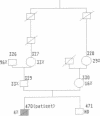
Abstract
We have determined the primary structure of a mutant insulin receptor of a leprechaun patient born from a consanguineous marriage. A characteristic feature of leprechaunism is an extreme resistance to insulin. In this patient the insulin resistance seems to result from an observed lack of insulin binding to intact cells. Solubilization of cells in non-ionic detergents leads to the appearance of insulin receptors which can bind insulin. However, the insulin-stimulated autophosphorylation of the receptor's beta subunit is markedly reduced. Cloning and sequencing of cDNA derived from insulin receptor mRNA of this patient revealed a leucine-to-proline mutation at position 233 in the alpha subunit. By means of DNA amplification we found that the patient is homozygous for this mutation and that the parents and two grandparents from the consanguineous line are heterozygous. The heterozygous individuals all show decreased insulin binding to cultured fibroblasts. In addition, they are mildly insulin resistant in vivo. These observations show a linkage between the leucine-to-proline mutation and the observed insulin resistance in this family. We therefore conclude that the mutation in the homozygous form is responsible for the extreme insulin resistance in the leprechaun patient. The mutation for the first time characterizes a region in the insulin receptor which seems to be involved in transmitting the insulin binding signal to the tyrosine kinase domain.
Full text
PDF

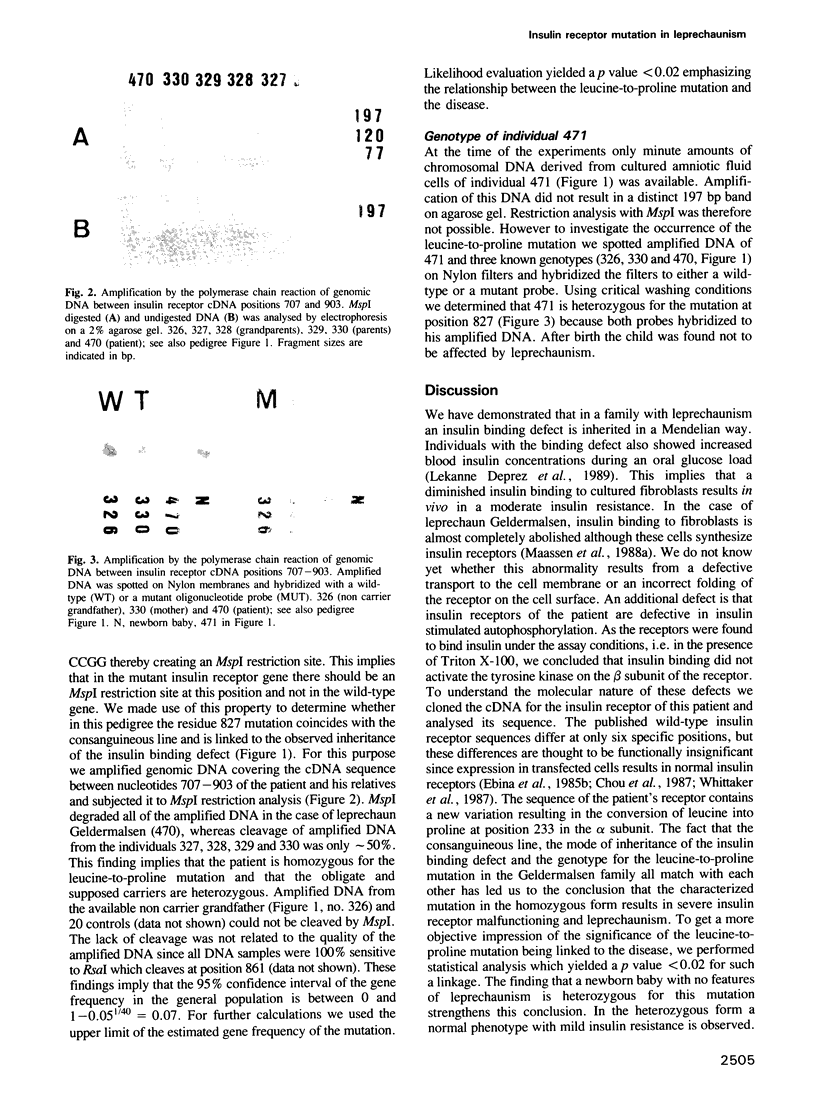


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. S. Cell biology. Reception and transmission. Nature. 1989 Jan 5;337(6202):12–12. doi: 10.1038/337012a0. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Edery M., Ellis L., Standring D., Beaudoin J., Roth R. A., Rutter W. J. Expression of a functional human insulin receptor from a cloned cDNA in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8014–8018. doi: 10.1073/pnas.82.23.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ellis L., Morgan D. O., Clauser E., Roth R. A., Rutter W. J. A membrane-anchored cytoplasmic domain of the human insulin receptor mediates a constitutively elevated insulin-independent uptake of 2-deoxyglucose. Mol Endocrinol. 1987 Jan;1(1):15–24. doi: 10.1210/mend-1-1-15. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Bevins C. L., Cama A., Ojamaa K., Marcus-Samuels B., Kadowaki H., Beitz L., McKeon C., Taylor S. I. Two mutant alleles of the insulin receptor gene in a patient with extreme insulin resistance. Science. 1988 May 6;240(4853):787–790. doi: 10.1126/science.2834824. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen J. A., Klinkhamer M. P., Odink R. J., Sips H., van der Zon G. C., Wieringa T., Krans H. M., Möller W. Improper expression of insulin receptors on fibroblasts from a leprechaun patient. Eur J Biochem. 1988 Mar 15;172(3):725–729. doi: 10.1111/j.1432-1033.1988.tb13949.x. [DOI] [PubMed] [Google Scholar]
- Maassen J. A., Klinkhamer M. P., van der Zon G. C., Sips H., Möller W., Krans H. M., Lindhout D., Beemer F. A. Fibroblasts from a leprechaun patient have defects in insulin binding and insulin receptor autophosphorylation. Diabetologia. 1988 Aug;31(8):612–617. doi: 10.1007/BF00264769. [DOI] [PubMed] [Google Scholar]
- Maassen J. A., Krans H. M., Möller W. The effect of insulin, serum and dexamethasone on mRNA levels for the insulin receptor in the human lymphoblastoic cell line IM-9. Biochim Biophys Acta. 1987 Aug 19;930(1):72–78. doi: 10.1016/0167-4889(87)90157-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. M., Haworth J. C., Degroot G. W., Trevenen C. L., Rechler M. M. A case of leprechaunism with severe hyperinsulinemia. Am J Dis Child. 1980 Feb;134(2):170–175. doi: 10.1001/archpedi.1980.02130140044014. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S., Seino M., Nishi S., Bell G. I. Structure of the human insulin receptor gene and characterization of its promoter. Proc Natl Acad Sci U S A. 1989 Jan;86(1):114–118. doi: 10.1073/pnas.86.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson S. E., White M. F., Kahn C. R. Tryptic activation of the insulin receptor. Proteolytic truncation of the alpha-subunit releases the beta-subunit from inhibitory control. J Biol Chem. 1988 Apr 5;263(10):4852–4860. [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Verlaan-de Vries M., Bogaard M. E., van den Elst H., van Boom J. H., van der Eb A. J., Bos J. L. A dot-blot screening procedure for mutated ras oncogenes using synthetic oligodeoxynucleotides. Gene. 1986;50(1-3):313–320. doi: 10.1016/0378-1119(86)90335-5. [DOI] [PubMed] [Google Scholar]
- Whittaker J., Okamoto A. K., Thys R., Bell G. I., Steiner D. F., Hofmann C. A. High-level expression of human insulin receptor cDNA in mouse NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5237–5241. doi: 10.1073/pnas.84.15.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip C. C., Hsu H., Patel R. G., Hawley D. M., Maddux B. A., Goldfine I. D. Localization of the insulin-binding site to the cysteine-rich region of the insulin receptor alpha-subunit. Biochem Biophys Res Commun. 1988 Nov 30;157(1):321–329. doi: 10.1016/s0006-291x(88)80050-0. [DOI] [PubMed] [Google Scholar]
- Yoshimasa Y., Seino S., Whittaker J., Kakehi T., Kosaki A., Kuzuya H., Imura H., Bell G. I., Steiner D. F. Insulin-resistant diabetes due to a point mutation that prevents insulin proreceptor processing. Science. 1988 May 6;240(4853):784–787. doi: 10.1126/science.3283938. [DOI] [PubMed] [Google Scholar]