To the Editor
Spinocerebellar ataxia type 1 (SCA-1) is a rare autosomal dominant neurodegenerative disease caused by a CAG triplet repeat expansion in the SCA-1 gene on chromosome 6, encoding for a protein called ataxin-1.1 SCA-1 typically produces a progressive cerebellar syndrome, with prominent ataxia, dysarthria, and bulbar palsy.2 Early ophthalmologic manifestations include saccadic hypermetria, gaze-evoked nystagmus, and rebound nystagmus, with saccadic slowing and ophthalmoplegia developing in the later stages of the disease.2,3 Decreased visual acuity, dyschromatopsia, and optic atrophy are less commonly reported, with attenuation of oscillatory potentials on full-field electroretinogram (ERG) also reported in six patients.4 We describe a patient with genetically-confirmed SCA-1 who developed progressive painless binocular visual loss, and had evidence of rod and cone photoreceptor dysfunction on full-field ERG.
Case Description
A 56-year-old Caucasian woman presented with an 8-year history of progressive painless binocular visual loss, blepharospasm, and cerebellar ataxia. There was a strong history of cerebellar ataxia with associated visual loss on the paternal side of her family.
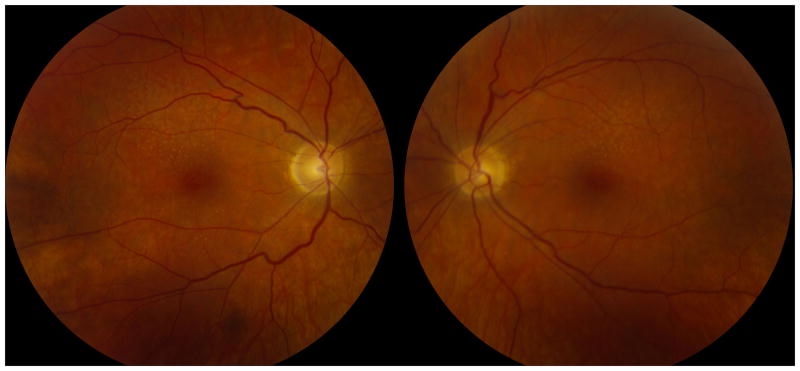
On examination, best-corrected visual acuities were 20/70 in both eyes. She identified only the control Ishihara color plate bilaterally. Confrontation visual fields revealed bilateral central scotomas. External examination revealed blepharospasm and occasional facial grimacing. Anterior segment examination was unremarkable. The pupils were normal. Ocular motor examination revealed slow saccades and impaired smooth pursuit, without nystagmus. General neurologic examination revealed dysarthria, head titubation, and appendicular, truncal, and gait ataxia. Funduscopic examination revealed absent foveal light reflexes, drusen and subtle pigmentary changes in the posterior poles, and retinal arteriolar attenuation (Figure 1). The optic discs were normal and no pigmentary changes were noted in the retinal peripheries (Figure 1).
Figure 1.
Posterior pole photographs from the right and left eyes demonstrate absent foveal light reflexes, superior greater than inferior drusen, subtle macular pigmentary changes, retinal arteriolar attenuation, but normal optic discs.
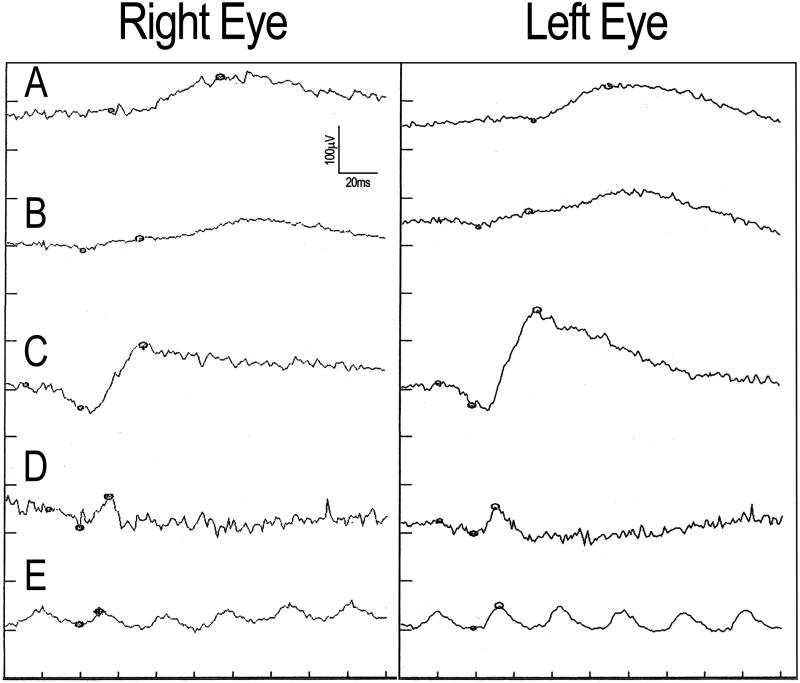
Goldmann visual fields showed central depression and constriction of all isopters. Brain MRI showed brainstem, cerebellar, and cervical spinal cord atrophy. Full-field ERG showed attenuated responses to all stimuli in both eyes, with prolonged implicit times for dim white flashes and maximal white b-waves (Figure 2 and eTable 1). Genetic testing revealed an increased CAG repeat number of 46 (normal <34) in one SCA-1 allele, confirming the diagnosis of SCA-1. Genetic testing for SCA-7 was negative. Other laboratory studies were unrevealing.
Figure 2.
Full-field electroretinogram tracings from the right eye (left panel) and left eye (right panel). Responses to (A) dim white, (B) red, (C) scotopic white, (D) photopic white, and (E) 30 Hz flicker stimuli are shown.
eTable 1. Full-field electroretinogram (ERG) findings.
| Stimulus | Right Eye | Left Eye | Normal 95% CI |
|---|---|---|---|
|
| |||
| Scotopic | |||
| Dim White 0.14 cd s/m2 | |||
| B-wave amplitude (μV) | 70.12* | 73.19* | 84-312 |
| B-wave implicit time (ms) | 92.80* | 88.80* | 62-83 |
| Red | |||
| B-wave amplitude 3.93 cd s/m2 | |||
| B-wave amplitude (μV) | 25.40* | 34.00* | 89-376 |
| B-wave implicit time (ms) | 50.40 | 47.20 | 41-51 |
| Scotopic white 2.11cd s/m2 | |||
| A-wave amplitude (μV) | 47.61* | 46.02* | 129-382 |
| A-wave implicit time (ms) | 20.00 | 17.60 | 15-22 |
| B-wave amplitude (μV) | 128.70* | 197.66* | 264-628 |
| B-wave implicit time (ms) | 52.80* | 52.00* | 33-47 |
|
| |||
| Photopic | |||
| White 2.11 cd s/m2 | |||
| B-wave amplitude (μV) | 64.90* | 57.34* | 72-305 |
| B-wave implicit time (ms) | 34.40* | 29.60 | 26-32 |
| 30 Hz flicker 2.11 cd s/m2 | |||
| Amplitude (μV) | 28.96* | 48.07* | 54-256 |
| Implicit time (ms) | 29.60 | 32.00 | 25-32 |
= abnormal finding;
CI = confidence intervals from a normal population
Comment
Prior to the era of molecular diagnosis, it was known that visual loss in the spinocerebellar ataxias (SCA) could result from primary optic neuropathies or, less commonly, retinal degeneration.5 Detailed studies of vision in the different SCA genotypes have not yet been performed, although it is well established that spinocerebellar ataxia type 7 (SCA-7) is the only genotype in which retinal degeneration commonly occurs.5 In a prior series of genetically confirmed SCA-1 patients, decreased visual acuity, dyschromatopsia, and optic atrophy were reported, but no other funduscopic abnormalities were noted.4 All six patients in that series had attenuated oscillatory potentials and some had decreased B-waves,4 possibly indicating inner retinal dysfunction. Another report described a patient with genetically-confirmed SCA-1 who had progressive visual loss and a pigmentary macular dystrophy,6 similar to that described in SCA-7.5 Full-field ERG revealed photoreceptor dysfunction and genetic testing for SCA-7 was negative, suggesting that a pigmentary macular dystrophy can occur in SCA-1.6 Our patient with genetically-confirmed SCA-1 presented with progressive binocular central visual loss and subtle funduscopic changes suggestive of retinal degeneration, without optic atrophy. Full-field ERG revealed rod and cone dysfunction. The presence of visual loss in other family members with cerebellar ataxia and presumably SCA-1, suggests that the visual loss was a manifestation of SCA-1 and not due to a second pathology. Our findings therefore suggest that visual loss in SCA-1 can be due to rod-cone dystrophy, and should prompt an ERG, even in the absence of obvious retinal changes.
Acknowledgments
This study was supported, in part, by a departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc, New York, New York, and by core grant UL1-RR025008 (Department of Ophthalmology) from the National Institute of Health, Bethesda, Maryland. Dr. Newman is a recipient of a Research to Prevent Blindness Lew R. Wasserman Merit Award.
Footnotes
Disclosure: The authors report no conflicts of interest
References
- 1.Banfi S, Servadio A, Chung MY, et al. Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat Genet. 1994;7(4):513–520. doi: 10.1038/ng0894-513. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki H, Fukazawa T, Yanagihara T, et al. Clinical features and natural history of spinocerebellar ataxia type 1. Acta Neurol Scand. 1996;93(1):64–71. doi: 10.1111/j.1600-0404.1996.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 3.Bürk K, Fetter M, Abele M, et al. Autosomal dominant cerebellar ataxia type I: oculomotor abnormalities in families with SCA1, SCA2, and SCA3. J Neurol. 1999;246(9):789–797. doi: 10.1007/s004150050456. [DOI] [PubMed] [Google Scholar]
- 4.Abe T, Abe K, Aoki M, Itoyama Y, Tamai M. Ocular changes in patients with spinocerebellar degeneration and repeated trinucleotide expansion of spinocerebellar ataxia type 1 gene. Arch Ophthalmol. 1997;115(2):231–236. doi: 10.1001/archopht.1997.01100150233013. [DOI] [PubMed] [Google Scholar]
- 5.Newman NJ. Hereditary optic neuropathies. In: Miller NR, Newman NJ, Biousse V, Kerrison JB, editors. Walsh & Hoyt's Clinical Neuro-Ophthalmology. 6th. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. p. 484. [Google Scholar]
- 6.Saito Y, Matsumura K, Shimizu S, et al. Pigmentary macular dystrophy in spinocerebellar ataxia type 1. J Neurol Neurosurg Psychiatry. 2006;77(11):1293. doi: 10.1136/jnnp.2006.092676. [DOI] [PMC free article] [PubMed] [Google Scholar]