Abstract
Alzheimer’s disease (AD) is the leading cause of dementia in the elderly. Although the etiology of AD remains unclear, microglia-mediated neuroinflammation is believed to play an important role in its pathogenesis. Microglial activation occurs in AD and is characterized by apparent phagocytic activity and by increased production and secretion of several cytokines, chemokines, reactive oxygen and nitrogen species, prostaglandin (PG)E2, and neurotrophic factors. Microglial activation can be neuroprotective through the release of neurotrophic factors and by phagocytosing Aβ, a critical neurotoxic component in AD brain. Concurrently, microglial activation causes elevated inflammatory responses that lead to paracrine damage to neurons. Therefore, a well-controlled microglial activation that diminishes microglial-mediated oxidative damage while promoting neuronal protection may be the key for AD therapy. Peroxisome proliferator-activated receptor gamma (PPARγ) has recently gained increasing attention in AD due to its function as a molecular target for nonsteroidal anti-inflammatory drugs (NSAIDs). In this review, we will discuss the role of PPARγ in microglial innate immunity in AD and how pharmacological manipulation of microglial activation using PPARγ ligands might facilitate the treatment of AD.
Keywords: Alzheimer’s disease, microglial activation, PPARγ, neuroinflammation, β-amyloid, therapy
1. INTRODUCTION
Alzheimer’s disease (AD) is the leading cause of neurodegenerative diseases that cause dementia, and afflicts approximately 25 million individuals worldwide. Senile plaques, which predominantly consist of β-amyloid (Aβ), are one of the pathological hallmarks in AD brain [1]. Although the pathogenesis of AD remains unclear, microglia are associated with Aβ plaques and are thought to play an important role in the pathogenesis of AD [2–7 for review].
Under physiological conditions, microglia actively monitor and respond to changes in the microenvironment [8,9]. Microglia become activated in several diseases. Activation is characterized by morphological changes and by production of various effectors that are critical for neuronal survival during pathological events. These effectors include cytokines, chemokines, reactive oxygen and nitrogen species, prostaglandins (PGs), and neurotrophic factors [10–13]. As a result of such functional changes, microglial activation in AD can be neuroprotective by phagocytosing Aβ and by releasing neurotrophic factors to promote neuronal survival. In contrast, microglial activation also accelerates oxidative stress by accumulating pro-inflammatory cytokines and reactive oxygen and nitrogen species, which in turn leads to exacerbation of AD pathogenesis. Indeed, Aβ is one of a handful of endogenous ligands known to activate microglial innate immunity, a contributor to AD pathogenesis, and has been used for microglial activation as a model in AD research. Hypotheses regarding the misfolding and aggregation of Aβ peptides that are responsible for triggering microglia-mediated inflammation in pathological conditions are under intensive investigation [14–21]. In addition, there are various stimulants used with differing inflammatory activation pathways. Specifically, lipopolysaccharide (LPS) is widely used as an inflammatory stimulant in microglial activation studies. Thus, in this review microglial activation refers to the state stimulated by any of the various stimulants cited in the literature.
Currently there is no cure for AD, and to date, FDA-approved treatments for AD provide symptomatic relief only. Therefore, to overcome these deficits a disease-modifying approach is needed [22–24]. There is an emerging consensus that understanding the regulation of microglial activation may be critical for AD therapy. Treatments devised to promote the beneficial effects of microglial activation, such as enhancing Aβ clearance, while diminishing neuroinflammation are a therapeutic target for AD. Several epidemiologic studies have demonstrated that non-steroidal anti-inflammatory drugs (NSAIDs) are efficacious in reducing the incidence and risk of AD, thereby perhaps suppressing processes of AD at very early stages [25–29]. Discrepant results have been reported from clinical trials of NSAIDs in patients with dementia or mild cognitive impairments from AD.
A postulated target of NSAIDs is peroxisome proliferator-activated receptor gamma (PPARγ) [30–33]. Activation of PPARγ has been shown to be anti-inflammatory and therefore may be capable of modulating microglial innate immunity. In addition, PPARγ activation exerts other beneficial functions such as control of energy metabolism through the modulation of mitochondrial function. PPARγ activation may promote normal mitochondrial functioning through PPARγ coactivator 1α (PGC-1α) [34]. Thus, PPARγ has received increasing attention in AD therapy. The main theme of this review will focus on PPARγ activation in microglial innate immunity in AD. Importantly, we will discuss how pharmacological manipulation of microglial activation using PPARγ ligands might complement treatment of AD.
2. ANTI-INFLAMMATORY PROPERTIES OF PPARγ
PPARγ and two other genetically distinct isoforms (PPARα, PPARβ/γ are highly related receptor isoforms encoded by three genes on chromosomes 3p25, 22q12-q13.1, 6p21.2-p21.1, respectively. All three are members of the PPAR subfamily of nuclear hormone receptor superfamily comprising steroid, thyroid, and retinoid receptors with approximately 75 proteins in the mammalian proteome [35]. There are four known PPARγ mRNA isoforms, PPARγ-1–4, generated from one single PPARγ gene with alternate promoter usage and splicing [36]. PPARγ-1, PPARγ-3, and PPARγ-4 encode the same protein, while PPARγ-2 possesses an additional exon (exon 2) comprised of 28 amino acids. Studies show that a P12A polymorphism at exon 2 of PPARγ gene is linked to type 2 diabetes and was recently found to confer a higher risk of AD in individuals 80 years or older [37].
PPARs are intimately involved in the regulation of gene expression for cell differentiation, apoptosis, glucose and lipid metabolism, inflammation, and carcinoma development [38–39]. Regulation of target gene expression by PPARs is ligand-dependent and requires binding to peroxisome proliferator response elements (PPREs) in the enhancer sites of regulated genes. PPARs have a highly conserved DNA-binding domain, encoding two zinc fingers, and a ligand binding site in its C-terminal region. Also, heterodimerization between PPARs and retinoid X receptors (RXRα, RXRβ, RXRγ) is required for the transcription activity. Upon binding of a ligand to the PPAR-RXR heterodimer complex, nuclear-receptor corepressor proteins are released from the complex, leading to binding of activated PPAR-RXR to PPREs of a target gene, which in turn downregulates or upregulates gene expression. Nevertheless, some regulation of gene expression by PPARs can be also PPRE independent through a poorly understood mechanism called receptor-dependent trans-repression that is believed to inhibit transcription factors, including nuclear factor kappa B (NF-κB), activator protein-1 (AP-1), and signal transducer and activator of transcription-1 (STAT-1) [40,41].
In brain, PPARs have been described in both neurons and glia, with PPARγ localized to specific regions [42,43]. Frontal cortex, basal ganglia, reticular formation, some cranial nerve nuclei, deep cerebellar nuclei, and cerebellar Golgi cells are rich in PPARγ and RXRs, while the hippocampal regions CA1 and CA3 are relatively low in those expressions. Given the pathological significance of the hippocampus in AD, the distinct expression patterns of the receptors may imply different susceptibility to the amyloidogenesis in various brain regions. All three PPARs are expressed in astrocytes, while PPARγ is the major form expressed in microglia, suggesting a role for PPARγ in microglia-mediated neuroinflammation. Not surprisingly, ligands for PPARγ, including both natural metabolites and synthetic compounds, seem to have different binding affinity for PPARγ as well as differing biological impacts. Details will be discussed in the next section.
The anti-inflammatory effects of PPARγ agonists on monocyte/macrophage activation were first studied by Ricote et al [44] and Jiang et al [45]. It was elucidated that PPARγ agonist 15-deoxy-delta (12,14)-prostaglandin J2 (15d-PGJ2) and a synthetic PPARγ agonist BRL49653 may attenuate interferon-γ (IFNγ)-stimulated activation of monocytes/macrophages as reflected in inducible nitric oxide synthase (iNOS), matrix metalloprotease-9 (MMP-9), and scavenger receptor type A. Agonist 15d-PGJ2 and/or troglitazone also led to inhibition of phorbol myristyl acetate (PMA)-induced pro-inflammatory cytokines, such as TNFα. Effector functions of microglia were reported by Petrova et al [46] and Bernardo et al [47]. Although they came to different mechanistic conclusions, their studies agreed that PPARγ agonists are capable of suppressing LPS-induced expressions of iNOS and pro-inflammatory cytokines. By measuring secretion of pro-inflammatory cytokines and cyclooxygenase-2 (COX-2) expression, subsequent studies by others also indicated that PPARγ agonists decreased Aβ- and LPS-stimulated microglial activation [48,49].
Microglia are postulated to play an important role in pathogenesis of several neurodegenerative diseases where they become activated, displaying innate immune responses and increased phagocytic activity. A robust innate immune response occurs in association with Aβ-containing plaques in brains of patients with AD, which may contribute to the pathogenesis of the disease [50–52]. However, as demonstrated by numerous examples, microglial activation is a double-edged sword and need not be exclusively deleterious [53]. In fact, double transgenic mice that overexpress an AD-causing mutant form of human APP as well as a natural inhibitor of complement C3 suggest that the innate immune response in AD may be beneficial in part by enhancing clearance of Aβ peptides from plaques through phagocytosis [54]. Thus, an emerging consensus hypothesizes that aggregated Aβ stimulates a presumably beneficial microglial phagocytic response while at the same time activating a neurotoxic glial innate immune response. If microglial phagocytosis fails to remove the aggregated Aβ, a protracted innate immune response becomes neurotoxic. This fact implies two apparently mutually exclusive therapeutic strategies: stimulate microglia to enhance Aβ clearance and suppress microglial activation to dampen bystander damage to neurons. As a result, completely suppressing microglial activation may not be effective in reversing AD pathogenesis. It is conceivable that clearance of the existing amyloid plaques in AD brain is critical in preventing and/or reversing the pathogenesis, since reducing exposure of neurotoxic Aβ to neurons is an ultimate goal for maintaining normal brain function. Recently, increasing research is focusing on PPARγ activation of microglia in AD because of its function as a target for NSAIDs, suggesting that PPARγ might have potential for modulating microglial activation in neurodegenerative diseases.
3. LIGAND-ACTIVATED PPARγ ACTIVITY IN REGULATION OF MICROGLIAL INNATE IMMUNITY
3.1. Most Common PPARγ Ligands
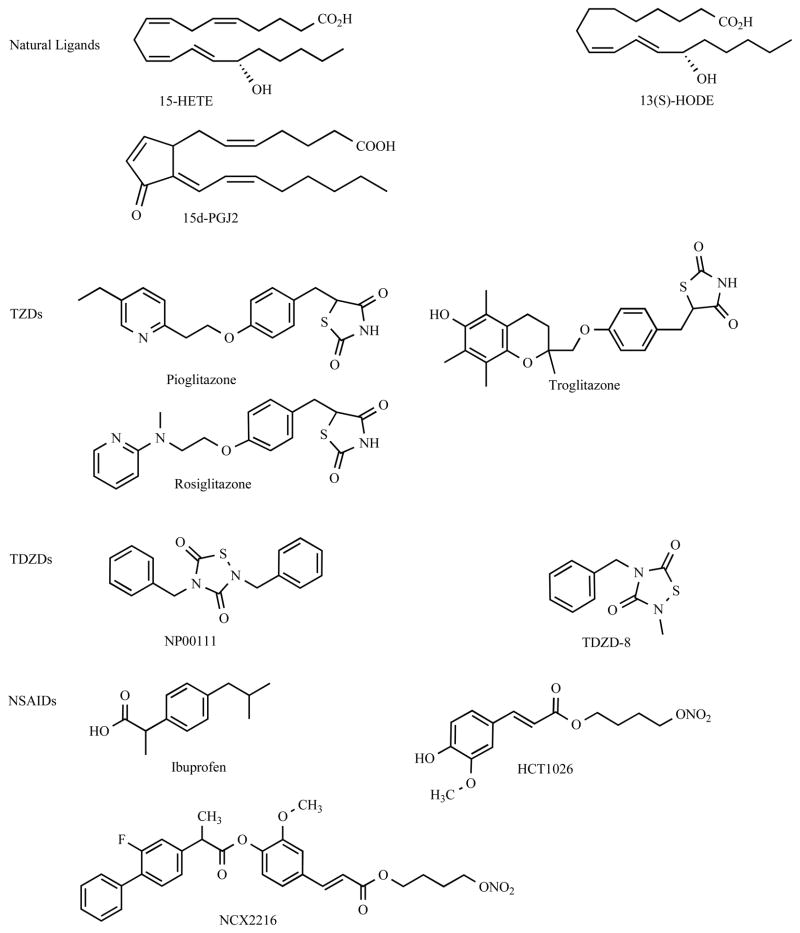
PPARγ activation requires ligand binding for subsequent regulation of gene transcription. Ligands for PPARγ include both synthetic compounds and endogenous metabolites with different binding affinities to the receptor [55]. Chemical structures of some PPARγ ligands are presented in Figure 1. The first known and probably the most potent endogenous PPARγ ligand is 15d-PGJ2, which is the PGD2 derivative from arachidonic acid [56]. Of note, studies report that naturally occurring 15d-PGJ2 may exert beneficial effects on anti-inflammation, dependent and/or independent of its function in activating PPARγ (vide infra). Other endogenous PPARγ ligands include gamolenic acid, arachidonic acid, docosahexanoic acid, eicosapentaenic acid, and some polyunsaturated fatty acid metabolites, such as certain hydroxyeicosatetraenoic acid (HETE) and hydroxyoctadecadienoic acid (HODE) [57]. HETE and HODE can be generated from oxidation of arachidonic acid and linoleic acid, respectively, found in oxidized low density lipoprotein (oxLDL) as well as in membranes. The 11-, 15-HETE and 9-, 13-HODE are among those capable of activating PPARγ with potency similar to 15d-PGJ2 [58,59].
Figure 1.
Chemical structures of PPARγ ligands.
For synthetic PPARγ ligands, the most widely known compounds are the thiazolidinediones (TZDs) that were initially developed for treatment of type II diabetes mellitus (DM). TZDs, also known as glitazones, include troglitazone (Rezulin), pioglitazone (Actos), rosiglitazone (Avandia), and ciglitazone. Troglitazone was the first member of this class to be approved by the FDA for clinical application, however it has subsequently been removed from the market due to its association with an increased risk of hepatitis [60]. In addition to their anti-diabetic potency, all of the above TZDs possess anti-inflammatory properties in vitro and in vivo. Pioglitazone and rosiglitazone, two current FDA approved TZDs for DM, are the most common drugs used in several studies for their anti-inflammatory properties, especially in regulation of microglial activation.
Some TZD-related derivatives that possess potent PPARγ activity are small heterocyclic thiadiazolidinones (TDZDs) [61]. Several TDZDs, including 2,4-dibenzyl-1,2,4-thiadiazolidine-3,5-dione (NP00111) and the related compounds, 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8), and many other synthetic TDZDs (Martinez-A), have been reported [62–64]. They are non-ATP competitive inhibitors of serine/threonine glycogen synthase kinase 3β (GSK-3β), although some of their neuroprotective functions can be dependent or independent of GSK-3β inhibition. In contrast to TZDs, TDZDs appear to have favorable oral bioavailability and blood-brain barrier (BBB) permeability and show neuroprotective effects against LPS- and kainic acid-induced inflammation. Thus, it has been postulated that TDZDs could be of better use in AD therapy. Other TZD derivatives, such as JTT-501, KRP-297, L764406, MCC-555, and some tyrosine-based PPARγ agonists, such as GW1929, GW7845, GI262570, L796449, and L805645 have also been designed and their actions in AD remain to be explored [65–71].
NSAIDs are classic drugs for inflammatory diseases and have been intensively studied in AD. Ibuprofen, indomethacin, and NO-releasing flurbiprofen (e.g. NCX2216 and HCT1026) are known to have PPARγ activity in cell culture models [29,36,72–74]. However, ibuprofen, indomethacin, and HCT1026 tend to completely suppress microglial activation without promoting phagocytic activity. Thus, these drugs may not effective for advanced disease and could be of better use in pre-clinical stages of the disease. In contrast, NCX2216 appears to have dynamic regulation of microglial activation, rather than complete inhibition, through the modulation of PPARγ activation. This may make it be ideal for conferring therapeutic benefit during the course of AD. The proposed interpretation will be discussed in detail below.
Another drug with newly indicated function in PPARγ activation is Telmisartan, an angiotensin receptor II type 1 (AT1) blocker used in treatment of hypertension [75,76]. It exerts a variety of pleiotropic functions, including antioxidative, anti-apoptotic, and anti-inflammatory effects. Telmisartan has been reported to attenuate TNFα and COX-2 expression, reduce oxidative damage, and suppress NADPH oxidase subunit p22 (phox) gene expression, most likely via its partial PPARγ agonist activity [77,78]. Although Telmisartan possesses anti-inflammatory and antioxidative stress properties in an intracerebral hemorrhage, the potential effect on regulation of microglial activation in AD is unknown.
3.2. Treatment of PPARγ Ligands on Microglial Immune Activation
Controlling microglial activation is a promising disease-modifying approach for AD therapy. As discussed above, various ligands are capable of activating PPARγ with different potencies. Increasing evidence indicates that treatment of PPARγ ligands appear to modulate microglial innate immunity, especially in regulation of pro-inflammatory cytokines such as interleukin (IL)-1α, IL-6, and tumor necrosis factor alpha-(TNFα), which have been implicated in the pathogenesis in AD and the regulation of amyloid peptide protein synthesis. In vitro, rosiglitazone, pioglitazone, and ciglitazone have been shown to suppress secretion of TNFα, IL-1β, and/or IL-6 in primary microglia induced by LPS or phorbol 12-myristate 13-acetate [79–81]. In some reports, TZDs also showed activity in inhibiting NO production and proinflammatory gene expressions, such as iNOS, COX-2, and MMP-9 [82–84]. In vivo, an acute oral pioglitazone treatment was reported to reduce the number of activated microglia and expression of COX-2 and iNOS in brain of APPV717I mice, an AD transgenic model [85]. A similar protective effect of pioglitazone was found in LPS-treated mice [84] and in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [86]. Molecular mechanisms underlying the beneficial effects of pioglitazone potentially involve inhibition of p38 and/or suppression of transcription factor NFκB, AP-1 and STATs [87]. Of note, the neuroprotective property of pioglitazone also functions directly by inhibiting oxidative stress in neurons. In addition, rosiglitazone increased IL-4, an anti-inflammatory cytokine that may suppress the activity of IL-1β [88].
The synthetic TZDs are more potent in activating PPARγ activity than endogenous 15d-PGJ2, which appears to be a relatively weak PPARγ agonist at low μM range [89,90]. However, evidence has shown that 15d-PGJ2 may be more effective in attenuating microglial immune responses than TZDs. 15d-PGJ2 has been shown to attenuate the expression of a variety of immune response genes in monocytes/macrophages through inhibition of I-κB kinase activation, alkylation of NF-κB-rel proteins, covalent modification and oligomerization of c-Jun which interferes with its DNA binding activities, and inhibition of AP-1 and phosphorylation of Janus kinase-STAT inflammatory signaling [91–95]. According to the literature and our unpublished data, the antiinflammatory effects of TZDs and 15d-PGJ2 are apparently involved in mechanisms independent of PPARγ activation, including pro-inflammatory cytokine release, iNOS and COX-2 expression and associated signaling. This was shown when these effects were not prevented by a PPARγ antagonist, GW9662. In another study, PGA2, a 15d-PGJ2-like cyclopentenone prostaglandin without apparent PPARγ binding affinity, was also reported to show ability of regulating microglial activation similar to 15d-PGJ2 [79]. Thus, these findings suggest that the beneficial effects of PPARγ ligand treatments are not necessarily achieved through activating PPARγ directly.
Of note, several in vivo models show that some TZDs have limited or no permeability across the BBB, suggesting a lack of direct impact on brain PPARγ: pioglitazone has poor permeability and rosiglitazone is reported not to cross the BBB at all. Another indicator of likely indirect brain effects of TZDs is found when evaluating corticosteroid levels. Because high levels of serum corticosteroids may impair cognitive function, TZDs may stimulate improvement of learning and memory in animals by reducing peripheral corticosteroids. Thus, the beneficial effects of these PPARγ ligands in animal models further implicate an indirect effect on the brain and may be due to multi-targeting in antiinflammation.
In contrast to TZDs, TDZDs are small heterocyclic thiadiazolidinones with favorable oral bioavailability, BBB permeability and show neuroprotective effects against LPS and kainic acid-induced inflammation. Thus, it has been postulated that TDZDs could be of better use in AD therapy. TDZDs are non-ATP competitive inhibitors of GSK-3β, which is critical in AD pathogenesis. However, some of their neuroprotective functions appear to be dependent or independent of GSK-3β inhibition. Intriguingly, the antiinflammatory effects of TDZDs, such as NP00111, NP01138, and NP031112, on pro-inflammatory cytokines and proteins are dependent of PPARγ activation because the beneficial effects were completely inhibited by the antagonist GW9662 [62,96].
Another intriguing aspect of PPARγ ligands in modulating microglial activation is with respect to NSAIDs. It is postulated that the potency of NSAIDs to stimulate PPARγ activity contributes to their ability to inhibit COX and iNOS activity and NFκB signaling, all of which is beneficial for AD therapy. In AD transgenic mice, ibuprofen and indomethacin did reduce Aβ load [85,97]. However, these conventional NSAIDs showed largely disappointing results in several AD human trials, with the beneficial effects even less potent in patients with advanced AD. These results suggest that the beneficial effects may be compound-specific.
Complete suppression of microglial activation may not be ideal for AD treatment because some functions of microglial activation may have neuroprotective effects [3, 98]. Recently, a NO-releasing flurbiprofen, NCX2216, has been shown to be effective on AD amyloid pathology and can either promote or inhibit microglial activation. NCX2216 and its related compound HCT1026 are R-enantiomer and NO-releasing derivatives of flurbiprofen. Unlike HCT1026, NCX2216 not only suppressed inflammation but also activated microglia in vivo and in vitro. Thus, NCX2216 may promote clearance of amyloid plaques. It is postulated that the presence of a NO-donating moiety with anti-oxidant activity, i.e., ferulic acid, may contribute to its dynamic regulation of microglial activation. The underlying mechanism may be explained by its unique nitration effects on PPARγ receptor [72]. It was found that NCX2216 possessed a long lasting effect on PPARγ and can induce nitration of the receptor, resulting in suppression of further PPARγ activation by itself or by endogenous ligands. The action prevents complete inhibition of microglial activation. As a result, NCX2216 transiently inhibited TNFα and NO production, while PGE2 and IL-1β are persistently inhibited. Thus, treatment of NCX2216 preserves certain functions of microglial activation that may have protective outcomes. One important function is microglial phagocytosis in Aβ clearance, which will be discussed below. The potential of NCX2216 in AD therapy is under investigation.
Taken together, the beneficial effects of PPARγ ligands are compound-specific and can be dependent and/or independent of PPARγ activation. The most potent PPARγ agonists are not necessarily the most effective compounds for regulation of microglial regulation. Therefore, the key to successful AD therapy may lie in modulating microglial activation, through dynamic regulation of PPARγ activation while preserving the beneficial function of microglial activation which diminishes neuroinflammation.
3.3. Regulation of Microglial Aβ Phagocytosis by Activation of PPARγ
In addition to the anti-inflammatory activity, another function of PPARγ is its ability to promote Aβ clearance. Aβ clearance is an action that reduces Aβ-activated neuroinflammation in brain and is critical to AD pathogenesis. It is hypothesized that microglial phagocytic activity plays an important role in reducing Aβ accumulation, which may be inhibited in AD brain. Phagocytosis is a complicated process [99,100 for review] and is a specialized immune function critical in protecting brain against neurotoxic agents during pathological events. It removes Aβ accumulation as well as apoptotic cells in AD. Entry of a substance into microglia by phagocytosis requires re-organization of the actin-based cytoskeleton involved in ligation of receptors with Aβ-forming phagosomes and phagolysosomes. Recognition of the ligation can be opsonin-dependent (via classical Fc receptors or complement receptors) or opsonin-independent (via non-classical mannose receptors or scavenger receptors). Fc receptors are various types of receptors for the Fc portion of IgG, such as FcγRI and FcγRII. CR3, the receptor for the complement protein C3bi, is the important complement receptor in this regard.
Aβ can bind to a variety of cell surface receptors, including the B-class scavenger receptor CD36, the alpha(6)beta(1)-integrin, the integrin-associated protein/CD47, receptor for advanced glycation endproducts (RAGE), serpin enzyme complex receptor, and heparin sulfate proteoglycans [101–105]. However, not all receptor bindings are involved in induction of microglial activation and/or phagocytosis. In microglia, binding between fibrillary Aβ and cell surface receptors, including the B-class scavenger receptor CD36, the alpha(6)beta(1)-integrin, and the integrin-associated protein/CD47, activate intracellular signaling cascades involved in phagocytic machinery [106]. It was reported that PPARγ activation induced Aβ clearance in primary murine mixed glia and cortical neuronal cultures. Other studies reported that the naturally occurring 15D-PGJ2 and synthetic TZDs, such as rosiglitazone, were able to transcriptionally induce expression of CD36 in macrophages [83]. The binding has been shown to drive a tyrosine kinase-based signaling cascade leading to induction of phagocytic activity. It was reported that phosphorylation of Vav, a guanine nucleotide exchange factor (GEF) for Rac1, appears to be required for Aβ-stimulated intracellular signaling events upstream of phagosome formation; Vav-deficient microglia showed attenuated phagocytic activity [107]. However, the effects of PPARγ agonists on these signaling events and expression of opsonin-dependent receptors in microglia are still unknown.
3.4. Regulation of PGE2 Expression by PPARγ Activation
Prostaglandin E2 (PGE2) is a product derived from arachidonic acid by cyclooxygenase (COX) and specific synthases. There are conflicting reports of PGE2 both mediating neurotoxicity and being neuroprotective, as well as both enhancing and suppressing macrophage phagocytosis. Increased PGE2 in the central nervous system, as occurs early in AD, may thus have both pro- and anti-inflammatory actions and may significantly modulate microglial phagocytosis. However, ongoing high levels of PGE2 appear to have adverse effects on AD pathogenesis. These reported differences indicate that regulation of PGE2 seems to be an appropriate target for AD therapy. Some nitric oxide-releasing NSAIDs derived from flurbiprofen, such as NCX2216 and HCT1026, are more potent than other NSAIDs in activating PPARγ. Intriguingly, these flurbiprofen derivatives have been shown to inhibit PGE2 synthesis in microglia, which action is associated with their ability in activating PPARγ [72].
PGE2 is a potent autocrine and paracrine factor that is distinct from other eicosanoid products of COX because of multiple G-protein coupled receptor subtypes of E prostanoid (EP), EP1-4, that are linked to functionally antagonistic second messenger systems. All EP receptor subtypes are expressed in brain. Recently, we reported that ablation of a one microglial PGE2 receptor, EP2, may enhance microglial Aβ phagocytosis while suppressing bystander damage to neurons from Aβ activation of microglial [108]. Data suggest that expression of microglial EP2 inhibits microglial phagocytic activity and is critical to microglia-mediated neuroinflammation. Furthermore, we also found that 15d-PGJ2 suppressed LPS-induced EP2 mRNA expression in microglia, suggesting a role of PPARβ in regulation of EP2-mediated neurotoxicity (unpublished data). This finding is consistent with other reports that PPARγ activation by TZDs inhibits EP2 expression in carcinoma cells [109]. Taken together, it appears that the beneficial effects of PPARγ activation may be involved in regulation of EP2 signaling.
3.5. Synthetic Ligands for PPARγ in Human Trial
Rosiglitazone use has recently been reported in two clinical studies with AD subjects. In the first, a small clinical study examining 30 subjects with mild AD or amnestic mild cognitive impairment, a 6-month treatment of rosiglitazone (4 mg daily) was associated with an improved memory and cognitive function as measure by delayed recall and selective attention [110]. The authors also reported that serum APP concentrations remained unchanged in subjects treated with rosiglitazone, while serum APP levels were reduced in the control group, consistent with the observation that Aβ decreases with progression of AD. Although using serum APP or Aβ levels to assess Aβ status in brain is still controversial, the data support the possible therapeutic use of PPARγ agonist for AD.
Another larger scale study of patients was reported by Risner et al [111]. A total of 336 mild-to-moderate AD patients treated with 3 different doses of rosiglitazone (2, 4, and 8 mg daily) and 106 patients receiving placebo completed the study. Following six months of treatment, outcomes were measured by AD Assessment Scale-Cognitive and Clinician’s Interview-Based Impression of Change Plus Caregiver Input global scores. This study suggests that the 8 mg dose of rosigliatazone may be associated with improved cognitive function in a subgroup defined by inheritance of common alleles of the apolipoprotein E gene (APOE), but with only marginal significance. Importantly, the data indicated that the beneficial effects were significantly impaired in those patients who inherited the ε4 allele of APOE. In contrast to those who inherited only ε2 and ε 3 alleles of APOE, ε4 allele-positive patients did not show improvement in response to drug. These studies support the beneficial effect of PPARγ agonist in AD therapy for at least some patients. However, the efficacy of the drug in treating AD is not satisfactory. Given the limited permeability of rosiglitazone across the BBB as discussed above, it is unclear whether rosiglitazone can modulate microglial activation in brain. Thus, there is plenty of room for improvement in future drug discovery.
4. SUMMARY
AD is the most commonly occurring dementia in elderly and is a devastating disease with massive neurodegeneration, which leads to major loss of brain function and eventually death. Because there is no cure for AD and current FDA-approved AD treatment is limited to symptomatic relief, a disease-modifying approach is needed. Although the etiology of AD remains to be clarified, microglia-mediated neuroinflammation has been implicated in the pathogenesis of AD. Even as it has become clear that the pathological impacts of microglial activation are complex, there is justifiable optimism that treatments devised to promote the beneficial effects of microglial activation, such as enhancing Aβ clearance while diminishing neuroinflammation, are an appropriate therapeutic target for AD. Due to its anti-inflammatory properties, PPARγ has conceivably gained increasing attention in AD research. Several in vivo and in vitro studies have indicated that activation of PPARγ by various ligands suppresses microglia-mediated neuroinflammation to different extents. However, it appears that efficacy in AD models is ligand specific and may be related to PPARγ activation. Indeed, activation of PPARγ is beneficial in down-regulation of pro-inflammatory gene expression as well as in control of microglial activation. To effectively counteract the disease, microglial activation needs to be finely regulated rather than completely inhibited in order to preserve the neuroprotective mechanisms while minimizing the microglia-mediated neuroinflammation. Finally, although the role of microglial activation is being examined predominantly in AD, a variety of neurodegenerative diseases share a similar presence of microglia-mediated inflammation and oxidative stress. The approaches or drugs developed by targeting microglial function in AD may also apply to other neurodegenerative diseases.
References
- 1.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 3.Streit WJ. Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Brain Res Rev. 2005;48:234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 5.Kitazawa M, Yamasaki TR, LaFerla FM. Microglia as a potential bridge between the amyloid beta-peptide and tau. Ann NY Acad Sci. 2004;1035:85–103. doi: 10.1196/annals.1332.006. [DOI] [PubMed] [Google Scholar]
- 6.Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 9.Fetler L, Amigorena S. Neuroscience. Brain under surveillance: the microglia patrol. Science. 2005;309:392–393. doi: 10.1126/science.1114852. [DOI] [PubMed] [Google Scholar]
- 10.Cooper NR, Kalaria RN, McGeer PL, Rogers J. Key issues in Alzheimer’s disease inflammation. Neurobiol Aging. 2000;21:451–453. doi: 10.1016/s0197-4580(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 11.El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 12.Husemann J, Loike JD, Kodama T, Silverstein SC. Scavenger receptor class B type I (SR-BI) mediates adhesion of neonatal murine microglia to fibrillar beta-amyloid. J Neuroimmunol. 2001;114:142–150. doi: 10.1016/s0165-5728(01)00239-9. [DOI] [PubMed] [Google Scholar]
- 13.Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Murphy GM, Jr, Brachova L, Yan SD, Walker DG, Shen Y, Rogers J. Inflammatory repertoire of Alzheimer’s disease and nondemented elderly microglia in vitro. Glia. 2001;35:72–79. doi: 10.1002/glia.1072. [DOI] [PubMed] [Google Scholar]
- 14.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 16.Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr Assembly of A beta amyloid protofibrils: an in vitro model for a possible early event in Alzheimer’s disease. Biochemistry. 1999;38:8972–8980. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- 17.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal longterm potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 18.Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK1/2 activation mediates Abeta oligomer-induced neurotoxicity via. caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 2006;281:20315–20325. doi: 10.1074/jbc.M601016200. [DOI] [PubMed] [Google Scholar]
- 19.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 20.Sturchler E, Galichet A, Weibel M, Leclerc E, Heizmann CW. Site-specific blockade of RAGE-Vd prevents amyloid-beta oligomer neurotoxicity. J Neurosci. 2008;28:5149–5158. doi: 10.1523/JNEUROSCI.4878-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nimmrich V, Grimm C, Draguhn A, Barghorn S, Lehmann A, Schoemaker H, Hillen H, Gross G, Ebert U, Bruehl C. Amyloid beta oligomers (A beta(1-42) globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J Neurosci. 2008;28:788–797. doi: 10.1523/JNEUROSCI.4771-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shie FS, Woltjer RL. Manipulation of microglial activation as a therapeutic strategy in Alzheimer’s disease. Curr Med Chem. 2007;14:2865–2871. doi: 10.2174/092986707782359981. [DOI] [PubMed] [Google Scholar]
- 23.Vellas B, Andrieu S, Sampaio C, Wilcock G European Task Force group. Disease-modifying trials in Alzheimer’s disease: a European task force consensus. Lancet Neurol. 2007;6:56–62. doi: 10.1016/S1474-4422(06)70677-9. [DOI] [PubMed] [Google Scholar]
- 24.Golde TE. Disease modifying therapy for AD? J Neurochem. 2006;99(3):689–707. doi: 10.1111/j.1471-4159.2006.04211.x. [DOI] [PubMed] [Google Scholar]
- 25.Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Klegeris A, McGeer PL. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr Alzheimer Res. 2005;2:355–365. doi: 10.2174/1567205054367883. [DOI] [PubMed] [Google Scholar]
- 28.Pasinetti GM. From epidemiology to therapeutic trials with antiinflammatory drugs in Alzheimer’s disease: the role of NSAIDs and cyclooxygenase in beta-amyloidosis and clinical dementia. J Alzheimers Dis. 2002;4:435–445. doi: 10.3233/jad-2002-4510. [DOI] [PubMed] [Google Scholar]
- 29.Townsend KP, Praticò D. Novel therapeutic opportunities for Alzheimer’s disease: focus on nonsteroidal anti-inflammatory drugs. FASEB J. 2005;19:1592–1601. doi: 10.1096/fj.04-3620rev. [DOI] [PubMed] [Google Scholar]
- 30.Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007;1771:1031–1045. doi: 10.1016/j.bbalip.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, Walter J, Klockgether T, van Leuven F, Heneka MT. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci USA. 2006;103:443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gasparini L, Ongini E, Wenk G. Non-steroidal antiinflammatory drugs (NSAIDs) in Alzheimer’s disease: old and new mechanisms of action. J Neurochem. 2004;91:521–536. doi: 10.1111/j.1471-4159.2004.02743.x. [DOI] [PubMed] [Google Scholar]
- 33.Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, Van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128(Pt 6):1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 34.Handschin C, Spiegelman BM. Peroxisome proliferator activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27(7):728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 35.Robinson-Rechavi M, Carpentier AS, Duffraisse M, Laudet V. How many nuclear hormone receptors are there in the human genome? Trends Genet. 2001;17:554–556. doi: 10.1016/s0168-9525(01)02417-9. [DOI] [PubMed] [Google Scholar]
- 36.Meirhaeghe A, Amouyel P. Impact of genetic variation of PPARgamma in humans. Mol Genet Metab. 2004;83:93–102. doi: 10.1016/j.ymgme.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Scacchi R, Pinto A, Gambina G, Rosano A, Corbo RM. The peroxisome proliferator-activated receptor gamma (PPARgamma2) Pro12Ala polymorphism is associated with higher risk for Alzheimer’s disease in octogenarians. Brain Res. 2007;1139:1–5. doi: 10.1016/j.brainres.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 38.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;6785:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 39.Wan YJ, Cai Y, Lungo W, Fu P, Locker J, French S, Sucov HM. Peroxisome proliferator-activated receptor alpha mediated pathways are altered in hepatocyte-specific retinoid X receptor alpha-deficient mice. J Biol Chem. 2000;275:28285–28290. doi: 10.1074/jbc.M000934200. [DOI] [PubMed] [Google Scholar]
- 40.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004;5:419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 41.Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. J Steroid Biochem Mol Biol. 2003;85(2–5):267–273. doi: 10.1016/s0960-0760(03)00214-0. [DOI] [PubMed] [Google Scholar]
- 42.Cabrero A, Laguna JC, Vazquez M. Peroxisome proliferator activated receptors and the control of inflammation. Curr Drug Targets Inflamm Allergy. 2002;1:243–248. doi: 10.2174/1568010023344616. [DOI] [PubMed] [Google Scholar]
- 43.Kainu T, Wikstrom AC, Gustafsson JA, Pelto-Huikko M. Localization of the peroxisome proliferator-activated receptor in the brain. Neuro report. 1994;5:2481–2485. doi: 10.1097/00001756-199412000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 45.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 46.Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Delta 12, 14-prostaglandin J2. Proc Natl Acad Sci USA. 1999;96(8):4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-Delta12, 14-prostaglandin J2 in the regulation of microglial functions. Eur J Neurosci. 2000;12(7):2215–2223. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- 48.Gasparini L, Ongini E, Wenk G. Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer’s disease: old and new mechanisms of action. J Neurochem. 2004;91(3):521–536. doi: 10.1111/j.1471-4159.2004.02743.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH, Baik EJ. Effects of peroxisome proliferator-activated receptor agonists on LPS-induced neuronal death in mixed cortical neurons: associated with iNOS and COX-2. Brain Res. 2002;941(1–2):1–10. doi: 10.1016/s0006-8993(02)02480-0. [DOI] [PubMed] [Google Scholar]
- 50.Pasinetti GM. Cyclooxygenase and inflammation in Alzheimer’s disease: experimental approaches and clinical interventions. J Neurosci Res. 1998;54:1–6. doi: 10.1002/(SICI)1097-4547(19981001)54:1<1::AID-JNR1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 51.McGeer PL, McGeer EG. Inflammation of the brain in Alzheimer’s disease: implications for therapy. J Leukoc Biol. 1999;65:409–415. doi: 10.1002/jlb.65.4.409. [DOI] [PubMed] [Google Scholar]
- 52.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 54.Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc Natl Acad Sci USA. 2002;99(16):10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43(4):527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 56.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 57.Lenz ML, Hughes H, Mitchell JR, Via DP, Guyton JR, Taylor AA, Gotto AM, Jr, Smith CV. Lipid hydroperoxy and hydroxy derivatives in copper-catalyzed oxidation of low density lipoprotein. J Lipid Res. 1990;31(6):1043–1050. [PubMed] [Google Scholar]
- 58.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93(2):229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 59.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400(6742):378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 60.Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96(2):519–525. doi: 10.1111/j.1572-0241.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 61.Ottmann G, Hooks H. 1, 3-Diethyleneguanidines. J Med Chem. 1966;9(6):962–964. doi: 10.1021/jm00324a042. [DOI] [PubMed] [Google Scholar]
- 62.Luna-Medina R, Cortes-Canteli M, Alonso M, Santos A, Martínez A, Perez-Castillo A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivatives through peroxisome proliferator-activated receptor gamma activation. J Biol Chem. 2005;280(22):21453–21462. doi: 10.1074/jbc.M414390200. [DOI] [PubMed] [Google Scholar]
- 63.Kim SD, Yang SI, Kim HC, Shin CY, Ko KH. Inhibition of GSK-3beta mediates expression of MMP-9 through ERK1/2 activation and translocation of NF-kappaB in rat primary astrocyte. Brain Res. 2007;1186:12–20. doi: 10.1016/j.brainres.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Martinez A, Alonso M, Castro A, Pérez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J Med Chem. 2002;45(6):1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 65.Shibata T, Matsui K, Nagao K, Shinkai H, Yonemori F, Wakitani K. Pharmacological profiles of a novel oral antidiabetic agent, JTT-501, an isoxazolidinedione derivative. Eur J Pharmacol. 1999;364(2–3):211–219. doi: 10.1016/s0014-2999(98)00832-2. [DOI] [PubMed] [Google Scholar]
- 66.Murakami K, Tobe K, Ide T, Mochizuki T, Ohashi M, Akanuma Y, Yazaki Y, Kadowaki T. A novel insulin sensitizer acts as a coligand for peroxisome proliferator-activated receptor alpha (PPAR-alpha) and PPAR-gamma: effect of PPAR-alpha activation on abnormal lipid metabolism in liver of Zucker fatty rats. Diabetes. 1998;47(12):1841–1847. doi: 10.2337/diabetes.47.12.1841. [DOI] [PubMed] [Google Scholar]
- 67.Elbrecht A, Chen Y, Adams A, Berger J, Griffin P, Klatt T, Zhang B, Menke J, Zhou G, Smith RG, Moller DE. L-764406 is a partial agonist of human peroxisome proliferator activated receptor gamma. The role of Cys313 in ligand binding. J Biol Chem. 1999;274(12):7913–7922. doi: 10.1074/jbc.274.12.7913. [DOI] [PubMed] [Google Scholar]
- 68.Berger J, Bailey P, Biswas C, Cullinan CA, Doebber TW, Hayes NS, Saperstein R, Smith RG, Leibowitz MD. Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-gamma: binding and activation correlate with antidiabetic actions in db/db mice. Endocrinology. 1996;137(10):4189–4195. doi: 10.1210/endo.137.10.8828476. [DOI] [PubMed] [Google Scholar]
- 69.Way JM, Görgün CZ, Tong Q, Uysal KT, Brown KK, Harrington WW, Oliver WR, Jr, Willson TM, Kliewer SA, Hotamisligil GS. Adipose tissue resistin expression is severely suppressed in obesity and stimulated by peroxisome proliferator activated receptor gamma agonists. J Biol Chem. 2001;276(28):25651–25653. doi: 10.1074/jbc.C100189200. [DOI] [PubMed] [Google Scholar]
- 70.Cobb JE, Blanchard SG, Boswell EG, Brown KK, Charifson PS, Cooper JP, Collins JL, Dezube M, Henke BR, Hull-Ryde EA, Lake DH, Lenhard JM, Oliver W, Jr, Oplinger J, Pentti M, Parks DJ, Plunket KD, Tong WQ. N-(2-Benzoylphenyl)-L-tyrosine PPARgamma agonists. 3. Structure activity relationship and optimization of the N-aryl substituent. J Med Chem. 1998;41(25):5055–5069. doi: 10.1021/jm980414r. [DOI] [PubMed] [Google Scholar]
- 71.Berger J, Tanen M, Elbrecht A, Hermanowski-Vosatka A, Moller DE, Wright SD, Thieringer R. Peroxisome proliferator activated receptor-gamma ligands inhibit adipocyte 11beta - hydroxysteroid dehydrogenase type 1 expression and activity. J Biol Chem. 2001;276(16):12629–12635. doi: 10.1074/jbc.M003592200. [DOI] [PubMed] [Google Scholar]
- 72.Bernardo A, Gasparini L, Ongini E, Minghetti L. Dynamic regulation of microglial functions by the non-steroidal antiinflammatory drug NCX 2216: implications for chronic treatments of neurodegenerative diseases. Neurobiol Dis. 2006;22(1):25–32. doi: 10.1016/j.nbd.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 73.Bernardo A, Ajmone-Cat MA, Gasparini L, Ongini E, Minghetti L. Nuclear receptor peroxisome proliferator-activated receptor-gamma is activated in rat microglial cells by the antiinflammatory drug HCT1026, a derivative of flurbiprofen. J Neurochem. 2005;92(4):895–903. doi: 10.1111/j.1471-4159.2004.02932.x. [DOI] [PubMed] [Google Scholar]
- 74.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272(6):3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 75.Jung KH, Chu K, Lee ST, Kim SJ, Song EC, Kim EH, Park DK, Sinn DI, Kim JM, Kim M, Roh JK. Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J Pharmacol Exp Ther. 2007;322(3):1051–1058. doi: 10.1124/jpet.107.120097. [DOI] [PubMed] [Google Scholar]
- 76.Unger T, Jakobsen A, Heroys J, Ralph A, Rees T, Shaw M. Targeting cardiovascular protection: the concept of dual reninangiotensin system control. Medscape J Med. 2008;10(Suppl):S4. [PMC free article] [PubMed] [Google Scholar]
- 77.Cianchetti S, Del Fiorentino A, Colognato R, Di Stefano R, Franzoni F, Pedrinelli R. Anti-inflammatory and anti-oxidant properties of telmisartan in cultured human umbilical vein endothelial cells. Atherosclerosis. 2008;198(1):22–28. doi: 10.1016/j.atherosclerosis.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 78.Takai S, Jin D, Kimura M, Kirimura K, Sakonjo H, Tanaka K, Miyazaki M. Inhibition of vascular angiotensin-converting enzyme by telmisartan via. the peroxisome proliferator-activated receptor gamma agonistic property in rats. Hypertens Res. 2007;30(12):1231–1237. doi: 10.1291/hypres.30.1231. [DOI] [PubMed] [Google Scholar]
- 79.Storer PD, Xu J, Chavis J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J Neuroimmunol. 2005;161(1–2):113–122. doi: 10.1016/j.jneuroim.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 80.Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor gamma agonist rosiglitazone. J Neurochem. 2006;97(2):435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- 81.Woster AP, Combs CK. Differential ability of a thiazolidinedione PPARgamma agonist to attenuate cytokine secretion in primary microglia and macrophage-like cells. J Neurochem. 2007;103(1):67–76. doi: 10.1111/j.1471-4159.2007.04706.x. [DOI] [PubMed] [Google Scholar]
- 82.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61(4):352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 83.Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000;20(2):558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xing B, Xin T, Hunter RL, Bing G. Pioglitazone inhibition of lipopolysaccharide-induced nitric oxide synthase is associated with altered activity of p38 MAP kinase and PI3K/Akt. J Neuroinflammation. 2008;5:4. doi: 10.1186/1742-2094-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci. 2003;23(20):7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88(2):494– 501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- 87.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2(10):748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 88.Loane DJ, Deighan BF, Clarke RM, Griffin RJ, Lynch AM, Lynch MA. Interleukin-4 mediates the neuroprotective effects of rosiglitazone in the aged brain. Neurobiol Aging. 2007 Oct 23; doi: 10.1016/j.neurobiolaging.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Jackson SM, Parhami F, Xi XP, Berliner JA, Hsueh WA, Law RE, Demer LL. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999;19(9):2094–2104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 90.Nosjean O, Boutin JA. Natural ligands of PPARgamma: are prostaglandin J(2) derivatives really playing the part? Cell Signal. 2002;1,4(7):573–583. doi: 10.1016/s0898-6568(01)00281-9. [DOI] [PubMed] [Google Scholar]
- 91.Cernuda-Morollón E, Rodríguez-Pascual F, Klatt P, Lamas S, Pérez-Sala D. PPAR agonists amplify iNOS expression while inhibiting NF-kappaB: implications for mesangial cell activation by cytokines. J Am Soc Nephrol. 2002;13(9):2223–2231. doi: 10.1097/01.asn.0000025786.87646.b1. [DOI] [PubMed] [Google Scholar]
- 92.Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-deoxydelta 12, 14-prostaglandin J2 inhibits multiple steps in the NFkappa B signaling pathway. Proc Natl Acad Sci USA. 2000;97(9):4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castrillo A, Mojena M, Hortelano S, Boscá L. Peroxisome proliferator-activated receptor-gamma-independent inhibition of macrophage activation by the non-thiazolidinedione agonist L-796, 449. Comparison with the effects of 15-deoxy-delta(12, 14)-prostaglandin J(2) J Biol Chem. 2001;276(36):34082–34088. doi: 10.1074/jbc.M102472200. [DOI] [PubMed] [Google Scholar]
- 94.Ward C, Dransfield I, Murray J, Farrow SN, Haslett C, Rossi AG. Prostaglandin D2 and its metabolites induce caspase dependent granulocyte apoptosis that is mediated via. inhibition of I kappa B alpha degradation using a peroxisome proliferator activated receptor-gamma-independent mechanism. J Immunol. 2002;168(12):6232–6243. doi: 10.4049/jimmunol.168.12.6232. [DOI] [PubMed] [Google Scholar]
- 95.Park EJ, Park SY, Joe EH, Jou I. 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem. 2003;278(17):14747–14752. doi: 10.1074/jbc.M210819200. [DOI] [PubMed] [Google Scholar]
- 96.Luna-Medina R, Cortes-Canteli M, Sanchez-Galiano S, Morales-Garcia JA, Martinez A, Santos A, Perez-Castillo A. NP031112, a thiadiazolidinone compound, prevents inflammation and neurodegeneration under excitotoxic conditions: potential therapeutic role in brain disorders. J Neurosci. 2007;27(21):5766–5776. doi: 10.1523/JNEUROSCI.1004-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci. 2000;20(15):5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Minghetti L, Ajmone-Cat MA, De Berardinis MA, De Simone R. Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain Res Brain Res Rev. 2005;48(2):251–256. doi: 10.1016/j.brainresrev.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 99.Greenberg S, Chang P, Wang DC, Xavier R, Seed B. Clustered syk tyrosine kinase domains trigger phagocytosis. Proc Natl Acad Sci USA. 1996;93(3):1103–1107. doi: 10.1073/pnas.93.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mitchison TJ. Evolution of a dynamic cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 1995;349(1329):299–304. doi: 10.1098/rstb.1995.0117. [DOI] [PubMed] [Google Scholar]
- 101.Boland K, Behrens M, Choi D, Manias K, Perlmutter DH. The serpin-enzyme complex receptor recognizes soluble, nontoxic amyloid-beta peptide but not aggregated, cytotoxic amyloid-beta peptide. J Biol Chem. 1996;271(30):18032–18044. doi: 10.1074/jbc.271.30.18032. [DOI] [PubMed] [Google Scholar]
- 102.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 103.El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382(6593):716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 104.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17(3):553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 105.Watson DJ, Lander AD, Selkoe DJ. Heparin-binding properties of the amyloidogenic peptides Abeta and amylin. Dependence on aggregation state and inhibition by Congo red. J Biol Chem. 1997;272(50):31617–31624. doi: 10.1074/jbc.272.50.31617. [DOI] [PubMed] [Google Scholar]
- 106.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta amyloid mediates microglial activation. J Neurosci. 2003;23(7):2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilkinson B, Koenigsknecht-Talboo J, Grommes C, Lee CY, Landreth G. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281(30):20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 108.Shie FS, Breyer RM, Montine TJ. Microglia lacking E Prostanoid Receptor subtype 2 have enhanced Abeta phagocytosis yet lack Abeta-activated neurotoxicity. Am J Pathol. 2005;166(4):1163–1172. doi: 10.1016/s0002-9440(10)62336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han S, Roman J. Suppression of prostaglandin E2 receptor subtype EP2 by PPARgamma ligands inhibits human lung carcinoma cell growth. Biochem Biophys Res Commun. 2004;314(4):1093–1099. doi: 10.1016/j.bbrc.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 110.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13(11):950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 111.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD Rosiglitazone in Alzheimer’s Disease Study Group. Efficacy of rosiglitazone in a genetically defined population with mild-to moderate Alzheimer’s disease. Pharmacogenom J. 2006;6(4):246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]