Abstract
Rationale
Ethanol is commonly used and abused during adolescence. Although adolescents display differential behavioral responses to ethanol, the mechanisms by which this occurs are not known. The PKC pathway has been implicated in mediating many ethanol-related effects in adults, as well as GABAA receptor regulation.
Objectives
The present study was designed to characterize cortical PKC isoform and GABAA receptor subunit expression during adolescence relative to adults as well as assess PKC involvement in ethanol action.
Results
Novel PKC isoforms were elevated, while PKCγ was lower during mid-adolescence relative to adults. Whole cell lysate and synaptosomal preparations correlated for all isoforms except PKC δ In parallel, synaptosomal GABAA receptor subunit expression was also developmentally regulated, with GABAAR δ and α4 being lower while α1 and γ2 were higher or similar, respectively, in adolescents compared to adults. Following acute ethanol exposure, synaptosomal novel and atypical PKC isoform expression was decreased only in adolescents. Behaviorally, inhibiting PKC with calphostin C, significantly increased ethanol-induced loss of righting reflex (LORR) in adolescents but not adults, whereas activating PKC with phorbol-dibutyrate was ineffective in adolescents but decreased LORR duration in adults. Further investigation revealed that inhibiting the cytosolic phospholipase A2/arachidonic acid (cPLA2/AA) pathway increased LORR duration in adolescents, but was ineffective in adults.
Conclusions
These data indicate that PKC isoforms are variably regulated during adolescence and may contribute to adolescent ethanol-related behavior. Furthermore, age-related differences in the cPLA2/AA pathway may contribute to age-related ethanol’s effects on novel and atypical PKC isoform expression and behavior.
Keywords: Ethanol, Protein Kinase C, GABAA Receptor, Phospholipase A2, Arachidonic Acid
INTRODUCTION
Alcohol (ethanol) is one of the most commonly used and abused drugs (NIAAA 2000), however, much remains unknown about its molecular mechanism of action, especially during adolescence. This remains problematic, as ethanol use often begins during this period (Johnston et al. 2012). Worse, the “National Survey on Drug Use and Health” reports increases for ethanol abuse or dependence if use begins before the age of 15 compared to later periods (NIDA 2011). Adolescence is a critical developmental period, and ethanol exposure during this time likely alters numerous neurochemical pathways that may accelerate alcohol-related problems. In fact, previous work indicates that adolescents readily display differential behavioral sensitivity to ethanol compared to adults, particularly to its aversive effects; such effects include decreased sensitivity to ethanol’s motor-ataxic (Ramirez & Spear 2010) and sedative/hypnotic effects (Silveri & Spear 1998), but increased sensitivity to its memory-impairing effects (Markwiese et al. 1998). Although much research has focused on behavioral disparities between adolescents and adults, less is known about the underlying molecular effects in adolescent ethanol responses.
In adults, ethanol is known to alter neurotransmission as well as many second messenger cascades (e.g., Darstein et al. 1998; Sabria et al. 2003; Tabakoff et al. 2001; Weiner & Valenzuela 2006). In particular, much work has focused on protein kinases such as protein kinase C (PKC). The PKC family includes 10 isoforms divided into 3 subfamilies: conventional, novel and atypical PKCs (Nishizuka 1992). Conventional PKC’s (cPKC) include PKC α, β and γ and require both calcium and diacylglycerol (DAG) for activation. Novel isoforms (nPKC) include PKC δ, ε, η, and θ and typically require DAG, but are calcium independent. Atypical isoforms (aPKC) include ζ and ι/λ and are calcium and DAG independent (Nishizuka 1992). Interestingly, novel and atypical PKC isoforms may also be activated by the cytoplasmic phospholipase A2/arachidonic acid (cPLA2/AA) pathway (e.g., Khan et al. 1995; Khan et al. 1994).
Prior knockout studies have shown that PKC isoforms contribute to ethanol behavioral responses. For instance, PKCγ or PKCδ knockouts are less sensitive to ethanol compared to their wild-type counterparts as assessed by loss of righting reflex (Bowers et al. 1999; Choi et al. 2008; Harris et al. 1995). Conversely, PKCε knockouts are more sensitive to ethanol’s sedative/hypnotic effects (Hodge et al. 1999). Along with behavior, acute ethanol exposure also alters PKC isoform expression and translocation in adults. For instance, moderate ethanol doses (2.0g/kg) increase β and ε, and decrease γ expression in cortical synaptosomal (P2) fractions after one hour in vivo (Kumar et al. 2006). Interestingly, these effects are dose-dependent as PKC isoform expression is unchanged following higher doses (Kumar et al. 2012). Therefore, it is possible that PKC regulation may contribute to adolescent ethanol responses.
PKC affects many synaptic proteins; however, regulation of basal neurotransmitter systems prominently affected by ethanol, such as GABAA receptors, likely influences ethanol age-related behavior (Kumar et al. 2010; Proctor et al. 2003). GABAA receptors are pentameric ligand-gated ion channels that mediate inhibitory neurotransmission in the central nervous system. Nineteen different subunits are capable of forming functional GABAARs, with the majority being 2α, 2β with either a γ or δ subunit (Olsen & Sieghart 2009). Importantly, PKC activity contributes to GABAA receptor function, trafficking and cell surface stability (Connolly et al. 1999; Herring et al. 2003; McDonald & Moss 1997; Moss et al. 1992). In particular, studies have found relationships between specific PKC isoforms and GABAAR subunits. For example, PKCδ interacts with the GABAAR δ subunit, whereas PKCγ may associate with the α1 and α4 subunits (Choi et al. 2008; Kumar et al. 2002). In fact, following ethanol exposure, the association of PKCγ with the GABAA α4 and α1 subunits increases, leading to their expression and internalization respectively (Kumar et al. 2002); an effect that is prevented by knockdown of PKCγ in cultured cortical neurons (Kumar et al. 2010; Werner et al. 2011). Additionally, PKCε-dependent phosphorylation of the GABAA γ2 subunit prevents ethanol mediated increases in GABAAR function (Qi et al. 2007). Notably, PKCδ regulates ethanol’s behavioral effects through enhancement of GABA-stimulated tonic inhibition (Choi et al. 2008). Thus, age-related differences in PKC expression may potentially influence GABAA receptor subtype expression.
In the present study, we investigated whether PKC isoform and GABAA receptor subunit expression were regulated during the adolescent period. In addition, we assessed whether PKC activity influenced ethanol’s sedative/hypnotic action and if ethanol affected PKC isoform synaptosomal expression in adolescents. Finally, we investigated the involvement of the cPLA2/AA pathway in ethanol-related behavior.
MATERIALS AND METHODS
Animals
Experiments were conducted in accordance with the National Institute of Health Guidelines under Institutional Animal Care and Use Committee-approved protocols at Binghamton University, State University of New York. Adolescent (P28–P42, 110–170 g) and adult (P75, 300–450 g) male Sprague-Dawley rats were ordered from Taconic (Germantown, NY, USA) or bred at Binghamton University. Rats were maintained on a standard 12 h light–dark schedule with lights on at 7:00 AM. For consistency with previous studies examining protein kinases and GABAA receptors, animal assessments began during the first four hours of the inactive period. Animals had ad libitum access to rat chow and water. Rats that underwent intracerebroventricular (i.c.v.) surgery, described below, were subsequently housed individually. Adult rats not surgically prepared were pair-housed, while adolescents were group housed with 3–4 other rats. All subjects had environmental enrichment.
Intracerebroventricular surgery
Stereotactic surgeries were performed to implant guide cannulae directed toward the lateral ventricles. Briefly, rats were anesthetized with 3.0% isoflurane and subsequently placed into a stereotactic frame. Guide cannulae (PlasticsOne, Roanoke, VA, USA) were implanted unilaterally into the lateral cerebral ventricle at coordinates AP −0.8 mm, L+ or −1.5 mm from bregma, and DV −2.5 mm for adults (Paxinos & Watson 2007) and AP −0.5 mm, L+1.2 mm from bregma, and DV −2.5 for adolescents. Cannulae were secured to the skull using three stainless steel screws and dental cement, protected with an internal guide and cap with the skin surrounding the surgical site sutured closed. Buprenex® was administered for postoperative care immediately following surgery, as well as 24 hours following cannulation. Animals were given a one-week recovery period prior to behavioral testing with cannula patency being assessed periodically during routine handling. Following sacrifice, India ink was used to determine i.c.v. cannula placements. Only animals with a positive indication of ink in their ventricles (98.9%) were used for subsequent analysis.
Pharmacological Agents
Ethanol (20% v/v in saline) was purchased from Pharmco (Brookfield, CT, USA). Calphostin C (CalC) and 4-beta-phorbol-12,13-dibutyrate (PDBu) were obtained from Sigma Chemical Co. (St. Louis, MO). CalC (500 pmol/rat) was dissolved in 2% DMSO/aCSF and was used to measure PKC inhibiton. PDBu (100 pmol/rat) was dissolved in 0.1% DMSO/aCSF and used to assess PKC activation. Arachidonyl trifluoromethyl ketone (AACOCF3) was purchased from Enzo Life Sciences (Farmingdale, NY) and dissolved in aCSF to assess cytoplasmic phospholipase A2/arachidonic acid pathway inhibition (5 nmol/rat). Appropriate DMSO/aCSF concentrations were used as vehicles for each drug condition.
Loss of righting reflex (LORR)
To measure the effect of PKC inhibition on ethanol-induced LORR, adolescent (P35) and adult (P75–P80) rats were administered CalC or 2% DMSO/aCSF i.c.v. 5 minutes prior to a hypnotic dose of ethanol (4.0 g/kg, intraperitoneally (i.p.)). To assess the effect of PKC activation on ethanol-induced LORR, PDBu or 0.1% DMSO/aCSF was injected i.c.v. 60 minutes prior to ethanol administration as described elsewhere (Kumar et al. 2005). To assess the involvement of arachidonic acid in modulating ethanol-induced LORR, the phospholipase A2 inhibitor AACOCF3 was administered i.c.v. 8 hours prior to ethanol injection. AACOCF3 dose and time was based on previous reports indicating effective inhibition of arachidonic acid (Shanker et al. 2004; Yeo et al. 2004). All i.c.v. injections were done at a flow rate of 1 µL/min. Following completion of drug delivery, the needles were left in place for an additional minute to mitigate backflow into the cannula. Following ethanol administration, rats were observed until they exhibited LORR via placement in a supine position in V-shaped troughs (90° angle). Animals remained in this supine position until they regained their righting reflex as assessed by the ability to right three times in a 60 second period. LORR duration was calculated by subtracting the time of onset of LORR from the time of recovery. Blood samples were taken from the tail immediately after rats regained the righting reflex and analyzed using an AM5 Alcohol Analyzer (Analox Instruments, Lunenburg, MA). Notably, PDBu, CalC and AACOCF3 do not elicit hypnotic effects in the absence of ethanol (Galeotti & Ghelardini 2011; Yeo et al. 2004). With the exception of PDBu-treated adolescents, only 1–2 rats per group failed to lose their righting reflex. All rats that failed to lose their righting reflex were excluded from analyses.
Tissue Collection
For assessment of PKC isoforms and GABAAR subunits during the adolescent period, rats were sacrificed at predetermined ages (P28, P35, P42 and P75). For acute ethanol studies, rats were injected with ethanol (3.5 g/kg, i.p.) or saline, and sacrificed at predetermined time points (30 m. and 60 m.). For both studies, the brain was rapidly removed from the skull, flash frozen, and stored at −80°C. Preparations are described below.
Sample Preparations
For all samples, cortical tissue was used in order to gain a better perspective in regions associated with loss of righting reflex and consciousness (Franks & Lieb 1990). For whole cell lysates (total expression), following dissection, cerebral cortices were homogenized in a mixture of 1% sodium dodecyl sulfate (SDS), 1mM ethylenediaminetetraacetic acid (EDTA), and 10mM of Tris, (Grosshans et al. 2002). For P2 synaptasomal samples, following dissection, cerebral cortical P2 fractions were homogenized in 0.32 M sucrose/PBS solution, and spun at low speed centrifugation (1,000g) followed by spinning the resulting supernatant at 12,000 × g for 20 min. The pellet (P2 fraction) was resuspended in phosphate buffered saline (PBS). Protein concentrations of all samples were quantified using a bicinchoninic acid method.
Western blot analysis
Protein samples from whole cell lysates and P2 synaptosomal fractions were subjected to sodium-dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using Novex Tris–Glycine gels (8–16%) and transferred to polyvinylidene difluoride (PVDF) membranes (Invitrogen, Carlsbad, CA, USA). Membranes were probed with antibodies for the following proteins: PKCβ, PKCε, PKCδ, (BD Biosciences, San Jose, CA, USA), PKCγ (Abcam Inc., Cambridge, MA, USA), GABAAR α1, α4 and γ2 (Millipore, Lake Temecula, CA, USA) and PKCζ, GABAAR δ (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Blots were subsequently exposed to an antibody directed against β-actin (Millipore) to verify equivalent protein loading and transfer. Secondary antibodies were obtained from Thermo Scientific (Waltham, MA). Samples were run in duplicate or triplicate and averaged. All bands were detected by enhanced chemiluminescence under non-saturating conditions (GE Healthcare, Piscataway, NJ, USA) and exposed to X-ray films and analyzed using NIH Image J.
Statistical analysis and Figure Design
For western blots, all comparisons were made within blots. For ethanol exposure time dependent studies, each group was compared to saline controls run in parallel. For age dependent studies, each group was compared to adult samples (P75). Analyses were conducted using one-way ANOVA with Dunnett’s post hoc test when appropriate. Pearson coefficient was used for correlational analyses. LORR data were assessed using Student’s t-test. For all experiments, p < 0.05 (α=0.05) was considered significant. All figures were generated using Prism (Graphpad, La Jolla, CA).
RESULTS
Total PKC isoform expression varies across adolescence
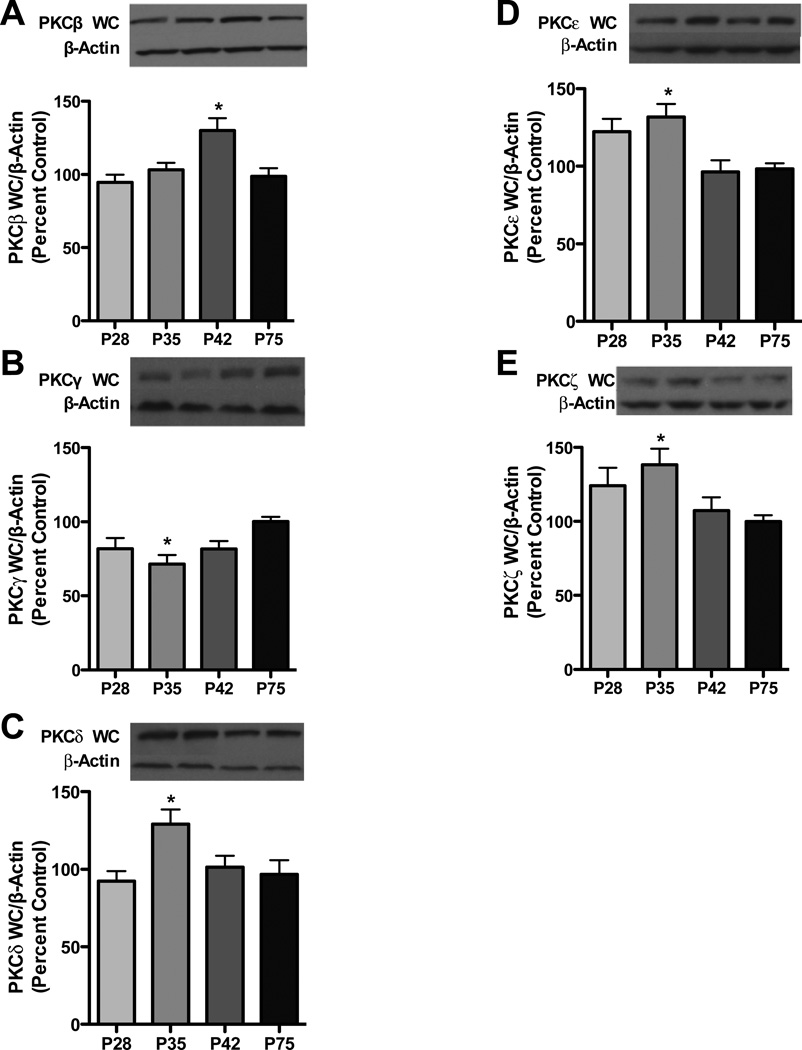
We first examined PKC isoform expression during early-adolescence (P28), mid-adolescence (P35), late-adolescence (P42) and adulthood (P75). Analysis of PKCβ revealed an effect of age [F3,56=6.324, p<0.001], with PKCβ being elevated by 31.2% during late adolescence relative to adults (Figure 1a). Analysis of PKCγ also revealed an effect of age [F3,56= 4.081, p<0.01], with PKCγ being 28.7% lower during mid adolescence relative to adults (Figure 1b). For novel PKCs, an effect of age was observed for both PKCδ [F3, 56=4.15, p<0.01] and PKCε [F3, 56= 6.057, p<0.001] with PKCδ levels being 32.4% higher during mid adolescence, and PKCε being 23.9% and 33.4% greater during early and during mid adolescence relative to adults (Figure 1c and d). The atypical isoform PKCζ also had an effect of age [F3,56=2.985, p<0.05], with PKCζ being elevated by 38.1% during mid-adolescence relative to adults (Figure 1e).
Figure 1. Total PKC isoform expression is variably expressed during adolescence.
Representative blots are shown for PKC β (a), PKC γ (b), PKC δ (c), PKCε (d) and PKC ζ (e). Data represent mean ± SEM *p < 0.05 compared to P75, n = 14–16/group.
Synaptosomal PKCδ isoforms expression does not parallel total expression across adolescence
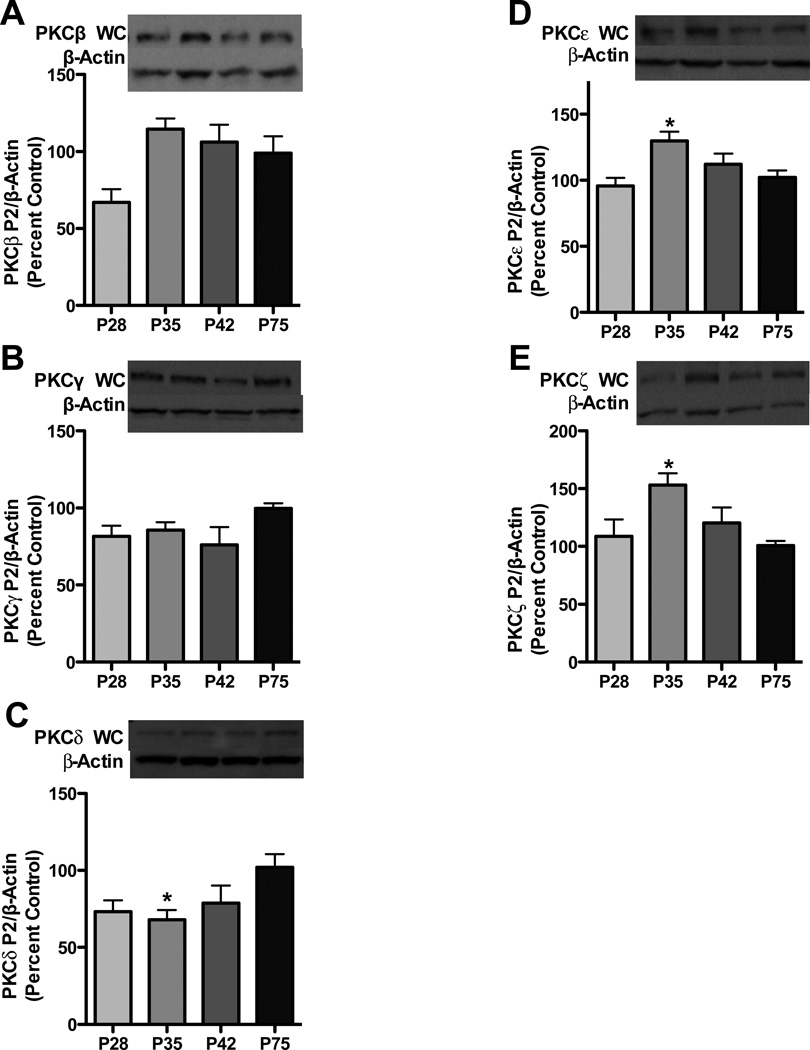
We next assessed synaptosomal PKC isoform expression in order to ascertain the relationship between membrane-associated PKC isoforms and total PKC expression across adolescence, as total expression does not necessarily give insight into synaptosomal receptor regulation. For conventional PKC isoforms,, an effect of age [F3,26=4.571, p<0.01] was observed for PKCβ, with a trend towards elevated levels during mid adolescence (Figure 2a); however, no effect was observed for PKCγ (Figure 2b). For novel PKCs, an effect of age was observed for both PKCδ [F3,26=3.13, p<0.05] and PKCε [F3, 27= 5.248, p<0.01]. PKCδ was 34.0% lower, while PKCε was 27.7% higher during mid adolescence relative to adults (Figure 2c and d). For PKCζ, an effect of age was observed [F3,26= 4.092, p>0.05]. PKCζ was elevated 52.3% during mid-adolescence relative to adults (Figure 2e). Furthermore, correlational analyses of total and synaptosoamal PKC expression revealed that PKCγ, -ε and -ζ were significantly correlated (r = 0.49, 0.44, 0.45, respectively, p<0.05) and PKCβ had a highly suggestive correlation (r=0.35, p=0.08). PKCδ synaptosomal expression did not correlate with total expression (r = 0.10, p>0.05).
Figure 2. Synaptosomal PKC isoform expression is variably regulated.
Representative blots are shown for PKC β (a), PKC γ (b) PKC δ (c), PKC ε (d) and PKC ζ (e). Data represent mean ± SEM, *p < 0.05 compared to P75, n = 7–8/group.
Synaptosomal GABAAR subunits are differentially expressed across adolescence
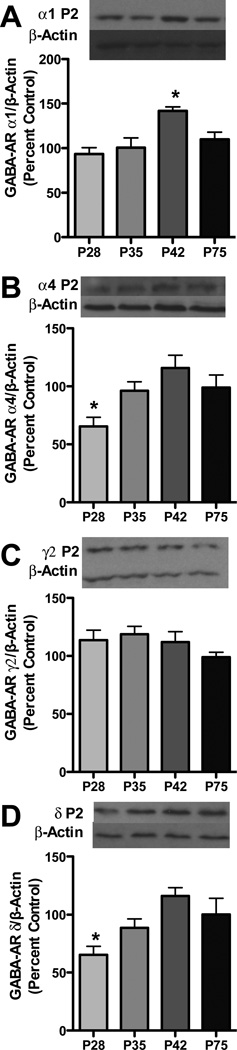
As PKC isoforms are known to regulate GABAARs, to further understand developmental differences in ethanol-sensitivity we assessed GABAAR subunit expression in synaptosomal preparations. For GABAAR α1 subunits, an effect of age [F3,26=6.971, p<0.01] was observed, with elevated α1 levels during late adolescence (Figure 3a). For GABAAR α4 and δ subunits, an effect of age was also observed [F3,26=4.891, p<0.01 and F3,26=5.387, p<0.01 respectively], with lower levels of both subunits during early adolescence (Figure 3b and d). No effect was observed for GABAAR γ2 subunits (Figure 3c).
Figure 3. Synaptosomal GABAAR subunits are developmentally regulated through adolescence.
Representative blots are shown for GABAAR α 1 (a), α 4 (b), γ 2 (c) and δ (d) subunits. Data represent mean ± SEM, *p < 0.05 compared to P75, n=7–8/group.
Modulating PKC activity differentially regulates ethanol-induced loss of righting reflex in adolescents and adults
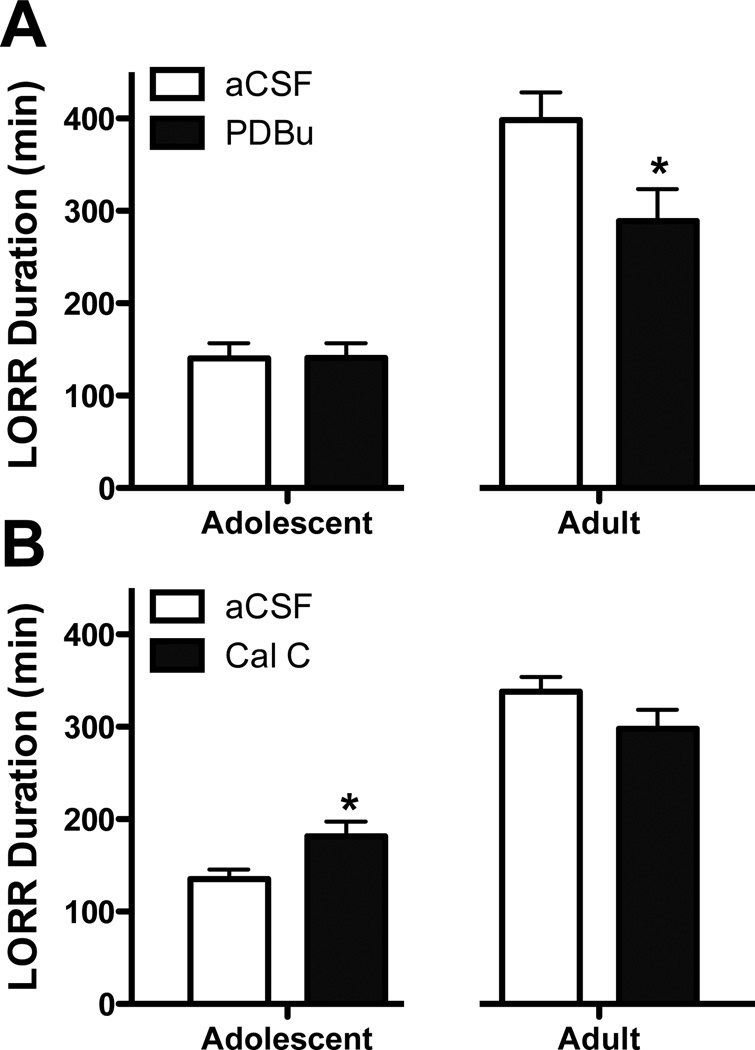
Due to the differences in basal PKC levels between adolescents and adults we wanted to determine whether altering PKC activity contributed to adolescent ethanol behavioral responses. Therefore, effects of altering PKC activity on the sedative/hypnotic effects of ethanol was investigated by determining the duration of ethanol-induced LORR in adolescents and adults following i.c.v. injection of the PKC activator PDBu or inhibitor CalC. For both, adolescents and adults were analyzed separately due to age-related differences in righting reflex responses. In adults, PDBu decreased the duration of ethanol-induced LORR by 27.7% (p<0.05). In contrast, adolescents showed no changes following i.c.v. administration of PDBu (Figure 4a). In order to determine if PDBu dose was also age dependent, we tested a 200 pmol/rat dose in adolescents. However, higher doses did not affect ethanol-induced LORR (167.7 ± 13.9 and 156.4 ± 18.0 for aCSF and PDBu, respectively; n = 11–13, p = 0.62). Interestingly, although not included in the analysis, approximately 27% of adolescents that were administered either 100 or 200pmol PDBu, (3 and 4 per group, respectively versus only 1–2 per other groups), failed to lose their righting reflex; however, this did not differ compared to aCSF-treated adolescents (100pmol, X2 = 2.59, p = 0.17; 200pmol, X2 = 1.93, p = 0.19). Interestingly, i.c.v. administration of CalC in adults had no effect on the duration of ethanol-induced LORR. In contrast, following CalC administration, adolescent’s duration of ethanol-induced LORR was increased by 33.8% (p<0.05, Fig. 4b). Adolescent CalC effects are likely independent of ethanol metabolism as a suggestive (but not significant) decrease in blood ethanol concentrations (BECs) was observed in CalC treated subjects (206.6 ± 16.4 vs 187.2 ± 6.3 mg/dL for aCSF and CalC, respectively). Similarly, a suggestive (but not significant) increase in BECs was also noted for PDBu treated adults (179.9 ± 9.3 and 203.0 ± 17.2 for aCSF and PDBu, respectively). BECs were almost identical in adolescent PDBu and adult CalC studies (190.8 ± .4.25 and 196.3 ± 8.4 for adolescent aCSF and PDBu, respectively; 165.0 ± 4.2 and 175.7 ± 8.5 for adult aCSF and CalC, respectively).
Figure 4. Modulating PKC activity differentially regulates ethanol-induced loss of righting reflex (LORR) in adolescents and adults.
Intracerebroventricular administration of PDBu decreased sleep time in adult rats (a) while CalC increased sleep time in adolescents (b). (n=8–9), *p<0.05, compared to age-matched controls.
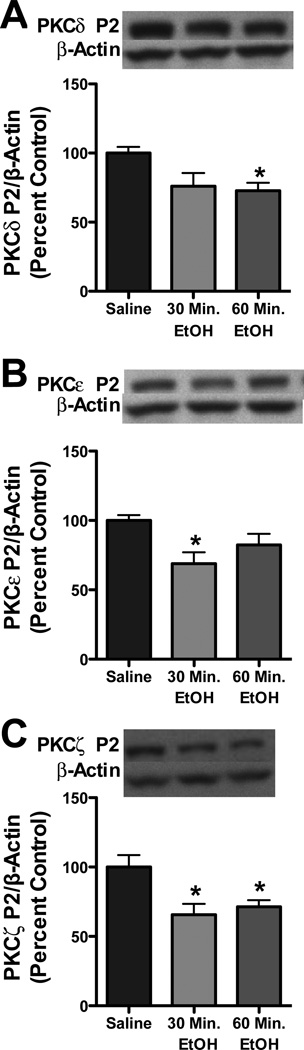
PKC isoform expression is decreased following acute ethanol exposure in adolescents
Synaptosomal PKC translocation is an indirect assessment of PKC activity. Given that previous studies demonstrate that PKC isoform expression is regulated following 2.0 g/kg ethanol exposure (Kumar et al., 2006), but not after 3.5 g/kg (Kumar et al., 2012), we wanted to investigate whether adolescent PKC isoform expression was differentially altered following a higher dose (3.5g/kg). Ethanol exposure decreased synaptosomal expression of novel and atypical PKC isoforms, but not conventional isoforms (Table 1) of PKC during adolescence. For PKCδ, an effect of time post ethanol was observed [F2,19=3.67, p<0.05]. Further analysis revealed that PKCδ was decreased 60 minutes following ethanol exposure by 27.3% (Figure 5a). In parallel, an effect of time post ethanol was observed for PKCε [F2,19=4.08, p<0.05]. Further analysis revealed that PKCε was decreased 30 minutes following ethanol exposure by 31.2% (Figure 5b). Similarly, an effect of time post ethanol administration was observed for PKCζ (F2,19=6.002, p<0.001). Further analysis revealed that PKCζ was decreased at both 30 and 60 minutes by 34.4% and 26.6%, respectively (Figure 5c). Consistent with previous reports, ethanol had no effect on PKC isoform expression in adult rats (Kumar et al., 2012) (Table 2)
Table 1. Adolescent conventional PKC isoform expression following ethanol exposure.
High dose acute ethanol administration does not alter conventional PKC isoform expression in adolescent rats (n=6–8/group).
| Saline | 30 Minute | 60 Minute | |
|---|---|---|---|
| PKC β | 100.0 ± 3.317 | 91.47 ± 5.792 | 83.48 ± 9.34 |
| PKC γ | 100.0 ± 8.882 | 89.65 ± 11.34 | 82.05 ± 8.26 |
Figure 5. Adolescent synaptosomal novel PKC isoforms are decreased following acute ethanol exposure.
Representative blots and graphic representation showing PKC δ (a), PKC ε (b) and PKC ζ (c) expression in cortical P2 fraction at 30 and 60 min following ethanol administration (n =8). *p<0.05 compared with saline.
Table 2. Adult PKC isoform expression following acute ethanol exposure.
High dose acute ethanol administration does not alter PKC isoform expression in adult rats (n=7–8/group).
| Saline | 30 Minute | 60 Minute | |
|---|---|---|---|
| PKC β | 100.0 ± 10.27 | 116.1 ± 13.98 | 101.8 ± 10.26 |
| PKC γ | 100.0 ± 14.39 | 97.08 ± 8.91 | 92.58 ± 15.17 |
| PKC δ | 100.0 ± 10.68 | 114.9 ± 16.10 | 110.2 ± 11.64 |
| PKC ε | 100.0 ± 7.49 | 82.42 ± 9.87 | 77.26 ± 9.45 |
| PKC ζ | 100.0 ± 8.217 | 90.15 ± 5.85 | 97.01 ± 11.82 |
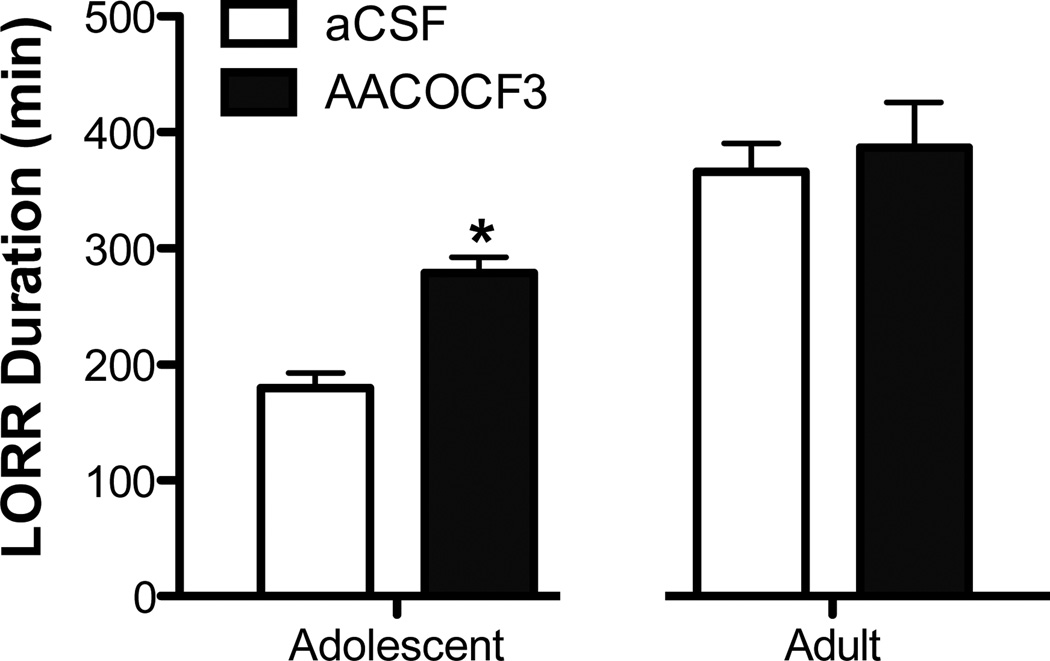
cPLA2/AA pathway contributes to adolescent ethanol-induced LORR
Since only novel isoforms of PKC were altered following ethanol exposure in adolescence, we investigated whether the cPLA2/AA contributed to ethanol behaviors. Arachidonic acid is typically freed from phospholipids through enzymatic cleavage by cytoplasmic phospholipase A2 and is known to preferentially activate novel and atypical isoforms of PKC. Therefore, to indirectly assess AA involvement, we centrally administered the cytoplasmic phospholipase A2 inhibitor AACOCF3. Similar to LORR above, adolescent and adult were analyzed separately due to differences in righting reflex durations. CalC, AACOCF3 increased the duration of ethanol-induced LORR in adolescents by 98.7 minutes (p<.001, 54.8%). In contrast, no effect was observed in adults (Figure 6). Results are likely not due to ethanol metabolism as BECs were reduced in AACOCF3 treated adolescents (p<0.05; 245.1 ± 12.3 vs 173.5 ± 18.6 for aCSF and AACOCF3, respectively). BECs were similar for adults (215.3 ± 20.7 and 216.6 ± 13.8, for aCSF and AACOCF3, respectively).
Figure 6. cPLA2/AA pathway contributes to adolescent ethanol-induced loss of righting reflex (LORR).
Intracerebroventricular administration of AACOCF3 increased sleep time in adolescent, but not adult rats. (n=8–9), *p<0.05, compared to age-matched controls.
DISCUSSION
The present study demonstrates that PKC isoforms are differentially regulated throughout ontogeny and may potentially contribute to adolescent synaptosomal GABAAR subunit expression and ethanol-related behavior. Analyses of whole-cell homogenates revealed that PKCβ, -δ, -ε and -ζ levels were all elevated at various adolescent ages whereas PKCγ was lower during adolescence in comparison to adults. Interestingly, analysis of synaptosomal fractions at identical times revealed differences in isoform expression that did not correlate with whole-cell lysate preparations. Although PKCβ, -γ, -ε and -ζ expression was correlated in both whole cell lysates and synaptosomes, PKCδ was lower in synaptosomal fractions but higher in whole-cell analysis during adolescence relative to adults. GABAAR synaptosomal expression also differed as α4 and δ subunits were lower, but α1 and γ2 subunits were either elevated or unchanged, respectively, during adolescence compared to adulthood. Behaviorally, PKC activity reciprocally modulated acute ethanol-induced LORR in adolescents compared to adults, and only adolescent novel and atypical PKC isoform translocation differed following ethanol exposure, thereby suggesting a putative role in adolescent ethanol sensitivity. Further analysis revealed that inhibiting the cPLA2/AA pathway produced a large increase in adolescent ethanol sensitivity while remaining ineffective in adults.
Data from this present study agree with previous ethanol behavioral assessments in PKC knockout mice. For example, reduced ethanol LORR sensitivity and lower PKCγ expression observed in adolescents parallels reduced ethanol LORR in PKCγ knockout mice (Bowers et al. 1999; Harris et al. 1995). Similarly, elevated adolescent PKCε expression may also contribute to decreased adolescent sensitivity as PKCε knockouts are more sensitive to ethanol LORR (Hodge et al. 1999). Age-related differences in PKCδ may also play a role. Although peak levels of total PKCδ during mid-adolescence initially contrast with reduced sensitivity in PKCδ knockout studies (Choi et al. 2008), reductions in adolescent synaptosomal PKCδ levels are consistent with the knockout results. Although it is unclear from the present results as to which factors contribute to the discrepancy in total versus synaptosomal PKCδ, further studies assessing subcellular localization will help address this difference, as synaptosomal preparations exclude peptides in other subcellular localizations such as cytoplasm. Further, given the current behavioral data supporting a potential role the cPLA2/AA pathway in adolescent ethanol sensitivity, characterization of PKC isoforms in other subcellular preparations will give valuable insight into their ontogenetic involvement. Such future assessment is critical as PKC isoforms are promiscuous in other intracellular molecular pathways in addition to regulation of receptors in synaptic regions. Interestingly, PKCβ was also altered through ontogeny, reaching peak levels during late adolescence. While the reason for this peak in relation to ethanol sensitivity is unclear as studies elsewhere suggest PKCβ is unrelated to ethanol-sensitivity (Kumar et al. 2010; Werner et al. 2011), it is possible that elevated PKCβ is reflective of hormonal changes related to puberty in late adolescence (Thomson et al. 1993; Wang et al. 2012) or glial-related mechanisms (Masliah et al. 1991; Russell & Acevedo-Duncan 2005).
PKC isoforms are well known modulators of GABAA receptors, from receptor association and regulation of surface expression (reviewed in: Kumar et al. 2009) to function (Brandon et al., 2000; Proctor et al., 2003) and can influence behavior (Tretter et al., 2009; Terunuma et al., 2008). Thus, although changes in total PKC observed across adolescence may contribute in part to ethanol-sensitivity, it’s likely that their regulation of GABAAR subtypes also contributes to basal and ethanol-related adolescent behavioral responses. For instance, GABAAR α1 and γ2 subunits are commonly localized synaptically and are involved in phasic inhibition, whereas GABAAR α4 and δ subunits are primarily localized extrasynaptically and are implicated in tonic inhibition (e.g., Choi et al. 2008; Prenosil et al. 2006). Consistent with prior studies, elevated adolescent GABAAR α1 (and potentially γ2) subunit expression observed here and elsewhere (Yu et al. 2006), likely contributes to increased phasic inhibition during development (Cohen et al. 2000; Hahm et al. 2005). Importantly, such increases may be due to region specific developmental trajectories as miniature inhibitory postsynaptic current amplitudes are similar between adolescents and adults in other brain regions (Cohen et al. 2000; Fleming et al. 2007; Hahm et al. 2005). In parallel to synaptic GABAARs, we also noted lower GABAAR α4 and δ subunit expression during early adolescence compared to adults, which is in agreement with reduced GABAAR tonic inhibition during this period (Fleming et al. 2007).
Ethanol potentiates both synaptic and extrasynaptic GABAARs. Given that extrasynaptic receptors are suggested to be markedly more sensitive to ethanol (Lovinger & Homanics 2007), it is possible that reduced adolescent ethanol responses include PKC isoform regulation of specific GABAAR subtypes. In fact, recent evidence suggests extrasynaptic receptors contribute to the sedative/hypnotic effects of ethanol and other GABAA receptor agents (Kretschmannova et al. 2013; Liang et al. 2009; Martin et al. 2011). Of particular interest, given that PKCδ colocalizes with extrasynaptic δ-containing GABAARs and influences tonic inhibition (Choi et al. 2008), reductions in basal synaptosomal PKCδ observed here may also contribute to decrements in adolescent GABAAR function. However, ethanol’s sedative/hypnotic effects are complex and are likely also driven by potentiation of synaptic receptors (Blednov et al., 2011). As such, PKCε phosphorylation of GABAAR γ2 subunits decreases ethanol potentiation (Qi et al. 2007); therefore increases in basal adolescent synaptosomal PKCεmay also influence adolescent ethanol-related responses. As such, developmental regulation of novel PKC isoform activity may contribute to adolescent ethanol-related effects. Additionally, lower levels of PKCγduring adolescence may also contribute to α and α4-containing GABAAR regulation (Kumar et al. 2010; Werner et al. 2011). Notably, lower levels of PKCγ observed here agrees with prior adolescent studies (Van Skike et al. 2010).
Albeit correlative, this interpretation should also be taken with caution, as it should be noted that the temporal variations in basal PKC isoform in the current study do not necessarily coincide with synaptosomal GABAA receptor subunit expression. Potentially, basal GABAA receptor synaptosomal expression may not be contingent on PKC as in vitro PKC knockdown and in vivo PKC knockout studies report normal GABAA subunit expression (Choi et al. 2008; Kumar et al. 2010; Werner et al. 2011). Rather, PKC’s regulatory effects on GABAA receptors become more prominent following ethanol exposure (Carlson et al. 2013; Kumar et al. 2010; Werner et al. 2011). Ultimately, although outside of scope of the current experiments, future studies investigating PKC regulation of adolescent GABAA-R expression and phosphorylation are necessary to better elucidate PKC isoforms in adolescent ethanol action. Additionally, synaptosomal analyses may exclude additional extrasynaptic receptor populations; this can be easily addressed by assessing GABAA receptor surface expression. It is also possible that fluctuations during specific adolescent time points are critical for interactions between specific PKC isoforms and GABAA receptor subtypes, thereby influencing ethanol responses; therefore caution should again be warranted with interpreting experimental results across all adolescent periods. Nonetheless, independent of basal expression levels, GABAA receptor function differs in PKC knockout models (Hodge 1999; Choi, 2008; Haris RA 1995; Proctor WR 2003) and may very well contribute to age-related differences in GABAA receptor electrophysiological responses. Taken together, regulation of GABAA receptor expression and function contributes to ethanol’s sedative-hypnotic effects and may contribute to adolescent responses. Finally, basal expression could also be related to other kinases. In fact, our recent analysis of protein kinase A regulatory subunits display reductions at similar time points (Gigante et al., submitted).
Behaviorally, we demonstrated that PKC activity differentially alters adolescent and adult responses to sedative/hypnotic doses of ethanol. Consistent with previous work, the non-selective PKC activator PDBu decreased adult sleep time (Ohsawa & Kamei 1997). In comparison, PDBu had no effect on adolescent LORR. Strikingly, administration of the PKC inhibitor CalC elicited reciprocal behavioral responses in adults and adolescents such that CalC increased adolescent sleep time, but was ineffective in adults. The latter is consistent with prior adult studies (Ohsawa & Kamei 1997). One possibility for these effects may be a greater baseline level of PKC activity in adolescents, such that further activation was ineffective, as noted after administering a higher dose. Conversely, the inability of CalC to increase adult sleep times may be due to a floor effect in adult PKC activity. Interestingly, Kumar et al., (2005) demonstrated that CalC pretreatment increases muscimol sleep time in rats. Although this initially may appear to contradict our results, subjects in that study were between the adolescent and adult ages used in the present study based on reported animal weights, and hence may be in a late-adolescent/early adult stage of development. Furthermore, their results assessing PKC activity on muscimol-induced LORR and adolescent studies elsewhere (Silveri & Spear 2002), along with the present study further supports PKC regulation of GABAARs in adolescent ethanol-related sedative/hypnotic effects. However, it should be noted that such behavioral effects should not be over-simplified to only fluctuations in PKC and GABAA receptors, as adolescents display reduced ethanol sensitivity across all adolescent ages that were molecularly characterized here relative to adults. Further, as PKC inhibition failed to restore adolescent LORR to adult levels, other age-dependent mechanisms are likely involved. Again, other kinases such as protein kinase A (Gigante et al., submitted) that also regulate GABAARs, may contribute to the remaining differences.
Apart from the behavioral data, adult synaptosomal PKC isoforms remained unaltered following acute high dose ethanol-administration, findings again consistent with previous reports (Kumar et al. 2012). However, unlike adults, ethanol reduced adolescent synaptosomal novel and atypical PKC isoforms. Such effects may possibly contribute to GABAAR function in response to ethanol exposure and eventual ethanol-related behavior. Along with data suggesting reduced tonic inhibition post ethanol administration in animals lacking PKCδ (Choi et al. 2008), our data highlights the possibility that lower levels of synaptosomal PKCδ isoform expression may be facilitating reduced tonic inhibition in cortical regions following ethanol administration in adolescence. However, ethanol’s effects on synaptosomal novel and atypical PKC expression may be region specific. Although adolescents display reduced hippocampal GABAAR tonic currents, ethanol potentiation of these extrasynaptic currents is greater in adolescents compared to adults (Fleming et al. 2007). Conversely, it is possible that similar effects may occur initially in adolescents, potentially due to increased synaptosomal PKCδ. Again, assessing adolescent synaptosomal GABAAR subunit regulation immediately following ethanol exposure may help address this issue. In either case, these findings further support a potential role for novel and atypical PKC isoforms contributing to differences between adolescent and adult ethanol responses.
Finally, we questioned whether novel and atypical PKCs were being differentially activated in adolescent and adults. As DAG activates both novel and conventional isoforms of PKC, it is unlikely that alterations in this pathway contribute to adolescent ethanol-related behavioral responses. However, fatty acids such as AA bind to a distinct site on PKC that is separate from the DAG and phorbol ester binding sites and can preferentially activate novel and atypical PKC isoforms (el Touny et al. 1990; Khan et al. 1995). These data led us to investigate the cPLA2/AA pathway in the sedative/hypnotic effects of ethanol. Inhibiting cPLA2 increased sleep time in adolescents, but not adults. Furthermore, cPLA2/AA inhibition appears to cause a greater magnitude of ethanol-induced increases in sleep time than a non-selective PKC inhibitor. Such results suggest that: 1) novel PKCs may have a selective role in adolescent ethanol-induced behavior; and 2) activation of other PKCs such as the conventional isoforms may have protective effects. Nonetheless, although cPLA2/AA activation of novel and atypical PKCs contributes to adolescent ethanol-related behavior, it still remains unclear whether adolescent effects are due to PKCδ, PKCε, and/or PKCζ. Coupled with the above results, future studies assessing whether these PKC isoforms do in fact translocate to the cytoplasm in response to cPLA2/AA activity as well as PKC-selective inhibitors in adolescent ethanol-related behavior will give further mechanistic insight into adolescent ethanol action. Apart from adolescent effects, it would be of interest to examine the contribution of the arachidonic acid pathway to ethanol tolerance in both ages as studies have shown arachidonic acid and cPLA2 to be decreased following ethanol exposure (Basavarajappa et al. 1998; Rubin 1989). However, cPLA2/AA inhibition does not exclusively modulate PKC, as AA activity also influences monoaminergic (Hellstrand et al. 2002; LaBelle & Polyak 1998; T. et al. 1997), cholinergic (Almeida et al. 1999) and histaminergic (Itoh et al. 2004) transmission. Future studies aim to investigate the specific relationship between AA and PKC in ethanol sensitivity through co-modulation of systems involved in the cPLA/AA cascade
In summary, the present study suggests that PKC isoforms, particularly novel PKCs, are differentially regulated during adolescence and may contribute to adolescent GABAAR biology and ethanol-related behavior.
Acknowledgements
This work was supported by the National Institute of Health grants AA017823 and AA019367 and the Developmental Exposure Alcohol Research Center. The authors would like to thank Laura Mickelson for her technical assistance. We would also like to thank Linda Spear and A. Leslie Morrow for their thoughtful comments and discussions during the drafting of this manuscript.
Nonstandard Abbreviations
- AA
arachidonic acid
- cPLA2
cytoplasmic phospholipase A2
- GABA
gamma-aminobutyric acid
- GABAAR
GABA type A receptor
- PKC
protein kinase C
Footnotes
The authors have now financial conflicts of interest to disclose.
REFERENCES
- Almeida R, RA C, Ribeiro JA. Facilitation by arachidonic acid of acetylcholine release from the rat hippocampus. Brain Res. 1999;826:104–111. doi: 10.1016/s0006-8993(99)01267-6. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Effect of chronic ethanol exposure on mouse brain arachidonic acid specific phospholipase A2. Biochem Pharmacol. 1998;55:515–521. doi: 10.1016/s0006-2952(97)00501-7. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Owen EH, Collins AC, Abeliovich A, Tonegawa S, Wehner JM. Decreased ethanol sensitivity and tolerance development in gamma-protein kinase C null mutant mice is dependent on genetic background. Alcohol Clin Exp Res. 1999;23:387–397. [PubMed] [Google Scholar]
- Carlson SL, Kumar S, Werner DF, Comerford CE, Morrow AL. Ethanol activation of PKA regulates GABAA alpha1 receptor function and trafficking in cultured cerebral cortical neurons. J Pharmacol Exp Ther. 2013;345:317–325. doi: 10.1124/jpet.112.201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Coulter DA. Protracted postnatal development of inhibitory synaptic transmission in rat hippocampal area CA1 neurons. J Neurophysiol. 2000;84:2465–2476. doi: 10.1152/jn.2000.84.5.2465. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Kittler JT, Thomas P, Uren JM, Brandon NJ, Smart TG, Moss SJ. Cell surface stability of gamma-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J Biol Chem. 1999;274:36565–36572. doi: 10.1074/jbc.274.51.36565. [DOI] [PubMed] [Google Scholar]
- Darstein M, Albrecht C, Lopez-Francos L, Knorle R, Holter SM, Spanagel R, Feuerstein TJ. Release and accumulation of neurotransmitters in the rat brain: acute effects of ethanol in vitro and effects of long-term voluntary ethanol intake. Alcohol Clin Exp Res. 1998;22:704–709. [PubMed] [Google Scholar]
- el Touny S, Khan W, Hannun Y. Regulation of platelet protein kinase C by oleic acid. Kinetic analysis of allosteric regulation and effects on autophosphorylation, phorbol ester binding, and susceptibility to inhibition. J Biol Chem. 1990;265:16437–16443. [PubMed] [Google Scholar]
- Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007;97:3806–3811. doi: 10.1152/jn.00101.2007. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Mechanisms of general anesthesia. Environmental health perspectives. 1990;87:199–205. doi: 10.1289/ehp.9087199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C. Antidepressant phenotype by inhibiting the phospholipase Cbeta(1)--protein kinase Cgamma pathway in the forced swim test. Neuropharmacology. 2011;60:937–943. doi: 10.1016/j.neuropharm.2011.01.037. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci STKE 2002. 2002:pl8. doi: 10.1126/stke.2002.137.pl8. [DOI] [PubMed] [Google Scholar]
- Hahm ET, Lee JJ, Min BI, Cho YW. Developmental change of GABAergic postsynaptic current in rat periaqueductal gray. Neurosci Lett. 2005;380:187–192. doi: 10.1016/j.neulet.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Harris RA, McQuilkin SJ, Paylor R, Abeliovich A, Tonegawa S, Wehner JM. Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of gamma-aminobutyrate type A receptors. Proc Natl Acad Sci U S A. 1995;92:3658–3662. doi: 10.1073/pnas.92.9.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand M, Eriksson E, Nilsson CL. Dopamine D(2) receptor-induced COX-2-mediated production of prostaglandin E(2) in D(2)-transfected Chinese hamster ovary cells without simultaneous administration of a Ca(2+)-mobilizing agent. Biochem Pharmacol. 2002;63:2151–2158. doi: 10.1016/s0006-2952(02)01020-1. [DOI] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABAA receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Sendo T, Yano T, Saito M, Kubota T, Oishi R. Comparison of cellular mechanisms underlying histamine release from rat mast cells induced by ionic and nonionic radiographic contrast media. Invest Radiol. 2004;39:455–461. doi: 10.1097/01.rli.0000128656.13658.60. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. Monitoring the future national results on adolescent drug use: overview of key findings 2011. [Google Scholar]
- Khan WA, Blobe GC, Hannun YA. Arachidonic acid and free fatty acids as second messengers and the role of protein kinase C. Cellular signalling. 1995;7:171–184. doi: 10.1016/0898-6568(94)00089-t. [DOI] [PubMed] [Google Scholar]
- Khan WA, Blobe GC, Richards AL, Hannun YA. Identification, partial purification, and characterization of a novel phospholipid-dependent and fatty acid-activated protein kinase from human platelets. J Biol Chem. 1994;269:9729–9735. [PubMed] [Google Scholar]
- Kretschmannova K, Hines RM, Revilla-Sanchez R, Terunuma M, Tretter V, Jurd R, Kelz MB, Moss SJ, Davies PA. Enhanced tonic inhibition influences the hypnotic and amnestic actions of the intravenous anesthetics etomidate and propofol. J Neurosci. 2013;33:7264–7273. doi: 10.1523/JNEUROSCI.5475-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Khisti RT, Morrow AL. Regulation of native GABAA receptors by PKC and protein phosphatase activity. Psychopharmacology (Berl) 2005;183:241–247. doi: 10.1007/s00213-005-0161-x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Lane BM, Morrow AL. Differential effects of systemic ethanol administration on protein kinase cepsilon, gamma, and beta isoform expression, membrane translocation, and target phosphorylation: reversal by chronic ethanol exposure. J Pharmacol Exp Ther. 2006;319:1366–1375. doi: 10.1124/jpet.106.110890. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Ren Q, Beckley JH, O'Buckley TK, Gigante ED, Santerre JL, Werner DF, Morrow AL. Ethanol activation of protein kinase A regulates GABAA receptor subunit expression in the cerebral cortex and contributes to ethanol-induced hypnosis. Frontiers in neuroscience. 2012;6:44. doi: 10.3389/fnins.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sieghart W, Morrow AL. Association of protein kinase C with GABAA receptors containing alpha1 and alpha4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J Neurochem. 2002;82:110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Suryanarayanan A, Boyd KN, Comerford CE, Lai MA, Ren Q, Morrow AL. Ethanol reduces GABAA alpha1 subunit receptor surface expression by a protein kinase Cgamma-dependent mechanism in cultured cerebral cortical neurons. Mol Pharmacol. 2010;77:793–803. doi: 10.1124/mol.109.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz T, Tolg R, Richardt G. Bradykinin B2-receptor-mediated stimulation of exocytotic noradrenaline release from cardiac sympathetic neurons. J Mol Cell Cardiol. 1997;9:2561–2569. doi: 10.1006/jmcc.1997.0492. [DOI] [PubMed] [Google Scholar]
- LaBelle EF, Polyak E. Norepinephrine stimulates arachidonic acid release from vascular smooth muscle via activation of cPLA2. Am J Physiol. 1998;4:1129–1137. doi: 10.1152/ajpcell.1998.274.4.C1129. [DOI] [PubMed] [Google Scholar]
- Liang J, Spigelman I, Olsen RW. Tolerance to sedative/hypnotic actions of GABAergic drugs correlates with tolerance to potentiation of extrasynaptic tonic currents of alcohol-dependent rats. J Neurophysiol. 2009;102:224–233. doi: 10.1152/jn.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Homanics GE. Tonic for what ails us? high-affinity GABAA receptors and alcohol. Alcohol. 2007;41:139–143. doi: 10.1016/j.alcohol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Martin LJ, Zurek AA, Bonin RP, Oh GH, Kim JH, Mount HT, Orser BA. The sedative but not the memory-blocking properties of ethanol are modulated by alpha5-subunit-containing gamma-aminobutyric acid type A receptors. Behav Brain Res. 2011;217:379–385. doi: 10.1016/j.bbr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Masliah E, Yoshida K, Shimohama S, Gage FH, Saitoh T. Differential expression of protein kinase C isozymes in rat glial cell cultures. Brain Res. 1991;549:106–111. doi: 10.1016/0006-8993(91)90605-u. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ. Conserved phosphorylation of the intracellular domains of GABAA receptor beta2 and beta3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology. 1997;36:1377–1385. doi: 10.1016/s0028-3908(97)00111-1. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Doherty CA, Huganir RL. Identification of the cAMP-dependent protein kinase and protein kinase C phosphorylation sites within the major intracellular domains of the beta 1, gamma 2S, and gamma 2L subunits of the gamma-aminobutyric acid type A receptor. J Biol Chem. 1992;267:14470–14476. [PubMed] [Google Scholar]
- NIAAA. 10th Special Report to the U.S. Congress on Alcohol and Health: Highlights From Current Research, National Institutes of Health. Washington DC: National Institute of Alcohol Abuse and Alcoholism; 2000. [Google Scholar]
- NIDA. NIDA Info Facts. National Institutes of Health - Department of Health; 2011. [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Ohsawa M, Kamei J. Pretreatment with the protein kinase C activator phorbol 12,13-dibutyrate attenuates the ethanol-induced loss of the righting reflex in mice: modification by diabetes. Brain Res. 1997;764:244–248. doi: 10.1016/s0006-8993(97)00601-x. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson CH. The Rat Brain in Stereotaxic Coordinates. London, UK: Elsevier; 2007. [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Poelchen W, Bowers BJ, Wehner JM, Messing RO, Dunwiddie TV. Ethanol differentially enhances hippocampal GABAA receptor-mediated responses in protein kinase C gamma (PKC gamma) and PKC epsilon null mice. J Pharmacol Exp Ther. 2003;305:264–270. doi: 10.1124/jpet.102.045450. [DOI] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou WH, Zhang C, Shokat KM, Messing RO. Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma2 subunits. J Biol Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Ramirez RL, Spear LP. Ontogeny of ethanol-induced motor impairment following acute ethanol: assessment via the negative geotaxis reflex in adolescent and adult rats. Pharmacol Biochem Behav. 2010;95:242–248. doi: 10.1016/j.pbb.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. Ethanol interferes with collagen-induced platelet activation by inhibition of arachidonic acid mobilization. Archives of biochemistry and biophysics. 1989;270:99–113. doi: 10.1016/0003-9861(89)90012-x. [DOI] [PubMed] [Google Scholar]
- Russell C, Acevedo-Duncan M. Effects of the PKC inhibitor PD 406976 on cell cycle progression, proliferation, PKC isozymes and apoptosis in glioma and SVG-transformed glial cells. Cell proliferation. 2005;38:87–106. doi: 10.1111/j.1365-2184.2005.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabria J, Torres D, Pasto M, Peralba JM, Allali-Hassani A, Pares X. Release of neurotransmitters from rat brain nerve terminals after chronic ethanol ingestion: differential effects in cortex and hippocampus. Addict Biol. 2003;8:287–294. doi: 10.1080/13556210310001602194. [DOI] [PubMed] [Google Scholar]
- Shanker G, Hampson RE, Aschner M. Methylmercury stimulates arachidonic acid release and cytosolic phospholipase A2 expression in primary neuronal cultures. Neurotoxicology. 2004;25:399–406. doi: 10.1016/j.neuro.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on ethanol sensitivity in immature and mature animals. Alcohol Clin Exp Res. 2002;26:449–456. [PubMed] [Google Scholar]
- Tabakoff B, Nelson E, Yoshimura M, Hellevuo K, Hoffman PL. Phosphorylation cascades control the actions of ethanol on cell cAMP signalling. J Biomed Sci. 2001;8:44–51. doi: 10.1007/BF02255970. [DOI] [PubMed] [Google Scholar]
- Thomson FJ, Johnson MS, Mitchell R, Wolbers WB, Ison AJ, MacEwan DJ. The differential effects of protein kinase C activators and inhibitors on rat anterior pituitary hormone release. Molecular and cellular endocrinology. 1993;94:223–234. doi: 10.1016/0303-7207(93)90171-f. [DOI] [PubMed] [Google Scholar]
- Van Skike CE, Botta P, Chin VS, Tokunaga S, McDaniel JM, Venard J, Diaz-Granados JL, Valenzuela CF, Matthews DB. Behavioral effects of ethanol in cerebellum are age dependent: potential system and molecular mechanisms. Alcohol Clin Exp Res. 2010;34:2070–2080. doi: 10.1111/j.1530-0277.2010.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen Q, Zhou J, Wen J, Bian F, Li G, Mu X, Han Y, Xia G, Zhang M. Specific protein kinase C isoforms alpha and betaI are involved in follicle-stimulating hormone-induced mouse follicle-enclosed oocytes meiotic resumption. PloS one. 2012;7:e45043. doi: 10.1371/journal.pone.0045043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111(3):533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Werner DF, Kumar S, Criswell HE, Suryanarayanan A, Fetzer JA, Comerford CE, Morrow AL. PKCgamma is required for ethanol-induced increases in GABAA receptor alpha4 subunit expression in cultured cerebral cortical neurons. J Neurochem. 2011;116:554–563. doi: 10.1111/j.1471-4159.2010.07140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo JF, Ong WY, Ling SF, Farooqui AA. Intracerebroventricular injection of phospholipases A2 inhibitors modulates allodynia after facial carrageenan injection in mice. Pain. 2004;112:148–155. doi: 10.1016/j.pain.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099:73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]