed with bevacizumab in metastatic CRC121, leading to approval by the FDA in this setting. Anti-angiogenic agents seems to slow tumor growth and progression but not prevent formation of new metastases; this might result from the complicated relationships among blood flow, hypoxia, and tumor growth.
Treating or preventing metastasis or cancer recurrence likely requires targeting of other factors within the tumor microenvironment or stem cell niche. For example, the Dnmt1 inhibitor decitabine and NSAIDs are thought to have therapeutic effects on the stroma. Folate was found to be chemopreventive agent in a mouse model of inflammatory gastric cancer, via its effects on CAF DNA methylation and biology122, as well as in human CRC. However, epigenetic modifiers also affect epithelial cells, so it is difficult to attribute their cancer prevention effects entirely to the stroma; their potential for side effects limit their use in the clinic.
Several therapeutic agents that are already in clinical use can increase anti-tumor immune responses and impair tumor-induced immune suppression. Treatment with sunitinib, a multi-target inhibitor of receptor tyrosine kinases, in combination with immunotherapy, decreased the number of MDSCs and Treg cells, increased intra-tumoral infiltration by tumor-specific T cells, reduced tumor volumes, and increased survival of tumor-bearing mice123. Furthermore, a general goal of most therapies is to prevent metastasis. Analyses of signaling events in CRC cells might identify tumors that are most likely to metastasize. Specifically, stromal TGFB expression in the tumor, both at the primary site and at a distant site were associated with metastatic potential. This observation addresses the paradox that there are high levels of TGFB within tumors, yet mutational inactivation of TGFB signaling in tumor cells. Inhibition of TGFB might therefore prevent CRC metastasis124.
It could be possible to target other specific proteins or cell types in the tumor microenvironment. Genetic disruption or pharmacological inhibition of the fibroblast activation protein, which is exclusively expressed on tumor-associated fibroblasts and pericytes in most human epithelial cancers, impairs the growth of subcutaneously transplanted tumors and reduces tumor cell proliferation in mice125. However, this approach is likely to cause side effects, because there are few specific markers of these cells. It is therefore necessary to identify new targets in the tumor microenvironment.
The effect of the host immune system on the outcome and response to therapy has led to the idea of an immune score that could be incorporated (by an international task force) into the routine diagnostic and prognostic assessment of patients with cancer 12. The goal should be to characterize the distinct populations of immune cells (beyond T cells) and other stromal cells, to analyze their associations with prognosis and response to therapy, and to incorporate this information with TNM classification. Such an immune score still needs to be proven feasible and then independently validated, to determine its true prognostic value for patients with GI cancers. Nonetheless, the host immune response, as well as increased numbers of lymph nodes, are associated with patient prognosis. Comprehensive assessments of patients’ immune response, disease stage, lymph node count and molecular features of tumors are necessary for complete evaluation126.
To improve surveillance of patients with intestinal polyps, intestinal metaplasia in the stomach, or BE, it will be important to define the bacterial factors that contribute to tumor formation and progression of preneoplastic lesions to cancer. It is not clear why only a small percentage of patients with inflammatory disorders such as BE or H pylori infection ultimately develop EAC or gastric cancer. Improving our understanding of the microbiome could provide entirely new therapeutic strategies for GI inflammation and cancer.
Many efforts are being made to develop new chemotherapeutic drugs that can specifically target CSCs. In this regard the discovery of the druggable kinase Dclk1 as a marker of CSCs might allow us to prevent tumor formation or progression without affecting normal tissue stem cells101. It remains to be determined if targeting only CSC is sufficient to inhibit tumor recurrence or metastasis, or if CSCs develop from normal stem cells. Therefore, it will be important to develop strategies to disrupt stromal pathways important for normal stem cell and CSC maintenance and homeostasis, such as the Wnt, Notch, and Hedgehog pathways127. These could induce terminal differentiation, deprive CSCs of their self-renew capacity, or inhibit dedifferentiation.
We are likely to see many new approaches to alter tumor microenvironment and the inflammatory niche that supports tumor growth. It should be a priority to translate our accumulating knowledge of the tumor microenvironment into clinical trials, such as those that combine drugs that target tumor stroma with conventional chemotherapies.
Acknowledgments
M.Q. is supported by the Max Eder Program of the Deutsche Krebshilfe (109789) and the BMBF grant 68310, T.C.W. is supported through NCI grants NCI 5UO1CA143056, NCI 1U54CA163111, and NCI 1UO1CA164337. F.R.G. is supported by the European Research Council (ROSCAN-281967), Deutsche Forschungsgemeinschaft (Gr-1916/5-1), and Deutsche Krebshilfe (108872).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Schwabe RF, Wang TC. Bacteria Deliver a Genotoxic Hit. Science. 2012;338:52–53. doi: 10.1126/science.1229905. [DOI] [PubMed] [Google Scholar]
- 3.Quante M, Wang TC. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology (Bethesda) 2008;23:350–9. doi: 10.1152/physiol.00031.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothwell PM, Fowkes FGR, Belch JFF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. The Lancet. 377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 5.Gonda TA, Kim YI, Salas MC, Gamble MV, Shibata W, Muthupalani S, Sohn KJ, Abrams JA, Fox JG, Wang TC, Tycko B. Folic acid increases global DNA methylation and reduces inflammation to prevent Helicobacter-associated gastric cancer in mice. Gastroenterology. 2012;142:824–833. doi: 10.1053/j.gastro.2011.12.058. e7. [DOI] [PubMed] [Google Scholar]
- 6.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–35. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–51. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 11.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B, Feyen N, Martens UM, Beckhove P, Gnjatic S, Schirmacher P, Herpel E, Weitz J, Grabe N, Jaeger D. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–7. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 12.Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emens LA, Silverstein SC, Khleif S, Marincola FM, Galon J. Toward integrative cancer immunotherapy: targeting the tumor microenvironment. J Transl Med. 2012;10:70. doi: 10.1186/1479-5876-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 15.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-[alpha] in gastric tumour cells. EMBO J. 2008;27:1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 17.Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wani S, Falk GW, Post J, Yerian L, Hall M, Wang A, Gupta N, Gaddam S, Singh M, Singh V, Chuang KY, Boolchand V, Gavini H, Kuczynski J, Sud P, Bansal A, Rastogi A, Mathur SC, Young P, Cash B, Goldblum J, Lieberman DA, Sampliner RE, Sharma P. Risk factors for progression of low-grade dysplasia in patients with Barrett's esophagus. Gastroenterology. 2011;141:1179–86. doi: 10.1053/j.gastro.2011.06.055. 1186 e1. [DOI] [PubMed] [Google Scholar]
- 19.Marx J. Cancer immunology Cancer's bulwark against immune attack: MDS cells. Science. 2008;319:154–6. doi: 10.1126/science.319.5860.154. [DOI] [PubMed] [Google Scholar]
- 20.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quante M, Wang TC. Stem cells in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol. 2009;6:724–37. doi: 10.1038/nrgastro.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–19. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arimura S, Matsunaga A, Kitamura T, Aoki K, Aoki M, Taketo MM. Reduced level of smoothened suppresses intestinal tumorigenesis by down-regulation of Wnt signaling. Gastroenterology. 2009;137:629–38. doi: 10.1053/j.gastro.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 24.Taketo MM. Role of bone marrow-derived cells in colon cancer: lessons from mouse model studies. J Gastroenterol. 2009;44:93–102. doi: 10.1007/s00535-008-2321-3. [DOI] [PubMed] [Google Scholar]
- 25.Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, Klein-Szanto A, Lee JS, Katz JP, Diehl JA, Reynolds AB, Vonderheide RH, Rustgi AK. Deletion of p120-Catenin Results in a Tumor Microenvironment with Inflammation and Cancer that Establishes It as a Tumor Suppressor Gene. Cancer Cell. 2011;19:470–83. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, Rao VP, Poutahidis T, Weissleder R, McNagny KM, Khazaie K. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A. 2007;104:19977–82. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, Weitz J, Koch M, Halverson AL, Bentrem DJ, Khazaie K. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci U S A. 2010;107:6430–5. doi: 10.1073/pnas.0913683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 29.Maniati E, Soper R, Hagemann T. Up for Mischief? IL-17/Th17 in the tumour microenvironment. Oncogene. 2010;29:5653–62. doi: 10.1038/onc.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:5540–4. doi: 10.1073/pnas.0912675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–22. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012 doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T, Fujimori T. Involvement of the IL-22/REG Ialpha axis in ulcerative colitis. Lab Invest. 2010;90:496–505. doi: 10.1038/labinvest.2009.147. [DOI] [PubMed] [Google Scholar]
- 34.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O’Connor W, Murphy AJ, Valenzuela DM, Yancopoulos GD, Booth CJ, Cho JH, Ouyang W, Abraham C, Flavell RA. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012 doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koller FL, Hwang DG, Dozier EA, Fingleton B. Epithelial interleukin-4 receptor expression promotes colon tumor growth. Carcinogenesis. 2010;31:1010–7. doi: 10.1093/carcin/bgq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H-W, Joyce JA. Alternative activation of tumor-associated macrophages by IL-4: Priming for protumoral functions. Cell Cycle. 2010;9:4824–4835. doi: 10.4161/cc.9.24.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsieh MH, Lin ZY, Huang CJ, Shih MC, Chuang WL. Management of bilateral adrenal metastases from hepatocellular carcinoma: a case report. Kaohsiung J Med Sci. 2005;21:371–6. doi: 10.1016/S1607-551X(09)70136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 40.Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K. T-Regulatory Cells Shift from a Protective Anti-Inflammatory to a Cancer-Promoting Proinflammatory Phenotype in Polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ, Carneiro F, Sobrinho-Simoes M. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–7. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 43.Tu S, Quante M, Bhagat G, Takaishi S, Cui G, Yang XD, Fox JG, Pritchard M, Wang T. Interferon-{gamma} inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T cell apoptosis. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massague J, Sancho E, Batlle E. Dependency of Colorectal Cancer on a TGF-beta-Driven Program in Stromal Cells for Metastasis Initiation. Cancer Cell. 2012;22:571–84. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maeshima AM, Niki T, Maeshima A, Yamada T, Kondo H, Matsuno Y. Modified scar grade: a prognostic indicator in small peripheral lung adenocarcinoma. Cancer. 2002;95:2546–54. doi: 10.1002/cncr.11006. [DOI] [PubMed] [Google Scholar]
- 47.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 48.Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514–20. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- 49.Hogan NM, Dwyer RM, Joyce MR, Kerin MJ. Mesenchymal stem cells in the colorectal tumor microenvironment: recent progress and implications. Int J Cancer. 2012;131:1–7. doi: 10.1002/ijc.27458. [DOI] [PubMed] [Google Scholar]
- 50.Henriksson ML, Edin S, Dahlin AM, Oldenborg PA, Oberg A, Van Guelpen B, Rutegard J, Stenling R, Palmqvist R. Colorectal cancer cells activate adjacent fibroblasts resulting in FGF1/FGFR3 signaling and increased invasion. Am J Pathol. 2011;178:1387–94. doi: 10.1016/j.ajpath.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dolznig H, Rupp C, Puri C, Haslinger C, Schweifer N, Wieser E, Kerjaschki D, Garin-Chesa P. Modeling colon adenocarcinomas in vitro a 3D co-culture system induces cancer-relevant pathways upon tumor cell and stromal fibroblast interaction. Am J Pathol. 2011;179:487–501. doi: 10.1016/j.ajpath.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koliaraki V, Roulis M, Kollias G. Tpl2 regulates intestinal myofibroblast HGF release to suppress colitis-associated tumorigenesis. J Clin Invest. 2012 doi: 10.1172/JCI63917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–9. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–4. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–41. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 56.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 57.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–46. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol. 2011;8:393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- 59.Läubli H, Spanaus K-S, Borsig L. Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood. 2009;114:4583–4591. doi: 10.1182/blood-2008-10-186585. [DOI] [PubMed] [Google Scholar]
- 60.Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A, Simonavicius N, Schneider C, Lang M, Sturzl M, Croner RS, Konrad A, Manz MG, Moch H, Aguzzi A, van Loo G, Pasparakis M, Prinz M, Borsig L, Heikenwalder M. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell. 2012;22:91–105. doi: 10.1016/j.ccr.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 61.Lundgren O, Jodal M, Jansson M, Ryberg AT, Svensson L. Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS One. 2011;6:e16295. doi: 10.1371/journal.pone.0016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, Ittmann MM, Rowley D. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 63.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 64.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 65.Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2012 doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guma M, Stepniak D, Shaked H, Spehlmann ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff MF, Karin M. Constitutive intestinal NF-kappaB does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med. 2011;208:1889–900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shaked H, Hofseth LJ, Chumanevich A, Chumanevich AA, Wang J, Wang Y, Taniguchi K, Guma M, Shenouda S, Clevers H, Harris CC, Karin M. Chronic epithelial NF-kappaB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1211509109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–28. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N, Baumann H, Judd LM, Giraud AS, Boussioutas A, Zhu HJ, Ernst M. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med. 2005;11:845–52. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- 72.Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, Matthews VB, Armes J, Bhathal PS, Hughes NR, Marcusson EG, Karras JG, Na S, Sedgwick JD, Hertzog PJ, Jenkins BJ. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–38. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, Dev A, Sievert W, Ooi CH, Ishikawa TO, Oshima H, Bhathal PS, Parker AE, Oshima M, Tan P, Jenkins BJ. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22:466–78. doi: 10.1016/j.ccr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 74.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 75.Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–23. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–93. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Achyut BR, Yang L. Transforming growth factor-beta in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141:1167–78. doi: 10.1053/j.gastro.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hahn JN, Falck VG, Jirik FR. Smad4 deficiency in T cells leads to the Th17-associated development of premalignant gastroduodenal lesions in mice. J Clin Invest. 2011;121:4030–42. doi: 10.1172/JCI45114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, Wolfraim L, Hong S, Mushinski E, Potter M, Kim SJ, Fu XY, Deng C, Letterio JJ. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–9. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 81.Rizzo A, Waldner MJ, Stolfi C, Sarra M, Fina D, Becker C, Neurath MF, Macdonald TT, Pallone F, Monteleone G, Fantini MC. Smad7 expression in T cells prevents colitis-associated cancer. Cancer Res. 2011;71:7423–32. doi: 10.1158/0008-5472.CAN-11-1895. [DOI] [PubMed] [Google Scholar]
- 82.Bhowmick NA. TGF- Signaling in Fibroblasts Modulates the Oncogenic Potential of Adjacent Epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 83.Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M, Takabayashi A, Minato N, Taketo MM. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467–75. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 84.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kinnebrew MA, Pamer EG. Innate immune signaling in defense against intestinal microbes. Immunological Reviews. 2012;245:113–131. doi: 10.1111/j.1600-065X.2011.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–7. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 87.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O'HUigin C, Marincola FM, Trinchieri G. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–36. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185:4912–20. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification, Cloning, and Characterization of a Novel Soluble Receptor That Binds IL-22 and Neutralizes Its Activity. The Journal of Immunology. 2001;166:7096–7103. doi: 10.4049/jimmunol.166.12.7096. [DOI] [PubMed] [Google Scholar]
- 90.Zeki SS, Graham TA, Wright NA. Stem cells and their implications for colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:90–100. doi: 10.1038/nrgastro.2010.211. [DOI] [PubMed] [Google Scholar]
- 91.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–7. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 93.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–20. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–84. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–9. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–26. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- 97.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 99.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 100.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D'Angelica M, Kemeny N, Lyden D, Rafii S. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–20. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, Isomura A, Kawada K, Sakai Y, Yanagita M, Kageyama R, Kawaguchi Y, Taketo MM, Yonehara S, Chiba T. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2012 doi: 10.1038/ng.2481. [DOI] [PubMed] [Google Scholar]
- 102.Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM, Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–9. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 103.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 104.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–8. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 105.van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–12. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 106.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal Tumorigenesis Initiated by Dedifferentiation and Acquisition of Stem-Cell-like Properties. Cell. 2012;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 107.Goodman AL, Gordon JI. Our unindicted coconspirators: human metabolism from a microbial perspective. Cell Metab. 2010;12:111–6. doi: 10.1016/j.cmet.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee CW, Rickman B, Rogers AB, Muthupalani S, Takaishi S, Yang P, Wang TC, Fox JG. Combination of sulindac and antimicrobial eradication of Helicobacter pylori prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res. 2009;69:8166–74. doi: 10.1158/0008-5472.CAN-08-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, Potter A, Varro A, Eibach D, Suerbaum S, Wang TC, Fox JG. Lack of commensal flora in H. pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–20. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, Liao X, Waldron L, Hoshida Y, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–54. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marchesi JR, Dutilh BE, Hall N, Peters WHM, Roelofs R, Boleij A, Tjalsma H. Towards the Human Colorectal Cancer Microbiome. PLoS One. 2011;6:e20447. doi: 10.1371/journal.pone.0020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320–9. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009 doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science. 2012 doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–51. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 116.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan T-J, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science. 2012 doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwitalla S, Ziegler P, Horst D, Becker V, Kerle I, Begus-Nahrmann Y, Lechel A, Rudolph KL, Langer RS, lotta-Huspenina J, Bader FG, da Costa OP, Neurath MF, Meining A, Kirchner T, Fr G. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell. 2013;23:93–106. doi: 10.1016/j.ccr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 118.Burn J, Gerdes A-M, Macrae F, Mecklin J-P, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, Bisgaard M-L, Dunlop MG, Ho JWC, Hodgson SV, Lindblom A, Lubinski J, Morrison PJ, Murday V, Ramesar R, Side L, Scott RJ, Thomas HJW, Vasen HF, Barker G, Crawford G, Elliott F, Movahedi M, Pylvanainen K, Wijnen JT, Fodde R, Lynch HT, Mathers JC, Bishop DT. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. The Lancet. 2011;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, Sun R, Nosho K, Meyerhardt JA, Giovannucci E, Fuchs CS, Chan AT, Ogino S. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F, Im SA, Bodoky G, Salazar R, Maindrault-Goebel F, Shacham-Shmueli E, Bajetta E, Makrutzki M, Shang A, Andre T, Hoff PM. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225–33. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 121.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 122.Kumar R, Wani SI, Chauhan NS, Sharma R, Sareen D. Cloning and characterization of an epoxide hydrolase from Cupriavidus metallidurans-CH34. Protein Expr Purif. 2011;79:49–59. doi: 10.1016/j.pep.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 123.Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer. 2012;130:1948–59. doi: 10.1002/ijc.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hammerle-Fickinger A, Riedmaier I, Becker C, Meyer HH, Pfaffl MW, Ulbrich SE. Validation of extraction methods for total RNA and miRNA from bovine blood prior to quantitative gene expression analyses. Biotechnol Lett. 2010;32:35–44. doi: 10.1007/s10529-009-0130-2. [DOI] [PubMed] [Google Scholar]
- 125.Santos AM, Jung J, Aziz N, Kissil JL, Puré E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119:3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, Chouchane L, Delrio P, Arndt H, Asslaber M, Maio M, Masucci GV, Mihm M, Vidal-Vanaclocha F, Allison JP, Gnjatic S, Hakansson L, Huber C, Singh-Jasuja H, Ottensmeier C, Zwierzina H, Laghi L, Grizzi F, Ohashi PS, Shaw PA, Clarke BA, Wouters BG, Kawakami Y, Hazama S, Okuno K, Wang E, O'Donnell-Tormey J, Lagorce C, Pawelec G, Nishimura MI, Hawkins R, Lapointe R, Lundqvist A, Khleif SN, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Palmqvist R, Nagtegaal ID, Wang Y, D'Arrigo C, Kopetz S, Sinicrope FA, Trinchieri G, Gajewski TF, Ascierto PA, Fox BA. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 128.Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kondoh S, Aoki M, Taketo MM. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci U S A. 2010;107:13063–8. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iotti G, Ferrari-Amorotti G, Rosafio C, Corradini F, Lidonnici MR, Ronchetti M, Bardini M, Zhang Y, Martinez R, Blasi F, Calabretta B. Expression of CCL9/MIP-1gamma is repressed by BCR/ABL and its restoration suppresses in vivo leukemogenesis of 32D–BCR/ABL cells. Oncogene. 2007;26:3482–91. doi: 10.1038/sj.onc.1210146. [DOI] [PubMed] [Google Scholar]
- 130.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJB, Sansom OJ. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. Journal of Clinical Investigation. 2012 doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, Egashira K, Mukaida N. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69:7884–92. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- 132.Shibata W, Ariyama H, Westphalen CB, Worthley DL, Muthupalani S, Asfaha S, Dubeykovskaya Z, Quante M, Fox JG, Wang TC. Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut. 2012 doi: 10.1136/gutjnl-2011-301824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, Camus M, Gillard M, Bruneval P, Fridman WH, Pages F, Trajanoski Z, Galon J. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138:1429–40. doi: 10.1053/j.gastro.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 134.Muthuswamy R, Berk E, Junecko BF, Zeh HJ, Zureikat AH, Normolle D, Luong TM, Reinhart TA, Bartlett DL, Kalinski P. NF-kappaB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res. 2012;72:3735–43. doi: 10.1158/0008-5472.CAN-11-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen HJ, Edwards R, Tucci S, Bu P, Milsom J, Lee S, Edelmann W, Gumus ZH, Shen X, Lipkin S. Chemokine 25-induced signaling suppresses colon cancer invasion and metastasis. J Clin Invest. 2012;122:3184–96. doi: 10.1172/JCI62110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Galle PR, Blessing M, Rose-John S, Neurath MF. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 137.Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, Vardam TD, Weis EL, Passanese J, Wang W-C, Gollnick SO, Dewhirst MW, Rose-John S, Repasky EA, Baumann H, Evans SS. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121:3846–3859. doi: 10.1172/JCI44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Asfaha S, Dubeykovskiy AN, Tomita H, Yang X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA, Muthupalani S, Ericksen R, Frucht H, Fox JG, Wang TC. Mice that Express Human Interleukin-8 Have Increased Mobilization of Immature Myeloid Cells, which Exacerbates Inflammation and Accelerates Colon Carcinogenesis. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, Lynch MP, Rueda BR, Chung DC. Induction of interleukin-8 preserves the angiogenic response in HIF-1 alpha-deficient colon cancer cells. Nat Med. 2005;11:992–7. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 140.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunology, Immunotherapy. 2011;60:909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–57. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 144.Popivanova B. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008:118. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu M, Lee DF, Chen CT, Yen CJ, Li LY, Lee HJ, Chang CJ, Chang WC, Hsu JM, Kuo HP, Xia W, Wei Y, Chiu PC, Chou CK, Du Y, Dhar D, Karin M, Chen CH, Hung MC. IKKalpha activation of NOTCH links tumorigenesis via FOXA2 suppression. Mol Cell. 2012;45:171–84. doi: 10.1016/j.molcel.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 148.De Wever O, Nguyen Q-D, Van Hoorde L, Bracke M, Bruyneel E, Gespach C, Mareel M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent proinvasive signals to human colon cancer cells through RhoA and Rac. The FASEB Journal. 2004 doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- 149.Goodwin AC, Shields CED, Wu S, Huso DL, Wu X, Murray-Stewart TR, Hacker-Prietz A, Rabizadeh S, Woster PM, Sears CL, Casero RA. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proceedings of the National Academy of Sciences. 2011;108:15354–15359. doi: 10.1073/pnas.1010203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boquoi A, Jover R, Chen T, Pennings M, Enders GH. Transgenic expression of VEGF in intestinal epithelium drives mesenchymal cell interactions and epithelial neoplasia. Gastroenterology. 2009;136:596–606. doi: 10.1053/j.gastro.2008.10.028. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Waldner MJ, Wirtz S, Jefremow A, Warntjen M, Neufert C, Atreya R, Becker C, Weigmann B, Vieth M, Rose-John S, Neurath MF. VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. J Exp Med. 2010;207:2855–68. doi: 10.1084/jem.20100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hsu S, Huang F, Friedman E. Platelet-derived growth factor-B increases colon cancer cell growth in vivo by a paracrine effect. Journal of Cellular Physiology. 1995;165:239–245. doi: 10.1002/jcp.1041650204. [DOI] [PubMed] [Google Scholar]
- 153.Kodama M, Kitadai Y, Sumida T, Ohnishi M, Ohara E, Tanaka M, Shinagawa K, Tanaka S, Yasui W, Chayama K. Expression of platelet-derived growth factor (PDGF)-B and PDGF-receptor beta is associated with lymphatic metastasis in human gastric carcinoma. Cancer Sci. 2010;101:1984–9. doi: 10.1111/j.1349-7006.2010.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McCarthy N. Tracing clones. Nat Rev Cancer. 2012:12. doi: 10.1038/nrc3354. [DOI] [PubMed] [Google Scholar]
- 155.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors[mdash]impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Denys H, Derycke L, Hendrix A, Westbroek W, Gheldof A, Narine K, Pauwels P, Gespach C, Bracke M, De Wever O. Differential impact of TGF-beta and EGF on fibroblast differentiation and invasion reciprocally promotes colon cancer cell invasion. Cancer Lett. 2008;266:263–74. doi: 10.1016/j.canlet.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 157.Dub xE EP, Yan F, Punit S, Girish N, McElroy SJ, Washington MK, Polk DB. Epidermal growth factor receptor inhibits colitis-associated cancer in mice. The Journal of Clinical Investigation. 2012;122:2780–2792. doi: 10.1172/JCI62888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrède J-P. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proceedings of the National Academy of Sciences. 2010;107:11537–11542. doi: 10.1073/pnas.1001261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Housseau F, Sears CL. EnterotoxigenicBacteroides fragilis (ETBF)-mediated colitis in Min (Apc+/−) mice: a human commensal-based murine model of colon carcinogenesis. Cell Cycle. 2010;9:3–5. doi: 10.4161/cc.9.1.10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X, Huycke MM. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology. 2007;132:551–61. doi: 10.1053/j.gastro.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 161.Arthur JC, Jobin C. The struggle within: microbial influences on colorectal cancer. Inflamm Bowel Dis. 2011;17:396–409. doi: 10.1002/ibd.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Garg P, Sarma D, Jeppsson S, Patel NR, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9 functions as a tumor suppressor in colitis-associated cancer. Cancer Res. 2010;70:792–801. doi: 10.1158/0008-5472.CAN-09-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kitamura T, Biyajima K, Aoki M, Oshima M, Taketo MM. Matrix metalloproteinase 7 is required for tumor formation, but dispensable for invasion and fibrosis in SMAD4-deficient intestinal adenocarcinomas. Lab Invest. 2009;89:98–105. doi: 10.1038/labinvest.2008.107. [DOI] [PubMed] [Google Scholar]
- 164.Baker AM, Bird D, Lang G, Cox TR, Erler JT. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2012 doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 165.Baker AM, Bird D, Welti JC, Gourlaouen M, Lang G, Murray GI, Reynolds AR, Cox TR, Erler JT. Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumor angiogenesis. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]