Abstract
Background
Villous atrophy (VA) with intraepithelial lymphocytosis is the histologic hallmark of coeliac disease (CD) but reported rates of mucosal recovery are variable.
Aim
To determine the impact of age and other demographic variables on the probability of persistent VA on follow-up biopsy.
Methods
We identified patients with VA on duodenal histology at all 28 Swedish pathology departments during the years spanning 1969–2008. We examined age, gender, calendar period, duration of disease, and educational attainment, to determine predictors of persistent VA.
Results
Of 7,648 patients with CD who underwent follow-up biopsy, persistent VA was present in 3,317 (43%; 95% CI 42%–44%). The effect of age on persistent VA varied according to time period; among those biopsied in the years spanning 2000–2008, the prevalence of persistent VA was 31%, and increasing age was associated with increasing rates of persistent VA (17% among those younger than 2 years compared to 56% among those ≥70 years). In contrast, persistent VA did not vary widely by age in earlier years. On multivariate analysis (restricted to the calendar period 2000–2008, 2–5 years after CD diagnosis), persistent VA was more common among males (OR 1.43; 95%CI 1.07–1.90) and less common among patients with higher educational attainment (OR for college degree versus <2 years of high school 0.52, 95%CI 0.35–0.78).
Conclusions
The prevalence of persistent VA has changed over time, with greater rates of healing in recent years. Social differences in persistent VA suggests that access and/or education regarding the gluten-free diet impacts mucosal healing.
Keywords: coeliac disease, epidemiology, histology
INTRODUCTION
Patients with coeliac disease (CD) exhibit villous atrophy (VA) and intraepithelial lymphocytosis on duodenal biopsy. This lesion develops as a consequence of gluten ingestion in genetically susceptible individuals, and adherence to the gluten-free diet results in clinical and histologic improvement.1 However, when follow-up duodenal biopsy is performed to document mucosal recovery, a substantial proportion of patients exhibit persistent VA.2–11 Reported rates of persistent VA are highly variable, and this heterogeneity is likely due to differences in study settings, follow-up time, availability of gluten-free food, and patient characteristics. Persistent VA may be associated with significant morbidity, including lymphoproliferative malignancies12 and possibly osteoporotic fracture.9 In addition to risk-stratifying patients, results of a follow-up biopsy can guide clinical management. In a recent study of patients with CD undergoing follow-up biopsy in the United Kingdom, those with persistent VA underwent targeted dietetic intervention, which led to a resolution of VA of 50% of such patients.13 While two studies have shown that rates of persistent VA in children are lower than in adults,3, 4 to our knowledge no study has examined whether the prevalence of persistent VA has changed over time.
In a population-based study of 7,648 Swedish patients with CD who underwent follow-up biopsy, the rate of persistent VA was 43%.14 We aimed to determine the independent contribution of age, gender, calendar year of follow-up biopsy, duration of disease, and educational attainment in determining the outcome of persistent VA among those patients.
METHODS
The characteristics of this data set have been described in detail elsewhere.14–16 In summary, patients with CD were identified via query of all 28 pathology departments in Sweden; the query was performed between October, 2006 and February, 2008, and consisted of patients diagnosed between July, 1969 and February, 2008. Patients were identified via Swedish SnoMed codes and CD was defined as the presence of VA, (Marsh 3). A previous validation study involving chart review found that 95% of patients with VA had a clinical diagnosis of CD, and that diagnoses apart from CD were uncommon.15
As was performed in a previous study evaluating mortality,14 we identified the subset of patients with CD who then underwent a follow-up biopsy. We excluded patients whose first follow-up biopsy was performed less than 6 months or beyond 5 years after their initial diagnosis of CD. Using the same Swedish SnoMed query, we then identified patients whose follow-up biopsy showed persistent VA. Patients with intraepithelial lymphocytosis but normal villi, or villous architecture that did not meet criteria for the Marsh 3 lesion were classified as healed.
We compared those patients with persistent VA to those with mucosal recovery with regard to patient age group in years. We also compared the rates of persistent VA to those with villous recovery according to the following categories: gender, duration of CD at the time of the follow-up biopsy (6 months to 1 year, between 1 and 2 years, and greater than 2 years) and the calendar period of the follow-up biopsy (≤1989, 1990–1999, and ≥2000). We also had access to data regarding educational attainment, which was obtained from the Swedish Education Register. We classified educational attainment in the following four categories: 9 years or less of primary and secondary school, 2 years of high school (typically preparation for manual or clerical work), 3 years of high school (theoretical programs), and college/university studies. In the case of children, the higher educational attainment of the subject’s parents was substituted.17
We used multivariate logistic regression to identify independent risk factors for persistent VA, adjusting for all of the covariates listed above, as well as the degree of VA on the initial diagnostic biopsy (partial versus subtotal/total VA). So as to be most applicable to present clinical circumstances, the multivariate analysis identifying independent predictors of persistent VA was restricted to those patients whose follow-up biopsy was performed in the most recent time period (2000–2008), and whose follow-up biopsy was performed between 2 and 5 years after their initial CD diagnosis. Effect modification was investigated via formal testing of interaction terms. P values lower than 0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.2 (Cary, NC). This project was approved by the Research Ethics Committee of the Karolinska Institute on June 14th, 2006.
RESULTS
Of the 7,648 patients with CD who underwent follow-up biopsy, 63% were female and the median age at follow-up biopsy was 27 years (Table 1). Nearly half (45%) of the patients were under the age of 20 years. Some 45% of the entire cohort had their follow-up biopsy between one and two years after initial diagnosis; the median duration of CD at the time of follow-up biopsy was 1.3 years. As was previously reported, persistent VA was present in 3,317 patients, 43% of those who underwent follow-up biopsy.14
Table 1.
Characteristics of patients with CD who underwent follow-up small intestinal biopsy 6 months to 5 years after initial diagnosis of CD (n=7,648).
| Characteristic | Number of patients (%) |
|---|---|
|
| |
| Age at follow-up biopsy (years) | |
| Mean/Median (SD) | 31/27 (24.8) |
| 0.5–2 | 883 (12) |
| 3–9 | 1,681 (22) |
| 10–19 | 843 (11) |
| 20–29 | 611 (8) |
| 30–39 | 722 (9) |
| 40–49 | 787 (10) |
| 50–59 | 894 (12) |
| 60–69 | 684 (9) |
| ≥70 | 543 (7) |
|
| |
| Male | 2,816 (37) |
| Female | 4,832 (63) |
|
| |
| Duration of CD at the time of repeat biopsy, years (mean/median) | 1.7/1.3 |
| 0.5–1 year | 2,024 (26) |
| Between 1 and 2 years | 3,429 (45) |
| 2–5 years | 2,195 (29) |
|
| |
| Calendar period of follow-up biopsy | |
| ≤1989 | 728 (10) |
| 1990–1999 | 2,891 (38) |
| ≥2000 | 4,029 (53) |
|
| |
| Education level | |
| < 2 years of HS | 2,017 (26) |
| 2 years of HS | 1,547 (20) |
| 3 years of HS | 1,614 (21) |
| College/University | 2,312 (30) |
| Unknown | 158 (2) |
|
| |
| Second biopsy result | |
| Mucosal recovery | 4,331 (57) |
| Persistent villous atrophy | 3,317 (43) |
CD: Coeliac disease. HS: High school. VA: Villous atrophy
Univariate predictors of persistent VA are listed in Table 2. Men had a slightly higher prevalence of persistent VA than women (45% versus 42%, p = 0.03). Calendar period of follow-up biopsy was associated with persistent VA; among those biopsied prior to the year 1990, 69% exhibited persistent VA as compared to 31% among those biopsied after the year 2000. We observed an interaction between age and calendar period at follow-up biopsy with regard to the prevalence of persistent VA (p for interaction term <0.0001). As shown in Table 3 and Figure 1, whereas the prevalence of persistent VA varied somewhat between age groups prior to 1990 without a clear trend (ranging from 58% among patients ≥70 years to 79% among patients 3–9 years), in the years spanning 2000–2008 the prevalence of persistent VA varied widely by age, with an increased prevalence of persistent VA in the oldest age group (ranging from 14% among children 3–9 years to 56% for adults ≥70 years).
Table 2.
Characteristics of patients with CD with control biopsy demonstrating recovery or persistent histologic changes.
| Characteristic | Improvement (n=4,331) | Persistent VA (n=3,317) | p value |
|---|---|---|---|
|
| |||
| Sex | 0.0255 | ||
| Male | 1,548 (55) | 1,268 (45) | |
| Female | 2,783 (58) | 2,049 (42) | |
|
| |||
| Duration of disease (years) | <0.0001 | ||
| 0.5–1 | 1,006 (50) | 1,018 (50) | |
| 1.01–2 | 2,101 (62) | 1,328 (39) | |
| 2.01–5 | 1,224 (56) | 971 (44) | |
|
| |||
| Duration of CD (mean/median years) | 1.73/1.36 | 1.69/1.30 | <0.0001 |
|
| |||
| Calendar period of follow-up biopsy | <0.0001 | ||
| ≤1989 | 225 (31) | 503 (69) | |
| 1990–1999 | 1,307 (45) | 1,584 (55) | |
| ≥2000 | 2,799 (69) | 1,230 (31) | |
|
| |||
| Education level | <0.0001 | ||
| < 2 years of HS | 975 (48) | 1042 (52) | |
| 2 years of HS | 858 (55) | 689 (45) | |
| 3 years of HS | 919 (57) | 695 (43) | |
| College/University | 1501 (65) | 811 (35) | |
| Unknown | 78 (49) | 80 (51) | |
|
| |||
| Histology on index biopsy* | <0.0001 | ||
| Partial villous atrophy | 643 (70) | 282 (30) | |
| Subtotal/total villous atrophy | 1,202 (58) | 853 (42) | |
| Non-specified villous atrophy | 2,486 (53) | 2,182 (47) | |
CD: Coeliac disease. HS: High school
The association between histology and index biopsy and the rate of persistent VA in this cohort was previously reported.14
Table 3.
Effect of age on the prevalence of persistent villous atrophy, stratified by year of follow-up biopsy. p value for interaction term of year and age <0.0001
| Pre-1990 | 1990–1999 | 2000–2008 | ||||
|---|---|---|---|---|---|---|
| Age at follow-up biopsy | Number of patients | Persistent VA (%) | Number of patients | Persistent VA (%) | Number of patients | Persistent VA (%) |
| 0.5–2 | 207 | 142 (69) | 509 | 267 (52) | 167 | 28 (17) |
| 3–9 | 135 | 106 (79) | 843 | 482 (57) | 703 | 101 (14) |
| 10–19 | 28 | 19 (68) | 171 | 80 (47) | 644 | 128 (20) |
| 20–29 | 39 | 27 (69) | 183 | 82 (45) | 389 | 80 (21) |
| 30–39 | 71 | 44 (62) | 197 | 90 (46) | 454 | 145 (32) |
| 40–49 | 88 | 60 (68) | 293 | 167 (57) | 406 | 158 (39) |
| 50–59 | 69 | 46 (67) | 326 | 201 (62) | 499 | 191 (38) |
| 60–69 | 48 | 34 (71) | 183 | 100 (55) | 453 | 222 (49) |
| ≥70 | 43 | 25 (58) | 186 | 115 (62) | 314 | 177 (56) |
Figure 1.
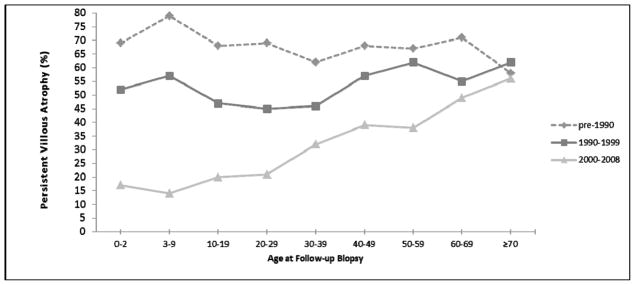
Effect of age on the prevalence of persistent villous atrophy, stratified by year of follow-up biopsy.
Educational attainment was associated with persistent VA; the highest prevalence of persistent VA were among those with less than two years of high school and the lowest prevalence was seen among those with a college or university degree (see Table 2 and Figure 2).
Figure 2.
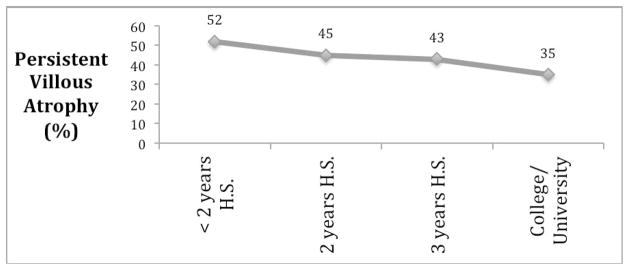
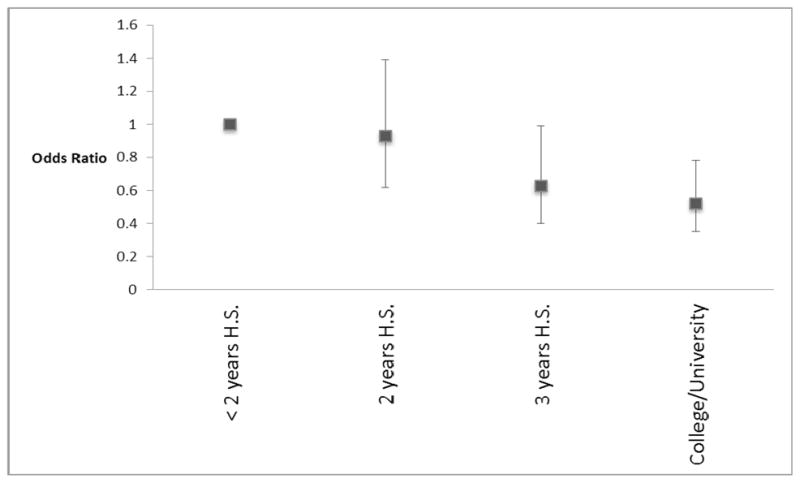
Figure 2A: Prevalence of persistent villous atrophy, stratified by educational attainment. H.S.=High School
Figure 2B: Association between educational attainment and persistent villous atrophy (adjusted for age, gender, duration of disease, and calendar period). Odds ratios <1 refer to lower odds of persistent villous atrophy. Corresponding 95% confidence intervals are shown by error bars.
The results of the multivariate model of predictors of persistent VA are shown in Table 4. In this model, restricted to those whose follow-up biopsy was performed during the years 2000–2008, 2–5 years after initial CD diagnosis, increased age was significantly associated with persistent VA (OR for ≥70 compared to 0.5–9 years 3.64; 95%CI 2.01–6.59). Male gender was associated with increased risk of persistent VA (OR 1.43; 95%CI 1.07–1.90). Educational attainment remained protective against persistent VA in the multivariate analysis (OR for college/university degree versus <2 years of high school 0.52; 95%CI 0.35–0.78).
Table 4.
Multivariate analysis of factors associated with persistent villous atrophy on follow-up biopsy.*
| Characteristic | OR for persistent villous atrophy (95% CI) | p value |
|---|---|---|
|
| ||
| Age at follow-up biopsy (years) | 0.0019 | |
| 0.5–9 | 1.0 (ref) | |
| 10–19 | 1.31 (0.81–2.10) | |
| 20–29 | 1.03 (0.56–1.89) | |
| 30–39 | 1.52 (0.89–2.58) | |
| 40–49 | 1.70 (0.98–2.94) | |
| 50–59 | 1.43 (0.85–2.40) | |
| 60–69 | 2.00 (1.19–3.38) | |
| ≥70 | 3.64 (2.01–6.59) | |
|
| ||
| Sex | 0.0155 | |
| Male | 1.43 (1.07–1.90) | |
| Female | 1.0 (ref) | |
|
| ||
| Education level | 0.0073 | |
| < 2 years of HS | 1.0 (ref) | |
| 2 years of HS | 0.93 (0.62–1.39) | |
| 3 years of HS | 0.63 (0.40–0.99) | |
| College/University | 0.52 (0.35–0.78) | |
|
| ||
| Histology on index biopsy | 0.4733 | |
| Partial villous atrophy | 1.0 (ref) | |
| Subtotal/total villous atrophy | 1.24 (0.80–1.90) | |
Restricted to patients whose follow-up biopsy was performed during the years 2000–2008, 2–5 years after initial CD diagnosis
DISCUSSION
In this population-based analysis of patients with CD undergoing follow-up biopsy, we found that the prevalence of persistent VA varied across age groups, gender, time period, and socio-demographic lines. The effect of age on the prevalence of persistent VA depended on the era of diagnosis and follow-up biopsy; younger patients were far more likely to heal than were older patients, but this phenomenon was limited to the most recent time period (2000–2008). In contrast, among patients who had a follow-up biopsy prior to 1990 or from 1990–1999, the prevalence of persistent VA was significantly greater and varied little by age.
Persistent VA on follow-up biopsy is common, though estimates of the prevalence of this phenomenon have varied considerably in the literature. Rates of persistent VA have ranged from 4%8 to 79%,2 though most studies reported rates between 24% and 47%.5–7, 10, 11, 13 This marked variability can be understood in part due to differences in demographics and length of follow-up. For example, in two studies, the prevalence of persistent VA declined with longer follow-up,8, 18 indicating that persistent VA may represent slow or gradual healing in some patients. A recent study of 391 patients undergoing follow-up biopsy found that persistent VA was present in 47% of patients; a subset of such patients underwent targeted dietetic intervention and half of these patients subsequently exhibited histologic recovery.13 To our knowledge, all previous studies examining rates of persistent VA have included fewer than 500 patients. Our study included 7,648 patients with follow-up histology, allowing for stratified analyses by more fine age categories and other characteristics.
Our findings are congruent with those of smaller studies that reported higher rates of persistent VA among adults than children.3, 4 However, the effect of age on the prevalence of persistent VA was not constant over this multi-decade population-based database. We found that the increased prevalence of persistent VA among older patients was limited to those whose follow-up biopsy was performed after 2000 (Figure 1), with higher overall rates of persistent VA prior to 2000 for all age groups. The reason for this effect modification is unknown, but there are several plausible explanations. The diagnosis of CD may be occurring more promptly in recent years due to the availability of serologic tests, and prompt diagnosis and institution of the gluten-free diet may result in greater healing rates than in those patients with a prolonged course of symptoms prior to diagnosis. In recent years, adults have reported a long mean duration of symptoms prior to CD diagnosis (11 years)19 and the improved recognition and prompt diagnosis rates may be disproportionately benefiting children. Indeed, in the United States older individuals are less likely than younger patients to be biopsied and tested for CD when undergoing endoscopy for symptoms suggestive of CD.20 As the elderly may have had more longstanding and untreated CD, their inflammation and atrophy may be more established and persistent. Another potential explanation is the greatly improved access to gluten-free foods in recent years, which may account for increased healing rates. If this improved availability is responsible for the era-varying healing rates observed in our study, it is less clear why older patients are not benefitting to the same extent as younger individuals. Patients with a more mild disease phenotype are likely being diagnosed in recent years, due to the widespread availability of serological testing and increased awareness of the protean manifestations of CD. This may account for the lower rates of persistent VA in recent years, with perhaps lower dietary adherence rates among adults.21
Given the changing role of follow-up biopsy over time, it remains possible that the observed interaction of era and age may reflect factors other than changes in adherence to the gluten-free diet. For example, a provocative gluten challenge after initial histologic recovery was widely considered a component of the diagnosis of CD up until the issuance of new guidelines in 1990.22 As a result of this change in guidelines and a likely shift in practice patterns, the indications of a third biopsy done in patients with CD are likely to be quite varied, i.e. including biopsies performed due to prior lack of recovery, in response to a gluten challenge, or prompted by a clinical worsening. It is for this reason that this study was restricted to the second (i.e. the first follow-up) biopsy and did not include subsequent biopsies. While these practice changes are unlikely to have a significant impact on changing indications for the first follow-up biopsy in various eras, explanations for the changes in rates of mucosal recovery over time remain speculative.
We found that the prevalence of persistent VA declines over time after diagnosis of CD, with the highest rates (50%) seen in those whose follow-up biopsy was performed within one year of the initial CD diagnosis. However, this prevalence did not continue to decline over time (see Table 2). Therefore, the phenomenon of gradual healing may explain persistent VA early on in the disease course, but is unlikely to be exerting a substantial effect beyond two years after diagnosis, where ongoing gluten ingestion may play a role.
Several of our findings can be attributed to adherence to the gluten-free diet. Those with the lowest educational attainment had the highest rates of persistent VA (52%), and this rate declined continuously with increasing education. In prior studies, the degree of self-rated social integration at school was strongly associated with dietary adherence,23 and higher scores on a gluten-free food quiz were likewise associated with greater adherence (though degree of educational attainment was not).24 While the relationship between education and mucosal recovery is likely due to effect of health literacy on adherence to the gluten-free diet, this may also be explained in part by the high cost of this diet,25 which may impact adherence. We found that men have a greater risk of persistent VA than women (OR1.43; 95%CI 1.07–1.90). While data on gender and dietary adherence in CD are limited, it appears that among adults, men are slightly less likely than women to exhibit good or excellent adherence to the gluten-free diet.24
This study has a number of strengths. It is the largest study to date measuring predictors of persistent VA, which allowed us to perform stratified analyses in multiple subgroups. The population-based method of identifying these CD patients enhances the generalizability of our findings. The validation of histology codes used in this study mitigates against bias due to misclassification.15 We also acknowledge several limitations to this study. Most prominently, adherence to the gluten-free diet was not measured in this analysis. In a chart review of 22 CD patients in this database with a follow-up biopsy and information on dietary adherence, persistent VA was more common in those with poor adherence (4/4, 100%) than those with good adherence (7/18, 39%, p=0.09).15 Lending further support to the notion that persistent VA correlates with adherence are the results of an analysis of CD serologies according to follow-up histology previously performed on this dataset.14 In a subset of these patients serological data were available; we previously reported that 545 patients were seropositive at initial diagnosis and had a follow-up serology on record within 6 months of their follow-up biopsy. Of these 545 patients, 224 (41%) had persistently elevated serologies at the time of their follow-up biopsy. The prevalence of persistent villous atrophy was greater among those with persistently elevated serologies (139/224, 62%) than among those whose serologies normalized (67/321, 21%, p<0.0001).14 Nevertheless, while prior studies have also found correlation between degree of dietary adherence and persistent VA,5, 6, 8, 10, 11 we do not know to what extent our findings of different rates of persistent VA among subgroups are driven by differential adherence, as opposed to biological mechanisms.
As this analysis was limited to the results of the first follow-up biopsy, we cannot distinguish between those patients who would exhibit long-term VA and those who would eventually heal. However, our finding of similar rates of persistent VA among those biopsied 1–2 years and 2–5 years after CD diagnosis suggests that gradual healing beyond two years is not a common phenomenon. While this study was population-based, its generalizability is limited in part because it consisted of those patients who underwent a follow-up biopsy in our pre-specified time frame of six months to five years after diagnosis. We have previously reported that those with a follow-up biopsy had a slightly decreased mortality rate compared to those who did not undergo a follow-up biopsy, but that this difference disappeared after excluding deaths within the first six months after CD diagnosis.14
This is the only study to our knowledge that reports rates of mucosal recovery over a multi-decade period. The role of the follow-up biopsy has changed over time, particularly for children. Prior to 1990, a follow-up biopsy to confirm healing was considered the standard of care, as was a third biopsy after a gluten challenge; with the 1990 publication of guidelines issued by the European Society of Pediatric Gastroenterology and Nutrition, 22 a third (gluten-challenge) biopsy was no longer deemed necessary, and a follow-up biopsy to confirm healing was advised only for scenarios when the response to the gluten-free diet was uncertain. Therefore it remains a possibility that the variable rates of healing over these different eras may be due in part to differing indications for the follow-up biopsy. However, this is unlikely to be driving the observed differences. First, the major variability between children and adults does not appear until the years 2000–2008, a decade after the most significant shift in pediatric guidelines. Second, it is unlikely that a biopsy during a gluten challenge is affecting rates of persistent VA prior to 1990, since the confirmatory gluten challenge was the patient’s third biopsy 22 and we restricted this analysis to patients’ first follow-up biopsy for this very reason. Nevertheless, the lack of clinical information contextualizing each biopsy (including the status of gluten exposure) is a limitation of this study.
In conclusion, we found that 43% of patients with CD exhibited persistent VA on follow-up biopsy. Rates of persistent VA varied markedly by age, but this variability was restricted to patients who underwent follow-up biopsy after the year 2000. Persistent VA was stable beyond two years after diagnosis of CD, arguing against “gradual healing” as a prominent cause of persistent VA beyond this time frame. Patients with lower educational attainment had the highest prevalence of persistent VA, indicating that adherence and/or cost are likely important causes of this phenomenon. Given the association between persistent VA and morbidity in CD9, 12 future studies should evaluate strategies to promote mucosal recovery in high-risk patients with CD.
Acknowledgments
Grant Support (Funding):
BL: The American Scandinavian Foundation, the Celiac Sprue Association, and the National Center for Advancing Translational Sciences, National Institutes of Health (UL1 TR000040)
JAM: The National Institutes of Health (DK057892).
AR-T: American College of Gastroenterology Junior Faculty Development Award
JFL: Örebro University Hospital, Karolinska Institutet, the Swedish Society of Medicine, the Swedish Research Council – Medicine (522-2A09-195) and the Swedish Celiac Society.
Abbreviations used in this article
- CD
Coeliac disease
- VA
Villous atrophy
- CI
Confidence Interval
- HR
Hazard Ratio
Footnotes
Details of ethics approval: This project (2006/633-31/4) was approved by the Research Ethics Committee of the Karolinska Institute, Sweden on June 14th, 2006.
Guarantor of the Article: Dr. Ludvigsson
Authors Contributions:
Study concept and design: BL, JAM, ART, PHRG, JFL
Acquisition of data: BL, JFL
Analysis and interpretation of data: BL, JAM, ART, PHRG, JFL
Drafting of the manuscript: BL, JFL
Critical revision of the manuscript for important intellectual content: BL, JAM, ART, PHRG, JFL
Statistical analysis: BL, JFL
Study supervision: JFL
All authors approve the final manuscript submitted and they approve the authorship list.
Competing Interests:
All authors declare that they have no conflicts of interest and nothing to declare.
References
- 1.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am J Gastroenterol. 2013;108(5):656–76. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SK, Lo W, Memeo L, Rotterdam H, Green PH. Duodenal histology in patients with celiac disease after treatment with a gluten-free diet. Gastrointest Endosc. 2003;57(2):187–91. doi: 10.1067/mge.2003.54. [DOI] [PubMed] [Google Scholar]
- 3.Wahab PJ, Meijer JW, Mulder CJ. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am J Clin Pathol. 2002;118(3):459–63. doi: 10.1309/EVXT-851X-WHLC-RLX9. [DOI] [PubMed] [Google Scholar]
- 4.Bardella MT, Velio P, Cesana BM, et al. Coeliac disease: a histological follow-up study. Histopathology. 2007;50(4):465–71. doi: 10.1111/j.1365-2559.2007.02621.x. [DOI] [PubMed] [Google Scholar]
- 5.Lanzini A, Lanzarotto F, Villanacci V, et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment Pharmacol Ther. 2009;29(12):1299–308. doi: 10.1111/j.1365-2036.2009.03992.x. [DOI] [PubMed] [Google Scholar]
- 6.Ciacci C, Cirillo M, Cavallaro R, Mazzacca G. Long-term follow-up of celiac adults on gluten-free diet: prevalence and correlates of intestinal damage. Digestion. 2002;66(3):178–85. doi: 10.1159/000066757. [DOI] [PubMed] [Google Scholar]
- 7.Selby WS, Painter D, Collins A, Faulkner-Hogg KB, Loblay RH. Persistent mucosal abnormalities in coeliac disease are not related to the ingestion of trace amounts of gluten. Scand J Gastroenterol. 1999;34(9):909–14. doi: 10.1080/003655299750025390. [DOI] [PubMed] [Google Scholar]
- 8.Collin P, Maki M, Kaukinen K. Complete small intestine mucosal recovery is obtainable in the treatment of celiac disease. Gastrointest Endosc. 2004;59(1):158–9. doi: 10.1016/s0016-5107(03)01311-7. [DOI] [PubMed] [Google Scholar]
- 9.Kaukinen K, Peraaho M, Lindfors K, et al. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment Pharmacol Ther. 2007;25(10):1237–45. doi: 10.1111/j.1365-2036.2007.03311.x. [DOI] [PubMed] [Google Scholar]
- 10.Rubio-Tapia A, Rahim MW, See JA, Lahr BD, Wu TT, Murray JA. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105(6):1412–20. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson JM, West NP, Robins GG, Howdle PD. Long-term histological follow-up of people with coeliac disease in a UK teaching hospital. QJM. 2010;103(7):511–7. doi: 10.1093/qjmed/hcq076. [DOI] [PubMed] [Google Scholar]
- 12.Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med. 2013;159(3):169–75. doi: 10.7326/0003-4819-159-3-201308060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharkey LM, Corbett G, Currie E, Lee J, Sweeney N, Woodward JM. Optimising delivery of care in coeliac disease - comparison of the benefits of repeat biopsy and serological follow-up. Aliment Pharmacol Ther. 2013;38(10):1278–91. doi: 10.1111/apt.12510. [DOI] [PubMed] [Google Scholar]
- 14.Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and mortality in coeliac disease. Aliment Pharmacol Ther. 2013;37(3):332–9. doi: 10.1111/apt.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009;9:19. doi: 10.1186/1471-230X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302(11):1171–8. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 17.Olen O, Bihagen E, Rasmussen F, Ludvigsson JF. Socioeconomic position and education in patients with coeliac disease. Dig Liver Dis. 2012;44(6):471–6. doi: 10.1016/j.dld.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137(1):88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green PHR, Stavropoulos SN, Panagi SG, et al. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96(1):126–31. doi: 10.1111/j.1572-0241.2001.03462.x. [DOI] [PubMed] [Google Scholar]
- 20.Lebwohl B, Tennyson CA, Holub JL, Lieberman DA, Neugut AI, Green PH. Sex and racial disparities in duodenal biopsy to evaluate for celiac disease. Gastrointest Endosc. 2012;76(4):779–85. doi: 10.1016/j.gie.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludvigsson JF, Rubio-Tapia A, van Dyke CT, et al. Increasing incidence of celiac disease in a north american population. Am J Gastroenterol. 2013;108(5):818–24. doi: 10.1038/ajg.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker-Smith JA, SG, JS, DS, JV Revised criteria for diagnosis of coeliac disease. Report of a Working Group of ESPGAN. Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Errichiello S, Esposito O, Di Mase R, et al. Celiac disease: predictors of compliance with a gluten-free diet in adolescents and young adults. J Pediatr Gastroenterol Nutr. 2010;50(1):54–60. doi: 10.1097/MPG.0b013e31819de82a. [DOI] [PubMed] [Google Scholar]
- 24.Leffler DA, Edwards-George J, Dennis M, et al. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Dig Dis Sci. 2008;53(6):1573–81. doi: 10.1007/s10620-007-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AR, Ng DL, Zivin J, Green PH. Economic burden of a gluten-free diet. Journal of human nutrition and dietetics: the official journal of the British Dietetic Association. 2007;20(5):423–30. doi: 10.1111/j.1365-277X.2007.00763.x. [DOI] [PubMed] [Google Scholar]


