Abstract
Context
Breast cancer (BC) is the most common cancer in women, with over 1 million new cases diagnosed every year worldwide. Over recent decades, considerable interest has emerged regarding whether vitamins and/or other supplements can lower the risk of BC. However, previous epidemiologic studies that investigated the association between intake of multivitamin and supplements of single vitamins and minerals and BC risk have reported conflicting results. Whether vitamins can actually reduce BC risk is still controversial.
Objective
This study examined whether multivitamin and calcium use was associated with BC incidence and DNA repair capacity (DRC).
Design
The research team designed an observational, case-control study.
Setting
All work was performed at the Ponce School of Medicine and Health Sciences under the direct supervision of principal investigator Dr Jaime Matta.
Participants
Participants were 836 women recruited primarily from the private practices of oncologists, gynecologists, and surgeons in Puerto Rico.
Intervention(s)
A total of 312 individuals in the breast cancer (BC) group and 524 individuals in the control group were compared for their multivitamin and calcium intake, DRC levels, and other covariates.
Outcome Measure(s)
Odds ratios (OR), adjusted using both crude analysis and multiple logistic regression, were used as measures of association between BC and DRC and other selected variables.
Results
The BC group had 30% reduced odds of taking multivitamins and calcium as compared to controls: (1) OR = 0.7 (95% CI, 0.4–1.0; P = .073) for multivitamins and (2) OR = 0.7 (95% CI, 0.4–1.2; P = .167) for calcium. Women with low DRC had 50% lower odds of taking calcium and 30% lower odds of currently taking vitamins OR = 0.5 (95% CI, 0.4–0.7; P = .001) for calcium and (2) OR = 0.7 (95% CI, 0.5–0.9.1; P = .047) for vitamins.
Conclusions
Although this study is a case-control study in which the risk of BC could not be assessed, results suggest that vitamin supplementation could be an independent protective factor for BC. Calcium intake appears to affect DRC in a positive way, because it was associated with a high DRC level, which in turn is associated with low odds for BC.
Worldwide, breast cancer (BC) is the most frequently occurring cancer in women. Each year, approximately 1.15 million cases and 410 000 deaths from BC are reported globally.1 Over recent decades, considerable interest has emerged regarding whether vitamins and/or other supplements can lower the risk of BC. The possibility that vitamins can offer a protective effect relates to their antioxidant properties that help (1) maintain cellular integrity, coenzyme/cofactor functions, and the functions of the immune and reproductive systems and (2) prevent DNA damage induced by free radicals.2,3 Animal studies indicate that calcium intake is associated with a reduction in the proliferation of mammary cells. Evidence exists that calcium may influence BC risk by maintaining the intracellular calcium concentration.4
The idea of being able to take a multivitamin or calcium to help reduce BC risk is an attractive one. Vitamins and minerals are widely accessible, do not require a prescription, are relatively inexpensive, and are convenient to take. More than half of all US adults take one or more nutritional supplements, the majority being multivitamins. People who currently have a chronic illness or are trying to prevent a recurrence of a serious illness (eg, cancer) are more likely to take vitamins than other populations of people.5 However, previous epidemiologic studies that investigated the association between intake of multivitamins and supplements of single vitamins and minerals and BC risk have reported conflicting results. Whether vitamins can actually reduce BC risk is still controversial.6–13
If such a simple measure as taking a multivitamin can indeed reduce BC risk, then research on the matter is worth pursuing. Being able to identify one modifiable factor that can significantly reduce risk, however, is the exception rather than the rule with many illnesses, including cancers. In a large case-control study of Puerto Rican women, Matta et al recently published findings on modifiable and nonmodifiable risk factors.14 Among the modifiable factors that were identified were intake of multivitamins and calcium. This identification was why the research team chose to study use of multivitamins and calcium, together with DNA repair capacity (DRC).
Because a hallmark of any cancer is faulty response to and repair of DNA damage, DRC was a logical covariate for the current study. Multiple studies have confirmed that low DRC correlates with increased cancer risk.15–17 The previously mentioned study by Matta et al showed that a low DRC was an important risk factor for BC.14 The study by Matta et al (2012) was carried out using the same cohort of women that was the basis of the ancillary study reported here.
People vary in their DNA-repair efficiency and capacity, and those inherent sensitivities to mutagens and carcinogens are increasingly being linked to polymorphisms in genes related to DNA repair.18,19 Although such small changes in gene sequencing as analyzed in that study may not cause cancer, they can predispose people to a higher risk of developing cancer. That predisposition means that modifying interactions among gene risk factors could play an important role in modifying cancer risk.19 If taking a multivitamin or calcium can decrease BC risk, then the topic is worthy of study from both health and financial standpoints.
The current investigation is the first of its kind to address all three of the following questions: (1) is multivitamin and/or calcium intake associated with BC, (2) is multivitamin and/or calcium intake associated with DRC, and (3) is intake of multivitamins and/or calcium independently associated with BC or indirectly associated with BC by potentially influencing DRC?
Methods
Studies on multivitamin use and cancer risk are inherently problematic. The population sampled needs to be sufficiently heterogeneous demographically and ethnically. The duration of the study needs to be long enough to yield useful data. The vitamins and minerals to be studied need to be selected for their biological plausibility. And ideally the best biomarkers need to be incorporated into the study’s design. The research team has addressed these issues with this study.
Participants
The population in the current study was a group of 836 adult, female Puerto Ricans, a genetically diverse population that is an admixture of European, African, and Amerindian ethnic groups.20 The women were recruited primarily from the private practices of oncologists, gynecologists, and surgeons. Participants represented 65 of the 78 counties on the island, as described by Matta et al.14 The ages of the participants ranged from 21 to 89 years. Like the United States and other countries, BC is more prevalent than most cancers within the female population of Puerto Rico and accounts for a disproportionate number of deaths.21
Participants in the BC group (n = 312) were patients with recently diagnosed and histopathologically confirmed, primary breast carcinoma tumors, who had not received chemotherapy, radiotherapy, or blood transfusions for any reason in the prior 5 years. Patients with a previous history of cancer or BC secondary to other types of cancer were not included. Pathology reports were used to confirm the BC diagnosis.
Control participants (n = 524), who were recruited concurrently with participants in the BC group, were women who had (1) received a negative result on a mammogram within the previous 6 months, (2) undergone a clinical breast examination by a gynecologist or other physician, and (3) not received any blood transfusions within the prior 5 years. Because Puerto Rico offers universal health insurance coverage, any healthy women who might develop BC would be treated in the same facilities where BC patients were recruited. This circumstance minimized potential selection bias.
The research team chose to study multivitamin intake, current and during the previous 5 years, based on results from previous studies.8,10,19,22 The team chose to study calcium intake because it regulates cell differentiation, proliferation, and apoptosis,23–26 functions that are deregulated in BC. Finally, inclusion of DRC was based on results of other researchers’ work,17,27,28 and on the current team’s previous studies on DRC as a biomarker for BC risk and recurrence.17,29
Data Collection
To facilitate comparison of the current study’s data to other studies’ results, the research team used a seven-page epidemiological questionnaire that was based on questionnaires used in previously published studies of BC.3,8,11,12,18,21,26,30,31 The current questionnaire was used to gather information on (1) age; (2) body-mass index (BMI); (3) family history of cancer; (4) genetic, gynecological, and hormonal data; (5) history of smoking and alcohol consumption; (6) environmental factors; (7) the use of multivitamin supplements and the use of single-vitamin supplements, β-carotene supplements, or calcium, currently and during the prior 5 years; and (8) other variables that could provide an estimate of BC risk, including civil status, schooling, and occupation. Blood samples for DRC analysis were collected in Vacutainer tubes (BD Franklin Lakes, NJ, USA) containing K2-EDTA anticoagulant. The tubes were kept at room temperature and gently mixed until processed in the laboratory using a host-cell reactivation (HCR) assay.
Measurement of DRC
The HCR assay to measure DRC levels in lymphocytes (white blood cells) is a standard procedure used in numerous molecular epidemiological studies of cancer.14,16,17,23,32–35 The assay used in this ancillary study was the same assay that Matta et al used in the team’s recently published study based on the same cohort of Puerto Rican women.14 The assay measures how well lymphocytes can repair damaged DNA when it is introduced into those cells. This measurement was done by genetically engineering a nonreplicating, plasmid expression vector to contain a gene that is not present in mammalian cells. That gene was exposed to precise doses of ultraviolet, electromagnetic radiation subtype C (UVC), creating damage that would be a direct measure of lymphocyte DRC in each participant. While this assay also measures total DRC, it primarily measures activity generated by one particular DNA-repair pathway—the nucleotide excision repair (NER) pathway.23 NER capacity is low in lymphocytes and tumors of BC patients.14,18,27,36
Statistical Analysis
Statistical analysis was performed using the SPSS 17 statistical package (SPSS, Chicago, IL, USA). The BC and control groups were compared regarding the distribution of selected variables, such as age, family history of BC, DRC, and the use of multivitamins and calcium supplements.
DRC was first analyzed as a continuous variable and then dichotomized into high and low levels by using the optimum cutoff point (3.7%) at which the highest sensitivity and specificity was reached in predicting BC, as suggested by Szklo and Nieto.24 Low- and high-DRC participants were compared regarding the distribution of the same selected variables as were used in the comparison of the BC and control groups. Participants in the two groups were combined in this part of the analysis because the research team did not find important effect modifications regarding the association of DRC with the rest of the covariates between the BC and control groups.
For the crude (unadjusted) analysis of diet-supplement intake and other selected covariates, the BC group was compared to controls, and participants with low DRC were compared to those with high DRC. For DRC as a continuous variable, after using the arcsine variable transformation to approximate normality, the research team used the mean difference to compare the BC and control groups. The 95% confidence intervals were used to assess the precision of the mean differences.
After the crude analysis of continuous variables, the team dichotomized them using the median of the entire sample, including both groups. The team used this criterion because it achieved a better sample distribution, and the sample power did not differ significantly in the associations under study from that obtained when using only the intervention group’s median or that of the controls. The sample power was assessed by using the Fleiss exact method with continuity correction for the difference in proportions between two populations (Epi Info v6).
For categorical variables, the odds ratio (OR) was used as a measure of association, and the 95% confidence interval of the OR was used to assess the precision of this estimate. The two-tailed Fisher’s exact test was used to measure the statistical significance of the crude OR.24 After the research team explored confounding and interaction effects among all of the study’s variables by means of the Mantel and Haenszel stratified analysis, it used multiple logistic regression to measure the adjusted OR. Potential interactions with covariates such as age group, menopause, and childbearing were further examined using multiple logistic regression, adjusting for all covariates simultaneously.24 These analyses to assess the association between BC and supplement intake were adjusted by the following variables: (1) DRC, (2) age, (3) BMI, (4) overweight condition or obesity, (5) educational level, (6) family history of BC, (7) age at menarche, (8) regularity of menstrual cycle, (9) oral contraceptive use, (10) childbearing, (11) age when first child was born, (12) breastfeeding practices, (13) age at onset of menopause, (14) postmenopausal hormone use, and (15) smoking and/or alcohol use.
Results
Demographic Variables
Table 1 includes demographic characteristics of the population studied. The group of BC cases included a larger proportion of older subjects than the control group. For this reason, the statistical comparisons between BC cases and controls were always adjusted by age. All variables used for adjustments in the models were included as well as confounders that were assessed through the study. When comparing DRC levels between the BC and control groups, 81.1% of the BC group exhibited a low DRC compared with 31.2% of the controls. Or alternatively, 68.8% of the control population showed a high DRC level vs 18.9% of the BC group.
Table 1.
Demographic Characteristics and Other Variables Used for Adjusting the Models (n = 836)
| Variable | Control Group n = 524 n (%) |
BC Group n = 312 n (%) |
|---|---|---|
| DRC | ||
| Low <4.39 | 159 (31.2) | 253 (81.1) |
| High >4.4 | 351 (68.8) | 59 (18.9) |
| BMI | ||
| Up to 24.99 kg/m2 | 179 (34.5) | 92 (29.6) |
| >25 kg/m2 | 340 (65.5) | 219 (70.4) |
| Education | ||
| 0 – 8 y | 8 (1.6) | 15 (5.8) |
| 9 – 12 y | 137 (27.4) | 102 (39.7) |
| 13+ y (some college, BA or BS, or graduate studies) | 355 (71.0) | 140 (54.5) |
| BC history in any family member | ||
| Yes | 75 (14.3) | 73 (23.4) |
| No | 449 (85.7) | 239 (76.6) |
| Age of menarche | ||
| 13 y or older | 226 (43.2) | 136 (44.0) |
| 12 y or younger | 297 (56.8) | 173 (56.0) |
| Regular menstrual periods | ||
| Yes | 307 (59.3) | 193 (61.9) |
| No | 211 (40.7) | 119 (38.1) |
| Oral contraceptive use | ||
| Yes | 279 (56.3) | 157 (50.6) |
| No | 217 (43.7) | 13 (49.4) |
| Childbearing | ||
| Nulliparous | 99 (18.9) | 51 (16.3) |
| 1–2 children | 250 (47.7) | 131 (42.0) |
| >3 children | 175 (33.4) | 130 (41.7) |
| Mother’s age at first birth | ||
| <19 y | 72 (17.0) | 53 (20.3) |
| 20–29 y | 283 (66.7) | 158 (60.5) |
| >30 y | 69 (16.3) | 50 (19.2) |
| Breastfeeding ever | ||
| Yes | 212 (45.9) | 112 (39.9) |
| No | 250 (54.1) | 169 (60.1) |
| Menopause | ||
| Yes | 92 (18.0) | 66 (22.0) |
| No | 418 (82.0) | 234 (78.0) |
| Menopause age | ||
| >50 y | 154 (47.8) | 118 (55.1) |
| <49 y | 168 (52.2) | 96 (44.9) |
| Hormone replacement therapy | ||
| Yes | 27 (5.2) | 8 (2.6) |
| No | 492 (94.8) | 298 (97.4) |
| Smoking | ||
| Yes | 46 (8.8) | 43 (13.8) |
| No | 478 (91.2) | 269 (86.2) |
| Alcohol consumption | ||
| Yes | 95 (18.1) | 64 (17.3) |
| No | 429 (81.9) | 258 (82.7) |
Note: Numbers of women in some categories do not add up to 100% of the population because the variable may not be applicable to all participating women (ie, breastfeeding and mother’s age at first birth are only applicable to those who have had at least one life birth), no response, or due to missing values.
Abbreviations: DRC = DNA repair capacity; BMI = body mass index; BC = breast cancer.
No major differences in BMI were found between the two groups, with 70.4% of the BC group and 65.5% of the controls having a BMI >25. The BC group had a lower educational level than controls: 71.0% of the controls had more than 12 years of education while the rate was 54.5% for the BC group. About 23.4% of the BC group reported a family history of BC as compared with 14.3% of the controls. No major differences were found on the smoking variable between the BC group and controls, and just 17.3% of the BC group reported alcohol use.
The control group showed a slightly higher percentage of participants (56.8%) with an age of menarche at 12 years old or younger than the BC did (56.0%). Irregular menstrual periods were found in 38.1% of the BC group whereas 40.7% of controls experienced them. The BC group reported a rate of use of oral contraceptives at 50.6%, with controls at 56.3%, and the BC group reported a larger number of children than controls, with 41.7% of that group bearing three children or more as opposed to 33.4% of controls. A higher percentage of the control group was between 20 and 29 years of age when they bore children, (66.7% vs 60.5% for controls). The BC group and controls showed no major difference in breastfeeding practices; just 39.9% of the BC group responded “yes” to this indicator while 45.9% did. In the BC group, 22.0% reported being menopausal while 18% of controls, with 44.9% of the BC group and 52.2% of controls being 49 years or younger at onset. In the BC group, 2.6% were receiving hormone replacement treatment (HRT) as opposed to 5.2% of controls.
Of the entire sample, 55% of the women were between 41 and 60 years old (Table 2). As age increased, the percentage of BC cases increased with a statistically significant linear trend, from 28.9% to 46.4% (χ2 for trend = 13.1, P < .001). Controls had a decrease in the DRC mean from the 20 to 40 age group to the 41 to 60 age group, falling from 6.5 to 5.9, with the mean rising to 6.3 at the highest age level (>60 y). A slight increase was seen in the BC group’s mean DRC from the 20 to 40 age group to the >60 years age group, from 2.6 to 2.8. The mean DRC difference between the BC group and the controls decreased with age, from 4.0 in youngest group to 3.1 in the oldest.
Table 2.
| Age Groups, y | BC Group n (%) | Control Group n (%) | Total n (%) | BC Group Mean DRC (95% CI) | Control Group Mean DRC (95% CI) | Mean DRC Difference (95% CI) |
|---|---|---|---|---|---|---|
| 20–40 | 37 (11.9) | 91 (17.4) | 128 (15.3) | 2.6 (1.9–3.2) | 6.5 (5.7–7.3) | 4.0 (2.6–5.3) |
| 41–60 | 160 (51.3) | 300 (57.2) | 460 (55.0) | 2.7 (2.4–3.1) | 6.3 (6.0–6.7) | 3.6 (3.1–4.1) |
| >60 | 115 (36.8) | 133 (25.4) | 248 (29.7) | 2.8 (2.5–3.2) | 5.9 (5.4–6.4) | 3.1 (2.4–3.7) |
| Total | 312 (100.0) | 524 (100.0) | 836 (100.0) | 2.8 (2.5–3.0) | 6.3 (6.0–6.5) | 3.5 (3.1–3.9) |
Abbreviations: BC = breast cancer; DRC = DNA repair capacity; CI = confidence interval.
Age distribution of women with BC and cancer-free women: χ2 test for trend = 13.1, P < .001.
Participants were recruited from 2006 to 2010.
Supplement Use
With regard to use of multivitamins and a calcium supplement the research team found no important effect modifications or statistically significant interactions with any of the studied variables in either comparison (BC group vs controls or low-DRC vs high-DRC women).
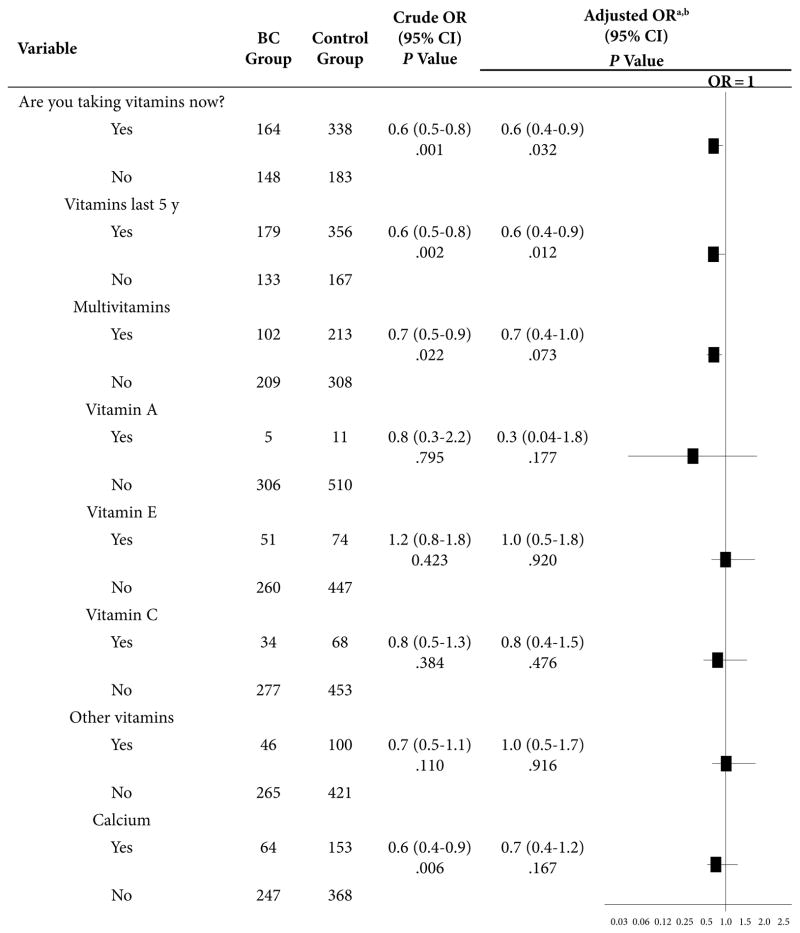
As shown in Table 3, multivitamin use was more frequently found among controls. For the BC group, the odds of taking multivitamins, both currently and in the prior 5 years, were 40% lower than in the control group. Both associations were statically significant (P = .032 and P = .012, respectively). Women with BC had 30% lower odds of taking multivitamins, with a borderline statistical association (P = .073) that became statistically significant (P = .030) when DRC was excluded from the logistics model. Intake of individual vitamins (A, E, C, and other vitamins) was not associated with BC.
Table 3.
Association Between BC and Intake of Vitamins and Calcium Supplements, Estimated by OR
|
Abbreviations: BC = breast cancer; CI = confidence interval; OR = odds ratio; DRC = DNA repair capacity; BMI = body mass index.
Adjusted by DRC, age, BMI, education, family history of BC, age at menarche, regularity of menstrual cycle, oral-contraceptive use, childbearing, age at birth of first child, breastfeeding, menopause, age at menopause, postmenopausal hormone use, and smoking and/or alcohol use.
When DRC was excluded from the model, the association between BC and taking multivitamin supplements was OR = 0.7 (95% CI, 0.5–0.9; P = .030); with calcium supplements, it was OR = 0.5 (95% CI, 0.3–0.7; P < 0.001).
The BC group had 50% lower odds of taking calcium supplements as compared to controls (P < .001). However, when adjusted by DRC level, the odds decreased to 30% (P = .167), denoting the important confounding effect of DRC in the association between calcium and BC.
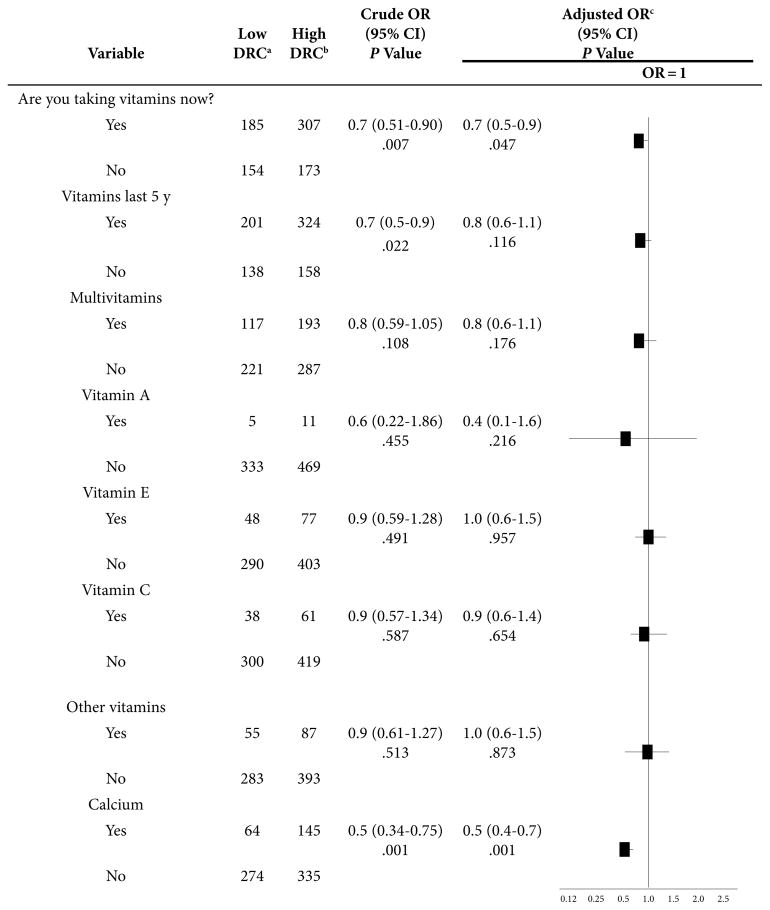
The statistical analysis to investigate the association between DRC (low/high) and intake of single vitamins vs multivitamin and calcium supplements (Table 4) was adjusted by the same group of variables used in Table 3. Women with low DRC had 30% and 50% lower odds of taking multivitamins and calcium supplements, respectively (P = .047 and P = .001) (Table 4). Women with low DRC had 60% lower odds of taking vitamin A supplements, but because only 16 women reported taking it as an individual supplement, the research team was unable to determine whether its effect was statistically significant. Current intake of other single vitamins showed no important associations with DRC levels.
Table 4.
Association Between Low DRC and Intake of Vitamin and Calcium Supplements in the BC Group and the Control Group
|
Abbreviations: BC = breast cancer; OR = odds ratio; DRC = DNA repair capacity; CI = confidence interval.
Low DRC—up to 3.7%.
High DRC—more than 3.7%.
Adjusted by age, BMI, education, family history of BC, age a menarche, regularity of menstrual cycle, oral-contraceptive use, childbearing, age at birth of first child, breastfeeding, menopause, age at menopause, postmenopausal hormone use, and smoking and/or alcohol use.
Discussion
This study evaluated the potential modulatory effect that intake of calcium and multivitamin supplements might have on DRC and BC. The results showed that women with BC had reduced odds of taking multivitamins and/or calcium compared to controls. Similar results were found when the team compared women with low and high DRC levels. Although this is a case-control study in which the risk or protective effects of variables cannot be assessed, the team can speculate that current use of multivitamin supplements may be an independent protective factor for BC. These results support those of other studies, including Nechuta’s 2011 work, “Vitamin Supplement Use During Breast Cancer Treatment and Survival: A Prospective Cohort Study,” which found that multivitamin consumption is associated with reduced BC risk, a reduced rate of BC recurrence, and a higher survival rate in the first six months of treatment for BC.3 The current study, as well as previous ones,3–5,8,12,40 found that multivitamins and calcium may be protective for BC, while single vitamins may not.22,25 The protective effect of multivitamin supplements against BC might be explained by different theories. The most popular theory involves the antioxidant effect, about which much has been published in the last few years. Antioxidants protect cells from the damage caused by free radicals that could cause cancer due to prolonged oxidative damage. Multivitamins function primarily as antioxidants, but how much each component of a multivitamin contributes to the antioxidant effect is unknown. The current study’s outcomes may reflect a dose-response effect. In the study, no single antioxidant vitamin (eg, A, C, or E) as an individual supplement produced a statistically significant protective effect against BC nor did one produce an increase in DRC levels, but the combined ingredients of a multivitamin achieved a measurable protective potential.40,41 It is known that vitamins function together optimally, due to coupled redox reactions.
Calcium has anticarcinogenic effects, regulating cell-structure differentiation, proliferation, and apoptosis.23–26 However, the protective effect of calcium supplements on BC decreased considerably when the association was adjusted by DRC. It is possible that calcium has a direct effect on DRC instead of BC. Increasing intake of calcium is associated with increased DRC levels; in turn, a higher DRC lowers the risk of BC. In other words, the important association for calcium may be with DRC.
Clearly, researchers have more to learn about calcium and its effect on risk of BC. Some evidence suggests that calcium intake may also be related to an increased risk of BC. However, Boyapati et al reported that a protective association between calcium and BC has been found in eight out of nine publications, although not all results were statistically significant.30 The association of calcium and BC should be confirmed with future studies. In addition, some studies have suggested that vitamin D may interact with or confound any positive effect that calcium has on reducing BC risk.26 However, other studies have shown contrary findings.12
The association between DRC (low/high) and intake of vitamins and calcium supplements in Table 3 includes all of the study’s participants (BC group and controls). Both groups were combined for this part of the analysis because no statistically significant differences occurred when they were kept separate (results not shown).
One of the limitations of this observational study and others like it is that the data obtained did not consider dietary factors. The research team did not collect information about the dietary intake of calcium or vitamins in fortified food that may have been part of the participants’ diets. In addition, the team did not obtain ingredient or dosing information on the multivitamins and calcium consumed. Interestingly, the NIH has proposed creating new, national multivitamin databases that “detail the exact composition of supplements, update them on a continuous basis, and assure their constant availability to the research community.”5 Finally, the self-reporting process could create a misclassification error that could underestimate the strength of an association. It is possible that participants didn’t think that certain data items were relevant to them, and therefore, either failed to report them or misreported them.
Conclusions
The research team’s results indicate that multivitamins and calcium supplements may be independent protective factors for BC and are associated with a high DRC. A high DRC may have a strong protective effect against BC, and part of the protective effect of calcium may be explained by an increased DRC. The research team believes that its recent findings could have a significant impact on the prevention of BC and could provide new mechanistic data concerning much publicized controversies over the role of multivitamins in cancer risk. Nevertheless, more studies using other populations are needed to confirm the team’s findings.
Acknowledgments
Thanks go to Rasa Hamilton, Moffitt Cancer Center, for editorial assistance and manuscript preparation. The research team also thanks Carmen Ortiz for revising the manuscript. Additional editorial assistance came from the PSMHS/RCMI Publications Office (G12 RR003050) through Mr Bob Ritchie and Lana Christian from CreateWrite Inc. Dr Michael J. Gonzalez provided critical remarks that strengthened the scientific aspects of this manuscript. This study was made possible by the volunteer participants and through their physicians, including R. Barnes, G. Bolaños, M. Echenique, J. González Cruz, J. Laboy, J. Ortiz Rosado, E. Ramirez Lizardi, Angel Romero, F. Sanchez Gaetan, A. Torres, and S. Santiago Medina.
Footnotes
Author Disclosure Statement
This work was supported by two grants, one from the NCI Diversity Training Branch of the Center to Reduce Cancer Health Disparities (CRCHD) through the MBRS SCORE Program (Grant SO6 GM008239-23 and 1SC1CA157250-01) and one from the PSMHS-Moffitt Cancer Center U56 Cancer Partnership (Grant 5U56 CA126379-04).
Contributor Information
Yeidyly Vergne, Student in the Public Health Program, Ponce School of Medicine and Health Sciences, Ponce, Puerto Rico.
Jaime Matta, Professor in the Department of Pharmacology, Physiology, and Toxicology, Ponce School of Medicine and Health Sciences.
Luisa Morales, Student in the Public Health Program and a laboratory supervisor in the Department of Pharmacology, Physiology, and Toxicology, Ponce School of Medicine and Health Sciences.
Wanda Vargas, Nurse in the Department of Pharmacology, Physiology, and Toxicology, Ponce School of Medicine and Health Sciences.
Carolina Alvarez-Garriga, Associate adjunct professor in the Public Health Program, Ponce School of Medicine and Health Sciences.
Manuel Bayona, Ajunct professor in the Public Health Program, Ponce School of Medicine and Health Sciences.
References
- 1.Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC. Relationship between low ultraviolet B irradiance and higher breast cancer risk in 107 countries. Breast J. 2008;14(3):255–260. doi: 10.1111/j.1524-4741.2008.00571.x. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez MJ, Miranda-Massari JR, Mora EM, et al. Orthomolecular oncology review: ascorbic acid and cancer 25 years later. Integr Cancer Ther. 2005;4(1):32–44. doi: 10.1177/1534735404273861. [DOI] [PubMed] [Google Scholar]
- 3.Dorjgochoo T, Shrubsole MJ, Shu XO, et al. Vitamin supplement use and risk for breast cancer: the Shanghai Breast Cancer Study. Breast Cancer Res Treat. 2008;111(2):269–278. doi: 10.1007/s10549-007-9772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1427–1437. doi: 10.1158/1055-9965.EPI-06-0075. [DOI] [PubMed] [Google Scholar]
- 5.NIH state-of-the-science conference statement on multivitamin/mineral supplements and chronic disease prevention. NIH Consens State Sci Statements. 2006;23(2):1–30. No authors listed. [PubMed] [Google Scholar]
- 6.Maruti SS, Ulrich CM, White E. Folate and one-carbon metabolism nutrients from supplements and diet in relation to breast cancer risk. Am J Clin Nutr. 2009;89(2):624–633. doi: 10.3945/ajcn.2008.26568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, et al. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2006;83(4):895–904. doi: 10.1093/ajcn/83.4.895. [DOI] [PubMed] [Google Scholar]
- 8.Ishitani K, Lin J, Manson JE, Buring JE, Zhang SM. A prospective study of multivitamin supplement use and risk of breast cancer. Am J Epidemiol. 2008;167(10):1197–1206. doi: 10.1093/aje/kwn027. [DOI] [PubMed] [Google Scholar]
- 9.Neuhouser ML, Patterson RE, Kristal AR, White E. Dietary supplements and cancer risk: epidemiological research and recommendations. In: Bendich A, Deckelbaum RJ, editors. Preventive Nutrition: The Comprehensive Guide for Health Professionals. 3. Totowa, NJ: Humana Press; 2005. pp. 89–122. [Google Scholar]
- 10.Neuhouser ML, Wassertheil-Smoller S, Thomson C, et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women’s Health Initiative cohorts. Arch Intern Med. 2009;169(3):294–304. doi: 10.1001/archinternmed.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feigelson HS, Jonas CR, Robertson AS, McCullough ML, Thun MJ, Calle EE. Alcohol, folate, methionine, and risk of incident breast cancer in the American Cancer Society Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2003;12(2):161–164. [PubMed] [Google Scholar]
- 12.Larsson SC, Akesson A, Bergkvist L, Wolk A. Multivitamin use and breast cancer incidence in a prospective cohort of Swedish women. Am J Clin Nutr. 2010;91(5):1268–1272. doi: 10.3945/ajcn.2009.28837. [DOI] [PubMed] [Google Scholar]
- 13.Meulepas JM, Newcomb PA, Burnett-Hartman AN, Hampton JM, Trentham-Dietz A. Multivitamin supplement use and risk of invasive breast cancer. Public Health Nutr. 2010;13(10):1540–1545. doi: 10.1017/S1368980009992187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matta J, Echenique M, Negron E, et al. The association of DNA repair with breast cancer risk in women: a comparative observational study. BMC Cancer Oct. 2012;12:490. doi: 10.1186/1471-2407-12-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Q, Li L, Chen DJ, editors. DNA Repair, Genetic Instability, and Cancer. Hackensack, NJ: World Scientific Publishing Co; 2007. [Google Scholar]
- 16.Matta JL, Villa JL, Ramos JM, et al. DNA repair and nonmelanoma skin cancer in Puerto Rican populations. J Am Acad Dermatol. 2003;49(3):433–439. doi: 10.1067/s0190-9622(03)00918-6. [DOI] [PubMed] [Google Scholar]
- 17.Ramos JM, Ruiz A, Colen R, Lopez ID, Grossman L, Matta JL. DNA repair and breast carcinoma susceptibility in women. Cancer. 2004;100(7):1352–1357. doi: 10.1002/cncr.20135. [DOI] [PubMed] [Google Scholar]
- 18.Grossman L, Wei Q. DNA Repair Capacity (DRC) as a biomarker of human variational responses to the environment. In: Vos JM, editor. DNA Repair Mechanisms: Impact on Human Diseases and Cancer. Austin, TX: R.G. Landes Company; 1995. pp. 329–348. [Google Scholar]
- 19.Wu PE, Shen CY. ‘Hide-then-hit’ to explain the importance of genotypic polymorphism of DNA repair genes in determining susceptibility to cancer. J Mol Cell Biol. 2011;3(1):59–65. doi: 10.1093/jmcb/mjq054. [DOI] [PubMed] [Google Scholar]
- 20.Via M, Gignoux CR, Roth LA, Fejerman L, Galanter J, Choudhry S, et al. History shaped the geographic distribution of genomic admixture on the island of Puerto Rico. Plos One. 2011;6(1):e16513. doi: 10.1371/journal.pone.0016513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hulka BS, Moorman PG. Breast cancer: hormones and other risk factors. Maturitas. 2001;38(1):103–113. doi: 10.1016/s0378-5122(00)00196-1. discussion 113–116. [DOI] [PubMed] [Google Scholar]
- 22.Berube S, Diorio C, Brisson J. Multivitamin-multimineral supplement use and mammographic breast density. Am J Clin Nutr. 2008;87(5):1400–1404. doi: 10.1093/ajcn/87.5.1400. [DOI] [PubMed] [Google Scholar]
- 23.Qiao Y, Spitz MR, Guo Z, et al. Rapid assessment of repair of ultraviolet DNA damage with a modified host-cell reactivation assay using a luciferase reporter gene and correlation with polymorphisms of DNA repair genes in normal human lymphocytes. Mutat Res. 2002;509(1–2):165–174. doi: 10.1016/s0027-5107(02)00219-1. [DOI] [PubMed] [Google Scholar]
- 24.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. 2. Sudbury, MA: Jones and Bartlett; 2007. [Google Scholar]
- 25.Pakseresht S, Ingle GK, Bahadur AK, et al. Risk factors with breast cancer among women in Delhi. Indian J Cancer. 2009;46(2):132–138. doi: 10.4103/0019-509x.49151. [DOI] [PubMed] [Google Scholar]
- 26.Anderson LN, Cotterchio M, Vieth R, Knight JA. Vitamin D and calcium intakes and breast cancer risk in pre- and postmenopausal women. Am J Clin Nutr. 2010;91(6):1699–1707. doi: 10.3945/ajcn.2009.28869. [DOI] [PubMed] [Google Scholar]
- 27.Helzlsouer KJ, Harris EL, Parshad R, Perry HR, Price FM, Sanford KK. DNA repair proficiency: potential susceptiblity factor for breast cancer. J Natl Cancer Inst. 1996;88(11):754–755. doi: 10.1093/jnci/88.11.754. [DOI] [PubMed] [Google Scholar]
- 28.Li SX, Sjolund A, Harris L, Sweasy JB. DNA repair and personalized breast cancer therapy. Environ Mol Mutagen. 2010;51(8–9):897–908. doi: 10.1002/em.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latimer JJ, Johnson JM, Kelly CM, et al. Nucleotide excision repair deficiency is intrinsic in sporadic stage I breast cancer. Proc Natl Acad Sci U S A. 2010;107(50):21725–21730. doi: 10.1073/pnas.0914772107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyapati SM, Shu XO, Jin F, et al. Dietary calcium intake and breast cancer risk among Chinese women in Shanghai. Nutr Cancer. 2003;46(1):38–43. doi: 10.1207/S15327914NC4601_05. [DOI] [PubMed] [Google Scholar]
- 31.Hall J, English DR, Artuso M, Armstrong BK, Winter M. DNA repair capacity as a risk factor for non-melanocytic skin cancer--a molecular epidemiological study. Int J Cancer. 1994;58(2):179–184. doi: 10.1002/ijc.2910580206. [DOI] [PubMed] [Google Scholar]
- 32.Wei Q, Matanoski GM, Farmer ER, Hedayati MA, Grossman L. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci U S A. 1993;90(4):1614–1618. doi: 10.1073/pnas.90.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng L, Eicher SA, Guo ZZ, Hong WK, Spitz MR, Wei Q. Reduced DNA repair capacity in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7(6):465–468. [PubMed] [Google Scholar]
- 34.Spitz MR, Wu X, Wang Y, et al. Modulation of nucleotide excision repair capacity by XPD polymorphisms in lung cancer patients. Cancer Res. 2001;61(4):1354–1357. [PubMed] [Google Scholar]
- 35.Landi MT, Baccarelli A, Tarone RE, et al. DNA repair, dysplastic nevi, and sunlight sensitivity in the development of cutaneous malignant melanoma. J Natl Cancer Inst. 2002;94(2):94–101. doi: 10.1093/jnci/94.2.94. [DOI] [PubMed] [Google Scholar]
- 36.Athas WF, Hedayati MA, Matanoski GM, Farmer ER, Grossman L. Development and field-test validation of an assay for DNA repair in circulating human lymphocytes. Cancer Res. 1991;51(21):5786–5793. [PubMed] [Google Scholar]
- 37.Jyothish B, Ankathil R, Chandini R, et al. DNA repair proficiency: a potential marker for identification of high risk members in breast cancer families. Cancer Lett. 1998;124(1):9–13. doi: 10.1016/s0304-3835(97)00419-9. [DOI] [PubMed] [Google Scholar]
- 38.Nechuta S, Lu W, Chen Z, et al. Vitamin supplement use during breast cancer treatment and survival: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20(2):262–271. doi: 10.1158/1055-9965.EPI-10-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: scientific review. JAMA. 2002;287(23):3116–3126. doi: 10.1001/jama.287.23.3116. [DOI] [PubMed] [Google Scholar]
- 40.Hart C, Cohen R, Norwood M, Stebbing J. The emerging harm of antioxidants in carcinogenesis. Future Oncol. 2012;8(5):535–548. doi: 10.2217/fon.12.45. [DOI] [PubMed] [Google Scholar]
- 41.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70(5):257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]


