Abstract
Purpose
Pharmacy commercial claims databases are widely used for pharmacoepidemiologic research. However, concerns have been raised that these databases may not fully capture claims for generic medication as a result of patients filling outside the context of their insurance. This has implications for many research activities and quality improvement programs. We sought to estimate the percentage of missing drug claims in US commercial claims data using a novel design.
Methods
Using a large US commercial insurance database, we examined the completeness of warfarin prescription claims among patients with atrial fibrillation receiving regular medical follow-up and testing to manage warfarin dosing. We examined 14 different 6-month cross sections. Each cross section was treated independently to identify patients with at least 2 outpatient diagnoses of atrial fibrillation, 2 international normalized ratio tests, and 1 pharmacy claim. Trends in the percentage of patients with prescription claims for generic and branded warfarin were compared by year and 6-month periods using chi-square tests and generalized linear models adjusting for patient characteristics.
Results
Out of 111,170 patients, the percentage of patients with any warfarin drug decreased slightly from 91.7% (95% CI: 91.0, 92.4) in early 2003 to 87.1% (95% CI: 86.7-87.6) in late 2009 (χ2=93.8, p<0.0001). Over the same interval, the proportion of patients with generic warfarin exposure appearing increased significantly, while the proportion of patients with branded warfarin exposure decreased significantly.
Conclusions
Our study supports the possibility that some prescriptions may not be captured in US commercial insurance databases.
Keywords: generic drugs, claims data, data quality
INTRODUCTION
Pharmacy claims databases are widely used in the United States to study drug safety and effectiveness, inappropriate medication use, medication adherence, health care disparities, clinical guideline adherence, and the effects of policy changes.1,2 While patients may be prescribed medications and not fill them, pharmacy claims are generally thought to be a good measure of actual drug exposure.3-5 However, it is possible that prescription medications being used by a patient may not be captured in claims databases.6
This loss of information could happen for various reasons. For example, patients could be paying out-of-pocket for inexpensive prescriptions, receiving samples from a physician, obtaining prescriptions through a spouse's pharmacy benefit or other dual pharmacy benefit such as through Veterans Affairs coverage, or receiving them via mail order from outside the US.7,8 Concerns have also been raised that very low-cost generic prescription drug programs may not be reporting pharmacy claims back to the insurer. Low-cost generic programs started to appear in in the United States beginning in late 20069,10 and are now currently offered by most chain pharmacies.11-13 Because the cost of the generic medications may be less than patients’ co-payments in some situations6, patients may choose to pay in cash without providing insurance information to the pharmacist. If this occurs, the transaction would not be processed by the pharmacy benefit manager, and a record of the prescription fill would not appear in the insurance pharmacy claims database.9
Widespread missing prescription claims, leading to misclassification of prescription drug exposure1, would have important implications for many of research activities.1,14-17 Despite the importance of this topic, the completeness of pharmacy claims data in US insurance databases has not been studied. We examined this issue directly by examining claims for warfarin among patients with atrial fibrillation who were receiving regular medical follow-up and testing explicitly to manage warfarin dosing.
METHODS
Warfarin is an anticoagulant that is often used chronically to prevent stroke in patients with atrial fibrillation. Patients taking warfarin need to be regularly monitored with international normalized ratio (INR) tests and have doses adjusted accordingly. In patients with atrial fibrillation, there is little reason that INRs would be regularly ordered on patients without concomitant warfarin use.18-20 In addition, given that these medications have a narrow therapeutic index, patients largely remain on one formulation once being initiated, minimizing the potential for missing claims in this population. Thus, there is limited potential for frequent switching between brand and generic formulations.20 Few therapeutic alternatives were also available at the time of study, as newer anticoagulants (e.g., dabigatran) were not available until late 2010.19 Using data from a large population of commercially-insured patients, we examined time trends in warfarin filling among patients with atrial fibrillation who were receiving regular INR tests.
Study Population and Data
The data for our study came from the TruvenHealth MarketScan Commercial Claims and Encounters Research Database for the years 2003-2009. These databases include de-identified patient-specific medical inpatient and outpatient claims, outpatient pharmaceutical data and enrollment data for approximately 20 million people annually from over 100 nationwide insurers.
We constructed 14 different 6-month non-overlapping cross-sectional cohorts, beginning from January-June 2003 through July-December 2009, hereafter referred to as early 2003 and late 2009, respectively. Within each 6-month cross section, we selected a cohort of patients with at least 2 International Classification of Diseases, 9th edition (ICD-9) codes for atrial fibrillation (AF) (ICD-9: 427.31) occurring on separate days, 2 Current Procedural Terminology (CPT) codes for an INR (CPT: 85610, 99363, or 99364) occurring on separate days and with continuous enrollment for the 6-month cross sections. We required the 2 ICD-9 and CPT codes to occur on separate days to ensure that the diagnoses and procedures were not being used to rule-out a particular condition. INR claims could have occurred at any point in the 6-month window, which allowed for prevalent use to be captured and for a conservative design. To ensure patients were using pharmacy benefits, we further restricted this cohort to individuals with at least 1 prescription fill for any medication during the 6-month cross section.
To eliminate potential reasons why a warfarin claim may not appear, enrollees were excluded from the 6-month window if they experienced 1 of the following in the 6-month calendar window: 1) hospitalization as determined through a claim in either the inpatient services or inpatient admission files; 2) hepatic-related diagnosis (ICD-9: 570.0, 571.0, 571.2, 571.4, 571.5, 571.9, 572.2, 572.4, 573.3); 3) vitamin K deficiency (ICD-9: 269.0, 286.7); 4) antiphospholipid syndrome (ICD-9: 795.79); 4) other coagulation deficiency (ICD-9: 286.0, 286.1, 286.2, 286.3, 286.4, 286.5, 286.52, 286.53, 286.59, 286.6, 286.7, 286.8); 5) cardiac ablation procedure (CPT procedure code: 93650, 93651); 6) outpatient prescription claims for either heparin or low-molecular weight heparin medications (dalteparin, fondaparinux, enoxaparin, tinzaparin). These exclusions were captured as the INR test may have been used diagnostically in these conditions without a concurrent warfarin prescribing process or to assess the effects of recent warfarin use despite its discontinuation.20 Patients with an inpatient admission were excluded because it was unknown whether missing warfarin claims from a hospitalization might result in INR testing in the outpatient setting. Otherwise, a missing warfarin claim could be misattributed. Use of prescription aspirin was also identified in the outpatient drug files as aspirin could potentially affect the use of anticoagulation and was used as an exclusion criterion in sensitivity analyses.20
For each 6-month calendar period, demographic and insurance plan variables were also identified including: age, region of residence, type of health benefit plan, and gender.
Medication Usage
Prescription drug use was identified through national drug codes (NDC) in the outpatient drug files, merged with the REDBOOK supplement. The cohort assembled based on the inclusion and exclusion criteria was merged with the outpatient drug files to identify those enrollees with warfarin or branded warfarin medication (including Coumadin, Bristol-Myers Squibb, Princeton NJ, and other branded generics not part of the four-dollar generic programs: Jantoven, Marevan, Lawarin, or Waran) exposure at any point in the 6-month calendar window, regardless of whether these occurred prior to or after the INR and AF diagnosis dates. For each prescription claim filled for either generic or branded warfarin, the patient out-of-pocket copay amounts were also captured. Use of aspirin (appearing in the generic name) was also captured from the outpatient drug files.
Statistical Analyses
The proportion of enrollees with at least 1 appearing claim for a generic or branded medication was calculated for each 6-month calendar window and calendar year. 95% confidence intervals were calculated for each proportion of patients. Descriptive statistics were calculated and assessed for all other variables. Chi-square (χ2) tests on the proportion of patients with prescription exposure were conducted to compare the cross sections over time. Multivariable generalized estimating equations were used to estimate the effect of the low-cost generic programs on the adjusted relative proportion of patients with warfarin use as a dependent variable. These models were adjusted for gender, age, region, and insurance plan type, and standard errors were computed robustly using an exchangeable correlation matrix to account for the within-patient correlation of outcomes. The baseline trend change in the proportion of patients with warfarin prescriptions compared with the first 6-month period (early 2003) and the level change following the low-cost generic programs were estimated. The major low-cost generic programs began in early 2007, so the post-period for the analysis was classified as the cross-sections beginning in 2007.21 Sensitivity analyses were also conducted on the inclusion and exclusion criteria to examine how the restrictions affected the results. The association between demographic characteristics and the proportion of patients with prescription exposure was also examined.
For those prescriptions that appeared within the calendar window periods, the day supply and the average copayment for a 30-day supply was calculated for both generic and brand medication per 6-month cross section. Of those prescriptions appearing, the proportion of generic warfarin prescriptions filled for $4.00 per 30 day supply and $10.00 per 90 day supply were also calculated. The proportion of generic prescriptions filled for $0.00 was also captured, indicating that the fill was covered by the insurance. As a secondary analysis, the percentage of mail order use over time per prescription was also assessed. The analyses on medication costs and mail order source were performed to assess trends and possible simultaneous changes in those prescriptions that do appear in the commercial claims databases.
All analyses were performed using SAS 9.2 (SAS institute, Cary, North Carolina). Statistical significance was determined as p<0.05. The UNC Institutional Review Board approved this study.
RESULTS
Between 2003 and 2009, we identified 111,170 continuously-enrolled patients with pharmacy insurance benefits having at least 2 outpatient AF diagnoses, 2 INRs, and 1 pharmacy claim within at least one 6-month period. In total, these individuals contributed to 264,206 6-month periods. When excluding patients for the potential concomitant restrictions, including hospitalization, vitamin K deficiency, antiphospholipid syndrome or other coagulation disorders, hepatic-related disorders, ablation procedure, or prescription fills for heparin or low-molecular weight heparins, 80,267 patients remained, contributing to 183,308 6-month periods. In the cohort of 80,267 individuals, the demographic characteristics and distributions per calendar year are displayed in Table 1. Individual plan composition changed somewhat, with the database containing more individuals with Preferred Provider Organization plans and fewer comprehensive or Health Maintenance Organization plans over time.
TABLE 1.
Demographic characteristics of the individuals* in the cohort per year (2003-2009)
| Characteristics | 2003 (N = 8,135) | 2004 (N = 11,305) | 2005 (N = 14,658) | 2006 (N = 14,512) | 2007 (N = 15,684) | 2008 (N = 25,477) | 2009 (N = 26,161) |
|---|---|---|---|---|---|---|---|
| Age, mean (SD), y | 57.2 (6.8) | 56.8 (6.4) | 56.8 (6.4) | 56.7 (6.4) | 57.0 (6.3) | 56.8 (6.4) | 57.0 (6.3) |
| Gender | |||||||
| Male, No. (%) | 5,708 (70.2) | 7,957 (70.4) | 10,388 (70.9) | 10,405 (71.7) | 11,117 (70.9) | 18,376 (72.1) | 18,919 (72.3) |
| Region (No., %) | |||||||
| Northeast | 1,292 (15.9) | 1,222 (10.8) | 1,620 (11.1) | 1,783 (12.3) | 1,762 (11.2) | 4,123 (16.2) | 3,399 (13.0) |
| North Central | 1,881 (23.1) | 2,566 (22.7) | 3,484 (23.8) | 3,829 (26.4) | 4,097 (26.1) | 7,958 (31.2) | 8,307 (31.8) |
| South | 2,886 (35.5) | 4,636 (41.0) | 6,111 (41.7) | 6,347 (43.7) | 6,905 (44.0) | 9,688 (38.0) | 10,474 (40.0) |
| West | 2,041 (25.1) | 2,787 (24.7) | 3,325 (22.7) | 2,454 (16.9) | 2,847 (18.2) | 3,617 (14.2) | 3,934 (15.0) |
| Plan (No., %) | |||||||
| Comprehensive | 1,403 (17.4) | 1,528 (13.7) | 1,550 (10.8) | 1,335 (9.4) | 680 (4.4) | 1,320 (5.3) | 961 (3.8) |
| HMO | 1,965 (24.3) | 2,145 (19.2) | 2,974 (20.6) | 2,079 (14.7) | 2,189 (14.3) | 4,384 (17.6) | 3,673 (14.4) |
| POS | 720 (8.9) | 1,313 (11.8) | 1,589 (11.0) | 1,545 (10.9) | 1,700 (11.1) | 2,137 (8.6) | 1,918 (7.5) |
| PPO | 3,655 (45.3) | 5,731 (51.3) | 7,784 (54.0) | 8,648 (61.2) | 10,145 (66.2) | 16,179 (64.8) | 17,972 (70.4) |
Characteristics are provided at the individual level per year, so column totals add to less than 183,308
Abbreviations: SD, Standard Deviation; HMO, Health Maintenance Organization; POS, Point of Service; PPO, Preferred Provider Organization
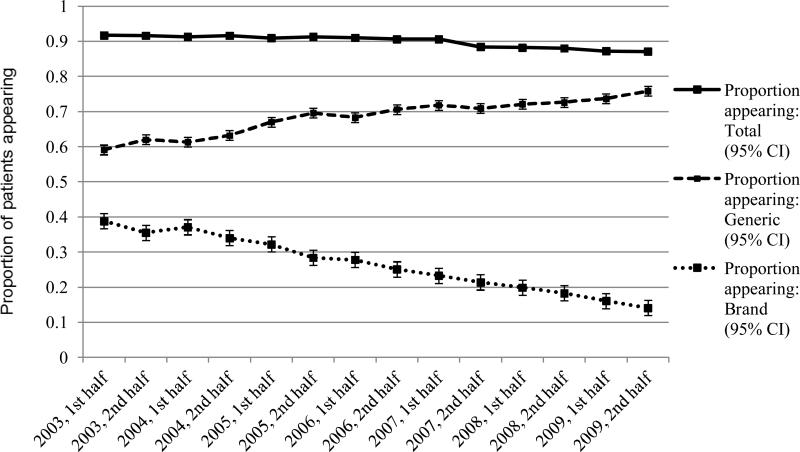
We present the proportion of patients identified as having warfarin exposure overall and across the 7 years in Table 2. Across all periods, the percentage of patients filling warfarin was 89.4% (95% CI: 89.2-89.5). Trends for exposure completeness for both generic and branded medication per 6-month calendar window are illustrated in Figure 1. Between 2003 and 2009, there was a significant decline in the percentage of patients with any warfarin exposure, from 91.7% (95% CI: 91.0, 92.4) in early 2003 to 87.1% (95% CI: 86.7-87.6) in late 2009 (χ2=93.8, p<0.0001). Over the same interval, the proportion of patients with generic warfarin exposure appearing increased significantly (χ2=649.3, p<0.0001), while the proportion of patients with branded warfarin exposure decreased significantly (χ2=1,833.5, p<0.0001). Adjusting for demographic characteristics and within-patient correlation, the multivariable models indicated that there was no significant trend between the 6-month cross-sections at baseline (β=−0.0004, p=0.49) for patient exposure to any type of warfarin. Adjusting for the baseline trend, a significant level change occurred in the post-period between 2006 and 2007 (β=−0.0058, p<0.0001) with an odds ratio of 0.994 (95% CI: 0.993-0.996). By comparison, there were significant trends between the 6-month cross-sections at baseline for brand (β=−0.0211, p<0.0001) and generic (β=0.0188, p<0.0001) warfarin, as illustrated in Figure 1. There were also significant level changes occurring for the proportion of patients with generic warfarin (β=−0.0092, p<0.0001) exposure in the post-period; no significant level change occurred for patients with brand warfarin (β=0.0020, p=0.14) exposure in the post-period.
TABLE 2.
Claims completeness for generic and branded warfarin for individuals in the yearly cohorts containing INR claims
| Year | No. of 6-month cross sections (N = 183,308) | Percent appearing: Generic (95% CI)* | Percent appearing: Brand (95% CI)* | Percent appearing: Total (95% CI) |
|---|---|---|---|---|
| 2003 | 12,472 | 60.6 (59.7-61.4) | 37.2 (36.3-38.0) | 91.6 (91.1-92.1) |
| 2004 | 17,666 | 62.3 (61.5-63.0) | 35.5 (34.8-36.2) | 91.5 (91.1-91.9) |
| 2005 | 23,173 | 68.4 (67.8-69.0) | 30.2 (29.6-30.8) | 91.1 (90.8-91.5) |
| 2006 | 23,056 | 69.5 (68.9-70.1) | 26.5 (25.9-27.0) | 90.8 (90.5-91.2) |
| 2007 | 24,552 | 71.3 (70.7-71.9) | 22.4 (21.8-22.9) | 89.5 (89.1-90.0) |
| 2008 | 40,579 | 72.4 (71.9-72.8) | 19.1 (18.7-19.5) | 88.1 (87.8-88.4) |
| 2009 | 41,810 | 74.7 (74.3-75.2) | 15.1 (14.8-15.4) | 87.2 (86.8-87.5) |
| Total | 183,308 | 70.1 (69.9-70.4) | 23.8 (23.4-24.2) | 89.4 (89.2-89.5) |
Columns may add up to more than the total
Abbreviations: INR: international normalized ratio; CI, Confidence interval
FIGURE 1.
Claims completeness for generic and branded warfarin in per total number of patients included in the 6-month cross-sectional cohorts
Abbreviation: CI, Confidence interval
Considering that to be included in the cohort, each individual had to have a minimum of 2 INR tests in the 6-month window, the mean (standard deviation [SD]) frequency of INR tests in each 6-month cross section varied from 6.17 (3.47) in early 2003 to 6.49 (3.59) in late 2009. These frequencies were also the minimum and maximum number of INR tests per individual recorded in any of the 14 6-month cross sections, suggesting little change in INR testing frequency over time. The association between demographic characteristics and proportion of patients with prescription exposure is displayed in Table 3.
TABLE 3.
Association between demographic characteristics and proportion of warfarin prescriptions* appearing in the individuals§ in the cohort per year (2003-2009)
| Characteristics | 2003 OR (95% CI) | 2004 OR (95% CI) | 2005 OR (95% CI) | 2006 OR (95% CI) | 2007 OR (95% CI) | 2008 OR (95% CI) | 2009 OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Age (ref=<60 years) | |||||||
| ≥60 years | 1.06 (0.91-1.24) | 1.14 (1.00-1.29) | 1.17 (1.04-1.31) | 1.06 (0.95-1.19) | 1.08 (0.98-1.19) | 1.02 (0.94-1.10) | 1.01 (0.94-1.09) |
| Gender (ref=male) | |||||||
| Female | 1.11 (0.95-1.31) | 0.93 (0.81-1.07) | 1.05 (0.93-1.18) | 1.07 (0.95-1.21) | 1.12 (1.01-1.25) | 1.08 (1.00-1.17) | 0.98 (0.90-1.06) |
| Region (ref=Northeast) | |||||||
| North Central | 1.00 (0.77-1.31) | 1.73 (1.38-2.16) | 1.38 (1.14-1.68) | 1.36 (1.13-1.63) | 1.09 (0.91-1.32) | 1.93 (1.74-2.14) | 1.05 (0.94-1.18) |
| South | 0.63 (0.50-0.80) | 1.30 (1.07-1.58) | 1.15 (0.97-1.37) | 1.14 (0.96-1.35) | 0.66 (0.56-0.78) | 1.82 (1.64-2.01) | 1.05 (0.94-1.17) |
| West | 0.95 (0.73-1.24) | 1.53 (1.23-1.89) | 1.19 (0.98-1.45) | 1.15 (0.94-1.40) | 0.93 (0.76-1.13) | 1.85 (1.62-2.10) | 1.13 (0.99-1.29) |
| Plan (ref=Comprehensive) | |||||||
| HMO | 1.21 (0.92-1.60) | 0.75 (0.58-0.97) | 0.71 (0.56-0.89) | 0.97 (0.74-1.26) | 1.17 (0.87-1.58) | 0.62 (0.51-0.75) | 1.00 (0.80-1.25) |
| POS | 0.96 (0.68-1.35) | 0.71 (0.84-0.95) | 0.73 (0.56-0.84) | 0.83 (0.63-1.08) | 1.05 (0.78-1.43) | 1.01 (0.80-1.27) | 0.88 (0.96-1.12) |
| PPO | 0.60 (0.48-0.75) | 0.58 (0.47-0.73) | 0.66 (0.53-0.81) | 0.67 (0.54-0.84) | 0.73 (0.56-0.95) | 0.79 (0.65-0.95) | 0.81 (0.66-0.99) |
Any warfarin prescription (generic or brand)
Characteristics are provided at the individual level per year, so column totals add to less than 183,308
Abbreviations: OR, Odds Ratio; CI, Confidence Interval; HMO, Health Maintenance Organization; POS, Point of Service; PPO, Preferred Provider Organization
In Table 4, we present results from the sensitivity analyses in which we reduced restrictions on the exclusion criteria (e.g., antiphospholipid syndrome, coagulation deficiencies). The proportion of patients with warfarin exposure appearing increased very slightly with the restrictions. In addition, excluding those with prescription use of any aspirin products also did not appreciably change the proportion of patients with warfarin exposure appearing. Furthermore, additional sensitivity analyses restricting the same analyses to 3-month calendar windows instead of 6-month windows did not change the proportion of patients with warfarin exposure appearing over time.
TABLE 4.
Restrictions on claims completeness for generic and branded warfarin for individuals in the 6-month calendar cohorts containing INR claims
| No. of 6-month cross sections remaining | Percent appearing: Generic (95% CI)* | Percent appearing: Brand (95% CI)* | Percent appearing: Total (95% CI) | |
|---|---|---|---|---|
| Restriction Criterion | ||||
| No Exclusion Criteria | 266,010 | 71.6 (71.4-71.8) | 22.9 (22.6-23.3) | 89.4 (89.2-89.5) |
| ≥ 1 prescription fill | 264,206 | 71.5 (71.3-71.7) | 22.9 (22.6-23.3) | 89.3 (89.2-89.4) |
| Hospitalization | 197,943 | 70.3 (70.1-70.6) | 23.7 (23.3-24.1) | 89.5 (89.3-89.6) |
| Hepatic-related diagnosis | 262,507 | 71.6 (71.4-71.8) | 23.0 (22.6-23.3) | 89.4 (89.2-89.5) |
| Vitamin K deficiency | 262,760 | 71.5 (71.3-71.7) | 23.0 (22.6-23.3) | 89.3 (89.2-89.4) |
| Anti-phospholipid syndrome | 263,821 | 71.5 (71.3-71.7) | 22.9 (22.6-23.3) | 89.3 (89.2-89.4) |
| Other coagulation deficiency | 255,628 | 71.4 (71.2-71.6) | 23.0 (22.6-23.3) | 89.2 (89.1-89.4) |
| Cardiac ablation procedure | 252,295 | 71.4 (71.2-71.6) | 23.0 (22.7-23.4) | 89.3 (89.1-89.4) |
| Outpatient claim for either heparin or LMWH | 245,117 | 70.8 (70.6-71.0) | 22.9 (22.6-23.3) | 88.8 (88.6-88.9) |
| All exclusion criteria | 183,308 | 70.1 (69.9-70.4) | 23.8 (23.4-24.2) | 89.4 (89.2-89.5) |
| All criteria + prescription aspirin use | 182,989 | 70.1 (69.9-70.4) | 23.8 (23.4-24.2) | 89.4 (89.2-89.5) |
Columns may add up to more than the total
Abbreviations: INR: international normalized ratio; CI, Confidence interval; LMWH: Low-molecular weight heparin
Among generic and branded warfarin prescriptions appearing, the mean copayment per 30-day supply of medication for generic medications decreased slightly over time from $4.93 in early 2003 to $3.86 in late 2009. By comparison, the mean branded medication copayment per 30-day supply remained fairly stable over time from $9.66 in early 2003 to $9.93 in late 2009. The mean (SD) day supply (e.g., 30 days) of medication dispensed per prescription also were similar between generic (38.9 [23.2] days) and branded medications (37.4 [22.0] days) in early 2003 and through late 2009 (42.5 [25.6] vs. 43.1 [25.7] days). The proportion of generic warfarin prescriptions filled for $0.00, $4.00 per 30 day supply, and $10.00 per 90 day supply are displayed in the Appendix 1. Concurrently, use of mail order pharmacy services for generic prescriptions also remained fairly stable over time, with 16.2% (95% CI: 14.6-17.9) of prescriptions delivered through mail order services in early 2003 compared with 15.9% (95% CI: 15.1-16.7) of prescriptions in late 2009.
DISCUSSION
To address emerging concerns regarding completeness of pharmacy data in insurance claims databases, our study sought to estimate the percentage of generic prescriptions that are captured in commercial claims databases in the United States. Using a commercial claims database, we estimated this quantity by examining generic warfarin prescribing among patients receiving regular INR testing to manage warfarin dosing through a design that enabled us to capture missing information because of a direct and specific monitoring test appearing in medical claims. Because there is little reason that regular INR tests would be ordered on atrial fibrillation patients without concomitant warfarin use outside of the exclusions applied,18-20 all patients in our cohort should have filled at least one prescription for warfarin during our study period.
We found that about one in ten patients who received regular INR testing did not have an associated warfarin claim. Our findings support the possibility that there exists a substantial amount of prescription medication use that is not captured in pharmacy claims data, which could be attributable to those filling outside the context of the pharmacy benefit for some medications but not for others, such as through spousal benefits or dual eligibility for multiple pharmacy benefit programs such as concomitant Veterans Affairs coverage. However, it is possible that some of these patients may not be filling prescriptions despite receiving specific testing to monitor medication dosing.3,4,22 Perhaps a strong desire to please their providers, observed in other contexts,23,24 could lead them to agree to regular INR testing, even though they are not taking the prescribed medication. This is probably not a common occurrence but may account for some of the claims that appear to be missing.
In addition, our study showed a small increase in the percentage of patients not filling warfarin over time, despite evidence of use as indicated by the presence of monitoring of INRs. It was interesting to note a significant decline in the percentage trend of patients with INR values matching to warfarin claims from 2007 to 2009 when generic discount programs became commonplace in community pharmacy settings, as indicated by the multivariable models. Missing data as a result of reduced prescription insurance billing has been noted as a potential concern but not formally tested in other papers.6 This could be a result of the introduction of the low-cost generic programs; however, other secular changes may be responsive for this trend such as increasing use of mail order pharmacies, which may in turn cause patients to circumnavigate their insurance benefit in the event of acute medication changes to fill at traditional pharmacies.25,26 These secular trends were not sensitive to study inclusion and exclusion criteria, indicating robustness of our results.
Our study was conservative by design. In the 6-month follow-up period, if a patient filled any medication that was captured, the patient would be classified as having non-missing data. It is possible that some of these patients may have had additional fills that were not captured or the timing of the INRs in the cross sections may have separated the fills and INRs over two different periods. However, as patients were not required to have remained adherent or have multiple fills over the 6-month period, if patients were to have one missing claim and one captured claim, they would be counted as having complete data. Therefore the percentage of missing claims could be somewhat higher.
No matter what the cause, a moderate rate of missing prescription claims could have important implications for research and quality improvement activities. In non-experimental studies of the safety and effectiveness of prescription medications, missing claims may cause exposure and covariates to be misclassified, leading to bias that may be difficult to predict or diagnose.1 In particular, non-user groups may consist of some patients who are treated and apparently new users may consist of patients who have previously treated. It is reasonable to conduct sensitivity analyses to see how varying degrees of misclassification of exposure and covariate may bias results. It also may be possible to minimize this problem through study design.
In studies of the appropriateness of prescribing, lack of complete pharmacy claims may cause some patients to appear to be under-treated. In studies of medication adherence, exposure misclassification could cause patients who are adherent to treatment, to appear to be non-adherent.6 These biases could potentially affect appropriate targeting of quality improvement activities designed to improve medication use.6 To reduce these problems, researchers conducting studies using administrative claims should consider restricting to those individuals using their pharmacy benefits and explore other approaches that can be used to identify patients who are less likely to have missing data. Researchers should also consider restricting or stratifying by generic and brand medication use to examine the robustness of their results, especially in drug utilization and medication adherence studies.
Our study is limited by generalizability. Our database contains information on a wide variety of commercially-insured patients in the United States; however, these results may not be generalizable to other types of insurance databases, such as Medicare or Medicaid.27 Medicaid claims in particular may not suffer from this problem since the Medicaid co-payment is required to be $3 or less in many states, thus incentivizing patients to provide insurance information to the pharmacy or purchase medications through their pharmacy benefit.26 On the other hand, we may not expect the same degree of completeness for other medications, as patients on warfarin may be even less likely to shop around, particularly if changing generics may lead to additional INR tests.20 For these other medications, we may perhaps even expect a smaller degree of completeness in prescription claims, as patients may be more likely to switch between pharmacies and potentially generic formulations.
CONCLUSION
The issue of completeness of pharmacy claims data is important for both research and quality improvement activities. We find evidence of missing prescriptions among patients being treated with warfarin for atrial fibrillation. Further investigation and monitoring is required to better understand the magnitude and nature of this potential problem across different databases and therapeutic areas.
Key Points.
Between 2003-2009, we estimated that 10.6% of commercially-insured patients with atrial fibrillation had missing drug information for generic or branded warfarin.
From early 2003 to early 2009, the percent of commercially-insured patients with atrial fibrillation with any warfarin exposure decreased from 91.7% (95% CI: 91.0, 92.4) to 87.1% (95% CI: 86.7-87.6).
Lack of complete pharmacy claims may impact medication adherence assessments and other observational studies using commercial pharmaceutical claims.
Additional research is warranted across different databases and therapeutic areas.
Acknowledgments
Disclosure of funding: This research was supported through unrestricted funding by Amgen Inc.
Conflict of interest: Dr. Brookhart has served as a scientific advisor for Amgen, Rockwell Medical, and Pfizer (with honoraria declined, donated, or paid to the institution); and has received consulting fees from DaVita Clinical Research, RxAnte, the Foundation for the National Institutes of Health, and World Health Information Consultants. Dr. Joel Farley has received consulting fees from Takeda, Daiichi Sankyo and Novartis. Dr. Mary Roth has been supported by the National Institutes of Health, National Institute on Aging Research Career Development Award. Drs. Balasubramanian, Critchlow and O'Malley are researchers at Amgen, Inc. Dr. Lauffenburger receives support from an Institutional National Service Award from the National Institute of Nursing Research (T32NR008856).
Appendix
APPENDIX 1.
Proportion of generic warfarin copayment amounts in the cross-sections per year (2003-2009)
| Copayment Amount, % | 2003 (N=51,701)* | 2004 (N=72,779)* | 2005 (N=98,825)* | 2006 (N=100,203)* | 2007 (N=111,371)* | 2008 (N=189,870)* | 2009 (N=195,234)* |
|---|---|---|---|---|---|---|---|
| $0.00 | 10.03 | 10.39 | 9.93 | 13.24 | 15.57 | 18.92 | 20.57 |
| $4.00 per 30 day supply | 1.03 | 1.22 | 0.22 | 0.89 | 3.96 | 8.20 | 8.80 |
| $10.00 per 90 day supply | 11.24 | 12.30 | 12.70 | 12.12 | 10.73 | 12.70 | 17.11 |
Number of total prescriptions for generic warfarin filled per year
Footnotes
This research has not been previously published but was presented at the 28th International Conference on Pharmacoepidemiology in Barcelona, Spain.
Contributor Information
Julie C. Lauffenburger, Division of Pharmaceutical Outcomes and Policy, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill.
Akhila Balasubramanian, Amgen Inc, Thousand Oaks, CA.
Joel F. Farley, Division of Pharmaceutical Outcomes and Policy, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill.
Cathy W. Critchlow, Amgen Inc, Thousand Oaks, CA.
Cynthia D. O'Malley, Amgen Inc, Thousand Oaks, CA.
Mary T. Roth, Division of Pharmaceutical Outcomes and Policy, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill.
Virginia Pate, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill.
M. Alan Brookhart, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill.
REFERENCES
- 1.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–37. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 3.Shrank WH, Choudhry NK, Fischer MA, et al. The epidemiology of prescriptions abandoned at the pharmacy. Ann Intern Med. 2010;153:633–40. doi: 10.7326/0003-4819-153-10-201011160-00005. [DOI] [PubMed] [Google Scholar]
- 4.Fischer MA, Choudhry NK, Brill G, et al. Trouble getting started: predictors of primary medication nonadherence. Am J Med. 2011;124:1081, e9–22. doi: 10.1016/j.amjmed.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Shah NR, Hirsch AG, Zacker C, et al. Factors associated with first-fill adherence rates for diabetic medications: a cohort study. J Gen Intern Med. 2009;24:233–7. doi: 10.1007/s11606-008-0870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhry NK, Shrank WH. Four-dollar generics - increased accessibility, impaired quality assurance. N Engl J Med. 2010;363:1885–7. doi: 10.1056/NEJMp1006189. [DOI] [PubMed] [Google Scholar]
- 7.Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008;46:394–402. doi: 10.1097/MLR.0b013e3181618ee0. [DOI] [PubMed] [Google Scholar]
- 8.Kesselheim AS, Choudhry NK. The international pharmaceutical market as a source of low-cost prescription drugs for U.S. patients. Ann Intern Med. 2008;148:614–9. doi: 10.7326/0003-4819-148-8-200804150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Czechowski JL, Tjia J, Triller DM. Deeply discounted medications: Implications of generic prescription drug wars. J Am Pharm Assoc. 2010;50:752–7. doi: 10.1331/JAPhA.2010.09114. [DOI] [PubMed] [Google Scholar]
- 10.Patel H, Dwibedi N, Omojasola A, et al. Impact of Generic Drug Discount Programs on Managed Care Organizations. Am J Pharm Benefits. 2011;3:45–53. [Google Scholar]
- 11.Dwibedi N, Sansgiry SS. Assessment of generic drug discount programs in large chaim pharmacies in the United States. Journal of Generic Medicines. 2009;6:363–368. [Google Scholar]
- 12.Gatwood J, Tungol A, Truong C, et al. Prevalence and predictors of utilization of community pharmacy generic drug discount programs. J Manag Care Pharm. 2011;17:449–55. doi: 10.18553/jmcp.2011.17.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhou L, Gellad WF. Potential Savings From Greater Use of $4 Generic Drugs. Arch Intern Med. 2011;171:468–9. doi: 10.1001/archinternmed.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polinski JM, Schneeweiss S, Levin R, et al. Completeness of retail pharmacy claims data: implications for pharmacoepidemiologic studies and pharmacy practice in elderly patients. Clin Ther. 2009;31:2048–59. doi: 10.1016/j.clinthera.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlahiotis A, Devine ST, Eichholz J, et al. Discontinuation rates and health care costs in adult patients starting generic versus brand SSRI or SNRI antidepressants in commercial health plans. J Manag Care Pharm. 2011;17:123–32. doi: 10.18553/jmcp.2011.17.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Yun H, Wright N, et al. Potential and Pitfalls of Using Large Administrative Claims Data to Study the Safety of Osteoporosis Therapies. Curr Rheumatol Rep. 2011;13:273–82. doi: 10.1007/s11926-011-0168-8. [DOI] [PubMed] [Google Scholar]
- 17.Desai NR, Shrank WH, Fischer MA, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med. 2012;125:302, e1–7. doi: 10.1016/j.amjmed.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095–106. doi: 10.1001/archinte.165.10.1095. [DOI] [PubMed] [Google Scholar]
- 19.Connolly SJ. Atrial fibrillation in 2010: advances in treatment and management. Nat Rev Cardiol. 2011;8:67–8. doi: 10.1038/nrcardio.2010.206. [DOI] [PubMed] [Google Scholar]
- 20.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133:160S–98S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 21.Dwibedi N, Patel H, Sansgiry SS. Equity and access to generic drugs: A comparison of generic drug discount programs offered by chain pharmacies in the United States. Value Health. 2009;12:A84–A. [Google Scholar]
- 22.Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14:553–60. doi: 10.18553/jmcp.2008.14.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garber MC, Nau DP, Erickson SR, et al. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care. 2004;42:649–52. doi: 10.1097/01.mlr.0000129496.05898.02. [DOI] [PubMed] [Google Scholar]
- 24.Wagner G, Miller LG. Is the influence of social desirability on patients' self-reported adherence overrated? J Acquir Immune Defic Syndr. 2004;35:203–4. doi: 10.1097/00126334-200402010-00016. [DOI] [PubMed] [Google Scholar]
- 25.IMS Health Channel distribution by U.S. dispensed prescriptions. 2010 Apr 6; Available at: www.imshealth.com/deployedfiles/ims/Global/Content/Corporate/Press%20Room/Top-Line%20Market%20Data%20&%20Trends/2011%20Top-line%20Market%20Data/Channels_by_RX.pdf. Accessed March 4, 2013.
- 26.Khandelwal N, Duncan I, Rubinstein E, et al. Medication adherence for 90-day quantities of medication dispensed through retail and mail order pharmacies. Am J Manag Care. 2011;17:e427–34. [PubMed] [Google Scholar]
- 27.Zhang Y, Gellad WF, Zhou L, Lin Y-J, Lave JR. Access to and Use of $4 Generic Programs in Medicare. J Gen Intern Med. 2012;27:1251–7. doi: 10.1007/s11606-012-1993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]